Abstract
Fungi in the sooty blotch and flyspeck (SBFS) complex cause blemishes on apple and pear fruit that result in economic losses for growers. The SBFS fungi colonise the epicuticular wax layer of pomaceous fruit but do not invade the cuticle. Fungi causing fuliginous and punctate mycelial types on apple are particularly difficult to identify based on morphological criteria because many species in the SBFS complex share the same mycelial phenotypes. We compared the morphology and nuclear ribosomal DNA phylogeny (ITS, LSU) of 11 fungal strains isolated from SBFS blemishes on apple obtained from two provinces in China and five states in the USA. Parsimony analysis, supported by cultural characteristics and morphology in vitro, provided support to delimit the isolates into three novel genera, representing five new species. Phaeothecoidiella, with two species, P. missouriensis and P. illinoisensis, is introduced as a new genus with pigmented endoconidia in the Dothideomycetes. Houjia (Capnodiales) is introduced for H. pomigena and H. yanglingensis. Although morphologically similar to Stanjehughesia (Chaetosphaeriaceae), Houjia is distinct in having solitary conidiogenous cells. Sporidesmajora (Capnodiales), based on S. pennsylvaniensis, is distinguished from Sporidesmium (Sordariomycetes) in having long, multiseptate conidiophores that frequently have a subconical, darkly pigmented apical cell, and very long, multi-euseptate conidia.
Keywords: anamorph, SBFS, taxonomy
INTRODUCTION
Fungi in the sooty blotch and flyspeck (SBFS) complex occur in humid temperate regions and blemish the appearance of pomaceous fruits, resulting in economic losses for growers. The SBFS fungi colonise the epicuticular wax layer of apple fruit. Colonies that form dark mycelial mats are referred to as ‘sooty blotch’, whereas ‘flyspeck’ designates clusters of shiny, black, round to ovoid, sclerotium-like bodies lacking a visible mycelial mat.
Progress in understanding the disease has been slowed by difficulty in isolating, maintaining and identifying SBFS fungi in culture, as well as paucity of fruiting structures on fruit and in culture (Hickey 1960). Prior to combining DNA sequencing with culture-based morphology studies, the SBFS complex was assumed to be comprised of fewer than five species (Colby 1920, Johnson et al. 1996, 1997). When anamorph states of purified isolates collected from blemished apples in the USA and China were studied and parsimony analysis of rDNA genotypes was conducted, it became clear that the SBFS complex comprised > 60 species of fungi, mostly within the Capnodiales (Batzer et al. 2005, 2008, Sun et al. 2008, Zhai et al. 2008, Díaz Arias et al. In press, Li et al. In press, Ma et al. In press).
Despite the recent discovery that the SBFS complex is highly diverse, little is known about the geographic range of individual species. Surveys in eastern USA revealed that some SBFS species were ubiquitous whereas others were localised to sub-regions (Díaz Arias et al. In press). The SBFS fungus Zygophiala cryptogama occurs in Asia and as well as North America (Batzer et al. 2008, Li et al. In press), and Peltaster fructicola and Schizothyrium pomi occur in both the USA and the Balkans (Ivanović et al. In press), but the range of most species in the complex has not been defined. However, recent surveys in China revealed several fungi that were highly similar genetically and morphologically to isolates from the USA (Sun & Gleason, unpubl. data). The aim of this study was to describe a novel group of closely related SBFS fungi in China and the USA by assessing nuclear ribosomal DNA sequences and morphology.
MATERIALS AND METHODS
Isolates
During the fall of 2007 apples infected with SBFS were collected from orchards in Jingning County, Gansu Province, and Lingboa, Henan Province, China. Thalli were transferred from colonies on the apple surface to potato-dextrose agar (PDA; Crous et al. 2009c) slants and cultured at 25 °C in darkness (Sun et al. 2003). Two isolates from China were selected for this study, along with nine isolates sampled in 2000 and 2005 from five orchards in Missouri, Illinois, Kentucky, Tennessee and Pennsylvania, USA (Batzer et al. 2005, Díaz Arias et al. In press). All isolates were purified and stored in glycerol at −80 °C at Iowa State University. Segments of apple peels exhibiting colonies with sooty blotch morphology were preserved by pressing the thallus and supporting peel between paper towels until dry. Specimens on apple peels were deposited at the Iowa State University Herbarium, Ames, Iowa.
