Abstract
Recent studies have found a wide range of ascomycetes to be associated with sooty blotch and flyspeck (SBFS) blemishes on the surfaces of pomaceous fruits, specifically apples. Based on collections of such fungi from apple orchards in Germany and Slovenia we introduce two novel genera according to analyses of morphological characters and nuclear ribosomal DNA sequences (large subunit and internal transcribed spacer regions). Microcyclosporella is represented by a single species, M. mali, and is presently known from Germany and Slovenia. Microcyclosporella is Pseudocercosporella-like in morphology, but genetically and morphologically distinct from Pseudocercosporella s.str., for which an epitype is designated based on a fresh collection of P. bakeri from Laos. Furthermore, Pseudocercosporella is shown to be paraphyletic within the Capnodiales. Microcyclospora gen. nov. is Pseudocercospora-like in morphology, but is genetically and morphologically distinct from Pseudocercospora s.str., which is based on P. vitis. Three species, Microcyclospora malicola, M. pomicola (both collected in Germany), and M. tardicrescens (collected in Slovenia) are described. Finally, a new species of Devriesia, D. pseudoamericana, is described from pome fruit surfaces collected in Germany. Devriesia is shown to be paraphyletic, and to represent several lineages of which only Devriesia s.str. is thermotolerant. Further collections are required, however, before the latter generic complex can be resolved.
Keywords: Devriesia, hyphomycetes, Malus, microfungi, Pseudocercospora, Pseudocercosporella, SBFS, taxonomy
INTRODUCTION
Sooty blotch and flyspeck (SBFS) is a fungal disease complex that causes a well known problem in apple fruit production (Schweinitz 1832, Colby 1920, Williamson & Sutton 2000). The number of fungal species causing sooty blotch and flyspeck of apples was previously underestimated (Johnson et al. 1997) and more than 60 taxa are currently known (Díaz Arias et al. 2010), of which the majority are either undescribed or taxonomically unresolved (Batzer et al. 2008, Sun et al. 2008, Schoch et al. 2009, Yang et al. 2010).
The general morphology type termed ‘sooty blotch’ describes species that form colonies on apples that are characterised by superficially spreading, more or less densely branched, dark olive-green, greyish, or brownish black hyphae or mycelial strands with or without sclerotium-like structures or fruiting bodies. Fungal growth of similar or differently shaded or branched colonies of the same or different fungal species can coalesce, resulting into a black or sooty appearance of parts of or the entire apple surface. Sooty blotch fungi are epiphytes and do not cause pre-harvest losses or fruit decay (Colby 1920). In some cases they can cause desiccation of apple fruits during post harvest and storage (R. Godec, Agricultural Institute of Slovenia, pers. comm.). However, sooty blotch reduces the market value of apples and has limited the growth in organic apple production (Williamson & Sutton 2000, Batzer et al. 2005, Yue et al. 2007).
Colby (1920) provided a thorough review on sooty blotch of pomaceous fruits, and accepted that a single species, namely Gloeodes (Dothidea) pomigena (= Phyllachora pomigena), was the responsible casual agent. According to the arrangement of spreading and branching mycelia, sooty blotch was classified into different types such as ramose, fuliginous, punctate, rimate and ridged honeycomb (Groves 1933, Batzer et al. 2005). Furthermore, various mostly dothidealean ascomycetes were identified and classified on the basis of overall morphological characters as species of Colletogloeum, Dissoconium, Peltaster, Pseudocercospora, Pseudocercosporella and Xenostigmina (Batzer et al. 2005, Crous et al. 2009b).
During inventories of SBFS fungi in Germany and Slovenia, a number of isolates were retrieved from apples showing sooty blotch that corresponded either to the ramose (RS) or a dense fuliginous (FG) phenotype. Molecular sequencing of phylogenetic marker genes identified these strains as sooty blotch isolates currently placed in genera such as Devriesia, Pseudocercospora or Pseudocercosporella. However, none of these identifications has until now been supported by sequences of reference strains of these genera, and therefore the aim of the present study was to compare these sooty blotch isolates with authentic reference strains, and to resolve their taxonomy.
MATERIAL AND METHODS
Sample collection and isolation of strains
Apples showing colonies of SBFS were collected from various orchards during summer and autumn of 2007 in Slovenia. Specimens were transported to the laboratory, where they were inspected under a NIKON SMZ 800 binocular. Parts of the apple peel characterised by a homogeneous pattern of sooty blotch were cut off from selected apples with a sterile scalpel and gently surface sterilised with cotton and 70 % ethanol. Pieces of sooty blotch mycelium were removed with a sterile scalpel and placed onto 2 % potato-dextrose agar (PDA; Crous et al. 2009d). Three to four isolations were made from the same sooty blotch colony. The growth habit on the natural substratum was photographically documented (×10–63 magnification). The remainder of the colony and subtending apple peel was dried down, pressed between layers of filter paper, and retained as voucher specimen. Petri dishes were incubated on the laboratory bench for 10–20 d, until colonies started to develop. Colonies were hyphal tipped, and transferred to clean PDA and synthetic nutrient-poor agar (SNA; Crous et al. 2009d) slants for preservation at 4 °C. The obtained strains were compared with a set of sooty blotch strains isolated from apples collected in Germany (Feldmann 2005).
DNA isolation, amplification and analyses
Genomic DNA was isolated from fungal mycelium grown on MEA, using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) according to the manufacturer’s protocols. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene (SSU), the internal transcribed spacer 1, the 5.8S rRNA gene, the internal transcribed spacer 2 (ITS) and the first 900 bases at the 5′ end of the 28S rRNA gene (LSU). The primers ITS4 (White et al. 1990) and LR0R (Rehner & Samuels 1994) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. Partial gene sequences were determined for translation elongation factor 1-α (TEF) as described by Crous et al. (2006c). The PCR conditions, sequence alignment and subsequent phylogenetic analysis followed the methods of Crous et al. (2006a, 2009a). Sequences were compared with the sequences available in NCBIs GenBank nucleotide (nr) database using a MegaBLAST search and results are discussed in the relevant species notes where applicable. Alignment gaps were treated as new character states. Sequence data were deposited in GenBank (Table 1) and alignments in TreeBASE (www.treebase.org).
Table 1.
Collection details and GenBank accession numbers of isolates for which novel sequences were generated in this study.
| Species | Strain no.1 | Substrate | Country | Collector | GenBank no. |
|||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | TEF | |||||
| Devriesia pseudoamericana | CPC 16174; SK 31.1b; CBS 126270 | Malus domestica fruit surface | Germany | S. Kern | GU570527 | GU570544 | – | HM177416 |
| Microcyclospora malicola | CPC 16172; SK 71fb; CBS 126138 | Malus domestica fruit surface | Germany | S. Kern | GU570537 | GU570549 | GU570557 | HM177426 |
| CPC 16186; JF 365-07; CBS 126139 | Malus domestica fruit surface | Slovenia | J. Frank | GU570538 | GU570550 | GU570558 | HM177427 | |
| Microcyclospora pomicola | CPC 16173; SK 51.2a; CBS 126140 | Malus domestica fruit surface | Germany | S. Kern | GU570539 | GU570551 | GU570559 | HM177428 |
| CPC 16175; SK 43.1a; CBS 126141 | Malus domestica fruit surface | Germany | S. Kern | GU570540 | – | – | HM177429 | |
| Microcyclospora tardicrescens | CPC 16187; JF 364-07; CBS 126142 | Malus domestica fruit surface | Slovenia | J. Frank | GU570541 | GU570552 | GU570560 | HM177430 |
| Microcyclosporella mali | CPC 16171; SK 63pgp; CBS 126129 | Malus domestica fruit surface | Germany | S. Kern | GU570528 | GU570545 | GU570555 | HM177417 |
| CPC 16177; JF 41-07; CBS 126130 | Malus domestica fruit surface | Slovenia | J. Frank | GU570529 | GU570546 | GU570556 | HM177418 | |
| CPC 16178; JF 406-07; CBS 126131 | Malus domestica fruit surface | Slovenia | J. Frank | GU570530 | – | – | HM177419 | |
| CPC 16180; JF 174-07; CBS 126132 | Malus domestica fruit surface | Slovenia | J. Frank | GU570531 | – | – | HM177420 | |
| CPC 16181; JF 85-07; CBS 126133 | Malus domestica fruit surface | Slovenia | J. Frank | GU570532 | – | – | HM177421 | |
| CPC 16182; JF 408-07; CBS 126134 | Malus domestica fruit surface | Slovenia | J. Frank | GU570533 | – | – | HM177422 | |
| CPC 16183; JF 407-07; CBS 126135 | Malus domestica fruit surface | Slovenia | J. Frank | GU570534 | – | – | HM177423 | |
| CPC 16184; JF 300-07; CBS 126136 | Malus domestica fruit surface | Slovenia | J. Frank | GU570535 | GU570547 | – | HM177424 | |
| CPC 16185; JF 176-07; CBS 126137 | Malus domestica fruit surface | Slovenia | J. Frank | GU570536 | GU570548 | – | HM177425 | |
| Pseudocercosporella bakeri | CPC 17570; CBS 125685 | Ipomoea sp. | Laos | P. Phengsintham | GU570542 | GU570553 | – | – |
| Schizothyrium pomi | CPC 16179; JF 175-07 | Malus domestica fruit surface | Slovenia | J. Frank | GU570543 | GU570554 | – | HM177431 |
1 CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of P.W. Crous, housed at CBS; SK: S. Kern isolation number (INRIS, Univ. Bonn); JF: J. Frank isolation number (Agricultural Institute of Slovenia).
