Abstract
Factor XII (FXII) and high molecular weight kininogen (HK) mutually block each other's binding to the urokinase plasminogen activator receptor (uPAR). We investigated if FXII stimulates cells by interacting with uPAR. FXII (3-62nM) with 0.05mM Zn2+ induces extracellular signal-related kinase 1/2 (ERK1/2; mitogen-activated protein kinase 44 [MAPK44] andMAPK42) and Akt (Ser473) phosphorylation in endothelial cells. FXII-induced phosphorylation of ERK1/2 or Akt is a zymogen activity, not an enzymatic event. ERK1/2 or Akt phosphorylation is blocked upstream by PD98059 or Wortmannin or LY294002, respectively. An uPAR signaling region for FXII is on domain 2 adjacent to uPAR's integrin binding site. Cleaved HK or peptides from HK's domain 5 blocks FXII-induced ERK1/2 and Akt phosphorylation. A β1 integrin peptide that binds uPAR, antibody 6S6 to β1 integrin, or the epidermal growth factor receptor (EGFR) inhibitor AG1478 blocks FXII-induced phosphorylation of ERK1/2 and Akt. FXII induces endothelial cell proliferation and 5-bromo-2′deoxy-uridine incorporation. FXII stimulates aortic sprouting in normal but not uPAR-deficient mouse aorta. FXII produces angiogenesis in matrigel plugs in normal but not uPAR-deficient mice. FXII knockout mice have reduced constitutive and wound-induced blood vessel number. In sum, FXII initiates signaling mediated by uPAR, β1 integrin, and the EGFR to induce human umbilical vein endothelial cell proliferation, growth, and angiogenesis.
Introduction
Factor XII (FXII) is known to initiate blood coagulation reactions by autoactivating on artificial surfaces.1 In vivo, several physiologic entities (collagen in injured vessels, polysomes from activated platelets, mRNA, or aggregated/misfolded proteins) promote FXII autoactivation leading to larger thrombus formation without influencing hemostasis.2–6 FXII also binds to a multiprotein receptor complex on endothelium in the intravascular compartment that consists of gC1qR, urokinase plasminogen activator receptor (uPAR), and cytokeratin 1.7 Single-chain urokinase (ScuPA) competes high molecular weight kininogen binding (HK) to domain 2 of the uPAR with a concentration that inhibits 50% (IC50) of 6μM.8 Furthermore, HK blocks biotin-FXII binding to cultured endothelial cells with an IC50 of 180nM versus FXII itself with an IC50 of 900nM7,9 Vitronectin also binds uPAR domain 2 to block FXII binding to human umbilical vein endothelial cells (HUVECs).7,9 These data suggest that FXII interacts with uPAR to mediate some activity.
FXII has been recognized to induce mitogen-activated protein kinase in HepG2 and vascular smooth muscle cells.11,12 uPAR mediates activation of extracellular-regulated kinases 1/2 (ERK1/2), stimulation of cancer cell proliferation, and the apoptotic effect of 2 chain HK (HKa).13,14 ScuPA induces phosphorylation of p44/42 mitogen-activated protein kinase (pERK1/2) in cultured HUVECs.15 Because ScuPA and FXII both contain an epidermal growth factor (EGF) domain, we examined if FXII induces ERK1/2 phosphorylation in HUVECs and if this pathway is blocked by HKa and related compounds.7,11,12,14 Our investigations indicate that FXII stimulates ERK1/2 and Akt phosphorylation through uPAR, specific integrins, and the EGF receptor (EGFR) leading to endothelial cell proliferation, growth, and angiogenesis. HKa or its domain 5 fragments block these events. These investigations characterize a zymogen FXII-initiated signaling pathway that leads to in vitro and in vivo angiogenesis. This activity for zymogen FXII is constitutive and independent of its activation to FXIIa and suggests a previously not recognized role for FXII in postnatal angiogenesis and injury repair.
Methods
Materials
Single-chain HK with a specific activity of 13 U/mg and HKa were purchased from Enzyme Research Laboratories Inc. Human FXII with specific activity of 46 U/mg, human factor XI (FXI) with a specific activity of 390 U/mg, and human FXIIa were purchased from Haematologic Technologies Inc. Human FXIIa also was prepared from FXII as described in the supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article). Biotin-FXII was prepared as previously reported.7 Soluble urokinase plasminogen activator receptor (SuPAR) were prepared, purified, and characterized as previously reported.8 Endothelial cell growth medium (CS-C Medium) was purchased from Cell Systems. HUVECs, trypsin–ethylenediaminetetraacetic acid, and trypsin-neutralizing solutions were purchased from Lonza. Electrochemiluminescence Western blotting detection reagents were purchased from GE Healthcare. Wortmannin, PD98059 (2′-amino-3′-methoxyflavone), LY294002 [2-(4-morholinyl)-8-phenyl-4H-1-benzopyran-4-one, AG1478 [4-(3-chloroanilino)-6,7-dimethoxyquinazoline], U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene], PP2 [[4-amino-5(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine]], PP3 [4-amino-7-phenylpyrazol[3,4-d]pyrimidine, APMSF (p-A-phenylmethylsulfonyl fluoride), or PFRCK (Pro-Phe-Arg-chloromethylketone) were purchased from Calbiochem. Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) were purchased from Antigenix America.
Peptides and antibodies
Peptides to domain 5 of HK were synthesized at the Carbohydrate Structure Facility of the University of Michigan and Multiple Peptide Systems (Table 1).16 Peptides comprising regions within domain 2 of uPAR and domain 1 of FXII were synthesized at Multiple Peptide Systems (Table 1).7,8 Each of these peptides are named for the first 3 amino acids using single letter nomenclature followed by the number of residues in the peptide.8,16 A scrambled peptide from uPAR P176 to T195 with the sequence H-NSFGCNHDFKGPTHNCLTNF-OH, a peptide on domain 2 of uPAR that binds integrins [H-130IQEGEEGRPKDDR142-OH (IQE13)], and a peptide from the β-propeller subunit of the β1 integrin that binds uPAR [H-224NLDSPEGGF232-OH (NLD9)] was prepared at NeoMPS.17,18
Table 1.
Peptides used to influence ERK1/2 and Akt phosphorylation
| Protein source | Domain | Sequence |
|---|---|---|
| uPAR* | ||
| ECI20 | 2 | H-116ECISCGSSDMSCERGRHQSL135-OH |
| QCR20 | 2 | H-136QCRSPEEQCLDVVTHWIQEG155-OH |
| EEG20 | 2 | H-156EEGRPKDDRHLRGCGYLPGC175-OH |
| LRG20 | 2 | H-166LRGCGYLPGCPGSNGFHNND185-OH |
| YLP20 | 2 | H-171YLPGCPGSNGFHNNDTFHFL190-OH |
| PGS20 | 2 | H-176PGSNGFHNNDTFHFLKCCNT195-OH |
| FHN20 | 2 | H-181FHNNDTFHFLKCCNTTKCNE200-OH |
| TKC19 | 2 | H-196TKCNEGPILELENLPQNGR214-OH |
| QCY21 | 3 | H-215QCYSCKGNSTHGCSSEETFLI235-OH |
| DCR20 | 3 | H-236DCRGPMNQCLATGTHEPKN255-OH |
| HK† | ||
| GKE19 | 5 | 402GKEQGHTRRHDWGHEKQRK420 |
| FKL20 | 5 | 459FKLDDDLEHQGGHVLDHGHK478 |
| GGH18 | 5 | 469GGHVLDHGHKHKHGHGHG486 |
| HVL19 | 5 | 471HVLDHGHKHKHGHGHGKHKNKGKK494 |
| HKH20 | 5 | 479HKHGHGHGKHKNKGKKNGKH498 |
| FXII‡ | ||
| YHK9 | 1 | 39YHKCTHKGR47 |
uPAR indicates urokinase plasminogen activator receptor; HK, high molecular weight kininogen; and FXII, factor XII.