Single-conidial isolates were established on malt extract agar (MEA; 20 g/L Biolab malt extract, 15 g/L Biolab agar) using the technique of Crous (1998). Cultures were plated onto fresh MEA, 2 % PDA and oatmeal agar (OA; Crous et al. 2009c), and subsequently incubated at 25 °C under near-ultraviolet light to promote sporulation. Reference strains are maintained in the culture collection of the Centraalbureau voor Schimmelcultures (CBS), Utrecht, the Netherlands, and at Iowa State University (Table 1). Descriptions, nomenclature, and illustrations were deposited in MycoBank (Crous et al. 2004).
Table 1.
Collection details and GenBank accession numbers of isolates for which novel sequences were generated in this study.
| Species | Strain no.1 | Substrate | Country | Collector | GenBank Accession number (ITS, LSU)2 |
|---|---|---|---|---|---|
| Houjia pomigena | CBS 125224; CPC 16109; CMG UIF2b | Malus sp. (fuliginous morphology on apple) | Illinois, USA | M. Gleason | AY598885, AY598925 |
| Houjia yanglingensis | CBS 125225; CPC 16114; CMG YHJB13 | Malus sp. (fuliginous morphology on apple) | Jingning, China | G.Y. Sun | GQ433628, GQ433631 |
| CBS 125226; CPC 16113; CMG LB20 | Malus sp. (fuliginous morphology on apple) | Lingbao, China | G.Y. Sun | GQ433629, GQ433630 | |
| CBS 125227; CPC 16111; CMG TN1 2.2F1d | Malus sp. (fuliginous morphology on apple) | Tennessee, USA | S. Bost | FJ438378, FJ147166 | |
| CBS 125228; CPC 16110; CMG KY3 13F1d | Malus sp. (fuliginous morphology on apple) | Kentucky, USA | J. Hartman | FJ438377, FJ147165 | |
| Phaeotheca fissurella | CBS 520.89; ATCC 44385; CBS 158.81; DAOM 178454; IMI 254604; UAMH 4245 | Pinus contorta | Canada | J. Petty | AJ244255, GU117900 |
| Phaeothecoidea intermedia | CBS 124994; CPC 13711 | Eucalyptus globulus | Australia | B.A. Summerell | GQ852754, GQ852628 |
| Phaeothecoidiella illinoisensis | CBS 118947; CMG UIE5 | Malus sp. (punctate morphology on apple) | Illinois, USA | M. Gleason | AY598879, AY598918 |
| CBS 125223; CPC 16115; CMG UIE3a | Malus sp. (punctate morphology on apple) | Illinois, USA | M. Gleason | GU117897, GU117901 | |
| CMG TN1_2.4E1d | Malus sp. (punctate morphology on apple) | Tennessee, USA | S. Bost | GU117898, GU117902 | |
| Phaeothecoidiella missouriensis | CBS 118959; CMG AHE7a | Malus sp. (punctate morphology on apple) | Missouri, USA | M. Gleason | GU117899, GU117903 |
| CBS 125222; CPC 16116; CMG AHE7c | Malus sp. (punctate morphology on apple) | Missouri, USA | M. Gleason | AY598878, AY598917 | |
| Sporidesmajora pennsylvaniensis | CBS 125229; CPC 16112; CMG PA1_9F1a | Malus sp. (fuliginous morphology on apple) | Pennsylvania, USA | J.W. Travis | FJ438379, FJ147167 |
1 ATCC: American Type Culture Collection, Virginia, USA; CBS: CBS Fungal Biodiversity Centre, Utrecht, The Netherlands; CMG: Culture collection of Mark Gleason, housed at Iowa State University, Ames, Iowa, USA; CPC: Culture collection of P.W. Crous, housed at CBS; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; UAMH: University of Alberta Microfungus Collection, Alberta, Canada.
