Abstract
The genus Ophiostoma (Ophiostomatales) has a global distribution and species are best known for their association with bark beetles (Curculionidae: Scolytinae) on conifers. An unusual assemblage of these fungi is closely associated with the African endemic plant genus Protea (Proteaceae). Protea-associated Ophiostoma species are ecologically atypical as they colonise the fruiting structures of various serotinous Protea species. Seven species have been described from this niche in South Africa. It has been speculated that novel species may be present in other African countries where these host plants also occur. This view was corroborated by recent collections of two unknown species from Protea caffra trees in Zambia. In the present study we evaluate the species delineation of these isolates using morphological comparisons with other Protea-associated species, differential growth studies and analyses of DNA sequence data for the β-tubulin and internal transcribed spacer (ITS1, 5.8S, ITS2) regions. As a result, the species O. protea-sedis sp. nov., and O. zambiensis sp. nov. are described here as new. This study brings the number of Protea-associated Ophiostoma species to nine and highlights the need for more inclusive surveys, including additional African countries and hosts, to elucidate species diversity in this uncharacteristic niche.
Keywords: β-tubulin, ITS, Ophiostoma, phylogeny, Protea, taxonomy
INTRODUCTION
The Proteaceae is a prominent Southern Hemisphere flowering plant family that includes c. 1 400 species (Rebelo 1995, Barker et al. 2007). It comprises five sub-families with the Proteoideae predominant in southern Africa (Weston & Barker 2006). The genus Protea is one of the principle members of the Proteoideae and has its origin in the Cape Floristic Region (CFR) at the southernmost tip of Africa (Reeves 2001, Barraclough & Reeves 2005). The genus has subsequently expanded its range from the CFR, migrated along the African escarpment and up into the tropics (Reeves 2001, Barraclough & Reeves 2005). Today the genus includes more than a hundred species, 80 % of which are confined to the CFR (Rourke 1998). The remainder is associated with various non-CFR vegetation types, ranging from tropical forests in west-central and eastern Africa, to savannah in the north-eastern parts of South Africa (Rourke 1998).
Protea species vary from low-growing shrubs (e.g. P. acaulos) to trees of up to 10 m tall (e.g. P. caffra) (Rebelo 1995). Their flowers are grouped in large inflorescences that form compact seed-storage structures after pollination. In serotinous species, these structures remain on the plants for at least one year and provide a moist, sheltered environment in which numerous arthropods and saprophytic fungi thrive (Coetzee & Giliomee 1985, Lee et al. 2003, 2005, Roets et al. 2006b). The ophiostomatoid fungi, including members of Gondwanamyces (Microascales) and Ophiostoma (Ophiostomatales), dominate fungal communities within such Protea infructescences (Roets et al. 2005).
Ophiostomatoid fungi are morphologically adapted to arthropod spore dispersal, producing sticky spores at the tips of conidiophores or of usually elongated ascomatal necks (Münch 1907, 1908, Francke-Grosmann 1967, Upadhyay 1981, Malloch & Blackwell 1993). The vectors of Ophiostoma include bark beetles and ambrosia beetles (Curculionidae: Scolytinae), longhorn beetles (Cerambycidae) and mites (Acari) (Barras & Perry 1975, Upadhyay 1981, Bridges & Moser 1983, 1986, Moser 1997, Jacobs & Wingfield 2001, Roets et al. 2007). The genus is best known as an associate of bark-beetles within larval galleries constructed in the phloem and wood of coniferous trees (Kirisits 2004). The interactions between these fungi and their vectors can be mutualistic (Francke-Grosmann 1967, Beaver 1989, Berryman 1989, Six & Paine 1998, Ayres et al. 2000, Jacobs & Wingfield 2001, Klepzig et al. 2001a, b).
Recent studies on the dispersal of Protea-associated Ophiostoma have shown that mites act as primary vectors of various species, while beetles play an intermediary role by carrying the mites between infructescences (Roets et al. 2006c, 2007, 2009b). Some of these mites feed exclusively on Ophiostoma, confirming that the association between these mites and the fungi they carry is also mutualistic (Roets et al. 2007). These ecological studies have led to the discovery of two new Ophiostoma species, both of which were first isolated from mites (Roets et al. 2008). Results further highlighted the need for extended surveys, including a greater number of host species and additional localities, to elucidate the total number of species associated with this Protea-infructescence niche.
Seven species of Ophiostoma have been described from Protea. These include Ophiostoma africanum, O. gemellus, O. palmiculminatum, O. phasma, O. protearum, O. splendens and the anamorphic Sporothrix variecibatus (Marais & Wingfield 1994, 1997, 2001, Roets et al. 2006a, 2008). All Protea-associated Ophiostoma species are known only from South Africa.
With the aim of gaining a more comprehensive understanding of the association between Ophiostoma and Protea, Roets et al. (2009b) conducted surveys including areas and hosts not previously considered. Collections from Zambia resulted in the isolation of two putatively novel Ophiostoma species from Protea caffra. Infructescence-associated Ophiostoma species are morphologically very similar. These fungi do not regularly produce sexual structures in culture making species delineation using mating studies difficult. Consequently, DNA sequence comparisons provide an important tool to confirm their identity. In the present study we evaluate the validity of the proposed new taxa (Roets et al. 2009b) based on morphological and DNA sequence-based comparisons with known Protea-associated Ophiostoma species.
MATERIALS AND METHODS
Isolates
Isolates of the putative new taxa (Ophiostoma sp. nov. 1 and Ophiostoma sp. nov. 3 (Roets et al. 2009b) were collected in Nchila, Zambia during October 2006. Three additional isolates of each of these species, not included in the study of Roets et al. (2009b) were included in the present study (Table 1). Isolates were maintained in Petri dishes containing 2 % malt extract agar (MEA, Biolab, Midrand, South Africa) at 4 °C. Representative cultures of the taxa considered in this study have been deposited in both the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands, and the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa. Herbarium specimens were deposited in the herbarium of the National Collection of Fungi, Pretoria, South Africa (PREM). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
Table 1.
Origin, hosts and GenBank accession numbers for fungal isolates associated with Protea species in Zambia and South Africa, used in phylogenetic analyses. Isolate and accession numbers of sequences obtained in this study are printed in bold type.