These peptides are described in Mahdi et al.8 The numbering of the amino acids shown represents the location of the amino acid in the mature uPAR protein. Note: the single letter code is used for amino acids.
These peptides are described in Hasan et al.16 The numbering of the amino acids shown represents the location of the amino acid in the mature HK protein.
This peptide is described in Mahdi et al.7 The numbering of the amino acids shown represents the location of the amino acid in the mature XII protein.
A monoclonal antibody (Mab) against uPAR (3B10FC) was generously provided by Dr Robert F Todd III, University of Michigan.7,8 Antibodies to ERK1/2 and phospho-p44/42 ERK1/2 (Thr202/Tyr204) were purchased from Cell Signaling. Antibodies to Akt and phospho-Akt (Ser473) were also obtained from Cell Signaling. Mabs to integrin β1 clones P4C10 and 6S6, integrin α5β1 clone HA5, and integrin α3 clone ASC-1 were purchased from Chemicon International. Monoclonal antibody to integrin α5 clone P1D6 was purchased from Upstate. Monoclonal antibody ΑIIB2 to integrin β1 was purchased from the Developmental Studies Hybridoma Bank.
Endothelial cell culture
HUVECs were cultured on gelatin in extracellular granular material according to the procedures of Lonza. In preparation for signaling experiments, cells between the first and fifth passage were subcultured onto gelatin-coated, 6-well plates 24 hour before the start of the experiment in CS-C complete medium from Cell Systems.8 In certain experiments, HUVECs were cultured in the same medium on type I collagen (BD Biosciences), fibronectin, fibrinogen (Enzyme Research Laboratories), or vitronectin (Molecular Innovations) at 1 to 2 μg/mL. Cell viability was determined with the use of trypan blue exclusion. Cell numbers were determined by counting on a hemocytometer. Mouse embryonic fibroblasts (MEFs) from SIN1−/− mice were provided by Dr Bing Su of Yale University and were cultured in Dulbecco modified Eagle medium with 15% fetal bovine serum.19
ERK1/2 and Akt phosphorylation studies
HUVECs in 6-well microtiter plates were washed in serum-free media (CS-C medium kit) without growth factors and starved for 2 or 16 hours (overnight) without difference in results followed by treatment for 2 more hours in the absence or presence of inhibitors. Unless stated otherwise, PD98059 was added at 100μM, Wortmannin at 30nM, LY294002 at 50μM, and peptides at 300μM. At the end of the incubation, the cells were usually incubated with 62nM FXII in the absence or presence of 50μM Zn2+, or vehicle for 5 to 7 minutes, and the reaction was stopped by washing with cold phosphate-buffered saline. In all cases, the cells were scraped into 100 μL of 2× Laemmli sample buffer from Bio-Rad, which was reduced with 5% β-mercaptoethanol. Equal amounts of cell lysates were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then electroblotting onto nitrocellulose membrane. The membranes were blocked with nonfat dried milk and were incubated overnight at 4°C with Mabs to ERK and phospho-p44/42 ERK1/2 (Thr202/Tyr204) or Akt or phospho-Akt (Ser473). The primary antibodies were detected with a horseradish peroxidase–conjugated anti–rabbit immunoglobulin G (1:2000) for 1 hour. The secondary antibody was detected by the electrochemiluminescence system (GE Healthcare). The blots were scanned with the use of Scion image software, and the intensity of inhibited samples was compared with uninhibited FXII-stimulated controls on the same immunoblot.
The preparation of FXIIa from FXII, FXIIa inactivation by APMSF or PFRCK, and FXII activation studies of a chromogenic substrate or FXI on HUVECs are described in the supplemental Methods. Endothelial cell proliferation and 5-bromo-2′-deoxy-uridine (BrdU) assays were performed with the procedures from Celltiter 96R Aqueous One Solution Cell Proliferation Assay (Promega) and Roche Applied Science, respectively (see supplemental Methods for details).
Aortic sprouting angiogenesis experiments
Thoracic aortas were isolated from 8- to 12-week-old C57BL/6 and uPAR knockout (KO) mice and transferred to a compartmentalized Felsen dish containing phenol-red free endothelial basal medium (Lonza) as reported and sectioned into 1-mm segments.20 All animal experiments were approved by Institutional Animal Care and Use Committee of Case Western Reserve University. uPAR KO mice were generously provided by Dr Tom Bugge of the National Institutes of Health.21 Each aortic segment was embedded into a 15-μL drop of rat-tail collagen I (1.81 mg/mL; BD Biosciences) in 12-well dishes (3 rings per well). Once the collagen was gelled, 1 mL of CS-C complete medium containing test reagents was added to each well. Aortic ring sprouts were photographed on days 4 to 6 with a Nikon SMZ-U microscope with a ED Plan Apo 1× FL lens. Sprouting was quantified by counting the number of sprout roots derived from the aortic section.
Matrigel angiogenesis assay
Growth factor-reduced matrigel was mixed with either basic FGF (800 ng/mL), murine VEGF (300 ng/mL), and heparin (50 μg/mL) or with FXII (240nM) and heparin (50 μg/mL) on ice as previously reported.22 The negative controls were matrigel and heparin (50 μg/mL) alone. Matrigel (0.5 mL) was placed subcutaneously in the lower abdomen flank on anesthetized and shaved wild-type C57BL/6 and uPAR KO mice. Ten days after injection, the matrigel plugs were recovered and weighed. Some plugs were photographed intact with a Nikon SMZ-U microscope with a ED Plan Apo 1× FL lens. Other plugs were frozen for sectioning for immunostains with antibody to von Willebrand factor or isotype-specific immunoglobulin G control followed by immunoperoxidase, and the number of vessels/10×-20× magnification fields was determined on a Nikon TE2000S microscope at 200× final magnification. Other frozen sections were stained with anti-CD31 (Santa Cruz Biotechnologies) at 1:500 and counterstained with DAPI (4,6 diamidino-2-phenylindole). Slides were visualized and photographed with a Nikon TE2000S fluorescent microscope at 20× magnification. The remaining plugs were mixed with 1 mL of phosphate-buffered saline pH 7.4 containing ethylenediaminetetraacetic acid 1mM and 25 μg/mL heparin and then homogenized completely for hemoglobin determination with Drabkin reagent.22
Wound angiogenesis assay
FXII KO generously by Dr Frank Castellino of the University of Notre Dame and wild-type mice had 5-mm punch biopsies performed on their backs between the forelimbs.23 The skin biopsies initially and after 9 days of wound healing were cut in half, embedded in OCT, and snap frozen in liquid nitrogen for sectioning. Each section for analysis was in the middle of the wound. The sectioned specimens were stained with anti-CD31 (Santa Cruz Biotechnologies) at 1:500 and counterstained with DAPI, and the number of vessels was counted. Slides were visualized and photographed with a Nikon TE2000S fluorescent microscope at 20× magnification.
Statistical analysis
Differences between inhibited samples and controls were determined by Student t test for nongrouped data. Significance is defined as a P value less than .05.