2 ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: 28S nrDNA.
Genomic DNA was isolated from fungal mycelium grown on MEA, using the UltraCleanTM Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA) according to the manufacturer’s protocols. The Primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene (SSU), the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region (ITS2) and the first 900 bases at the 5′ end of the 28S rRNA gene (LSU). The primers ITS4 (White et al. 1990) and LR0R (Rehner & Samuels 1994) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. The PCR conditions, sequence alignment and subsequent phylogenetic analysis followed the methods of Crous et al. (2006). Alignment gaps were treated as new character states. Novel sequence data were deposited in GenBank (Table 1) and the alignment in TreeBASE (www.treebase.org).
Morphology
Signs of SBFS on preserved apple peels are described, including mycelial growth patterns and fruiting body size and density. Morphological descriptions are based on cultures sporulating on synthetic nutrient-poor agar (SNA; Crous et al. 2009c) in vivo. Wherever possible, 30 measurements (×1 000 magnification) were made of all taxonomically informative structures mounted in lactic acid, with the extremes of spore measurements given in parentheses. Colony colours (surface and reverse) were assessed after 1 mo on MEA, PDA and OA at 25 °C in the dark, using the colour charts of Rayner (1970).
RESULTS
Phylogenetic analysis
Amplification products of approximately 1 700 bases were obtained for the isolates listed in Table 1. The LSU region of these sequences was used to obtain additional sequences from GenBank, which were added to the alignment. Due to the inclusion of the shorter LSU sequences of ‘AHE7c’ (AY598917), Stomiopeltis sp. (AY598919) and Stomiopeltis versicolor (FJ147163) in the alignment, it was not possible to subject the full length of the determined LSU sequences (Table 1) to the analysis. The manually adjusted LSU alignment contained 85 sequences (including the outgroup sequence) and, of the 568 characters used in the phylogenetic analysis, 271 were parsimony-informative, 62 were variable and parsimony-uninformative, and 235 were constant. Fourteen equally most parsimonious trees (TL = 1 737 steps; CI = 0.349; RI = 0.749; RC = 0.261), the first of which is shown in Fig. 1, were obtained from parsimony analysis of the LSU alignment. The results of the phylogenetic analysis are highlighted below under the taxonomic notes or in the Discussion, where applicable. Although the ITS sequences were not used in phylogenetic analyses, they were lodged in GenBank for future studies and identification purposes.
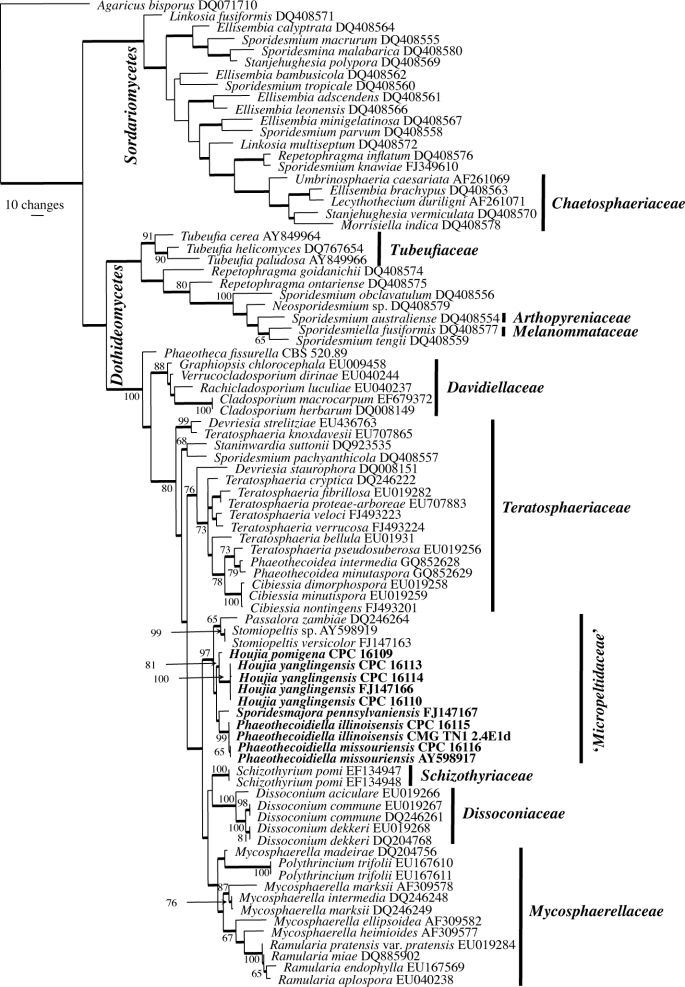
Fig. 1.