| Species | Isolate no. |
Host | Origin | Collector | GenBank no. |
||
|---|---|---|---|---|---|---|---|
| CBS | CMW | ITS | β-tubulin | ||||
| Ophiostoma africanum | 116374 | 1822 | Protea dracomontana | KZ-Natal, South Africa | M.J. Wingfield | DQ316197 | DQ316159 |
| 116376 | 1812 | Protea dracomontana | KZ-Natal, South Africa | M.J. Wingfield | DQ316198 | DQ316160 | |
| 116566 | 1104 | P. caffra | Gauteng, South Africa | Unknown | DQ316200 | DQ316162 | |
| 116571 | 823 | P. gaguedi | Unknown | M.J. Wingfield | DQ316199 | DQ296073 | |
| O. gemellus | 121957 | 23054 | P. caffra | Gauteng, South Africa | F. Roets | DQ821557 | DQ821551 |
| 121958 | 23056 | P. caffra | Gauteng, South Africa | F. Roets | DQ821558 | DQ821552 | |
| 121959 | 23057 | Tarsonemus sp. from P. caffra | Gauteng, South Africa | F. Roets | DQ821560 | DQ821554 | |
| 23055 | P. caffra | Gauteng, South Africa | F. Roets | DQ821559 | DQ821553 | ||
| 23058 | Tarsonemus sp. from P. caffra | Gauteng, South Africa | F. Roets | DQ821561 | DQ821555 | ||
| 23059 | Tarsonemus sp. from P. caffra | Gauteng, South Africa | F. Roets | DQ821562 | DQ821556 | ||
| O. palmiculminatum | 119590 | 20677 | P. repens | Western Cape, South Africa | F. Roets | DQ316191 | DQ821543 |
| 119591 | 20693 | P. repens | Western Cape, South Africa | F. Roets | DQ316192 | DQ821544 | |
| 119592 | 20694 | P. repens | Western Cape, South Africa | F. Roets | DQ821546 | ||
| 119593 | 20697 | P. repens | Western Cape, South Africa | F. Roets | DQ821547 | ||
| 20695 | P. repens | Western Cape, South Africa | F. Roets | DQ821545 | |||
| 20696 | P. repens | Western Cape, South Africa | F. Roets | DQ821548 | |||
| 23049 | Trichouropoda sp. from P. repens | Western Cape, South Africa | F. Roets | DQ821563 | DQ821550 | ||
| O. phasma | 119721 | 20676 | P. laurifolia | Western Cape, South Africa | F. Roets | DQ316219 | DQ316181 |
| 20683 | P. laurifolia | Western Cape, South Africa | F. Roets | DQ316227 | DQ316189 | ||
| 20684 | P. laurifolia | Western Cape, South Africa | F. Roets | DQ316218 | DQ316180 | ||
| 20692 | P. laurifolia | Western Cape, South Africa | F. Roets | DQ316228 | DQ316190 | ||
| 20698 | P. laurifolia | Western Cape, South Africa | F. Roets | DQ316222 | DQ316184 | ||
| 20699 | P. laurifolia | Western Cape, South Africa | F. Roets | DQ316220 | DQ316182 | ||
| O. protearum | 116567 | 1103 | P. caffra | Gauteng, South Africa | M.J. Wingfield | DQ316203 | DQ316165 |
| 116568 | 1102 | P. caffra | Gauteng, South Africa | M.J. Wingfield | DQ316202 | DQ296072 | |
| 116654 | 1107 | P. caffra | Gauteng, South Africa | M.J. Wingfield | DQ316201 | DQ316163 | |
| Ophiostomasp. nov. 2 = | 124909 | 28600 | P. caffra | Nchila, Zambia | F. Roets | EU660448 | EU660463 |
| (O. protea-sedis sp. nov.) | 124910 | 28601 | P. caffra | Nchila, Zambia | F. Roets | EU660449 | EU660464 |
| 124911 | 29074 | P. caffra | Nchila, Zambia | F. Roets | EU660450 | EU660465 | |
| 29075 | P. caffra | Nchila, Zambia | F. Roets | EU660451 | EU660466 | ||
| 29076 | P. caffra | Nchila, Zambia | F. Roets | EU660452 | EU660467 | ||
| O. splendens | 116377 | 873 | P. repens | Unknown | M.J. Wingfield | DQ316214 | DQ316176 |
| 116378 | Unknown | Unknown | Unknown | DQ316208 | DQ316170 | ||
| 116379 | 896 | P. repens | Unknown | M.J. Wingfield | DQ316207 | DQ316169 | |
| 116569 | 872 | P. repens | Unknown | M.J. Wingfield | DQ316215 | DQ296071 | |
| 2753 | P. neriifolia | Western Cape, South Africa | G.J. Marais | DQ316206 | DQ316168 | ||
| 20674 | P. repens | Western Cape, South Africa | F. Roets | DQ316204 | DQ316166 | ||
| 20675 | P. repens | Western Cape, South Africa | F. Roets | DQ316205 | DQ316167 | ||
| 20678 | P. repens | Western Cape, South Africa | F. Roets | DQ316210 | DQ316172 | ||
| 20679 | P. repens | Western Cape, South Africa | F. Roets | DQ316212 | DQ316174 | ||
| 20680 | P. repens | Western Cape, South Africa | F. Roets | DQ316211 | DQ316173 | ||
| 20685 | P. repens | Western Cape, South Africa | F. Roets | DQ316213 | DQ316175 | ||
| 20691 | P. repens | Western Cape, South Africa | F. Roets | DQ316209 | DQ316171 | ||
| Ophiostoma sp. nov. 1 = | 124912 | 28604 | P. caffra | Nchila, Zambia | F. Roets | EU660453 | EU660473 |
| (O. zambiensis sp. nov.) | 124913 | 28605 | P. caffra | Nchila, Zambia | F. Roets | EU660454 | EU660474 |
| 124914 | 29077 | P. caffra | Nchila, Zambia | F. Roets | EU660455 | EU660475 | |
| 29078 | P. caffra | Nchila, Zambia | F. Roets | EU660456 | EU660476 | ||
| 29079 | P. caffra | Nchila, Zambia | F. Roets | EU660457 | EU660477 | ||
| Sporothrix variecibatus | 121960 | 23060 | Protea longifolia | Western Cape, South Africa | F. Roets | DQ821569 | DQ821573 |
| 121961 | 23051 | Trichouropoda sp. from Protea repens | Western Cape, South Africa | F. Roets | DQ821568 | DQ821539 | |
| 121962 | 2543 | Eucalyptus leaf litter | Western Cape, South Africa | P.W. Crous | DQ821567 | DQ821572 | |
Morphology and growth in culture
Isolates of Ophiostoma sp. nov. 1 and Ophiostoma sp. nov. 2 (Table 1) were grown in the dark for 5 d at 25 °C on 2 % MEA (Biolab, Midrand, South Africa). Ascomata collected from colonized infructescences and conidiogenous cells formed in culture were mounted on microscope slides in lactophenol (Stephens 1974). Specimens were studied using a Nikon Eclipse E600 light microscope (Nikon Corporation, Tokyo, Japan) with differential interference contrast. Fifty measurements of each taxonomically informative structure were made and, where appropriate, means (± standard deviation) calculated. Photographic images were captured using a Nikon DXM1200 digital camera (Nikon Corporation, Tokyo, Japan).
The growth of isolates of the two putative new Ophiostoma species was compared to that of closely related species. Based on results of previous molecular analyses (Roets et al. 2009b) three isolates of each of O. africanum, O. gemellus, O. palmiculminatum, O. protearum and O. splendens were thus included. For these initial studies, conidia were removed from actively growing 1 wk old cultures using a sterile needle with a small piece of agar at the tip and transferred to the centre of fresh dishes containing 20 mL MEA. Plates were incubated at 25 °C for 8 d in the dark, after which mean (± standard deviation) colony diameters (three measurements per isolate) were determined.
Species that are morphologically closely related to Ophiostoma sp. nov. 1 and Ophiostoma sp. nov. 2 from P. cafra in Zambia, respectively, and that had similar growth rates at 25 °C were used in subsequent growth studies to test for significant differences in growth response at different temperatures. For these studies three isolates each of Ophiostoma sp. nov. 1, O. gemellus, O. palmiculminatum, O. splendens and Ophiostoma sp. nov. 2 were included. Mycelium-covered agar discs (5 mm diam) were excised from the edges of actively growing 1 wk old cultures and transferred to the centers of fresh MEA dishes. Plates were incubated in the dark at temperatures ranging from 5–35 °C at 5 °C intervals. Colony diameters were measured after 2 d and again after an additional 8 d of growth. The mean diameter of additional growth (three measurements per isolate) and the mean growth diameter (± standard deviation) for each isolate were calculated. A factorial one-way analysis of variance (ANOVA) with Sigma-restricted parameterization was used to analyze the data using Statistica 8 (Statsoft Corp., Tulsa, Oklahoma). Significant differences between growth rates of these fungal species are reported when statistically different at the 99.9 % level (P ≤ 0.001).