Results
FXII stimulates ERK1/2 and Akt phosphorylation on HUVECs
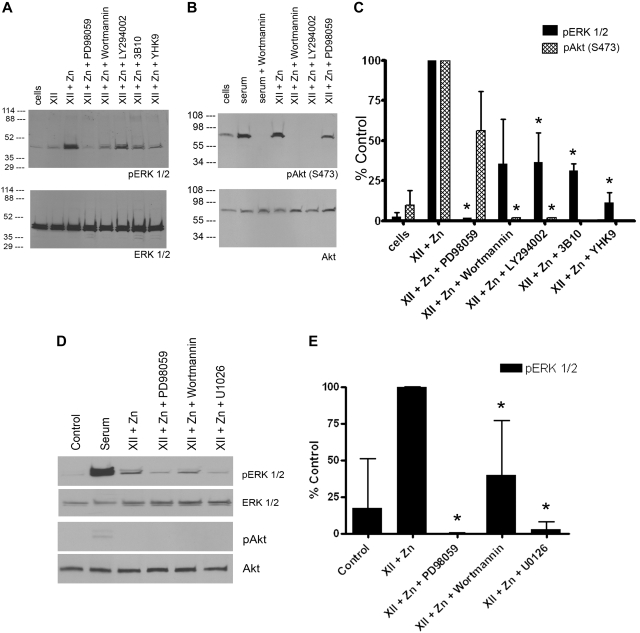
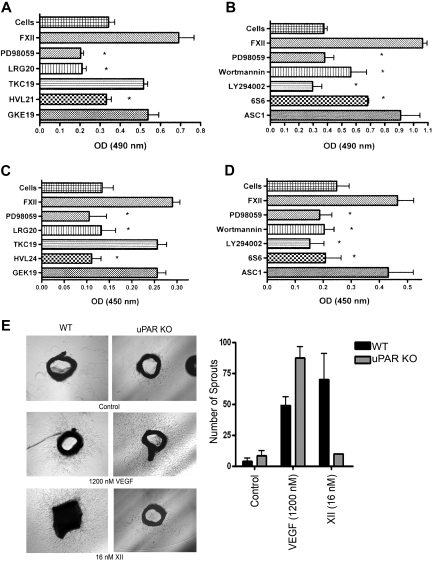
FXII (3-60nM) in the presence of 50μM Zn2+, but not FXII alone, induced ERK1/2 phosphorylation in serum-starved HUVECs (supplemental Figure 1). Subsequent experiments used 60 to 62nM FXII in the presence of 50μM Zn2+ (Figure 1A,C). Sixty-two nanomolar FXII is 12% of normal plasma FXII concentration. FXII-induced ERK1/2 phosphorylation was blocked by the mitogen-activated protein/ERK kinase 1 (MEK1) inhibitor PD98059 and reduced 70% by phosphoinositol-3 (PI3) kinase inhibitor LY294002 (P < .03). Wortmann also reduced ERK1/2 phosphorylation similarly, but this was not significant, probably because of the higher variation in the experiments. Monoclonal antibody 3B10 to the FXII binding region on domain 2 of uPAR or a peptide (YHK9) to amino acids Y39 to R47 in FXII's fibronectin type II domain that blocks FXII binding to HUVECs also reduced ERK1/2 phosphorylation 70% (P < .001) and 85% (P < .001), respectively (Table 1; Figure 1A,C).7,8
Figure 1.
FXII stimulates ERK1/2 or Akt phosphorylation on HUVECs cultured on gelatin. (A) Immunoblots that used an antibody to phospho-ERK1/2 (pERK1/2) and antibody to total ERK1/2 (ERK1/2). (B) Immunoblots that used an antibody to phospho-Akt [pAkt (S473)] and total Akt (Akt). (A) ERK1/2 phosphorylation: lane 1, the control (cells) is untreated serum-starved cells. In lanes 2 and 3, 62nM FXII-treated cells in the absence or presence of 50μM Zn2+ are shown. The effect of PD98059, Wortmannin, LY294002, 0.6μM Mab 3B10, or 300μM peptide YHK9 on 62nM FXII plus 50μM Zn2+-treated cells is shown in lanes 4-8, respectively. (B) Akt phosphorylation: lane 1 is serum-starved cells (cells). In lanes 2 and 3, 10% serum-treated cells (serum) alone with Wortmannin. In lanes 4-7, 62nM FXII plus 50μM Zn2+-treated cells in the absence or presence of Wortmannin, LY294002, or PD98059, respectively, is shown. (A-B) The figures are representative immunoblots of 3 or more experiments. (C) Bar graphs of the means ± SD of scans of 3 or more immunoblots shown in panels A and B for both pERK1/2 and pAkt. (C) An asterisk over a bar graph indicates P ≤ .05 compared with FXII-stimulated HUVECs alone. (D) A single immunoblot is shown with SIN1−/− MEFs treated with FXII plus 50μM Zn2+ in the absence or presence of PD98059, Wortmannin, or 50μM U1026 immunoblotted for pERK1/2, total ERK, pAkt, or total Akt. (E) Bar graphs of the means ± SD of scans of 4 immunoblots shown in panel D for pERK1/2. (E) An asterisk over a bar graph indicates P ≤ .05 compared with FXII-stimulated HUVECs alone.
FXII stimulated phosphorylation of Akt S473 in HUVECs that was blocked by Wortmannin or LY294002 (Figure 1B-C). Preliminary experiments showed that LY294002 at 3 to 50μM inhibited FXII- and 50μM Zn2+-induced PI3 kinase leading to Akt phosphorylation. We used 50μM LY294002 in all investigations. FXII-induced Akt phosphorylation was partially blocked by PD98059 but did not reach statistical significance (P > .15; Figure 1B-C). FXII-induced ERK1/2 and Akt phosphorylation was blocked by PD98059 and LY294002, respectively, when HUVECs were cultured on fibronectin, fibrinogen, or vitronectin or by PD98059 and Wortmannin when HUVECs were cultured on collagen or gelatin (supplemental Figure 2).
Investigations next determined whether the ERK1/2 phosphorylation pathway was distinct from the Akt pathway. Stimulated MEFs made from SIN1−/− embryos cannot phosphorylate Akt S473 because SIN1 is essential for mammalian target of rapamycin (mTOR), mLST8, and rictor (mTORC2) assembly.19 FXII-induced ERK1/2 phosphorylation in SIN1−/− MEFs was blocked by PD98059 (P < .001), Wortmannin (P < .007), or U0126 (P < .001; Figure 1D-E). These data indicated that the ERK1/2 phosphorylation pathway is independent of phosphorylation of Akt S473.