2 ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: 28S nrDNA; SSU: 18S nrDNA (not used in phylogenetic analyses due to limited resolution); TEF: Translation elongation factor 1-α.
Morphology
Isolates were established on 2 % malt extract agar (MEA), PDA, SNA and oatmeal agar (OA; Crous et al. 2009d), and subsequently incubated at 25 °C under near-ultraviolet light to promote sporulation. Preparations from cultured fungal colonies were mounted on glass slides with clear lactic acid for microscopic examination after 7 d of incubation. Thirty measurements per relevant microscopic structure were gathered where possible. Colony colours on MEA and OA (surface and reverse) were determined using the colour charts of Rayner (1970) after 1 mo at 25 °C in the dark. Reference strains are maintained in the culture collection of the Centraalbureau voor Schimmelcultures (CBS-KNAW), Utrecht, the Netherlands, the working collection (CPC) of P.W. Crous, and at the Agricultural Institute of Slovenia (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
RESULTS
Phylogenetic analysis
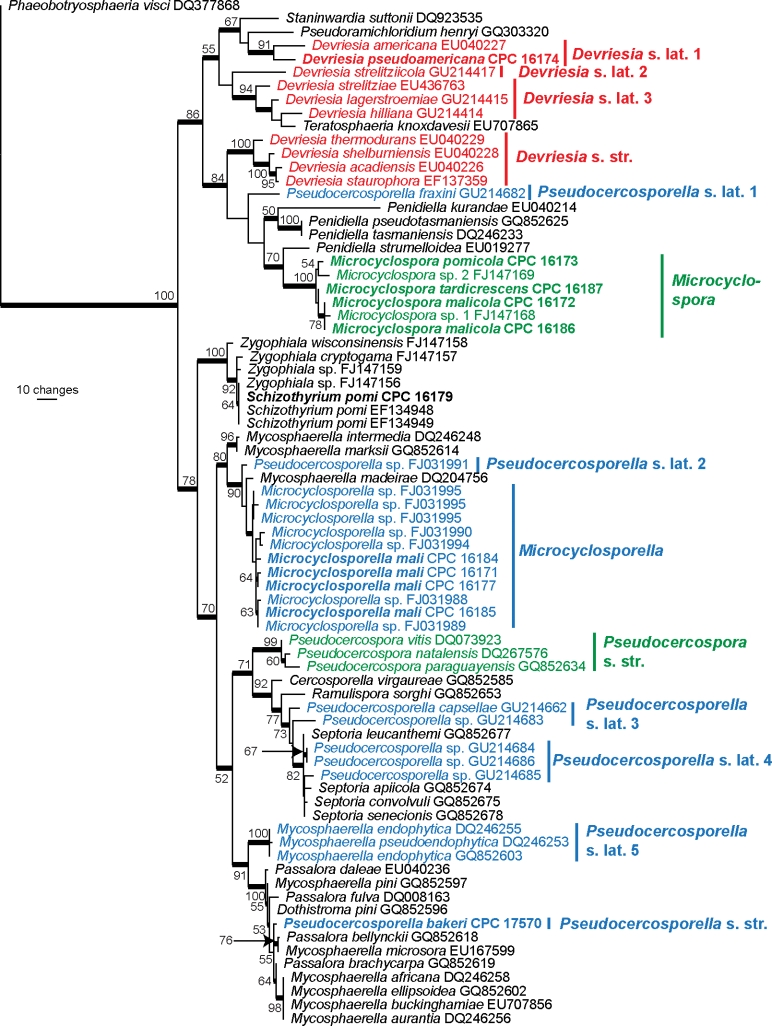
Approximately 1 700 bases, spanning the ITS and LSU regions, were obtained for isolates listed in Table 1. The LSU region was used in the phylogenetic analysis for the generic placement (Fig. 1) and ITS to determine species-level relationships (Fig. 2; see notes under species descriptions).
Fig. 1.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows 10 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Novel sequences generated for this study are shown in bold. Branches present in the strict consensus tree are thickened and important lineages are colour-coded. The tree was rooted to a sequence of Phaeobotryosphaeria visci (GenBank accession DQ377868).
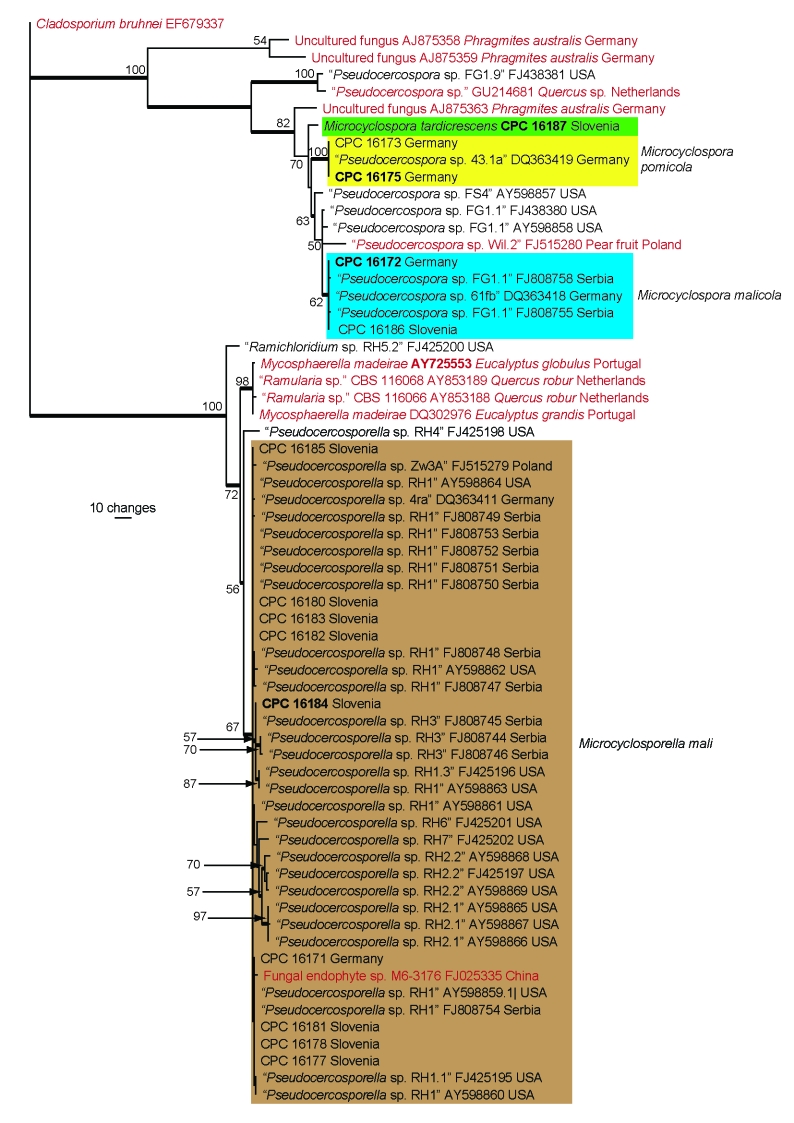
Fig. 2.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the ITS sequence alignment. The scale bar shows 10 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. The four novel species described in this study are indicated by coloured boxes and sequences from non-apple hosts as red text. Strain numbers of epitype cultures are shown in bold and branches present in the strict consensus tree are thickened. The tree was rooted to a sequence of Cladosporium bruhnei (GenBank accession EF679337).