DNA isolation, amplification and sequencing
Genomic DNA was extracted from fungal mycelium using a Sigma GenElute™ plant genomic DNA miniprep kit (Sigma-Aldrich Chemie CMBH, Steinheim, Germany) following the manufacturer’s instructions. The primers ITS1-F (Gardes & Bruns 1993) and ITS4 (White et al. 1990) were used for amplification of the internal transcribed spacers and 5.8S rDNA regions (ITS). For amplification of the β-tubulin gene region the primers T1 (O’Donnell & Cigelnik 1997) and Bt2b (Glass & Donaldson 1995) were used. PCR reaction mixtures and amplification conditions followed those of Roets et al. (2006a, 2009b). PCR products were purified using the Wizard® SV gel and PCR clean up system (Promega, Madison, Wisconsin, USA) following the manufacturer’s instructions. The amplicons were sequenced using the Big Dye™ Terminator v3.0 cycle sequencing premix kit (Applied Biosystems, Foster City, CA, USA) and analyzed on an ABI PRISM™ 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
ITS and β-tubulin DNA sequences for four isolates, two of each of the two putative new species from P. caffra in Zambia, generated by Roets et al. (2009b) were included in the present study. DNA sequence data for all other Ophiostoma spp. included in phylogenetic reconstructions were obtained from NCBI GenBank (www.ncbi.nlm.nih.gov/), and accession numbers are presented in the phylogenetic trees. Sequences were aligned using the L-INS-I strategy in the online version of MAFFT v6 (Katoh & Toh 2008).
The ITS sequence dataset included several Ophiostoma and Sporothrix species to illustrate the phylogenetic positions of the species from Protea in relation to other species and species groups in the Ophiostomatales. Three Grosmannia species were designated as outgroup taxa in the ITS analyses.
Sequence data of the β-tubulin gene were used to distinguish between closely related species and were analyzed in two datasets. The first dataset included several Ophiostoma and Sporothrix species from the S. schenckii–O. stenoceras complex, and all available β-tubulin sequences of species associated with Protea. The region included in the analyses spanned β-tubulin exons 4, 5 and 6, as well as intron 5. In all the species included in this dataset, intron 4 was absent. In the second β-tubulin dataset, an extended fragment was analyzed, consisting of exons 3–6, as well as introns 3 and 5. Data of the longer fragment are available for fewer species, thus fewer species were included in the analyses.
All three datasets were subjected to maximum parsimony (MP), Bayesian inference (BI) and maximum likelihood (ML) analyses. The protocols followed for each analysis are described below. Isolate numbers for all species from Protea (including those from previous studies) are given in the phylogenetic trees. GenBank accession numbers and all other information regarding the Protea isolates are presented in Table 1. GenBank accession numbers of all non-Protea species of Ophiostoma are presented in the phylogenetic trees.
Maximum parsimony
Five thousand random stepwise addition heuristic searches were performed using the software package PAUP (Phylogenetic Analysis Using Parsimony) v4.0b10 (Swofford 2003) with tree-bisection-reconnection (TBR) branch swapping active. All characters were unordered and of equal weight and alignment gaps were treated as a fifth character state. Ten trees were saved per replicate and branches of zero length were collapsed. Confidence levels were estimated by performing 1 000 bootstrap replicates (Felsenstein 1985) with fast-stepwise addition.
Bayesian inference
Bayesian inference, based on a Markov Chain Monte Carlo (MCMC) approach, was performed in the software package MrBayes v3.1.2 (Ronquist & Huelsenbeck 2003). Evolutionary models were determined using MrModeltest v2.2 (Nylander 2004) based on the Akaike Information Criterion (AIC). Base equilibrium frequencies, instantaneous substitution rates and among-site rate variation were estimated independently on shared topologies. Two independent Markov chains were initiated from a random starting tree where after runs of 5 000 000 generations (sample frequency of 100) were performed. Burnin values were determined using Tracer v1.4 (http://tree.bio.ed.ac.uk/software/tracer/), and all sampled trees lower than the burnin values were discarded. The remaining trees were pooled into a 50 % majority rule consensus tree.
Maximum likelihood
Maximum likelihood (ML) analyses were conducted in the online version of PhyML v3.0 (Guindon & Gascuel 2003). The best fit substitution models were determined by AIC in Modeltest v3.7 (Posada & Crandall 1998). Confidence values were estimated using bootstrap analysis (1 000 replicates).
RESULTS
Morphology and growth in culture
Morphological comparisons revealed close similarities between O. africanum, O. protearum, O. splendens and Ophiostoma sp. nov. 1 (hereafter O. zambiensis). Ophiostoma protearum differs from the other species in that it has hyphal ornamentation on the ascomatal bases. Ophiostoma protearum and O. africanum have ostiolar hyphae, while these structures are not found in O. splendens or O. zambiensis from P. caffra in Zambia. Morphological distinction between O. splendens and O. zambiensis is not unambiguous, as most characters overlap in their dimensions. Ascospores of O. zambiensis are on average slightly shorter and wider (4 × 2 μm) than those of O. splendens (5.7 × 1.1 μm).
Based on morphology, Ophiostoma sp. nov. 2 (hereafter O. protea-sedis) is closely related to O. gemellus and O. palmiculminatum. The size-ranges of most morphological characters overlap between these three species. All three species form fairly large ascomata with long necks that have ostiolar hyphae at the tips. These ostiolar hyphae vary slightly in size between the three species. The ostiolar hyphae of O. gemellus are 32–42 μm (35 ± 3) long, while those of O. palmiculminatum are 10–25 μm (16 ± 5) long. The ostiolar hyphae of O. protea-sedis are intermediate (25–27 μm (25 ± 1)) in length between O. gemellus and O. palmiculminatum. Another morphological character that aids delineation between these species is the presence of hyphal ornamentation on the ascomatal bases. Ophiostoma gemellus lacks hyphal ornamentation on the ascomatal base, while O. palmiculminatum usually has very sparse hyphae originating from these structures. Ophiostoma protea-sedis, in contrast, usually has very dense hyphal ornamentation on the ascomatal bases. In addition to these morphological differences, conidia of O. gemellus are usually strongly curved, while those of O. palmiculminatum and O. protea-sedis are clavate. Conidia of O. protea-sedis and O. palmiculminatum can be distinguished by their respective dimensions. Those of O. protea-sedis are generally slightly wider than those of O. palmiculminatum.
Cultures of O. africanum, O. gemellus, O. palmiculminatum, O. protearum, O. splendens and the two putative new Ophiostoma spp. from P. caffra in Zambia showed differences in their growth rates. Mean colony diameters (± standard deviation) for O. africanum, O. protearum, O. splendens and O. zambiensis were 25.8 mm (± 1.1), 25.1 mm (± 0.3), 30.2 mm (± 0.4) and 29.7 mm (± 0.7), respectively, after 8 d in the dark at 25 °C. Mean colony diameters (± standard deviation) for O. gemellus, O. palmiculminatum and O. protea-sedis were 25.2 mm (± 0.6), 23.3 mm (± 0.4) and 21.1 mm (± 1.2), respectively, after 8 d in the dark at 25 °C.