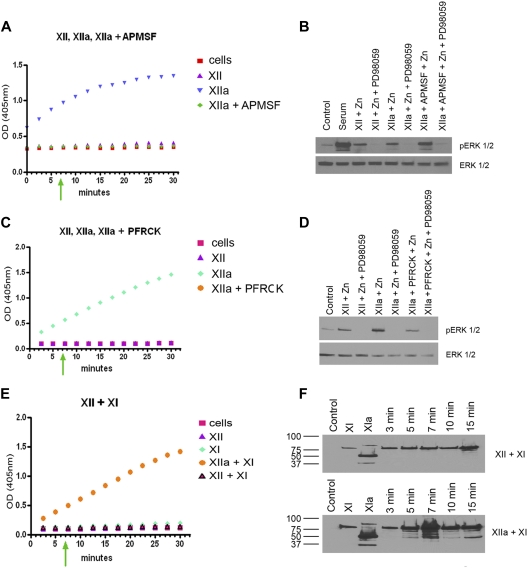
Role of FXII versus FXIIa in signaling events
We examined if FXII-induced ERK1/2 phosphorylation was induced by zymogen FXII or protease FXIIa. Sixty nanomolar FXII incubated with HUVEC monolayers did not develop amidolytic activity over 30 minutes (Figure 2A,C). Sixty nanomolar FXII, FXIIa, APMSF-treated FXIIa, or PFRCK-treated FXIIa, the latter 2 having no amidolytic activity, stimulated ERK1/2 phosphorylation after 7 minutes of incubation with HUVECs (Figure 2A-D). Because FXI is a natural substrate of FXIIa with a lower Km of activation than a chromogenic substrate, we examined if FXII autoactivated on HUVECs to activate FXI. Thirty nanomolar FXI and 30nM FXI plus 60nM FXII incubated on HUVEC monolayers also did not develop amidolytic activity over 10 minutes of incubation (Figure 2E). An immunoblot of FXI plus FXII incubated on monolayers of HUVECs indicated that FXI remained a zymogen after longer than 10 minutes of incubation (Figure 2F top). At 15 minutes, there was slight activation of FXI (Figure 2F top). Alternatively, when FXI was incubated with 60nM FXIIa, FXI changed to FXIa by 5 minutes as seen on reduced sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Figure 2F bottom). These combined studies indicated that zymogen FXII does not autoactivate during its HUVEC incubation time (5-7 minutes) for induction of ERK1/2 and Akt phosphorylation, and noncatalytic forms of FXII alone initiate ERK1/2 phosphorylation.
Figure 2.
The role of FXII vs FXIIa in ERK1/2 phosphorylation. (A) The hydrolytic activity of 60nM FXII, FXIIa, or APMSF-treated FXIIa (FXIIa + APMSF) on 0.8mM H-D-Pro-Phe-Arg-pNA HCl is shown. (B) Serum-starved HUVECs alone (Control), serum-treated HUVECs (Serum), 60nM FXII or FXIIa in the presence of 50μM Zn2+ in the absence or presence of 50μM PD98059, or 60nM APMSF-treated FXIIa in the presence of 50μM Zn2+ in the absence or presence of 50μM PD98059 is shown on ERK1/2 phosphorylation. (C) The hydrolytic activity of 60nM FXII, FXIIa, or PFRCK-treated FXIIa (FXIIa + PFRCK) on 0.8mM H-D-Pro-Phe-Arg-pNA HCl is shown. (D) Serum-starved HUVECs alone (Control), serum-treated HUVECs (Serum), 60nM FXII or FXIIa in the presence of Zn2+ in the absence or presence of PD98059, or 60nM PFRCK-treated FXIIa in the presence of Zn2+ in the absence or presence of PD98059 is shown on ERK1/2 phosphorylation. (E) The hydrolytic activity of 60nM FXII, 30nM FXI, 60nM FXIIa + 30nM FXI, or 60nM FXII + 30nM FXI on 0.8mM pyroGly-Pro-Arg-pNA.HCl is shown. (F top) Lane 1 indicates Control; lane 2, 30nM FXI incubated over HUVECs for 7 minutes; lane 3, 30nM FXIa incubated over HUVECs for 7 minutes; lanes 4-8, 60nM FXII + 30nM FXI incubated over HUVEC monolayers for 3-15 minutes. (F bottom) Lane 1 indicates Control; lane 2, 30nM FXI incubated over HUVECs for 7 minutes; lane 3, 30nM FXIa incubated over HUVECs for 7 minutes; lanes 4-8, 60nM FXIIa + 30nM FXI incubated over HUVEC monolayers for 3-15 minutes. Both figures are immunoblots of reduced sodium dodecyl sulfate–polyacrylamide gel electrophoresis using antibody to FXI. In all panels on the left, the green arrow indicates the time of incubation of FXII or FXI in their various forms with HUVECs for the immunoblot in the adjacent panels on the right.
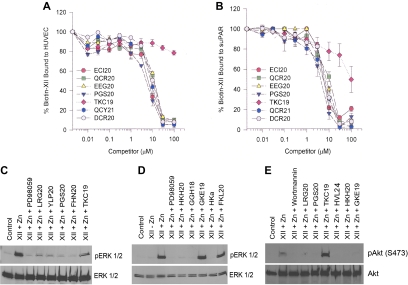
Role of uPAR in FXII-induced ERK1/2 and Akt phosphorylation
Because FXII binds to domain 2 of uPAR and Mab 3B10 to uPAR domain 2 blocks ERK1/2 phosphorylation, we determined if FXII-induced signaling events are mediated by uPAR domain 2.7,8 Investigations mapped FXII binding to domain 2 of uPAR.7,8 Sequential peptides ECI20, QCR20, EEG20, and PGD20, but not TKC19, from domain 2 of uPAR blocked FXII binding in a concentration-dependent manner to HUVECs or SuPAR (Figure 3A-B; Table 1). Peptides QCY21 and DCR20 from domain 3 of uPAR also blocked FXII binding to HUVECs or SuPAR.8 uPAR domain 2 peptides blocked FXII phosphorylation of ERK1/2 (Figure 3C). Sequential peptides LRG20, YLP20, PGS20, and FHN20 from uPAR domain 2 blocked FXII-induced phosphorylation of ERK1/2. Peptide TKC19 was less inhibitory (Figure 3C; Table 1).
Figure 3.
Mapping FXII binding and signalling on uPAR. (A-B) Biotin-FXII binding to HUVECs or SuPAR. Biotin-FXII (20nM) in the presence of 50μM Zn2+ was incubated with cultured HUVEC monolayers (A) in microtiter plates in HEPES (N-2-hydroxyethylpiperazine-n′-2-ethanesulfonic acid) buffer as previously reported7 in the absence or presence of increasing concentrations of overlapping uPAR domain 2 peptides ECI20, QCR20, EEG20, PGS20, or TKC19 (see Table 1) or uPAR domain 3 peptides OCY21 or DCR20.8 Similar experiments were performed with microtiter plates coated with 1 μg/mL SuPAR in 0.1M Na2CO3, pH 9.6 buffer (B). (A-B) Means ± SDs of 3 or more competition inhibition binding experiments. (C-E) Mapping the regions on uPAR or high molecular weight kininogen that compete with FXII binding to HUVECs to prevent FXII stimulation of ERK1/2 or Akt phosphorylation. (C-D) The regions on uPAR or HK that influence FXII-induced ERK1/2 phosphorylation are examined. (E) The regions on both uPAR and HK that influence FXII-induced Akt phosphorylation are examined. In all panels “Control” represents serum-starved cells alone. (C-D) FXII (62nM) and 50μM Zn2+ were incubated with serum-starved HUVECs in the absence or presence of 50μM PD98059 or 300μM peptide LRG20, YLP20, PGS20, FHN20, or TKC19 from uPAR (C) or 300μM peptide HKH20, GGH18, GKE19, or FKL20 or HKa (D). (E) FXII (62nM) and 50μM Zn2+ were incubated with serum-starved HUVECs in the absence or presence of 30nM Wortmannin or 300μM peptide LRG20, PGS20, TKC19, HVL24, HKH20, or GKE19 (Table 1). This figure is 1 experiment under these conditions and with these peptides.
We also examined if peptides of HK's domain 5 or plasma kallikrein-cleaved HK (HKa) that bind uPAR domain 2 blocked FXII-induced phosphorylation of ERK1/2 (Figure 3D).16 Peptides HKH20 and GGH18 from HK's domain 5 and HKa itself blocked FXII-induced phosphorylation of ERK1/2 (Figure 3D). In contrast, 2 other peptides, GKE19 and FKL20, from a more N-terminal region of HK's domain 5 that do not block HK binding to cells, did not interfere with FXII plus Zn2+ phosphorylation of ERK1/2 (Figure 3D; Table 1).16 FXII increased Akt phosphorylation also was blocked by peptides LRG20 or PGS20 to uPAR's domain 2 and peptides to domain 5 of HK (peptides HVL24, HKH20, GKE19; Figure 3E). These combined studies indicate that uPAR domain 2 is a FXII binding and signaling region on this receptor.