The manually adjusted LSU alignment contained 76 taxa (including the Phaeobotryosphaeria visci outgroup sequence) and, of the 731 characters used in the phylogenetic analysis, 171 were parsimony-informative, 95 were variable and parsimony-uninformative and 465 were constant. Only the first 1 000 equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 1 (TL = 758, CI = 0.493, RI = 0.844, RC = 0.416). The phylogenetic tree of the LSU region (Fig. 1) showed the isolates obtained in this study to cluster in three lineages, namely Devriesia s.l. and two novel genera described below, the first phylogenetically related to Penidiella and the second to a clade containing Mycosphaerella intermedia, Mycosphaerella marksii and Mycosphaerella madeirae.
Only ITS sequences obtained from the GenBank nucleotide database with an identity of 95 % and higher to the four novel species described in this study were added to the alignment. The manually adjusted ITS alignment contained 64 taxa (including the Cladosporium bruhnei outgroup sequence) and, of the 489 characters used in the phylogenetic analysis, 165 were parsimony-informative, 71 were variable and parsimony-uninformative and 253 were constant. Only the first 1 000 equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 2 (TL = 450, CI = 0.747, RI = 0.949, RC = 0.709). The phylogenetic tree of the ITS region (Fig. 1) showed the novel species obtained in this study to cluster with other sequences in GenBank lodged as ‘Pseudocercospora sp.’ and ‘Pseudocercosporella sp.’ and these could represent identical or closely related cryptic species (see species notes below).
Taxonomy
Several taxonomic novelties were found to be associated with SBFS blemishes on apple surfaces (Fig. 3) that do not match any species presently described, though some could be linked to taxa deposited under preliminary names in GenBank. These genera and species are described as new below.
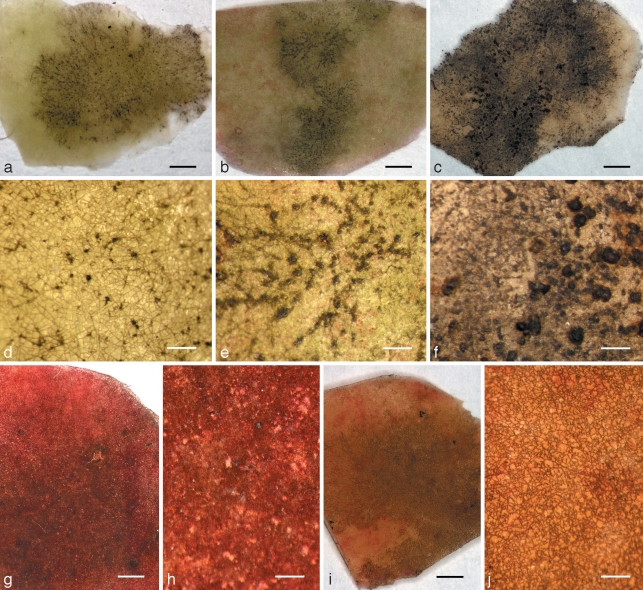
Fig. 3.
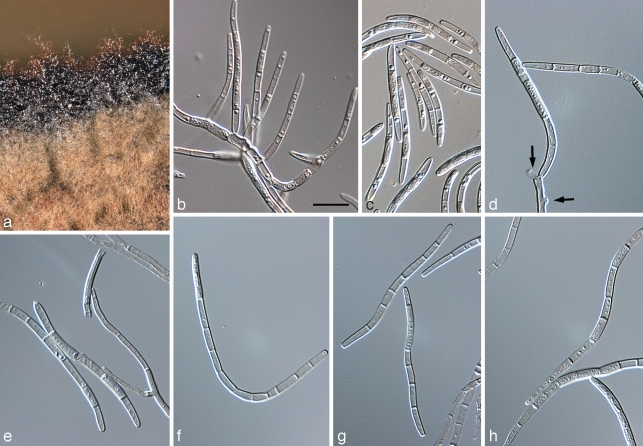
Colonies on apple surfaces of Microcyclosporella mali causing a ramose and Microcyclospora spp. causing a fuliginous sooty blotch phenotype. a–f. Microcyclosporella mali (a, d, CBS 126137; b, e, CBS 126130; c, f, CBS 126134); g, h. Microcyclospora malicola (CBS 126139); i, j. Microcyclospora tardicrescens (CBS 126142). –– Scale bars: a–c, g, i = 1 mm; d–f, h, j = 200 μm.
Devriesia pseudoamericana Jana Frank, B. Oertel, Schroers & Crous, sp. nov. — MycoBank MB516839; Fig. 4
Fig. 4.
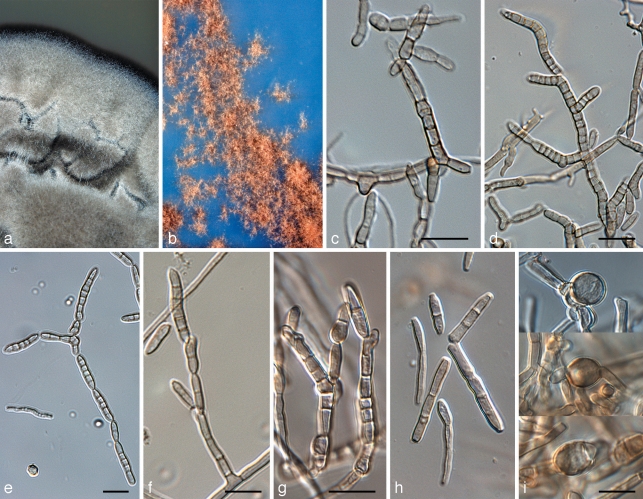
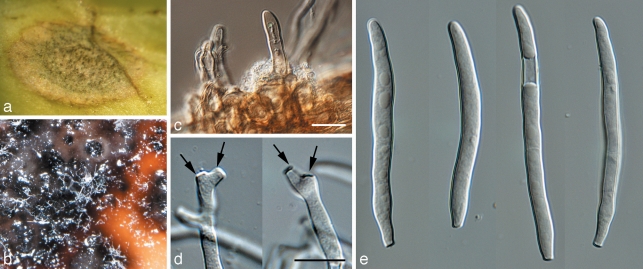
Devriesia pseudoamericana (CPC 16174). a. Colony on MEA; b. colony on SNA; c, d, f, g. conidiophores; e, h. conidia in chains; i. chlamydospores. — Scale bars = 10 μm.
Teleomorph. Unknown.
Devriesiae americanae morphologice similis, sed conidiis longioribus, (7–)10–20(–30) × 2–3 μm.
Etymology. Named after its morphological similarity, and close phylogenetic relationship to D. americana.
Colonies on OA. Mycelium consisting of branched, septate, brown, finely verruculose, 2–3 μm wide hyphae; chlamydospores intercalary, globose, 5–7 μm diam, brown, smooth. Conidiophores terminal and lateral on hyphae, highly variable in length, at times macronematous, but also micronematous, reduced to conidiogenous cells; cylindrical, straight to curved, brown, smooth, 10–50 × 2–3 μm, 0–6-septate. Conidiogenous cells terminal and lateral on conidiophores, 5–10 × 2–3 μm, proliferating sympodially with terminal and lateral polyblastic loci; scars 1–1.5 μm wide, flattened, somewhat darkened and thickened, not refractive. Conidia brown, finely verruculose, subcylindrical, at times somewhat swollen, appearing narrowly ellipsoid, straight to curved or once geniculate, irregular, apex obtuse, base truncate, 0–10-septate, (7–)10–20(–30) × 2–3 μm, occurring in irregular branched chains; hila somewhat thickened and darkened, 1–1.5 μm diam. Conidia on SNA poorly developed, much narrower, with less septa and more fusoid-ellipsoidal.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA spreading, with sparse to moderate aerial mycelium; surface folded, irregular, crumpled, at times breaking the agar surface, olivaceous-grey, with thin, iron-grey margin; reverse iron-grey; colonies reaching up to 25 mm diam after 1 mo. On OA spreading, flattened, with sparse aerial mycelium in middle of colony; aerial mycelium pale olivaceous-grey, outer region olivaceous-grey; colonies reaching 25 mm diam after 1 mo. On SNA appearing woolly, erumpent, not spreading, smoke-grey, with sparse aerial mycelium, reaching 8 mm diam after 1 mo. Cultures of D. pseudoamericana do not grow at 37 °C.