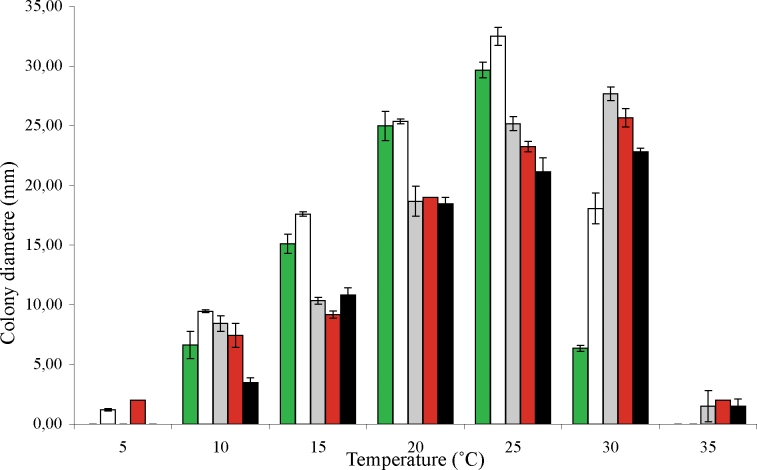
Further growth tests (at different temperatures) focused on comparisons between O. splendens and O. zambiensis and comparisons between O. gemellus, O. palmiculminatum and O. protea-sedis as these species are morphologically closely related to the Zambian isolates and had similar growth rates at 25 °C. Cultures of the two Ophiostoma species from P. caffra in Zambia grew optimally on MEA at 30 °C and 25 °C, respectively (Fig. 1). Ophiostoma zambiensis grew significantly slower than O. splendens at the tested temperatures (F = 52.99; P ≤ 0.001). Ophiostoma splendens and O. zambiensis also reacted differently towards different temperatures (F = 16604, P ≤ 0.001). Ophiostoma protea-sedis grew significantly slower than O. gemellus (F = 18.72, P ≤ 0.001) and O. palmiculminatum (F = 15.52, P ≤ 0.001) when growth rates of these species at different temperatures are compared (Fig. 1). In addition, O. protea-sedis and O. gemellus reacted significantly different to the different temperatures (F = 12149, P ≤ 0.001). Ophiostoma protea-sedis and O. palmiculminatum differed significantly in their reaction to different growth temperatures (F = 18417, P ≤ 0.001).
Fig. 1.
Mean growth on MEA (three isolates per tested species, ± standard deviation) of O. splendens (white bars), O. zambiensis (green bars), O. gemellus (grey bars), O. palmiculminatum (red bars), and O. protea-sedis (black bars) at a range of temperatures after 8 d in the dark.
DNA isolation, amplification and sequencing
Amplified fragments obtained using the primers ITS1-F and ITS4 were 547 bp for O. protea-sedis and 541 bp long for O. zambiensis. Amplification of extracted genomic DNA with the primers T1 and Bt2b resulted in fragment lengths of 999 bp for O. protea-sedis and 879 bp for O. zambiensis.
Phylogenetic analyses
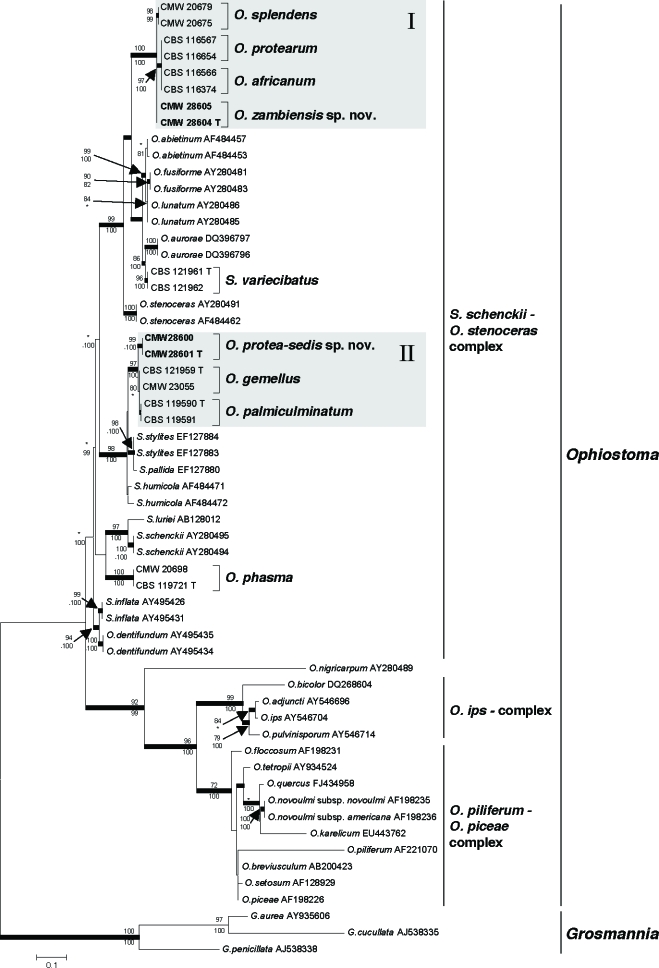
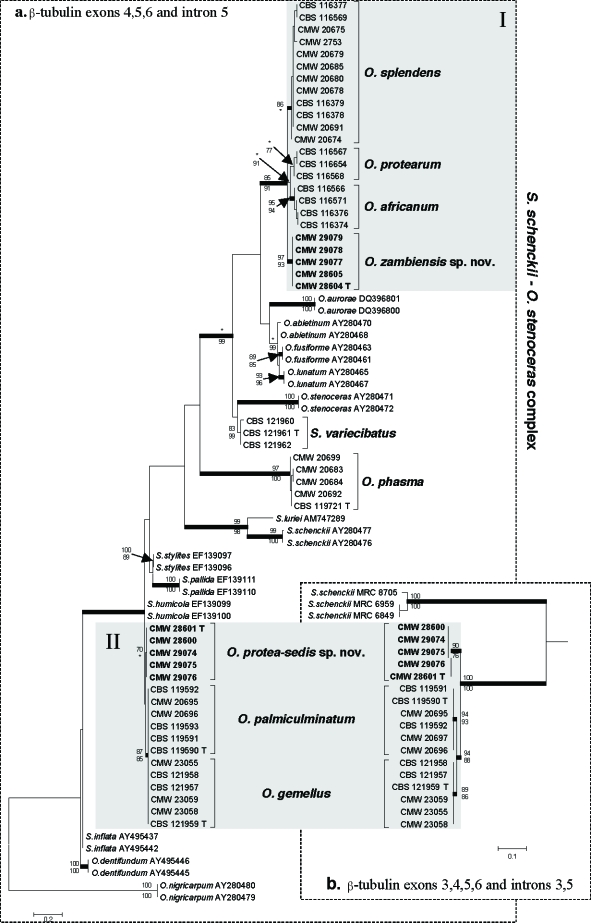
Parameters used and statistical values obtained from the various analyses of the ITS, β-tubulin and extended β-tubulin datasets, are presented in Table 2. The three aligned datasets were respectively 725, 269 and 600 base pairs in length. Phylograms obtained with ML for the three datasets are presented in Fig. 2, 3a, b.