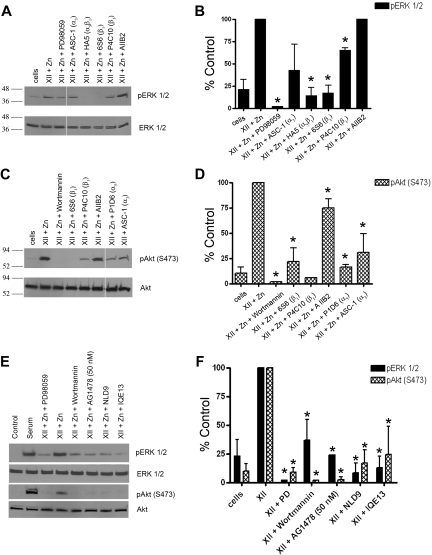
Demonstration of a FXII signaling pathway between uPAR and integrins and the EGFR
Investigations next determined how uPAR communicates FXII signals. Mabs to β1 integrins (HA5, 6S6, and P4C10, respectively) inhibited (P < .001) FXII-induced phosphorylation of ERK1/2 (Figure 4A-B). ASC-1 blocked FXII-induced phosphorylation of ERK1/2, but it did not reach significance (P = .12; Figure 4A-B). Mabs 6S6 and P4C10 (P ≤ .02) to β1 integrin blocked Akt phosphorylation (Figure 4C-D). Similarly, antibodies to α5 (P1D6) or ASC-1 also significantly reduced (P < .007) FXII-induced Akt phosphorylation (Figure 4C-D). Antibody AIIB2 did not reduce ERK1/2 phosphorylation but produced a significant 27% reduction (P = .02) in Akt phosphorylation. A peptide from uPAR domain 2 that binds integrins (IQE13) or a peptide from the β-propeller subunit of the β1 integrin (NLD9) additionally blocked (P < .001) FXII-induced phosphorylation of ERK1/2 or Akt (Figure 4E-F).17,18
Figure 4.
Influence of integrins and EGFR on FXII signalling. (A-D) Influence of integrin antibodies on FXII-induced ERK1/2 or Akt phosphorylation. (A) An antibody to phospho or total ERK1/2 was used; (C) an antibody to phospho (S473) and total Akt was used. In all panels the lane labeled “cells” is serum-starved HUVECs. (A) In lanes 2 and 3, HUVECs were incubated with 62nM FXII plus 50μM Zn2+ alone or in the presence of 100μM PD98059. In lanes 4-8, cells were incubated with 62nM FXII plus 50μM Zn2+ in the presence of 188nM ASC-1, HA5, 6S6, or P4C10 or 63nM AIIB2, respectively. The integrin the antibody is directed toward is indicated on the figure in parentheses; Mab AIIB2 is directed to β1 integrin. (A) The space in the immunoblot indicates nonconsecutive lanes of the same gel. (B) A graphic representation of 3 or more experiments (means ± SD) shown in panel A. (C) Scan of nonconsecutive lanes on the same immunoblot. In lanes 2 and 3, HUVECs were incubated with 62nM FXII plus 50μM Zn2+ alone or in the presence of 30nM Wortmannin. In lanes 4-8, cells were incubated with 62nM FXII plus 50μM Zn2+ in the presence of 188nM Mabs 6S6, P4C10, P1D6, ASC-1, or 63nM AIIB2, respectively. (D) Graphic representation of 3 or more experiments (means ± SDs) in panel C. (B,D) An asterisk over a bar graph indicates P ≤ .05. (E-F) Influence of peptides to the uPAR-integrin interaction sites or an EGFR inhibitor on FXII-induced phosphorylation of ERK1/2 or Akt. (E) Serum-starved HUVECs were treated with 62nM FXII and 50μM Zn2+ alone or in the absence or presence of 50μM PD98059, Wortmannin, 50nM AG1478, 300μM peptide NLD9 or peptide IQE13, respectively, and examined for ERK1/2 or Akt phosphorylation. (E) Representative experiment; (F) bar graphs of the means ± SDs of 3 or more experiments of panel E. (F) An asterisk over a bar graph indicates P ≤ .05 compared with FXII-treated HUVECs alone.
EGFR transduces uPAR signaling.24 The specific EGFR inhibitor AG1478 at 50nM blocked (P < .001) FXII-induced phosphorylation of ERK1/2 and Akt (Figure 4E-F). These findings were confirmed with the EGFR inhibitor PP3 (100μM), but not the src inhibitor PP2 (100μM), that also blocked FXII-induced phosphorylation of ERK1/2 or Akt (supplemental Figure 3). The combined findings indicated that both β1 and other integrins and the EGFR contribute to the transduction of FXII signaling.
FXII stimulates HUVEC proliferation and growth
FXII stimulated endothelial cell proliferation in a concentration-dependent manner from 60 to 240nM. FXII-induced cell proliferation was blocked by PD98059 (P < .003) and peptides LRG20 (P < .004) from FXII binding site on domain 2 of uPAR or HVL24 (P < .011) from the cell binding site on HK's domain 5, but not peptides GKE19 or TKC19 (P = .25) that do not interfere with ScuPA or HK binding to uPAR, respectively (Figure 5A).8,16 Further, FXII-induced proliferation was blocked by Wortmannin (P < .012), LY294002 (P < .001), and Mab 6S6 (P < .001) to β1 integrin, but not by Mab ASC-1 (Figure 5B).
Figure 5.
FXII-induced cell proliferation and aortic sprouting. (A-B) FXII-induced cell proliferation. HUVECs were prepared for cell proliferation assays as described in supplemental Methods. (A) The lane labeled “Cells” represents the proliferation of untreated HUVECs alone. HUVECs are stimulated with 240nM FXII plus 50μM Zn2+ in the absence or presence of 100μM PD98059 or 300μM peptide LRG20, TKC19, HVL21, or GKE19, respectively. The figure is the means ± SDs of 4 independent experiments. The asterisk indicates P ≤ .011. (B) HUVECs are stimulated with 240nM FXII plus 50μM Zn2+ in the absence or presence of 100μM PD98059, 30nM Wortmannin, 50μM LY294002, 188nM 6S6 or ASC-1, respectively. The figure is the means ± SDs of 4 independent experiments. The asterisk indicates P ≤ .012. (C-D) FXII-induced cell 5-bromo-2′-deoxy-uridine incorporation. HUVECs were prepared for cell growth assays as determined by BrdU incorporation described in supplemental Methods. (C) The lane labeled “Cells” represents the “growth” of serum-starved HUVECs alone. HUVEC growth is stimulated with 240nM FXII plus 50μM Zn2+ in the absence or presence of 100μM PD98059 or 300μM peptides LRG20, TKC19, HVL24, or GKE19, respectively (Table 1). The figure is the means ± SDs of 4 independent experiments. The asterisk indicates a P < .05. (D) HUVEC growth is stimulated with 240nM FXII plus 50μM Zn2+ in the absence or presence of 100μM PD98059, 30nM Wortmannin, 50μM LY294002, 188nM 6S6 or ASC-1, respectively. The figure is the means ± SDs of 4 independent experiments. The asterisk indicates P ≤ .032. (E) The ability of agonists to stimulate sprouting in aorta from a wild-type (WT) or uPAR KO mouse. Sprouting was stimulated with 1200nM VEGF or 16nM FXII. The bar graph is the means ± SDs number of sprouts from the aorta of WT or uPAR KO mice after each stimulus. The figures are representative of 3 experiments at each of the conditions, except that FXII treatment of uPAR KO aorta is the mean of 2 identical experiments.