Specimens examined. Germany, Baden-Württemberg, Experimental Station Bavendorf, University of Stuttgart-Hohenheim, on Malus domestica fruit surface, 23 Oct. 1996, S. Kern, CBS H-20411 holotype, culture ex-type 31.1b = CPC 16174 = CBS 126270.
Notes — Members of Devriesia s.l. share morphological similarities in their conidiophores, branched conidial chains, somewhat darkened conidial hila and scars, as well as their terminal and intercalary chlamydospores formed in culture. According to LSU rDNA based phylogenetic analyses (Fig. 1), the genus is, however, paraphyletic. Devriesia comprises at least four lineages, of which three are distantly related to D. staurophora, the type species of the genus. The others are represented by i) D. pseudoamericana and D. americana (CBS 117726); ii) D. strelitziicola; and iii) D. hilliana, D. lagerstroemiae (Crous et al. 2009b), D. strelitziae (Arzanlou et al. 2008) and Teratosphaeria knoxdavesii (Crous et al. 2008), which lacks a known anamorph in culture. The ITS sequences of D. pseudoamericana and D. americana (GenBank AY251068) have an 88 % (441/496 nucleotides) identity. An ITS sequence lodged in GenBank (FN549915) as Devriesia sp. matches D. pseudoamericana with 99 % (463/467 nucleotides, 4/467 gaps) and appears to be the same species. The strain (CBS 529.82) from which that sequence derived is lodged in the CBS-KNAW database as Septonema ochraceum isolated from needles of Picea abies in the Netherlands. More collections are needed to determine whether this implies a wider host range for D. pseudo-americana. No type material is available for Septonema ochraceum and Koukol (2010) failed to find strains that could be regarded as representative of the species using fresh collections and cultures lodged in public collections under that name. Koukol (2010) included CBS 529.82 in his study and also observed the relationship of this strain to D. americana but failed to find morphological differences between these strains.
Taxa presently accommodated in different lineages of Devriesia s.l. share a different ecology to members of Devriesia s.str. (Seifert et al. 2004), which usually occur in soil, and are thermotolerant (able to grow at high temperatures, namely to survive exposure to 75 °C for 30 min; Samson et al. 2000). In contrast, members of Devriesia s.l. are usually associated with leaf spots, or occur on dead plant debris as saprobes, and are not able to grow above 37 °C (Crous et al. 2007b, 2009b, Koukol 2010). In spite of Devriesia being paraphyletic, we refrain from describing novel genera formally because more taxa and strains have to be added first for resolving possible synapomorphies supporting their phylogenetic concept morphologically.
Microcyclospora Jana Frank, Schroers & Crous, gen. nov. — MycoBank MB516842
Hyphomycetes. Mycelium ex hyphis ramosis, septatis, pallide brunneis, levibus, 2–3 μm latis compositum. Conidiophora in cellulis conidiogenis reducta, in hyphis lateraliter integrata, mono- vel polyblastica, subdenticulata, pallide brunnea, levia. Conidia scolecospora, cylindracea, recta vel diverse curvata, flexuosa, apice obtuso, basi truncata, uni- ad multiseptata, ad septa leniter constricta, levia, pallide brunnea, guttulata, aggregata in massa mucosa; hila inconspicua, neque incrassata neque fuscata; in cultura cum formatione microcyclica conidiorum.
Type species. Microcyclospora pomicola Jana Frank, Schroers & Crous, sp. nov.
Etymology. Named after its resemblance to Pseudocercospora, and prominent microcyclic conidiation.
Hyphomycetous. Mycelium consisting of branched, septate, pale brown, smooth, 2–3 μm wide hyphae. Conidiophores reduced to conidiogenous cells, integrated, mono- to polyblastic, lateral on hyphae, subdenticulate, 1 μm wide, 1–2 μm tall, pale brown, smooth. Conidia scolecosporous, cylindrical, straight to variously curved, flexuous, apex obtuse, base truncate, 1–multi-septate, somewhat constricted at septa, smooth, pale brown, guttulate, aggregated in mucoid masses; hila not thickened or darkened; microcyclic conidiation observed in culture.
Notes — Members of the genus Microcyclospora have in the past been accommodated in Pseudocercospora based on their pigmented conidiophores and pigmented, scolecosporous, transversely septate conidia (Batzer et al. 2005). Microcyclospora can be distinguished from Pseudocercospora s.str. (Crous et al. 2006b, 2007a) in that conidiophores are mostly reduced to solitary conidiogenous cells on hyphae which appear to be mono- to polyblastic (non fasciculate), conidia that occur in mucoid masses, and commonly undergo microcyclic conidiation. The closest phylogenetic neighbours of Microcyclospora are species of Penidiella (Fig. 1). Based on comparisons of our ITS sequences to those available on GenBank, more species than the three described here can be predicted in this genus (data not shown).
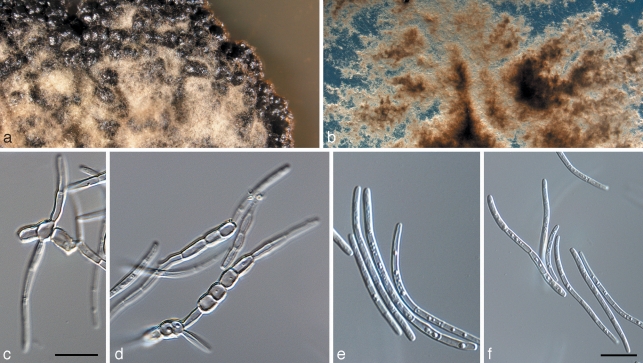
Microcyclospora malicola Jana Frank, Schroers & Crous, sp. nov. — MycoBank MB516843; Fig. 5
Fig. 5.
Microcyclospora malicola (CPC 16172). a. Colony on MEA; b, d. conidiogenous loci (arrows); c, e–h. conidia. — Scale bar = 10 μm.
Teleomorph. Unknown.
Mycelium ex hyphis ramosis, septatis, pallide brunneis, levibus, 2–3 μm latis compositum. Conidiophora in cellulis conidiogenis reducta, in hyphis lateraliter integrata, mono- vel polyblastica, subdenticulata, 1 μm lata, 1–2 μm alta, pallide brunnea, levia. Conidia scolecospora, cylindracea, recta vel diverse curvata, flexuosa, apice obtuso, basi truncata, (1–)5–7(–13)-septata, levia, pallide brunnea, guttulata, (30–)45–75(–120) × (2–)2.5(–3) μm; hila inconspicua.
Etymology. Named after its host, Malus.
Colonies on SNA. Mycelium consisting of branched, septate, pale brown, smooth, 2–3 μm wide hyphae. Conidiophores reduced to conidiogenous cells, integrated, lateral on hyphae, mono- to polyblastic, subdenticulate, 1 μm wide, 1–2 μm tall, pale brown, smooth. Conidia scolecosporous, cylindrical, straight to variously curved, flexuous, apex obtuse, base truncate, (1–)5–7(–13)-septate, somewhat constricted at septa, smooth, pale brown, guttulate, (30–)45–75(–120) × (2–)2.5 (–3) μm, after 7 d, and similar after 1 mo; hila not thickened nor darkened; microcyclic conidiation observed in culture.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA flat, spreading, with moderate, smoke-grey aerial mycelium that collapses with age, becoming iron-grey; margins smooth, regular, lobate; reverse iron-grey; colonies reaching 5–6 mm diam after 7 d, and up to 40 mm diam after 1 mo. On OA similar, aerial mycelium sparse, collapsing, becoming iron-grey; colonies reaching 5–6 mm diam after 7 d, and up 50 mm diam after 1 mo. Microcyclic conidiation commonly observed on all media in culture.
Specimens examined. Germany, Nordrhein-Westfalen, Obstpark Schloss Türnich, Kerpen-Türnich, on Malus domestica fruit surface, 30 Nov. 1997, S. Kern, CBS H-20410 holotype, culture ex-type 71fb = CPC 16172 = CBS 126138. – Slovenia, Harije near Ilirska Bistrica, on M. domestica fruit surface, 16 Oct. 2007, J. Frank, 365-07 = CPC 16186 = CBS 126139.