Table 2.
Statistics resulting from the different phylogenetic analyses.
| Dataset | β-tubulin |
Maximum Parsimony |
Maxinum Likelihood |
MrBayes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| exons | introns | PICa | No. of trees | Tree length | CIb | RIc | HId | Subst.e model | Pinvarf | Gammag | Subst.e model | Burn-in | |
| ITS | – | – | 382 | 96 | 1432 | 0.624 | 0.864 | 0.376 | GTR+I+G | 0.3504 | 1.4792 | GTR+I+G | 500 |
| β-tubulin | 4–6 | 5 | 116 | 84 | 455 | 0.642 | 0.936 | 0.358 | GTR+I+G | 0.5396 | 1.7180 | GTR+I+G | 100 |
| β-tubulin | 3–6 | 3, 5 | 303 | 1 | 517 | 0.899 | 0.973 | 0.101 | HKY+I | 0.5246 | – | HKY+I | 100 |
a PIC = number of parsimony informative characters; b CI = consistency index; c RI = retention index; d HI = homoplasy index;
e Subst.model = best fit substitution model; f Pinvar = proportion of invariable sites; g Gamma = Gamma distribution shape parameter.
Fig. 2.
Phylogram obtained from maximum likelihood (ML) analysis of the ribosomal ITS data. Isolate numbers of sequences obtained in the present study are printed in bold type. Isolates from Protea are indicated by square brackets and their species names printed in bold type. Bootstrap support values (> 70 %) for ML and MP are respectively presented above and below branching points. Branches supported by posterior probabilities > 90 % obtained with Bayesian inference, are printed in bold. Trees were rooted against the three species of Grosmannia.
Fig. 3.
Phylograms obtained from maximum likelihood (ML) analysis of the shorter (a) and extended (b) β-tubulin gene regions. Isolate numbers of sequences obtained in the present study are printed in bold type. Isolates from Protea are indicated by square brackets and their species names printed in bold type. Bootstrap support values (> 70 %) for ML and MP are respectively presented above and below branching points. Branches supported by posterior probabilities > 90 % obtained with Bayesian inference, are printed in bold. Two isolates of Ophiostoma nigricarpum was used as outgroup in (a), while the tree obtained in (b) was midpoint-rooted.
Trees generated by analyses of the ITS dataset (Fig. 2) revealed that the two putative new taxa from Zambia grouped in two distinct clades (hereafter referred to as Group I and Group II) in the S. schenckii–O. stenoceras complex of the genus Ophiostoma. Group I included O. africanum, O. protearum, O. splendens and the new species, O. zambiensis. The two isolates of O. zambiensis did not form a clade with strong statistical support, and ITS sequences also failed to distinguish between O. africanum and O. protearum as has been reported previously (Roets et al. 2006a). Group II isolates included O. gemellus, O. palmiculminatum and the second putative new species from Zambia, O. protea-sedis. The two isolates of O. protea-sedis had good statistical support, but this was not the case for O. gemellus or O. palmiculminatum (Roets et al. 2008).
The trees obtained from the β-tubulin dataset that included exons 4–6, and intron 5 (Fig. 3a), showed the same two clades (Groups I and II) containing the species from Protea. The four species in Group I, including O. zambiensis, grouped separately with good statistical support. These included O. africanum, O. protearum, (both from the Gauteng Province) and O. splendens from the Western Cape Province. In Group II, O. protea-sedis had some level of support from ML analyses, separating it from O. gemellus and O. palmiculminatum, but this was not convincing. Neither could the latter two species be separated based on this region, as was shown previously (Roets et al. 2007). This lack of resolution necessitated analyses of an extended region of the β-tubulin gene used previously to distinguish between O. gemellus and O. palmiculminatum (Roets et al. 2007). This analysis also incorporated exon 3 and intron 3 of the β-tubulin gene (Fig. 3b). Apart from S. schenckii and the species in Group II, intron 3 is absent from all the other Protea-associated species. Analyses of this extended region distinguished clearly, with strong support (Fig. 3b), between O. gemellus from the Gauteng Province, O. palmiculminatum from the Western Cape Province of South Africa, and O. protea-sedis from Zambia.
Taxonomy
Both the analyses of phylogenetic data in Roets et al. (2009b) and in the present study provided strong support for the hypothesis that the Ophiostoma isolates from P. caffra infructescences in Zambia represent two undescribed species. They are described here as follows:
Ophiostoma zambiensis Roets, M.J. Wingf. & Z.W. de Beer, sp. nov. — MycoBank MB513284; Fig. 4a–h
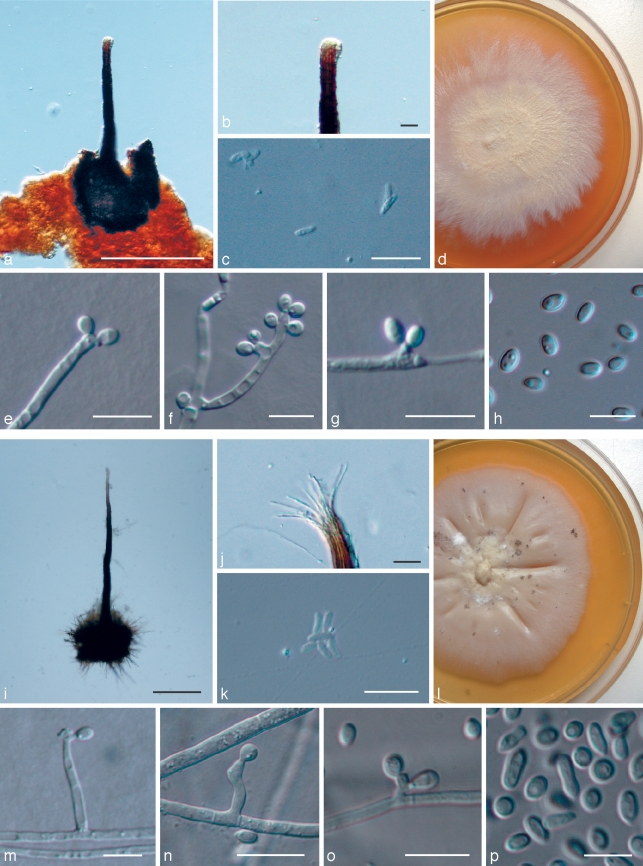
Fig. 4.
Ophiostoma from Zambian P. caffra. a–h: O. zambiensis. a. Perithecium; b. perithecial tip; c. ascospores; d. 3 wk old colony on MEA; e–h. conidiogenous cells, conidiophores and conidia. — i–p: O. protea-sedis. i. Perithecium; j. perithecial tip; k. ascospores; l. 3 wk old colony on MEA; m–p. conidiogenous cells, conidiophores and conidia. — Scale bars: a, i = 100 μm; all others = 10 μm.
Asomata superficialia, basi globosa, atro, 90–120 μm diam, nonnumquam paucis hyphis circumdata, collo atro, 135–165 × 17–27 μm, sursum ad 10–18 μm angustato, nonnumquam paucis hyphis ostiolaribus. Asci evanescentes. Ascosporae allantoideae, unicellulares, hyalinae, vagina gelatinosa carentes, 4 × 2 μm, aggregatae incoloratae. Anamorphe Sporothrix sp., conidiis clavatis 3.5–5 × 2 μm.