FXII independently stimulated BrdU incorporation in a concentration-dependent manner from 60 to 240nM. FXII-induced growth of cultured endothelial cells was blocked by PD98059 (P < .011), peptides LRG (P < .011) or HVL24 (P < .002), but not peptides GKE19 or TKC19 (P = .071; Figure 5C). FXII-stimulated BrdU incorporation also was blocked by Wortmannin (P < .018), LY294002 (P < .015), and Mab 6S6 (P ≤ .032), but not Mab ASC-1 (P = .58; Figure 5D). In sum, these investigations indicated that cell proliferation and BrdU incorporation induced by FXII were mediated by similar pathways as ERK1/2 and Akt phosphorylation.
FXII-induced angiogenesis
In aortas from wild-type mice, VEGF induced 45 plus or minus 11 sprouts (mean ± SD), which was not significantly different (P ≥ .33) than that induced by FXII (70 ± 36 sprouts; Figure 5E). Alternatively, in aortas of uPAR KO mice, media alone (control) induced 8 plus or minus 6 sprouts. FXII treatment produced a mean of 10 sprouts in aortas from KO mice (P = .8 vs control; Figure 5E). VEGF induced 87 plus or minus 16 sprouts in uPAR KO aortas. FXII-induced sprouting in wild-type aortas was inhibited by PD98059, LY294002, AG1478, or HKa (supplemental Figure 4). FXII-induced sprouting in wild-type aorta was not dependent on enzymatic activity because 16nM FXII, FXIIa, or APMSF-treated FXIIa induced aortic sprouting that was blocked by PD98059 (supplemental Figure 5). These combined findings indicate that uPAR is important for FXII to induce sprouting ex vivo by a pathway(s) similar to its signaling mechanism. Further nonenzymatically active FXII is sufficient to induce sprouting.
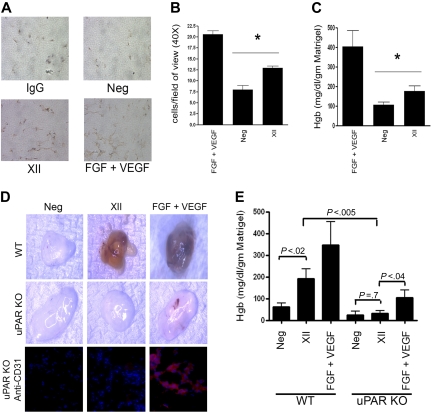
FXII induced angiogenesis in vivo in matrigel plugs in C57Bl/6 mice (Figure 6). On immunoperoxidase stain with an antibody to von Willebrand factor, matrigel plugs stimulated with 240nM FXII have increased new vessel formation 13 plus or minus 2.49 cells/field of view versus 8 plus or minus 2.87 cells/field of view of the control (Neg) matrigel plug (P < .008; Figure 6A-B). Additional investigations indicated that the hemoglobin content of the FXII-treated matrigel plug (177 ± 25 mg/dL/g of matrigel; FXII) was significantly greater (P < .023) than the control (106 ± 16 mg/dL/g of matrigel; Neg) matrigel plug (Figure 6C). In uPAR KO mice, FXII treatment did not produce any angiogenesis, whereas FGF plus VEGF did (Figure 6D-E). The degree of angiogenesis in uPAR KO mice stimulated by FGF plus VEGF as seen in plugs and by hemoglobin content is less than that seen in wild-type mice. When the matrigel plug sections were stained for CD31, no CD31 staining was seen in FXII-treated samples similar to no treatment (Figure 6D). These findings indicate in vivo that the presence of uPAR is essential for FXII-induced angiogenesis.
Figure 6.
FXII stimulates angiogenesis in matrigel plugs. Matrigel plugs were prepared with FGF + VEGF, FXII, or nothing and implanted in C57BL6 mice as indicated in “Methods.” All plugs did contain heparin. (A) Representative histology with an antibody to VWF detected with immunoperoxidase. The panel labeled IgG (immunoglobulin G) is an isotype-specific control antibody to the antibody to VWF detected with immunoperoxidase. “Neg” represents matrigel plugs with no growth factor stimulation; “FXII” represents matrigel plugs treated with FXII, and “FGF + VEGF” represents vessel formation stimulated by these combined growth factors. (B) The bar graph represents the means ± SDs of 10 high-power field of new vessel formation stimulated by FGF + VEGF, unstimulated (Neg), or FXII. (C) Represents the means ± SDs (n = 10 plugs) hemoglobin content/g of matrigel under each of the conditions: FGF + VEGF–treated plugs, untreated plugs (Neg), or FXII-treated plugs. (B-C) The asterisk indicates that there is a significant (P < .05) increase in the cells/field of view or hemoglobin content, respectively, compared with control in the FXII-treated samples. (D) Photographs of excised representative matrigel plugs placed in wild-type (WT) or uPAR KO mice that were not stimulated (Neg), treated with FXII (FXII), or treated with FGF + VEGF. The bottom row is anti-CD31 staining of representative sections from an untreated (Neg), FXII-treated (FXII), or FGF + VEGF-treated matrigel plugs that were placed into uPAR KO mice. (E) Represents the means ± SDs (n ≥ 3 plugs) hemoglobin content/g of matrigel under each of the conditions: Neg, no treatment; FXII, FXII treatment; FGF + VEGF, FGF + VEGF treatment in wild-type (WT) or uPAR KO (KO) mice. The significance between conditions is shown on the figure.
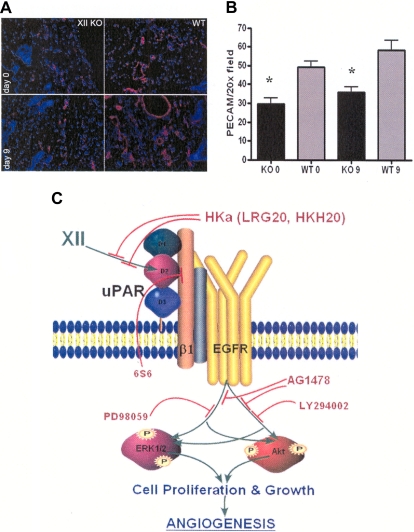
Last, angiogenesis experiments were performed in FXII KO mice. Skin punch biopsies were evaluated for the number of CD31 staining (red) vessels on initial biopsy (day 0) and 9 days after the wounding (day 9). There was reduced day 0 constitutive and day 9 wound platelet endothelial cell adhesion molecule staining of vessels in the skin of the FXII KO mice (Figure 7A). The wild-type mice on initial biopsy had 49 plus or minus 3 platelet endothelial cell adhesion molecule staining vessels versus FXII KO mice with 30 plus or minus 3 vessels (P < .005; Figure 7B). Likewise, after injury on day 9, skin from the initial wound site had 58 plus or minus 5 vessels in wild-type mice versus 36 plus or minus 3 vessels in the FXII KO mice (P < .009; Figure 7B).
Figure 7.