Notes — Considerable morphological variation was observed within M. malicola, and it was initially expected that isolate CPC 16186 could represent a distinct species to isolate CPC 16172 based on differences in conidial dimensions and growth rate in culture. However, based on sequence data of the ITS region they were identical, thus we chose to treat them as representative of a single taxon until more strains have been collected. A megablast search of NCBIs GenBank nucleotide database revealed six additional accessions with high identity to M. malicola (Fig. 2), currently filed at GenBank under ‘Pseudocercospora’. The strains from which GenBank FJ808758 and FJ808755 were generated probably represent M. malicola originating from Serbia, while sequence DQ363418 represents another strain from Germany. The three other accessions (GenBank FJ438380, AY598858 and AY598857 from the USA) probably represent currently undescribed Microcyclospora species because they accumulate up to 6 additional substitutions in their ITS sequences when compared with M. malicola. The TEF sequence of the type strain of M. malicola is 90 % identical (281/310 bases and 6 gaps) to the second strain of the species sequenced in this study and 87 % identical (119/136 bases and 2 gaps) and 84 % identical (245/289 bases and 13 gaps) to the sequences of the ex-type strains of M. pomicola and M. tardicrescens, respectively. It is quite possible that M. malicola represents a species complex, but this can only be resolved once more strains of the species are collected.
Microcyclospora pomicola Jana Frank, B. Oertel, Schroers & Crous, sp. nov. — MycoBank MB516844; Fig. 6
Fig. 6.
Microcyclospora pomicola (CPC 16175). a. Colony on OA; b, c. conidiogenous loci (arrows); d, e. conidia. — Scale bars = 10 μm.
Teleomorph. Unknown.
Microcyclosporae malicolae morphologice valde similis, sed dissimilitudinibus in sequentibus nucleotidium (ITS) in positionibus diversis sequentis culturae typicae distinguitur: 136 (T/C), 142 (C/T), 181 (A/G), 187 (T/C), 258 (T/A), 265 (C/T), 271 (A/G), 278 (A/G), 279 (A/T), 556 (A/C), 562 (G/A), 569 (C/T), 570 (T/C), 578 (G/C), extra A inter 581 et 582.
Etymology. Named after its occurrence on pome fruit (apples).
Colonies on SNA. Mycelium consisting of branched, septate, pale brown, smooth, 2–3 μm wide hyphae. Conidiophores reduced to conidiogenous cells, integrated, lateral on hyphae, mono- to polyblastic, subdenticulate, 1 μm wide, 1–2 μm tall, pale brown, smooth. Conidia scolecosporous, cylindrical, straight to variously curved, flexuous, apex obtuse, base truncate, somewhat constricted at septa, smooth, pale brown, guttulate, (15–)35–55(–65) × (2–)2.5(–3) μm, (3–)4–7-septate after 7 d, (15–)50–75(–120) × (2–)2.5–3 μm, 1–13-septate after 1 mo; hila not thickened nor darkened; microcyclic conidiation observed in culture.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA spreading, with sparse to moderate aerial mycelium; surface smooth due to collapsing, wet aerial mycelium; margin regular, lobate; surface olivaceous-grey, becoming iron-grey due to collapsing aerial mycelium; reverse iron-grey; colonies reaching 6–8 mm diam after 7 d, and up to 40 mm diam after 1 mo. On OA flattened, submerged, spreading with sparse aerial mycelium and even, smooth margins; colony growth rate similar to that observed on MEA.
Specimens examined. Germany, Baden-Württemberg, Friedrichshafen, on Malus domestica fruit surface, 5 Nov. 1996, S. Kern, CBS H-20412 holotype, culture ex-type 43.1a = CPC 16175 = CBS 126141; Baden-Württemberg, Friedrichshafen, on M. domestica fruit surface, 5 Nov. 1996, S. Kern, 51.2a = CPC 16173 = CBS 126140.
Notes — The TEF sequence of the type strain of M. pomicola is 90 % identical (351/387 bases and 136 gaps) to the second strain of the species sequenced in this study, and 87 % identical (119/136 bases and 2 gaps) and 84 % identical (150/177 bases and 11 gaps) to the sequences of the ex-type strains of M. malicola and M. tardicrescens, respectively. It is quite possible that M. pomicola represents a species complex, but this can only be resolved once more strains of the species are collected.
Microcyclospora tardicrescens Jana Frank, Schroers & Crous, sp. nov. — MycoBank MB516845; Fig. 7
Fig. 7.
Microcyclospora tardicrescens (CPC 16187). a. Colony on MEA; b. colony on SNA; c, d. conidiogenous cells giving rise to conidia; e, f. conidia. — Scale bars = 10 μm.
Teleomorph. Unknown.
Microcyclosporae malicolae morphologice similis, sed conidiis minoribus, (15–)35–55(–60) × (1.5–)2(–2.5) μm, in cultura tarde crescenti et tamen signis nucleotidium singulariter affixis in ITS sequentibus culturae typicae in positionibus 190 (C), 213 (quod additur C), 557 (C), 559 (T) distinguitur.
Etymology. Named after its slow growth rate in culture.
Colonies on SNA. Mycelium consisting of branched, septate, pale brown, smooth, 2–3 μm wide hyphae. Conidiophores reduced to conidiogenous cells, integrated, lateral on hyphae, mono- to polyblastic, subdenticulate, 1 μm wide, 1–2 μm tall, pale brown, smooth. Conidia scolecosporous, cylindrical, straight to variously curved, flexuous, apex obtuse, base truncate, 1–6-septate, somewhat constricted at septa, smooth, pale brown, guttulate, (15–)35–55(–60) × (1.5–)2(–2.5) μm; hila not thickened nor darkened; microcyclic conidiation observed in culture; older conidia develop intercalary chlamydospores that are medium brown, up to 5 μm wide.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA erumpent, with sparse smoke-grey aerial mycelium; surface irregular, umbonate, iron-grey; margin lobate; reverse iron-grey; colonies reaching up to 3 mm after 7 d, and 20 mm diam after 1 mo. On OA similar, slimy, iron-grey, lacking aerial mycelium; colonies reaching up to 2 mm after 7 d, and 12 mm diam after 1 mo.
Specimen examined. Slovenia, Harije near Ilirska Bistrica, on Malus domestica fruit surface, 16 Oct. 2007, J. Frank, CBS H-20414 holotype, culture ex-type 364-07 = CPC 16187 = CBS 126142.
Notes — After 30 d on SNA, M. malicola and M. pomicola have larger conidia (15–120 × 2–3 μm), than M. tardicrescens (15–60 × 1.5–2.5 μm). Furthermore, M. tardicrescens also forms intercalary chlamydospores, which are lacking in the other two species, and has a slower growth rate in culture.
Although M. malicola and M. pomicola are similar in morphology and culture characteristics after 30 d, they can be distinguished after 7 d on SNA. Conidia of M. malicola are longer, (30–)45–75(–120) μm, 1–13-septate, than those of M. pomicola, (15–)35–55(–65) μm, 3–7-septate. Furthermore, after 7 d on MEA, colonies of M. malicola are 5–6 mm diam, while those of M. pomicola grow somewhat faster, reaching 6–8 mm diam. The TEF sequence of the ex-type strain of M. tardicrescens is 84 % identical (245/289 bases and 13 gaps) and 84 % identical (150/177 bases and 11 gaps) to the sequences of the ex-type strains of M. pomicola and M. malicola, respectively.
Microcyclosporella Jana Frank, Schroers & Crous, gen. nov. — MycoBank MB516840
Hyphomycetes. Mycelium ex hyphis pallide brunneis, levibus vel subtiliter verruculosis, ramosis, septatis, 2–3.5 μm latis compositum, interdum strato mucoso, locis conidiogenis ex transverse integratis, truncatis. Conidiophora saepe in cellulis conidiogenis reducta. Cellulae conidiogenae integratae, intercalares in hyphis, raro terminales, cylindraceae vel doliiformes, pallide brunneae, sed hyalinae in partibus sentinoidibus coloniae, leves, mono- vel polyblasticae, sympodiales, locis conidiogenis inconspicuis, truncatis, non incrassatis, non refractivis, pallide brunneis vel hyalinis. Conidia hyalina, levia, subcylindracea, anguste obclavata vel fusiformia, apice acute rotundato, basi obconice truncata, guttulata, 0–6 transverse septata, vulgo cum formatione microcyclica conidiorum.