Etymology. The epithet zambiensis refers to Zambia, the country from which this species was collected.
Ascomata superficial on host tissue. Ascomatal bases globose, dark, 90–120 μm (101 ± 13) diam, without hyphal ornamentation. Ascomatal necks black, 135–165 μm (155 ± 12) long, 17–27 μm (23 ± 5) wide at the base, 10–18 μm (13 ± 4) wide at the apex. Ostiolar hyphae absent. Asci evanescent. Ascospores allantoid, 1-celled, hyaline, sheaths absent, 4 μm long, 2 μm wide, collecting in a hyaline gelatinous droplet at the apex of the neck.
Culture of the Sporothrix anamorph on MEA 29.7 mm (± 0.7) diam after 8 d at 25 °C in the dark, cream coloured, effuse, circular with an entire edge, surface smooth. Growth reduced at temperatures below and above the optimum of 25 °C. Hyphae superficial on 2 % MEA plates. Sporulation profuse on MEA. Conidiogenous cells 3–12 μm long, 2–3 μm wide, arising directly from hyphae and from 2–43 μm long aerial conidiophores, proliferating sympodially, hyaline, becoming denticulate. Denticles 0.5–1.5 μm (1 ± 0.5) long, usually in an apical crown of 3–10, scattered, solitary or in nodes. Conidia holoblastic, hyaline, 1-celled, clavate, smooth, thin-walled, 3.5–5 μm (4 ± 0.5) long and 2 μm wide. Conidia formed singly, but aggregate to form slimy masses.
Specimens examined. Zambia, Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, holotype Herb. PREM 60350, culture ex-type CMW 28604 = CBS 124912; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, paratype Herb. PREM 60351, culture ex-type CMW 28605 = CBS 124913; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, paratype Herb. PREM 60352, culture ex-type CMW 29077 = CBS 124914; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, paratype Herb. PREM 60353, culture ex-type CMW 29079; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, culture CMW 29078.
Notes — Based on morphological characteristics, O. zambiensis is closely related to O. africanum, O. protearum and O. splendens. Ophiostoma protearum can be distinguished from O. splendens, O. zambiensis and O. africanum by the presence of hyphal ornamentation at the ascomatal base, structures absent for all the other species. The only morphological character that distinguishes O. zambiensis from O. africanum is the presence of short ostiolar hyphae in the latter species, while these structures are absent in O. zambiensis. Except for slightly smaller and wider conidia in O. zambiensis, no other consistent morphological characters distinguish this species from O. splendens. The ITS and β-tubulin gene nucleotide sequences of these four species differ markedly.
Ophiostoma protea-sedis Roets, M.J. Wingf. & Z.W. de Beer, sp. nov. — MycoBank MB513285; Fig. 4i–p
Asomata superficialia, basi globosa, atro, 130–300 μm diam, hyphis circumdata 40–70 μm, collo atro, 540–850 × 30–60 μm, sursum ad 11–13 μm angustato, 8–12 hyphis ostiolaribus rectis vel curvatis, hyalinis vel subhyalinis, 25–27 μm longis palmam fingentibus ornato. Asci evanescentes. Ascosporae allantoideae, unicellulares, hyalinae, vagina gelatinosa carentes, 4–5 × 1–2 μm, aggregatae incoloratae. Anamorphe Sporothrix sp., conidiis clavatis 3.5–7 × 2–3.5 μm.
Etymology. The epithet proteasedis (protea = host genus, sedis = home) refers to the close association between this species and its plant host.
Ascomata superficial on host tissue. Ascomatal bases globose, black, 130–300 μm (229 ± 66) diam, usually with dense hyphal ornamentation, hyphae 40–70 μm long. Ascomatal necks black, 540–850 μm (677 ± 95) long, 30–60 μm (41 ± 11) wide at the base, 11–13 μm (12 ± 1) wide at the apex. 8–12 Ostiolar hyphae usually present, divergent, somewhat curved, hyaline to subhyaline, 25–27 μm (25 ± 1) long. Asci evanescent. Ascospores allantoid, 1-celled, hyaline, sheaths absent, 4–5 μm (4 ± 1) long, 1–2 μm wide collecting in a hyaline gelatinous droplet at the apex of the neck.
Culture of the Sporothrix anamorph on MEA 21.1 mm (± 1.2) diam after 8 d at 25 °C in the dark, cream coloured, effuse, circular with an entire edge, surface smooth. Growth reduced at temperatures below and above the optimum of 30 °C. Hyphae superficial on 2 % MEA plates. Sporulation profuse on MEA. Conidiogenous cells 1–42 μm long, 1–2 μm wide, arising directly from hyphae and from 5–63 μm long aerial conidiophores, proliferating sympodially, hyaline becoming denticulate. Denticles 0.5–1 μm long, usually in an apical crown of 7–14, scattered, solitary or in nodes. Conidia holoblastic, hyaline, 1-celled, clavate, smooth, thin-walled, 3.5–7 μm (4 ± 1) long and 2–3.5 μm (3) wide. Conidia formed singly, but aggregate to form slimy masses.
Specimens examined. Zambia, Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, holotype Herb. PREM 60354, culture ex-type CMW 28601= CBS 124910; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, paratype Herb. PREM 60355, culture ex-type CMW 29074 = CBS 124911; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, paratype Herb. PREM 60357, culture ex-type CMW 28600 = CBS 124909; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, paratype Herb. PREM 60356, culture ex-type CMW 29076; Nchila, from the infructescences of Protea caffra, Oct. 2006, F. Roets, culture CMW 29075.
Notes — Based on morphological characteristics, O. protea-sedis is closely related to O. palmiculminatum and O. gemellus. The only reliable means to distinguish between these species is by comparison of their respective ITS and β-tubulin gene nucleotide sequences.
DISCUSSION
This study confirms an earlier contention by Roets et al. (2009b) that isolates of Ophiostoma collected from P. caffra infructescences in Zambia represent two new taxa. Ophiostoma protea-sedis and O. zambiensis represent the first Protea-associated members of the genus described from a country other than South Africa. These species were recognised based on morphological characteristics as well as comparisons of DNA sequence data and comparisons of growth in culture with closely related species. With the description of O. protea-sedis and O. zambiensis, nine Ophiostoma species are now known to occur in the unusual Protea-infructescences niche.
Most species of Ophiostoma from Protea infructescences are morphologically very similar and the size ranges for some characters overlap. Ophiostoma phasma and the anamorphic S. variecibatus are morphologically well-defined and can readily be distinguished from other Protea-associated species based on morphology alone. These species are also phylogenetically well-separated from other Protea-associated species. The remaining species reside in two well-defined groups based on morphology, physiology and phylogenetic inference. The first of these groups (Group I) comprises species that form slightly smaller ascomatal bases and have much shorter necks (135–167 μm) than species in Group II. These species (O. africanum, O. protearum, O. splendens and O. zambiensis) grow optimally at 25 °C. Species in the second group (Protea-host Group II), produce fairly large ascomatal bases and very long necks (up to 850 μm as for O. protea-sedis). All species in this group (O. gemellus, O. palmiculminatum and O. protea-sedis) grow optimally at 30 °C, rather than 25 °C. Differentiating species residing in these two groups based on morphology alone is tenuous. The only reliable method to readily distinguish between them is by comparison of DNA sequence data.