The first of 14 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows 10 changes, and bootstrap support values > 64 % from 1 000 replicates are shown at the nodes. Novel species from Malus treated in this study are indicated in bold. Branches present in the strict consensus tree are thickened. Classes are shown to the left of the tree and families, where known, to the right. The tree was rooted to a sequence of Agaricus bisporus (GenBank accession DQ071710).
Taxonomy
The present study resulted in discovery of several novel genera and species that are associated with SBFS on apples. These taxa are treated below:
Phaeothecoidiella Batzer & Crous, gen. nov. — MycoBank MB514394
Mycelio ex hyphis brunneis, laevibus vel subtiliter exasperatis, ramosis, atro-brunneis, cum vagina crassa mucilagina; endoconidiis phragmosporioidibus, in hyphis evolutis, pallide vel modice brunneis, aseptatis, tenuitunicatis, subcylindraceis vel late ellipsoideis, sed deinde (post liberationem) subglobosis, atro-brunneis, verruculosis, interdum cum septo tenui inconspicuo.
Type species. Phaeothecoidiella missouriensis Batzer & Crous, sp. nov.
Etymology. Named after the genus Phaeothecoidea, which it resembles.
Hyphomycetous. Mycelium consisting of brown, smooth to finely roughened, branched, regularly septate hyphae, becoming wider, dark brown, with irregular warts, encased in a thick mucilaginous sheath. Endoconidia developing like phragmospores inside hyphae, pale to medium brown, aseptate, thin-walled, subcylindrical to broadly ellipsoid but becoming more globose, dark brown and roughened upon release; at times with a thin, inconspicuous septum.
Notes — Phaeothecoidiella resembles three other genera with pigmented endoconidia in the Dothideomycetes, namely Phaeotheca, which clusters basal to the Davidiellaceae, Hyphospora (teleomorph: Cumminutispora, Planistromellaceae), and Phaeothecoidea, which resides in the Teratosphaeriaceae (see Crous et al. 2009a). Morphologically there is little to choose among these genera, although species of Phaeothecoidiella tend to have endoconidia developing like phragmospores inside hyphae, rarely arranged in clusters inside hyphal cells. Furthermore, hyphae are covered in a prominent mucilaginous sheath, suggesting that taxa could occur elsewhere in nature as true sooty moulds (Hughes 1976), probably being opportunistic on the surface of apple fruit.
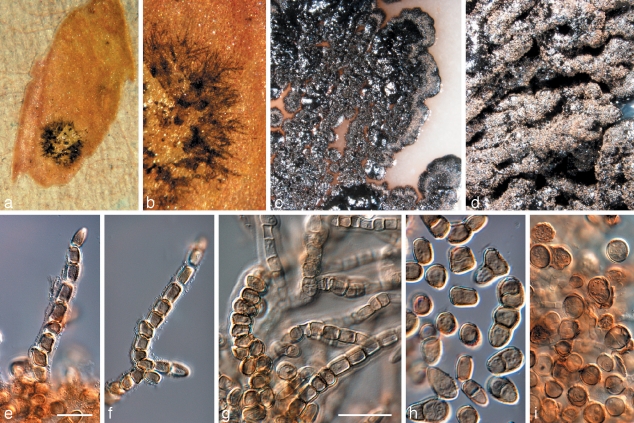
Phaeothecoidiella illinoisensis Batzer & Crous, sp. nov. —MycoBank MB514395; Fig. 2
Fig. 2.