Type species. Microcyclosporella mali Jana Frank, Schroers & Crous, sp. nov.
Etymology. Named after its resemblance to Pseudocercosporella, and its prominent microcyclic conidiation.
Hyphomycetous. Mycelium consisting of pale brown, smooth to finely verruculose, branched, septate, 2–3.5 μm wide hyphae, at times covered in a mucoid layer, with integrated, lateral, truncate conidiogenous loci. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells integrated, intercalary on hyphae, rarely terminal, cylindrical to doliiform, pale brown, but hyaline if occurring in yeast-like sectors of colonies, smooth, mono- or polyblastic, proliferating sympodially; loci inconspicuous, truncate, unthickened, not darkened, pale brown to hyaline. Conidia hyaline, smooth, subcylindrical to narrowly obclavate or narrowly fusoid with acutely rounded apex and obconically truncate base, guttulate, 0–6 transversely septate; microcyclic conidiation common.
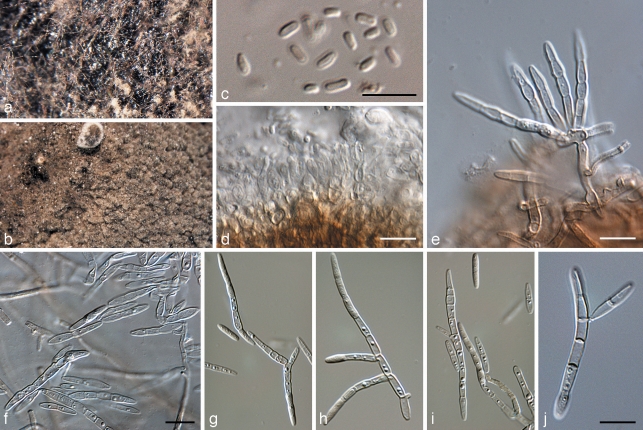
Microcyclosporella mali Jana Frank, Schroers & Crous, sp. nov. — MycoBank MB516841; Fig. 8
Fig. 8.
Microcyclosporella mali (CPC 16184). a. Colony on OA; b. colony on MEA; c. spermatia; d. spermatogenous cells; e. conidiophore with conidia; f–j. conidia with microcyclic conidiation. — Scale bars = 10 μm.
Teleomorph. Unknown.
Mycelium ex hyphis pallide brunneis, levibus vel subtiliter verruculosis, ramosis, septatis, 2–3.5 μm latis compositum. Conidiophora saepe in cellulis conidiogenis reducta. Cellulae conidiogenae integratae, intercalares in hyphis, raro terminales, cylindraceae vel doliiformes, hyalinae vel pallide brunneae, leves, mono- vel polyblasticae, sympodiales. Conidia hyalina, levia, subcylindracea, anguste obclavata vel fusiformia, apice acute rotundato, basi obconice truncata, guttulata, aseptata usque ad multiseptata, hilis inconspicuis.
Etymology. Named after its host, Malus.
Colonies on SNA. Mycelium consisting of pale brown, smooth to finely verruculose, branched, septate, 2–3.5 μm wide hyphae, at times covered in a mucoid layer, with integrated, lateral, truncate conidiogenous loci. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells integrated, intercalary on hyphae, 5–7 × 1–2 μm, rarely terminal, cylindrical to doliiform, hyaline to pale brown, smooth, mono- or polyblastic, proliferating sympodially; loci inconspicuous, truncate, unthickened, not darkened, 1–2 μm diam; conidiogenous cells pale brown if integrated on mycelium, but hyaline when occurring in slimy, yeast-like parts of colonies, which give rise to conidia via microcyclic conidiation as well as via hyphal loci. Conidia hyaline, smooth, subcylindrical to narrowly obclavate or narrowly fusoid with acutely rounded apex and obconically truncate base, guttulate, (0–)3–6(–10)-septate, (15–)17–25(–40) × 2.5(–3) μm; conidial hila unthickened, not darkened, 1–2 μm diam; microcyclic conidiation common, sporulating profusely on SNA.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA with sparse aerial mycelium and even, lobate to somewhat feathery margins; surface folded, crumpled, olivaceous-grey with patches of iron-grey and pale olivaceous-grey; reverse olivaceous-black; colonies reaching up to 30 mm diam after 1 mo. On OA similar, except surface not folded and crumpled, but more flattened and spreading; colonies reaching 40 mm diam after 1 mo. On SNA immersed, with sparse grey-olivaceous mycelium and feathery margins, reaching 30 mm diam after 1 mo; colonies developing patches that appear yeast-like, white and slimy. Isolate CBS 126136 exhibited the ability to form microconidia or spermatia in culture, which were hyaline, bacilliform, aseptate, 2–3 × 1–1.5 μm (Fig. 8). This was rarely observed, however, and the formation and role of these structures remain unresolved.
Specimens examined. Slovenia, Ptujska Gora, on Malus domestica fruit surface, 7 Aug. 2007, J. Frank, CBS H-20413 holotype, culture ex-type 300-07 = CPC 16184 = CBS 126136; Senožeti near Dolsko, on M. domestica fruit surface, 12 July 2007, J. Frank, 41-07 = CPC 16177 = CBS 126130; Planina near Rakek, on M. domestica fruit surface, 16 Oct. 2007, J. Frank, 85-07 = CPC 16181 = CBS 126133; Lutverci, on M. domestica fruit surface, 1 Aug. 2007, J. Frank, 406-07 = CPC 16178 = CBS 126131; Čikečka vas, on M. domestica fruit surface, 1 Aug. 2007, J. Frank, 408-07 = CPC 16182 = CBS 126134; Mirna, on M. domestica fruit surface, 17 Oct. 2007, J. Frank, 176-07 = CPC 16185 = CBS 126137; Mirna, on M. domestica fruit surface, 17 Oct. 2007, J. Frank, 174-07 = CPC 16180 = CBS 126132; Čikečka vas, on M. domestica fruit surface, 1 Aug. 2007, J. Frank, 407-07 = CPC 16183 = CBS 126135. – Germany, Baden-Württemberg, Experimental Station Bavendorf, University of Stuttgart-Hohenheim, on M. domestica fruit surface, 30 Nov. 1997, S. Kern, 63pgp = CPC 16171 = CBS 126129.
Notes — Members of the genus Microcyclosporella have thus far been referred to as representative of Pseudocercosporella due to their hyaline conidiophores, and transversely septate, hyaline scolecosporous conidia with inconspicuous hila (Batzer et al. 2005). Although the genus Pseudocercosporella has been shown to be polyphyletic within the Mycosphaerellaceae (Crous 2009, Crous et al. 2003, 2009b, c), it has thus far not been possible to resolve the correct placement of the SBFS isolates, as the type species of Pseudocercosporella, P. ipomoeae (= P. bakeri, see Braun 1995), has not been known from culture. In the present study this matter has finally been resolved, as a fresh collection from its centre of origin has allowed us to designate an epitype for P. bakeri (see below). The closest phylogenetic sisters of Microcyclosporella are Mycosphaerella intermedia, Mycosphaerella marksii and Mycosphaerella madeirae (Fig. 1). A megablast search of NCBIs GenBank nucleotide database revealed several accessions with high identity to M. mali (Fig. 2). Although these accessions are present in GenBank under the name ‘Pseudocercosporella’, they represent M. mali or potentially other cryptic species related to Microcyclosporella. If many of these accessions are indeed representative of M. mali, it would expand its geographic distribution to include Poland, Serbia and the USA. These accessions share most or all of the nucleotide sequence variation of M. mali s.str. with two exceptions, namely ‘Pseudocercosporella sp. RH6′ (GenBank FJ425201, showing 11 substitutions in the ITS compared with M. mali) and ‘Pseudocercosporella sp. RH2.2′ (GenBank FJ425197, showing 10 substitutions), which could represent novel species related to M. mali. The lower identity of the accession from Poland (GenBank FJ515279, 5 substitutions in the ITS compared with M. mali) could be ascribed to three deletions at the beginning of the sequence that is most likely due to low quality raw sequence in this area; for the rest the sequence fits many of the other sequences for M. mali (Fig. 2). The TEF sequences of the isolates of M. mali sequenced in this study differed at five fixed nucleotide positions over the 324 bases, which essentially splits the species into two groups, namely CPC 16171, 16178, 16181 and 16184 vs CPC 16177, 16180, 16182, 16183 and 16185. However, based on the current sampling it is unclear whether this only constitutes intraspecific variation.