The two distinct groups of Ophiostoma species residing in Protea infructescences have varied host plant preferences. Ophiostoma gemellus and O. protea-sedis are confined to P. caffra, while O. palmiculminatum is restricted to the infructescences of P. repens (Roets et al. 2009b). Protea caffra also hosts O. protearum and O. zambiensis, while O. africanum is associated with various different Protea species (P. caffra, P. dracomontana and P. gaguedi). Ophiostoma splendens is also associated with many different Protea species (P. burchellii, P. coronata, P. laurifolia, P. lepidocarpodendron, P. longifolia, P. lorifolia, P. neriifolia and P. repens) (Roets et al. 2009b). Based on these host preferences and the distribution of the hosts, Roets et al. (2009b) concluded that the Protea-associated Ophiostoma species probably originated in tropical Africa and migrated southwards to the tip of Africa. This migration of Ophiostoma into the CFR was probably facilitated by vector arthropods (Roets et al. 2009b). This contrasts with the hypothesised origin of the genus Protea in the Cape region of South Africa and its subsequent migration into the tropics following the Great Rift Valley (Barraclough & Reeves 2005).
The specific evolutionary origin of Protea-associated Ophiostoma species remains unclear. The closest sister species of Protea-host Group I includes O. abietinum, O. aurorae, O. fusiforme, O. lunatum, O. stenoceras and S. variecibatus (de Beer et al. 2003, Roets et al. 2008). These species have been isolated from a large diversity of niches that include wood, leaves, soil, bark beetles and even human tissues. The sister species of Protea-host Group II includes S. humicola, S. pallida, and S. stylites. These species have only been isolated from soil and from the bases of planted wooden utility poles (de Meyer et al. 2008). Interestingly, the latter two species have been reported only from South Africa (de Meyer et al. 2008). A soil-linked origin for Ophiostoma species in Protea-host Group II seems plausible. It is also possible that species currently known only from Protea infructescences may eventually also be isolated from soil samples in the habitats where the hosts occur. The proposed evolutionary migratory pathway for Ophiostoma on Protea hosts (Roets et al. 2009b) may be a reflection of an underlying soil-dictated pattern and not because of movement on the Protea host species alone. Studies focused on intensive soil sampling from a range of different Ophiostoma host-Protea localities are required to corroborate this hypothesis.
Various Protea-associated Ophiostoma species are primarily dispersed by mites, while beetles play an intermediary role and carry the mites between infructescences (Roets et al. 2007, 2009a). It has also been shown that these mites may have a mutualistic association with their fungal partners, as they can feed and reproduce on a diet consisting solely of Ophiostoma (Roets et al. 2007). It is not known whether mites also disperse O. protea-sedis and O. zambiensis in Zambia, but this seems likely, because the closely related O. gemellus, O. palmiculminatum and O. splendens have confirmed relationships with mites (Roets et al. 2007, 2009a). Identifying the mites and also the secondary dispersal agents of these fungi (beetles) will lead to a more comprehensive understanding of the co-evolution between these organisms.
The dispersal of soil-inhabiting Ophiostoma species has not yet been investigated, but a large diversity of soil-borne mites may well be involved. Should this be verified, mites may well have facilitated a jump from a soil niche to the Protea infructescence niche. Such a jump could have occurred either early in the evolutionary history of the fungi with subsequent speciation on the host genus (Roets et al. 2009b) or, if these species are also detected from soil samples, continuous current movement between soil and infructescences is likely. This could then provide an alternative explanation of how the isolated Protea infructescence niche could be re-populated with Ophiostoma species in the first flowering season after fire, other than by long-distance dispersal facilitated by insects (Roets et al. 2009a).
Future studies should focus on clarifying natural host ranges and number of Ophiostoma species found in the unusual Protea-infructescence niche. It is likely that more cryptic species remain to be discovered when new hosts and hosts from a wider geographical range are evaluated. Future studies should also include the evaluation of soil and other niches for the presence of these fungi in order to elucidate the evolutionary origin of Protea-associated Ophiostoma. Investigations into the ecology of these fungi, focussed on the interactions between specific fungi, their mite and beetle vectors and their influence on Protea ecology will undoubtedly be a fruitful field for future study.
Acknowledgments
We thank the Western Cape Nature Conservation Board for issuing the necessary collecting permits. We also thank the National Research Foundation (NRF) and the NRF/DST Centre of Excellence in Tree Health Biotechnology (CTHB) for funding, as well as colleagues at the Forestry and Agricultural Biotechnology Institute (FABI) and the Centraalbureau voor Schimmelcultures (CBS) for making cultures available for study. We appreciate assistance from Prof. J. Roux in the collection of specimens from Zambia.
REFERENCES
- Ayres MP, Wilkens RT, Ruel JJ, Vallery E. 2000. Fungal relationships and the nitrogen budget of phloem-feeding bark beetles (Coleoptera: Scolytidae). Ecology 81: 2198 – 2210 . [Google Scholar]
- Barker NP, Weston PH, Rutschmann F, Sauquet H. 2007. Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. Journal of Biogeography 34: 2012 – 2027 . [Google Scholar]
- Barraclough TG, Reeves G. 2005. The causes of speciation in flowering plant lineages: species-level DNA trees in the African genus Protea. In: Bakker FT, Chatrou LW, Gravendeel B, Pelser PB. (eds), Plant species-level systematics: new perspectives on patterns and process Koeltz, Königstein: 31 – 46 . [Google Scholar]
- Barras SJ, Perry TJ. 1975. Interrelationships among microorganisms, bark or ambrosia beetles, and woody host tissue: an annotated bibliography, 1956–1974. USDA Forest Service General Technical Report SO-10 Southern Forest Experiment Station, New Orleans, USA: . [Google Scholar]
- Beaver RA. 1989. Insect-fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins NM, Hammond PM, Webber JF. (eds), Insect-fungus interactions: 121–143 Academic Press, London: . [Google Scholar]
- Beer ZW de, Harrington TC, Vismer HF, Wingfield BD, Wingfield MJ. 2003. Phylogeny of the Ophiostoma stenoceras - Sporothrix schenckii complex. Mycologia 95: 434 – 441 . [PubMed] [Google Scholar]
- Berryman AA. 1989. Adaptive pathways in Scolytid-fungus associations. In: Wilding N, Collins NM, Hammond PM, Webber JF. (eds), Insect-fungus interactions: 145–159 Academic Press, London: . [Google Scholar]
- Bridges JR, Moser JC. 1983. Role of two phoretic mites in transmission of bluestain fungus, Ceratocystis minor. Ecological Entomology 8: 9 – 12 . [Google Scholar]
- Bridges JR, Moser JC. 1986. Relationship of phoretic mites (Acari: Tarsonemidae) to the bluestaining fungus, Ceratocystis minor, in trees infested by southern pine beetle (Coleoptera: Scolytidae). Environmental Entomology 15: 951 – 953 . [Google Scholar]