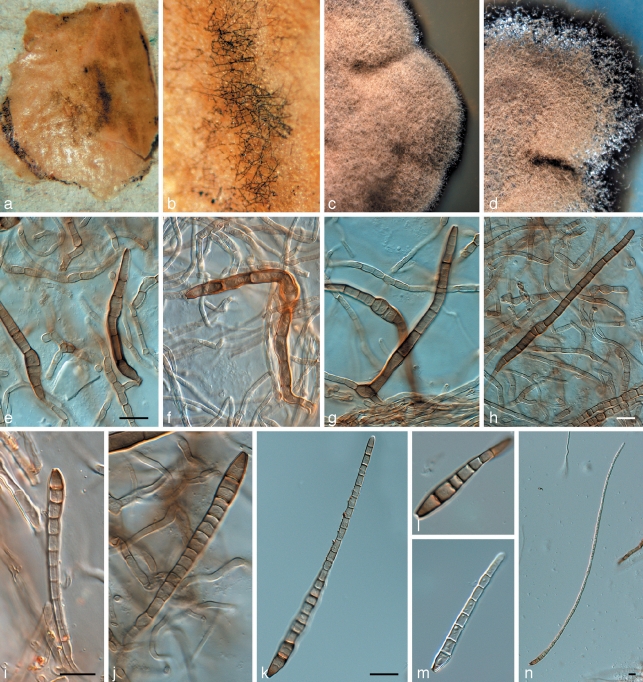
Phaeothecoidiella illinoisensis (CPC 16115). a. Colony on apple surface; b. colony on SNA; c. colony on OA; d, e. hyphae with endoconidia; f, g. endoconidia. — Scale bars = 10 μm.
Mycelio ex hyphis brunneis, laevibus vel subtiliter exasperatis, ramosis, 3–4 μm latis, cum vagina mucilaginosa, ad 1.5 μm lata; endoconidiis raro in fasciculis in cellulis hypharum, pallid vel modice brunneis, aseptatis, globosis vel ellipsoideis, laevibus vel exasperatis, 5–8 × 4–5 μm.
Etymology. Named after its type locality, Illinois, USA.
On SNA. Mycelium consisting of brown, smooth to finely roughened, branched, regularly septate, 3–4 μm diam hyphae, becoming wider and dark-brown with endoconidia, and covered in a mucilaginous sheath, up to 1.5 μm thick. Endoconidia developing like phragmospores inside hyphae, rarely in clusters inside hyphal cells, pale to medium brown, aseptate, globose to ellipsoid, smooth to roughened, 5–8 × 4–5 μm; older conidia becoming more globose to broadly ellipsoid, thin-walled, pale brown to brown, 6–10 μm diam.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA erumpent, slimy, with sparse aerial mycelium and even, lobate margin; surface iron-grey, reaching 18 mm diam. Similar on PDA and OA, reaching up to 10 mm diam.
Appearance on apple — Punctate mycelia type; dark brown to black colonies; reaching 2.5 mm diam with even, feathered margins; shiny black sclerotium-like bodies scattered on the surface at a density of 15 per mm2 and ranging from 28 μm to 60 μm diam.
Specimen examined. USA, Illinois, on fruit surface of apple, Sept. 2000, M. Gleason, CBS H-20323 holotype, cultures ex-type CPC 16115 = UIE3a = CBS 125223.