Pseudocercosporella Deighton, Mycol. Pap. 133: 38. 1973
Type species. Pseudocercosporella ipomoeae Deighton.
Colonies in vivo. Mycelium consisting of primary internal and secondary external hyphae, hyaline to pale brown, septate, branched, smooth; stromata lacking or weakly to well-developed, substomatal to intraepidermal. Conidiophores solitary to fasciculate, emerging through stomata or erumpent through the cuticle, arising from inner hyphae or from stromata, sometimes formed as lateral branches of superficial hyphae, or forming crustose to subglobose sporodochia; conidiophores rarely branched, straight and subcylindric to geniculate-sinuous, hyaline, occasionally faintly pigmented, reduced to conidiogenous cells, or septate. Conidiogenous cells integrated, terminal, mono- to polyblastic, sympodial; conidiogenous loci inconspicuous, unthickened, hyaline. Conidia formed singly, rarely in simple or branched chains, subcylindrical, filiform, somewhat obclavate, euseptate, 1–multi-septate, hyaline, thin-walled, apex obtuse to subacute, base subtruncate, hilum unthickened, not darkened, nor refractive. Adapted from Braun (1995).
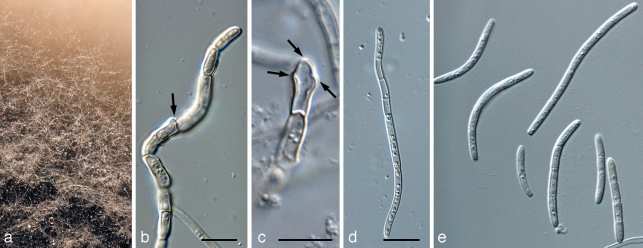
Pseudocercosporella bakeri (Syd. & P. Syd.) Deighton, Mycol. Pap. 133: 41. 1973 — Fig. 9
Fig. 9.
Pseudocercosporella bakeri (CPC 17570). a. Leaf spot on Ipomoeae sp. with visible sporulation; b. colony on OA; c. conidiophores in vivo; d. conidiophores in vitro (arrows denote loci); e. conidia in vitro. — Scale bars = 10 μm.
Basionym. Cylindrosporium bakeri Syd. & P. Syd., Ann. Mycol. 14: 372. 1916.
= Ramularia ipomoeae F. Stevens, Bull. Bernice P. Bishop Mus. 19: 150. 1925.
= Cercosporella ipomoeae Sawada, Rep. Dept. Agric. Gov. Res. Inst. Formosa 86: 161. 1943, nom. inval.! (Art. 36.1).
= Cercosporella ipomoeicola Sawada, Special Publ. Coll. Agric. Natn. Taiwan Univ. 8: 192. 1959, nom. inval.! (Art. 36.1).
= Pseudocercosporella ipomoeae Deighton, Mycol. Pap. 133: 39. 1973.
Leaf spots amphigenous, subcircular, angular to irregular, 2–20 mm diam, pale greenish, becoming pale brown, finally greyish white; margin indefinite or with a narrow brown border. Caespituli hypophyllous, punctiform to effuse, whitish. Mycelium internal, consisting of hyaline, septate, sparingly branched, 1.5–3.5 μm wide hyphae; stromata absent or small, intraepidermal, 10–40 μm diam, consisting of swollen, hyaline hyphal cells. Conidiophores usually aggregated, occasionally subfasciculate, up to about 20, arising from inner hyphae or hyphal aggregations, erumpent through the cuticle, short, erect, subcylindrical, conical, straight, curved to geniculate-sinuous, hyaline, (2.5–)20–40 × (2–)2.5–4(–6) μm, 0–2-septate. Conidiogenous cells terminal, integrated, (2.5–)10–20 × 3.5–4 μm; conidiogenous loci usually more or less truncate, 1.5–3 μm diam, unthickened, not darkened. Conidia solitary, subcylindrical, somewhat acicular to slightly obclavate, (35–)40–65 × (2.5–)3.5–4 μm, (1–)3-septate in vivo, not constricted, hyaline, smooth, apex obtuse, base truncate or slightly obconically truncate, unthickened (adapted from Braun 1995). In vitro on OA, conidia (30–)46–60(–75) × (3.5–)4(–4.5) μm, (1–)3–7-septate, subcylindrical, guttulate, hyaline, smooth, at times narrowly obclavate, tapering in apical part to acutely rounded apex, and in basal part to long obconically subtruncate or subcylindrical base; base truncate, 2–3 μm wide, but with marginal thickening along the rim, which is also seen on scars on conidiogenous cells (but not observed in vivo).
Specimens examined. Philippines, Los Baños, Ipomoea sp., Dec. 1915, Baker 4029, lectotype of Cylindrosporium bakeri (S). – Laos, Vientiane Capital, Xaythany District, Xay Villiage, on Ipomoea sp., 8 Sept. 2009, P. Phengsintham, CBS H-20409 epitype designated here, culture ex-epitype CPC 17570 = CBS 125685.
Notes — Pseudocercosporella ipomoeae was described as a new species by Deighton (1973) based on its shorter, narrower conidia. However, an examination of type materials and additional collections of P. bakeri and P. ipomoeae, led Braun (1995) to the conclusion that they represented a single taxon. As shown in the present study, conidial dimensions vary considerably from host material to culture, and hence we support the conclusion of Braun (1995), and treat this as a single species, P. bakeri, for which an epitype is designated. This species clusters as a close sister to the ‘Dothistroma clade’ (Clade 7 in Crous et al. 2009c).
DISCUSSION
The present study treats three genera of fungi associated with SBFS of apple in Germany and Slovenia, namely Devriesia, Microcyclospora and Microcyclosporella. However, based on sequence similarity to related taxa associated with SBFS in public databases (Batzer et al. 2005, Díaz Arias et al. 2010), it is clear that these fungi have a much wider distribution with apples (see sequences from other hosts currently in GenBank; Fig. 2), and that many other species await description in the new genera introduced in this paper.
One genus newly linked to the SBFS complex on apples is Devriesia. As discussed previously, however, the genus Devriesia is paraphyletic (Crous et al. 2007b, Koukol 2010), and further taxa need to be collected to provide more robust clades, and help delineate the morphological features needed to separate the non-thermotolerant genera from Devriesia s.str., which seems to be primarily adapted to burnt soil environments although its type species, D. staurophora, has been also isolated from dead leaves of Pinus sylvestris (Seifert et al. 2004). Devriesia s.str., however, is not commonly associated with leaf spots and blemishes on fruit surfaces as is the case in other lineages in Devriesia s.l.
The newly introduced genus Microcyclosporella shows clear similarities with genera such as Pseudocercosporella and Ramulispora. Deighton (1973) established the genus Pseudocercosporella for anamorphs of the Mycosphaerella complex that were Cercosporella-like, but had unthickened and inconspicuous conidial scars. The four cercosporoid species known to be associated with eyespot disease of cereals (Nirenberg 1981, Robbertse et al. 1995, Lucas et al. 2000) were subsequently included in Pseudocercosporella, even though von Arx (1983) preferred to place them in Ramulispora. The genus Ramulispora is based on R. sorghi, which causes sooty stripe of sorghum, due to the abundant production of microsclerotia on the leaf surface (Braun 1995). In a subsequent study, Crous et al. (2003) showed the eyespot fungi of wheat to represent a separate genus, Helgardia, which has apothecial teleomorphs in Oculimacula (Helotiales, Dermateaceae), while Ramulispora represents a genus in the Mycosphaerellaceae (Crous et al. 2009b, c), distinct from Pseudocercosporella. Interestingly enough, both Ramulispora and Helgardia exhibit microcyclic conidiation (Robbertse et al. 1995), as does the newly introduced Microcyclosporella. It appears that this character is of less taxonomic value at the generic level, and probably more ecologically relevant for pathogens that sporulate on superficial plant surfaces (wheat stems, sorghum leaves and apples), facilitating onward splash dispersal.