Angiogenesis in FXII KO mice. (A left) Representative immunofluorescent stain with anti-CD31 of the punch biopsies at 20× magnification of skin at days 0 and 9 of the FXII KO or wild-type mice. (B) Bar graph of the means ± SDs of 4 or more slides of anti-CD31–stained vessels (platelet endothelial cell adhesion molecule) in the FXII KO or wild-type (WT) mice at day 0 (0) or day 9 (9). (C) Model of FXII signaling in HUVECs. FXII binds to domain 2 of uPAR on HUVEC membranes. Binding is blocked by plasma HKa or peptide HKH20 from domain 5 of HK or LRG20 from domain 2 of uPAR. FXII engagement induces uPAR's communication to the cell through a β1 integrin. Mab 6S6 to β1 integrin blocks intracellular signaling. Cell stimulation through the integrin or uPAR is mediated through the EGFR because the specific EGFR inhibitor AG1478 blocks FXII-initiated signaling. The MEK inhibitor PD98059 blocks FXII-induced ERK1/2 phosphorylation. Alternatively, LY294002, a PI3 kinase inhibitor, blocks FXII-induced Akt phosphorylation. Cross talk between ERK1/2 and Akt systems also occurs. Inhibition of either of these pathways blocks cell growth, proliferation, or angiogenesis arising from FXII treatment of HUVECs and aortic segments.
Discussion
These investigations show that when HUVECs are stimulated with FXII plus Zn2+ 44/42 mitogen-activated protein kinase (ERK1/2) and PI3′-kinase/Akt (Ser473) are phosphorylated like VEGF-induced cell activation.25 There is considerable cross talk between phosphorylation of ERK1/2 and Akt with the MEK inhibitor PD98059 and the PI3 kinase inhibitors Wortmannin or LY294002. ERK1/2 phosphorylation regulates Akt S473 phosphorylation through mTORC2.19 In all cases, PD98059 inhibits ERK1/2 phosphorylation and Wortmannin or LY294002 inhibits Akt S473 phosphorylation. Neither Wortmannin nor LY294002 are specific PI3 kinase inhibitors. Wortmannin inhibits PI3 kinase and polo-like kinase 1 with equal affinity.26 LY294002 inhibits PI3 kinase, mTOR, DNA-PK, casein kinase 2, and Pim-1.27 In cells of hematopoietic origin, inhibition of PI3 kinase influences ERK1/2 phosphorylation sequentially through phospholipase C γ (PLCγ), Raf-1, and MEK.28 Our investigations indicate that FXII stimulation of ERK1/2 phosphorylation is independent of Akt because in SIN1−/− MEFs 44/42 mitogen-activated protein kinase is phosphorylated in the absence of Akt S473 phosphorylation.19 However, cross talk with the PI3 kinase pathway is evident with SIN1−/− MEFs. Wortmannin, a PI3 kinase inhibitor, substantially inhibits ERK1/2 phosphorylation even in the absence of Akt S473 phosphorylation.
The finding that antibody 3B10 blocks phosphorylation of pERK1/2 by FXII indicates that uPAR participates in these events. 3B10 reacts with the uPAR domain 2 binding sites for ScuPA, HK, FXII, and vitronectin.8,10 Overlapping peptides from uPAR's domain 2 block FXII binding to HUVECs, and SuPAR and FXII-induced phosphorylation of pERK1/2 and Akt. FXII also interacts at site(s) on uPAR where HK binds.7,10 Peptides derived from the endothelial cell binding domain 5 of HK block FXII-induced ERK1/2 and Akt phosphorylation.16 The inhibitory effects of these peptides are overlapping, not identical. The HK domain 5 peptide GKE19 blocks FXII-induced phosphorylation of Akt, but not ERK1/2. GKE19 also does not block cell proliferation or growth. These data suggest that ERK1/2 phosphorylation is more important for cell proliferation and growth than Akt in this system.
FXII signal transduction occurs through integrins. An uPAR domain 2 peptide that is known to interact with integrins and adjacent to the FXII binding region blocks FXII-induced signaling.17 Supporting this finding, several Mabs to β1 integrin suppress the phosphorylation of ERK1/2 or Akt induced by FXII. The β-propeller subunit of β1 integrin is involved in the signaling complex with uPAR.18 The variability among several antibodies to inhibit signaling must be related to their specificity to this region or how they change the conformation of the integrin interaction with uPAR.18,29,30 Antibodies to integrins influence phosphorylation of Akt more than ERK1/2. For example, FXII-induced ERK1/2 phosphorylation is blocked by integrin antibodies 6S6 or HA5 or P4C10 to a lessor extent, but not ASC-1 or AIIB2. FXII-induced phosphorylation of Akt is blocked by antibodies 6S6, P4C10, P1D6, ASC-1, or AIIB2. Antibody epitopes or induced conformation changes on β1 integrin influence these phosphorylation events.31 Other integrins also influence FXII signaling because the α5 antibody P1D6 or α3 antibody ASC-1 is inhibitory. This assessment is supported by the finding that FXII stimulates phosphorylation of ERK1/2 on various matrices: collagen, gelatin, fibrinogen, fibronectin, or vitronectin.
FXII does not have to be enzymatically active to trigger ERK1/2 or Akt phosphorylation. The zymogen or active site inhibited FXIIa is as able to induce phosphorylation on ERK1/2 and Akt as the enzymatic form. How the EGFR participates in this process is not completely known, but activation of the EGFR is associated with cell proliferation.32,33 α5β1 Integrin associates with the EGFR when uPAR is up-regulated.32,33 The cooperation between uPAR and EGFR after FXII binding may prime the cell for mitogenesis and proliferation.24,34,35
FXII stimulates cell proliferation under similar conditions that promote ERK1/2 and Akt phosphorylation. Although there is a difference in cell density and incubation times used for the phosphorylation versus the cell proliferation assays, the same chemical, peptide, and antibody inhibitors interfere with the results. The ability of HK to interfere with FXII-mediated signaling through uPAR explains at least some of the antiproliferation, antiangiogenic, and apoptotic activity associated with activated forms of HK.36
In sum, these investigations indicate a newly recognized activity for zymogen FXII. FXII binds to uPAR to stimulate ERK1/2 and Akt phosphorylation through β1 integrin and the EGFR (Figure 7C). The downstream consequences of these pathways are FXII-induced cell proliferation and growth leading to angiogenesis. It is presently not known what the specific physiologic significance of these activities may be. Because the FXII deficiency is not lethal, it is not essential for developmental angiogenesis. However, FXII may have a role in postnatal angiogenesis after injury, inflammation, or tumor cell growth. The present experiments show that FXII stimulates signaling at 12.5% of its plasma concentration and aortic sprouting at 3.2% of plasma concentration. These data suggest that it is a constitutive growth factor. The fact that skin biopsies of FXII-deleted mice have fewer vessels than wild-type mice supports that notion. These activities may be viewed as independent of FXII's roles in pathologic thrombus formation and inflammation.6 However, in the context of a formed thrombus or pathologic aggregated protein, FXII and its active forms also could contribute to the repair process by stimulating angiogenesis. Further, we have observed that oral thrombin inhibitors reduce the size of new vessel formation in developing tumors.37 The presence of FXII in the tumors could stimulate tumor cell growth as well as contribute to the extent of thrombin formation promoting vessel growth.
Acknowledgments
We thank Dr Balazs Halmos for his advice on experimental inhibition of the EGFR and Dr Marvin Nieman for his critical review of the manuscript. We also thank Drs Frank Castellino and Tom Bugge for generously providing FXII and uPAR knockout mice for investigation. Additional thanks to Dr Bing Su for providing SIN1−/− MEFs.