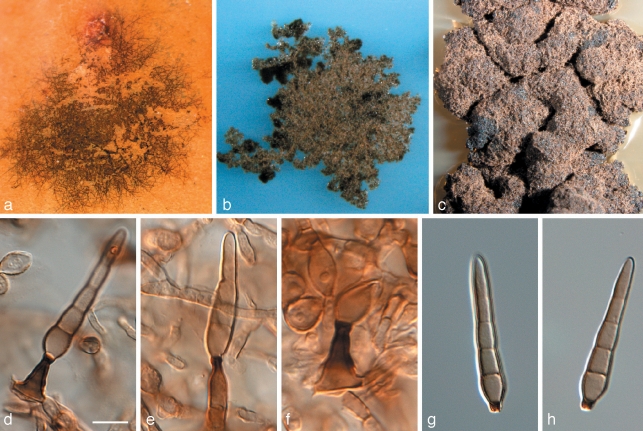
Phaeothecoidiella missouriensis Batzer & Crous, sp. nov. — MycoBank MB514396; Fig. 3
Fig. 3.
Phaeothecoidiella missouriensis (CPC 16116). a, b. Colonies on apple surface; c. colony on OA; d. colony on SNA; e–g. hyphae with phragmo- and endoconidia (note sheath); h, i. conidia. — Scale bars = 10 μm.
Mycelio ex hyphis brunneis, laevibus vel subtiliter exasperatis, ramosis, 2–4 μm latis, cum vagina crassa mucilagina, ad 2 μm lata; endoconidiis medio-brunneis, aseptatis, 3–7 × 3–5 μm, tenuitunicatis, subcylindraceis; conidiis veteribus ad 12 μm longis et 8 μm latis.
Etymology. Named after its type locality, Missouri, USA.
On SNA. Mycelium consisting of brown, smooth to finely roughened, branched, regularly septate, 2–4 μm diam hyphae, becoming wider, dark brown, with irregular warts, up to 1 μm high, or encased in a thick, mucilaginous sheath, up to 2 μm thick. Endoconidia developing like phragmospores inside hyphae, medium brown, aseptate, 3–7 × 3–5 μm, thin-walled, subcylindrical, but becoming more globose, dark brown and roughened upon release, up to 12 μm long and 8 μm wide, at times with a thin, inconspicuous septum.
Cultural characteristics — Colonies after 1 mo at 25 °C in the dark on MEA erumpent, spreading, with sparse aerial mycelium and uneven, crenate margin; surface uneven, folded, iron-grey, reaching up to 20 mm diam. Similar on PDA, reaching up to 15 mm diam, as well as on OA, reaching up to 10 mm diam.
Appearance on apple — Punctate mycelial type (Batzer et al. 2005); dark brown to black colonies; diameter reaching 2 mm with irregular feathered margins; shiny black sclerotium-like bodies scattered on the surface at a density of 10 per mm2 and ranging in diameter from 17 μm to 47 μm.
Specimens examined. USA, Missouri, on fruit surface of apple, Sept. 2000, M. Gleason, CBS H-20322 holotype, cultures ex-type CPC 16116 = AHE7c = CBS 125222; AHE7a = CBS 118959.
Notes — Both species of Phaeothecoidiella have endoconidia that resemble phragmospores inside hyphae, and are rarely arranged in clusters. Conidia of P. missouriensis are 3–7 × 3–5 μm, while those of P. illinoisensis are somewhat larger, 5–8 × 4–5 μm. It would be difficult, however, to separate these taxa without the aid of molecular data (Table 1).
Houjia G.Y. Sun & Crous, gen. nov. — MycoBank MB514397
Mycelio ex hyphis ramosis, septatis, brunneis, exasperatis vel verrucatis, ad septa saepe constrictis; conidiophoris in cellulis conidiogenis reductis, intercalaribus, atro-brunneis, subcylindraceis vel cuneiformibus, crassitunicatis, latitudine maximo basali, apice truncato; formatione conidiorum holoblastica vel phialidica; conidiis late ellipsoidibus-subcylindraceis vel obclavatis, basi truncata, apice obtuse rotundato, transverse et oblique septatis, ad septa leniter constrictis, subtiliter verruculosis, medio-brunneis, tenuitunicatis, basi cuneiforme attenuata, hilo truncato.