- Coetzee JH, Giliomee JH. 1985. Insects in association with the inflorescence of Protea repens (Proteaceae) and their role in pollination. Journal of the Entomological Society of Southern Africa 48: 303 – 314 . [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 . [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenetics: an approach using the bootstrap. Evolution 39: 783 – 791 . [DOI] [PubMed] [Google Scholar]
- Francke-Grosmann H. 1967. Ectosymbiosis in wood-inhabiting insects. In: Henry SM. (ed), Symbiosis, Vol II: 171–180. Academic Press, New York: . [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113 – 118 . [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environmental Microbiology 61: 1323 – 1330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696 – 704 . [DOI] [PubMed] [Google Scholar]
- Jacobs K, Wingfield MJ. 2001. Leptographium species: Tree pathogens, insect associates, and agents of blue-stain APS Press, Minnesota: . [Google Scholar]
- Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286 – 298 . [DOI] [PubMed] [Google Scholar]
- Kirisits T. 2004. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans H. (eds), Bark and wood boring insects in living trees in Europe, a synthesis: 1–55 Kluwer Academic Press, The Netherlands: . [Google Scholar]
- Klepzig KD, Moser JC, Lombardero MJ, Ayres MP . 2001a. Symbiosis and competition: Complex interactions among beetles, fungi and mites. Symbiosis 30: 83 – 96 . [Google Scholar]
- Klepzig KD, Moser JC, Lombardero MJ, Ayres MP, Hofstetter RW, Walkinshaw CJ . 2001b. Mutualism and antagonism: Ecological interactions among bark beetles, mites and fungi. In: Jeger MJ, Spence NJ. (eds), Biotic interactions in plant-pathogen associations: 237–267 CAB International, Cambridge: . [Google Scholar]
- Lee S, Roets F, Crous PW. 2005. Biodiversity of saprobic microfungi associated with the infructescences of Protea species in South Africa. Fungal Diversity 19: 69 – 78 . [Google Scholar]
- Lee S, Taylor J, Groenewald JZ, Crous PW, Roets F. 2003. Rhyncostomatoid fungi occurring on Proteaceae including two new species. Mycologia 95: 902 – 910 . [PubMed] [Google Scholar]
- Malloch D, Blackwell M. 1993. Dispersal biology of the ophiostomatoid fungi. In: Wingfield MJ, Seifert KA, Webber JF. (eds), Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity: 195–206 APS Press, St. Paul: . [Google Scholar]
- Marais GJ, Wingfield MJ. 1994. Fungi associated with infructescences of Protea species in South Africa, including a new species of Ophiostoma. Mycological Research 98: 396 – 374 . [Google Scholar]
- Marais GJ, Wingfield MJ. 1997. Ophiostoma protearum sp. nov. associated with Protea caffra infructescences. Canadian Journal of Botany 75: 362 – 367 . [Google Scholar]
- Marais GJ, Wingfield MJ. 2001. Ophiostoma africanum sp. nov., and a key to ophiostomatoid species from Protea infructescences. Mycological Research 105: 240 – 246 . [Google Scholar]
- Meyer EM de, Beer ZW de, Summerbell RC, Moharram AM, Hoog GS de, Vismer HF, Wingfield MJ. 2008. Taxonomy and phylogeny of new wood- and soil-inhabiting Sporothrix species in the Ophiostoma stenoceras - Sporothrix schenckii complex. Mycologia 100: 647 – 661 . [DOI] [PubMed] [Google Scholar]
- Moser JC. 1997. Phoretic mites and their hyperphoretic fungi associated with flying Ips typographys japonicus Niijima (Coleoptera: Scolytidae) in Japan. Journal of Applied Entomology 121: 425 – 428 . [Google Scholar]
- Münch E. 1907. Die Blaufäule des Nadelholzes I–II. Naturwissenschaftliche Zeitschrift für Forst- und Landwirtschaft 5: 531 – 573 . [Google Scholar]
- Münch E. 1908. Die Blaufäule des Nadelholzes III–VII. Naturwissenschaftliche Zeitschrift für Forst- und Landwirtschaft 6: 32 – 47 , 297 – 323 . [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author Evolutionary Biology Centre, Uppsala University, Sweden: . [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103 – 116 . [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817 – 818 . [DOI] [PubMed] [Google Scholar]
- Rebelo T. 1995. Proteas of South Africa Fernwood Press, Cape Town: . [Google Scholar]
- Reeves G. 2001. Radiation and macro-evolutionary ecology of the African genus Protea L. PhD thesis, Imperial College of Science, Technology and Medicine and NERC Centre for Population Biology, University of London, UK: . [Google Scholar]
- Roets F, Beer ZW de, Dreyer LL, Zipfel R, Crous PW, Wingfield MJ . 2006a. Multigene phylogeny for Ophiostoma spp. reveals two new species from Protea infructescences. Studies in Mycology 55: 199 – 212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roets F, Beer ZW de, Wingfield MJ, Crous PW, Dreyer LL. 2008. Ophiostoma gemellus and Sporothrix variecibatus from mites infesting Protea infructescences in South Africa. Mycologia 100: 496 – 510 . [DOI] [PubMed] [Google Scholar]
- Roets F, Crous PW, Dreyer LL. 2005. Seasonal trends in colonization of Protea infructescences by Gondwanamyces and Ophiostoma spp. South African Journal of Botany 71: 307 – 311 . [Google Scholar]
- Roets F, Crous PW, Wingfield MJ, Dreyer LL. 2007. Discovery of fungus-mite-mutualism within a unique niche of the Cape Floral Kingdom. Environmental Entomology 36: 1226 – 1237 . [DOI] [PubMed] [Google Scholar]
- Roets F, Dreyer LL, Crous PW, Wingfield MJ . 2009a. Mite-mediated hyperphoretic dispersal of Ophiostoma spp. from the infructescences of South African Protea spp. Environmental Entomology 38: 143 – 152 . [DOI] [PubMed] [Google Scholar]
- Roets F, Dreyer LL, Geertsema HG, Crous PW . 2006b. Arthropod communities in Proteaceae infructescences: seasonal variation and the influence of infructescence phenology. African Entomology 14: 257 – 265 . [Google Scholar]
- Roets F, Wingfield MJ, Crous PW, Dreyer LL . 2009b. Fungal radiation in the Cape floristic region: an analysis based on Gondwanamyces and Ophiostoma. Molecular Phylogenetics and Evolution 51: 111 – 119 [DOI] [PubMed] [Google Scholar]
- Roets F, Wingfield MJ, Dreyer LL, Crous PW, Bellstedt DU. 2006c. A PCR-based method to detect Ophiostoma and Gondwanamyces from the surface of insects colonising Protea flowers. Canadian Journal of Botany 84: 989 – 994 . [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 . [DOI] [PubMed] [Google Scholar]
- Rourke JP. 1998. A review of the systematics and phylogeny of the African Proteaceae. Australian Systematic Botany 11: 267 – 285 . [Google Scholar]
- Six DL, Paine TD. 1998. Effects of mycangial fungi on host tree species progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae). Environmental Entomology 27: 1393 – 1401 . [Google Scholar]
- Stephens RB. 1974. Mycology guidebook University of Washington, Seattle, USA: . [Google Scholar]
- Swofford DL. 2003. PAUP* (Phylogenetic Analysis Using Parsimony), version 4.0b10 Sinauer Associates, Massachusetts: . [Google Scholar]
- Upadhyay HP. 1981. A monograph of Ceratocystis and Ceratocystiopsis University of Georgia Press, Athens, Georgia: . [Google Scholar]
- Weston PH, Barker NP. 2006. A new generic classification of the Proteaceae with an annotated checklist of genera. Telopea 11: 314 – 344 . [Google Scholar]
- White TJ, Bruns TD, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 New York Academic Press, New York: . [Google Scholar]