This work was supported by grant HL052775 (A.H.S.) Schmaier and an AHA Scientific Development Grant (N004313; Z.S.-M.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.A.L., F.M., Z.S.-M., R.G.S., G.A., and W.M.Z. performed experiments; A.H.S. analyzed data with help from G.A.L and F.M.; W.M.Z. and K.R.M. contributed to the matrigel experiments; and A.H.S. did the overall planning and manuscript writing with contributions by G.A.L., R.G.S., and W.M.Z.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alvin H. Schmaier, Department of Medicine, Case Western Reserve University, 10900 Euclid Ave, WRB 2-130, Cleveland, OH 44106; e-mail: schmaier@case.edu.
References
- 1.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood coagulation. Science. 1964;145:1310–1320. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 2.van der Meijden PEJ, Munnix ICA, Auger JM, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114(4):881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 3.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103(4):903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104(15):6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas C, Govers-Riemslag JW, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118(9):3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renné T, Pozgajova M, Gruner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202(2):271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahdi F, Shariat-Madar Z, Figueroa CD, Schmaier AH. Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin on endothelial cell membranes. Blood. 2002;99(10):3585–3596. doi: 10.1182/blood.v99.10.3585. [DOI] [PubMed] [Google Scholar]
- 8.Mahdi F, Shariat-Madar Z, Kuo A, Carinato M, Cines DB, Schmaier AH. Mapping the interaction between high molecular weight kininogen and the urokinase plasminogen activator receptor. J Biol Chem. 2004;279(16):16621–16628. doi: 10.1074/jbc.M313850200. [DOI] [PubMed] [Google Scholar]
- 9.Reddigari S, Shibayama Y, Brunnee T, Kaplan A. Human Hageman factor (factor XII) and high molecular weight kininogen compete for the same binding site on human umbilical vein endothelial cells. J Biol Chem. 1993;268(16):11982–11987. [PubMed] [Google Scholar]
- 10.Li Y, Lawrence DA, Zhang L. Sequences within domain II of the urokinase receptor critical for differential ligand recognition. J Biol. Chem. 2003;278(32):29925–29932. doi: 10.1074/jbc.M300751200. [DOI] [PubMed] [Google Scholar]
- 11.Gordon EM, Venkatesan N, Salazar R, et al. Factor XII-induced mitogenesis is mediated via a distinct signal transduction pathway that activates a mitogen-activated protein kinase. Proc Natl Acad Sci. U S A. 1996;93(5):2174–2179. doi: 10.1073/pnas.93.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernando AN, Fernand LP, Fukuda Y, Kaplan AP. Assembly, activation, and signaling by kinin-forming proteins on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289(1):H251–H257. doi: 10.1152/ajpheart.00206.2004. [DOI] [PubMed] [Google Scholar]
- 13.Jo M, Thomas KS, Wu L, Gonias SL. Soluble urokinase-type plasminogen activator receptor inhibits cancer cell growth and invasion by direct urokinase-independent effects on cell signaling. J Biol Chem. 2003;278(47):46692–46698. doi: 10.1074/jbc.M308808200. [DOI] [PubMed] [Google Scholar]
- 14.Cao DJ, Guo Y-L, Colman RW. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ Res. 2004;94(9):1227–1234. doi: 10.1161/01.RES.0000126567.75232.46. [DOI] [PubMed] [Google Scholar]
- 15.Tang H, Kerins DM, Hao Q, Inagami T, Vaughan DE. The urokinase-type plasminogen activator receptor mediates tyrosine phosphorylation of focal adhesion proteins and activation of mitogen-activates protein kinase in cultured endothelial cells. J Biol Chem. 1998;273(29):18268–18272. doi: 10.1074/jbc.273.29.18268. [DOI] [PubMed] [Google Scholar]
- 16.Hasan AAK, Cines DB, Herwald H, Schmaier AH, Muller-Esterl W. Mapping the cell binding site on high molecular weight kininogen's domain 5. J Biol Chem. 1995;270(33):19256–19261. doi: 10.1074/jbc.270.33.19256. [DOI] [PubMed] [Google Scholar]
- 17.Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem. 2005;280(26):24792–24803. doi: 10.1074/jbc.M413954200. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Czekay R-P, Robillard L, et al. Regulation of alpha5beta1 integrin conformation and function by urokinase receptor binding. J Cell Biol. 2005;168(3):501–511. doi: 10.1083/jcb.200404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127(1):125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Blacher S, Devy L, Burbridge MF, et al. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4(2):133–142. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- 21.Bugge TH, Suh TT, Flick MJ, et al. The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J Biol Chem. 1995;270(28):16886–16894. doi: 10.1074/jbc.270.28.16886. [DOI] [PubMed] [Google Scholar]
- 22.Guan X, Juarez JC, Qi X, et al. Histidine-proline rich glycoprotein (HPRG) binds and transduces anti-angiogenic signals through cell surface tropomyosin on endothelial cells. Thromb Haemost. 2004;92(2):403–412. doi: 10.1160/TH04-02-0073. [DOI] [PubMed] [Google Scholar]
- 23.Iwaki T, Castellino FJ. Plasma levels of bradykinin are suppressed in factor XII-deficient mice. Thromb Haemost. 2006;95(6):1003–1010. doi: 10.1160/TH06-03-0128. [DOI] [PubMed] [Google Scholar]
- 24.Jo M, Thomas KS, O'Donnell DM, Gonias SL. Epidermal growth factor receptor-dependent and –independent cell-signaling pathways originating from the urokinase receptor. J Biol Chem. 2003;278(3):1642–1646. doi: 10.1074/jbc.M210877200. [DOI] [PubMed] [Google Scholar]
- 25.Gerber H-P, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase, Chem Biol. 2005;12(1):99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404(1):15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Séverin S, Ghevaert C, Mazharian A. The mitogen-activated protein kinase signaling pathways: role in megakaryocyte differentiation. J Thromb Haemost. 2010;8(1):17–26. doi: 10.1111/j.1538-7836.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Tang C-H, Kim Y, et al. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282(6):3929–3939. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 30.Tang M-L, Vararattanavech A, Tan S-M. Urokinase-type plasminogen activator receptor induces conformational changes in the integrin alphaMbeta2 headpiece and reorientation of its transmembrane domains. J Biol Chem. 2008;283(37):25392–25403. doi: 10.1074/jbc.M802311200. [DOI] [PubMed] [Google Scholar]
- 31.Green JA, Berrier AL, Pankov R, Yamada KM. Beta1 integrin cytoplasmic domain residues selectively modulate fibronectin matrix assembly and cell spreading through talin and Akt-1. J Biol Chem. 2009;284(12):8148–8159. doi: 10.1074/jbc.M805934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaurasia P, Aguirre-Ghiso JA, Liang OD, Gardsvoll H, Ploug M, Ossowski L. A region in urokinase plasminogen receptor domain III controlling a functional association with alpha5beta1 integrin and tumor growth. J Biol Chem. 2006;281(26):14852–14863. doi: 10.1074/jbc.M512311200. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Aguirre Ghiso JA, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1(5):445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 34.Jo M, Thomas KS, Marozkina N, et al. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determined the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem. 2005;280(17):17449–17457. doi: 10.1074/jbc.M413141200. [DOI] [PubMed] [Google Scholar]
- 35.Jo M, Thomas KS, Takimoto S, et al. Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Oncogene. 2007;26(18):2585–2594. doi: 10.1038/sj.onc.1210066. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JC, Claffey K, Sakthivel R, et al. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. FASEB J. 2000;14(15):2589–2600. doi: 10.1096/fj.99-1025com. [DOI] [PubMed] [Google Scholar]
- 37.Nieman MT, LaRusch GA, Fang C, Zhou Y, Schmaier AH. Oral thrombostatin FM19 inhibits prostate cancer. Thromb Haemost. doi: 10.1160/TH09-08-0570. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]