Type species. Houjia yanglingensis G.Y. Sun & Crous, sp. nov.
Etymology. Named after Hou Ji, the creator of the Yellow River Farming Civilization in central China and a famous agriculture educator 4 000 years ago, who gave lectures in Yangling, China.
Hyphomycetous. Mycelium consisting of branched, septate, brown, roughened to warty hyphae, frequently constricted at septa. Conidiophores reduced to conidiogenous cells, intercalary, dark-brown, subcylindrical to cuneiform, thick-walled, widest at the base, apex truncate; conidiogenesis unclear, varying from holoblastic to phialidic. Conidia broadly ellipsoid to subcylindrical or obclavate, base truncate, apex obtusely rounded, transversely and obliquely septate, slightly constricted at septa, finely verruculose, medium brown, thin-walled, tapering to a cuneiform basal part with a truncate hilum.
Notes — Morphologically the genus resembles Janetia, Stanjehughesia and Linkosia. It can be distinguished from Stanjehughesia by having solitary conidiogenous cells that are not aggregated in clusters (Hughes & Illman 1974). Furthermore, phylogenetically it clusters in the Capnodiales, whereas the type strain of Stanjehughesia, S. hormiscioides, has Umbrinosphaeria caesariata as teleomorph (Réblová & Winka 2001), and resides in the Chaetosphaeriaceae. Houjia is distinct from Linkosia by having euseptate conidia, and from Janetia by having monoblastic conidiogenous cells (Wu & Zhuang 2005).
Hughes (1983) was one of the first to remark on the fact that sporidesmium-like taxa that were foliicolous and have monoblastic conidiogenous cells needed to be placed elsewhere. At this time he chose to widen the concept of Janetia, placing these taxa there. His description of Janetia capnophila appears to be allied to Houjia. The latter taxon occurred on leaves of a range of diverse plant hosts, and was associated with various species of sooty moulds.
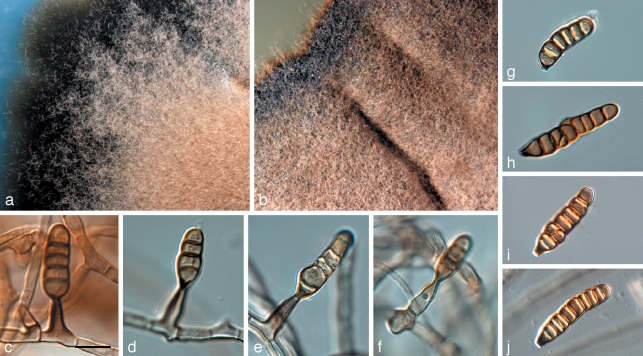
Houjia pomigena Batzer & Crous, sp. nov. — MycoBank MB514398; Fig. 4
Fig. 4.
Houjia pomigena (CPC 16109). a. Colony on apple surface; b. colony on SNA; c. colony on OA; d–f. conidiogenous cells giving rise to conidia; g, h. conidia. — Scale bar = 10 μm.
Mycelio ex hyphis ramosis, septatis, brunneis, exasperates vel verrucatis, 2–3 μm latis; cellulis conidiogenis intercalaribus, atro-brunneis, subcylindraceis vel cuneiformibus, crassitunicatis, 12–16 × 5–9 μm; conidiis obclavatis, basi truncate, apice obtuse rotundata, 4(–5)-septata, ad septa leniter constrictis, subtiliter verruculosis, medio-brunneis, tenuitunicatis, (30–)38–40(–47) × (5–)6–7(–8) μm.
Etymology. Named after the fruit that it blemishes.