The genus Pseudocercospora has recently been shown to include taxa that vary greatly in their conidiomatal morphology, ranging from solitary conidiogenous loci, synnemata, sporodochia or fascicles. Furthermore, conidia were shown to include taxa that are transversely euseptate, but also with some muriform septa, or containing a mixture of eu- and distoseptation. Conidial hila and scars vary from being inconspicuous, to being slightly thickened along the rim (Stewart et al. 1999), and conidia, although solitary, could in some cases also occur in unbranched chains (Braun 1995), though this has rarely been observed to date (Crous et al. 2006b). This new circumscription led to several genera being reduced to synonymy with Pseudocercospora, most notably Cercostigmina (Taylor et al. 2003), Phaeoisariopsis and Stigmina (Crous & Corlett 1998, Crous et al. 2006b). Species associated with SBFS on apple have thus far been placed in Pseudocercospora based on the pigmentation observed in their conidia and conidiophores, and their transversely septate scolecosporous conidia with unthickened hila. Because these taxa have been shown to cluster apart from Pseudocercospora s.str. (based on P. vitis) in the present study, a new genus, Microcyclospora, has been introduced to accommodate the SBFS species. Morphologically, Microcyclospora can be distinguished from Pseudocercospora s.str. in that conidiophores are never fasciculate, but are reduced to solitary conidiogenous loci on hyphae, and that conidia occur in mucoid masses, which prominently undergo microcyclic conidiation.
Little is presently known about the ecology, epidemiology and host ranges of the SBFS fungi, and more sampling on other substrates or crops growing in the vicinity of apple orchards needs to occur to enable us to resolve these aspects. For Schizothyrium pomi for instance, up to 78 different plant hosts have been reported, of which many occurred in close vicinity of apple orchards in temperate North American climates (Baker et al. 1977). However, no modern approaches have been applied until now to test this hypothesis, while molecular analyses indicated that a collection of North American Schizothyrium pomi strains comprised more than 10 genotypes, of which four were elevated to species level (Batzer et al. 2008). These results clearly suggest that all published geographic and host distribution records of SBFS fungi will have to be treated with caution until they have been re-evaluated based on the new molecular approach currently employed to resolve species and generic boundaries.
Acknowledgments
Parts of this study were supported by the Slovenian Research Agency (ARRS) in the form of the Young Researcher grant 1000-06-310056 to J.F. We are grateful to A. van Iperen, M. Vermaas, M. Starink (CBS, Utrecht) and J. Wolter-Sadlers (INRES, Bonn) for providing technical assistance.
REFERENCES
- Arx JA von . 1983. Mycosphaerella and its anamorphs. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Series C 86, 1: 15 – 54 . [Google Scholar]
- Arzanlou M, Crous PW, Groenewald JZ . 2008. Devriesia strelitziae. Fungal Planet No. 22 CBS, Utrecht, Netherlands: . [Google Scholar]
- Baker KF, Davis LH, Durbin RD, Snyder WC . 1977. Greasy blotch of carnation and flyspeck disease of apple: diseases caused by Zygophiala jamaicensis. Phytopathology 67: 580 – 588 . [Google Scholar]
- Batzer JC, Díaz-Arias MM, Harrington TC, Gleason ML, Groenewald JZ, Crous PW . 2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 246 – 258 . [DOI] [PubMed] [Google Scholar]
- Batzer JC, Gleason ML, Harrington TC, Tiffany LH . 2005. Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia 97: 1268 – 1286 . [DOI] [PubMed] [Google Scholar]
- Braun U . 1995. A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes) Vol. 1 IHW Verlag, Eching, Germany [Google Scholar]
- Colby AS . 1920. Sooty blotch of pomaceous fruits. Transactions of the Illinois State Academy of Science 13: 139 – 179 . [Google Scholar]
- Crous PW . 2009. Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Diversity 38: 1 – 24 . [Google Scholar]
- Crous PW, Braun U, Groenewald JZ . 2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ . 2007b. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33 – 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Corlett M . 1998. Reassessment of Mycosphaerella spp. and their anamorphs occurring on Platanus. Canadian Journal of Botany 76: 1523 – 1532 . [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G . 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 . [Google Scholar]
- Crous PW, Groenewald JZ, Gams W . 2003. Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology 109: 841 – 850 . [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD . 2006a. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213 – 226 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ . 2009a. Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Liebenberg MM, Braun U, Groenewald JZ . 2006b. Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology 55: 163 – 173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, Hoog GS de, Groenewald JZ . 2009b. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17 – 47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ . 2006c. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ . 2009c. Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99 – 118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ . 2008. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkleij GJM, Groenewald JZ, Samson RA. (eds). 2009d. Fungal Biodiversity. CBS Laboratory Manual Series 1 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: . [Google Scholar]
- Deighton FC . 1973. Studies on Cercospora and allied genera. IV. Cercosporella Sacc., Pseudocercosporella gen. nov. and Pseudocercosporidium gen. nov. Mycological Papers 133: 1 – 62 . [Google Scholar]
- Díaz Arias MM, Batzer JC, Harrington TC, Wong AW, Bost SC. et al. 2010. Diversity and biogeography of sooty blotch and flyspeck fungi on apple in the eastern and midwestern United States. Phytopathology 100: 345 – 355 . [DOI] [PubMed] [Google Scholar]
- Feldmann T . 2005. Biological, chemical and physiological studies on epiphytic asco- and deuteromycetes as causal organisms of sooty blotch and pink rot in apple fruit Dissertation, University of Bonn; . [Google Scholar]
- Groves AB . 1933. A study of the sooty blotch disease of apples and the causal fungus Gloeodes pomigena. Virginia Agricultural Experiment Station, Technical Bulletin 50: 1 – 43 . [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG . 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183 – 189 . [DOI] [PubMed] [Google Scholar]
- Johnson EM, Sutton TB, Hodges CS . 1997. Etiology of apple sooty blotch disease in North Carolina. Phytopathology 78: 88 – 95 . [DOI] [PubMed] [Google Scholar]
- Koukol O . 2010. Revision of “Septonema ochraceum” revealed three new species of Venturiaceae and Herpotrichiellaceae. Mycological Progress: doi 10.1007/s11557-009-0645-x . [Google Scholar]
- Lucas JA, Dyer P, Murray TD . 2000. Pathogenicity, host specificity, and population biology of Tapesia spp., causal agents of eyespot disease of cereals. Advances in Botanical Research 33: 226 – 258 . [Google Scholar]
- Nirenberg HI . 1981. Differentiation of Pseudocercosporella strains causing foot rot disease of cereals. 1. Morphology. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 88: 241 – 248 . [Google Scholar]
- Rayner RW . 1970. A mycological colour chart CMI and British Mycological Society, Kew, Surrey, England: . [Google Scholar]
- Rehner SA, Samuels GJ . 1994. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625 – 634 . [Google Scholar]
- Robbertse B, Campbell GF, Crous PW . 1995. Revision of Pseudocercosporella-like species causing eyespot disease of wheat. South African Journal of Botany 61: 43 – 48 . [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O . 2000. Introduction to food-borne fungi 6th ed Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: . [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZS, Boehm EWA, Burgess TI , et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1 – 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinitz LD . 1832. Synopsis Fungorum in America Boreali media degentium. Transactions of the American Philosophical Society NS 4: 232 . [Google Scholar]
- Seifert KA, Nickerson NL, Corlett M, Jackson ED, Louis-Seize G, Davies RJ . 2004. Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany 82: 914 – 926 . [Google Scholar]
- Stewart EL, Liu Z, Crous PW, Szabo L . 1999. Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research 103: 1491 – 1499 . [Google Scholar]
- Sun GY, Zhang R, Li H, Gleason ML . 2008. Diversity of fungi causing flyspeck-like signs on apple in China. Phytopathology 98: S153 . [Google Scholar]
- Taylor JE, Groenewald JZ, Crous PW . 2003. A phylogenetic analysis of Mycosphaerellaceae leaf spot pathogens of Proteaceae. Mycological Research 107: 653 – 658 . [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M . 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238 – 4246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J . 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA: . [Google Scholar]
- Williamson SM, Sutton TB . 2000. Sooty blotch and flyspeck of apple: Etiology, biology and control. Plant Disease 84: 714 – 724 . [DOI] [PubMed] [Google Scholar]
- Yang HL, Sun GY, Batzer JC, Crous PW, Groenewald JZ, Gleason ML . 2010. Novel fungal genera and species associated with the sooty blotch and flyspeck complex on apple in China and the USA. Persoonia 24: 29 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Jensen HH, Mueller DS, Nonnecke GR, Bonnet D, Gleason ML . 2007. Estimating consumers’ valuation of organic and cosmetically damaged apples. HortScience 42: 1366 – 1371 . [Google Scholar]