On SNA. Mycelium consisting of branched, septate, brown, roughened to warty, 2–3 μm wide hyphae, frequently constricted at septa. Conidiophores reduced to conidiogenous cells, intercalary, dark-brown, subcylindrical to cuneiform, thick-walled, widest at the base, 12–16 × 5–9 μm, apex truncate, 3 μm wide, at times appearing somewhat cup-shaped with what could be periclinal thickening, but conidiogenesis unclear, varying from holoblastic to phialidic. Conidia obclavate, base truncate, apex obtusely rounded, 4(–5)-septate, slightly constricted at septa, finely verruculose, medium brown, thin-walled, widest in middle of basal cell, from where it tapers to a cuneiform basal part with a truncate hilum that is dark brown, 3 μm wide and long, appearing more thick-walled than the rest of the conidium, (30–)38–40(–47) × (5–)6–7(–8) μm.
Cultural characteristics — Colonies after 1 mo at 25 °C in the dark on PDA leaden-black, erumpent, folded, lacking aerial mycelium, and with lobate margins, reaching 5 mm diam. On SNA similar, but more flattened, reaching 5 mm diam. On MEA similar, but more erumpent, reaching 10 mm diam. On OA similar, reaching 15 mm diam.
Appearance on apple — Fuliginous mycelial type; lacking sclerotium-like bodies; densely arranged ropey mycelia, arborescent margins; circular shaped, brown colonies reaching 10 mm diam.
Specimen examined. USA, Illinois, on fruit surface of apple, Sept. 2000, M. Gleason, CBS H-20324 holotype, cultures ex-type CPC 16109 = UIF2b = CBS 125224.
Houjia yanglingensis G.Y. Sun & Crous sp. nov. — MycoBank MB514399; Fig. 5
Fig. 5.
Houjia yanglingensis (CPC 16114). a. Colony on SNA; b. colony on MEA; c–f. conidiogenous cells giving rise to conidia; g–j. conidia. — Scale bar = 10 μm.
Mycelio ex hyphis ramosis, septatis, medi-brunneis, subtiliter exasperates, 2–3 μm latis; cellulis conidiogenis solitariis, intercalaribus, erectis, subcylindraceis, brunneis, laevibus, 0–1-septatis, 10–15 × 5–6 μm; conidiis brunneis, laevibus, rectis vel leniter curvatis, (18–)25–28(–30) × (5–)6–7(–8) μm, muriforme septatis, 3–6(–10) transverse et 1–4 oblique septatis.
Etymology. Named after its type locality, the city in China where it was first isolated.
On SNA. Mycelium consisting of branched, septate, medium brown, finely roughened, 2–3 μm wide hyphae. Conidiophores solitary, intercalary, erect, subcylindrical, brown, smooth, 0–1-septate, 10–15 × 5–6 μm. Conidiogenous cells brown, smooth, subcylindrical, tapering towards a subtruncate apex, 1.5–2 μm wide, 6–11 × 3–4 μm. Conidia brown, smooth, straight to slightly curved, (18–)25–28(–30) × (5–)6–7(–8) μm, muriformly septate, with 3–6(–10) transverse (eusepta or distosepta), and 1–4 oblique septa, slightly constricted at septa, broadly ellipsoid to subcylindrical or obclavate, widest in the middle; apex obtuse, tapering towards a cuneiform basal part with subtruncate base, 1.5–2 μm wide, 1–2 μm long.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark on MEA erumpent, spreading, with sparse aerial mycelium and folded surface, with even, lobate margins; colonies iron-grey, reaching up to 30 mm diam. On PDA similar, with moderate aerial mycelium, reaching 30 mm diam. On OA similar, outer region more olivaceous-grey in colour, reaching 35 mm diam.
Appearance on apple — Fuliginous mycelial type; lacking sclerotium-like bodies; irregularly shaped brown colonies with even margins reaching 5 mm diam.
Specimens examined. China, Jingning County, Gansu Province, on fruit surface of apple, Oct. 2007, G.Y. Sun, CBS H-20325 holotype, cultures ex-type CPC 16114 = JN13 = CBS 125225; Lingbao County, Henan Province, on fruit surface of apple, Oct. 2007, G.Y. Sun, cultures CPC 16113 = LB20 = CBS 125226. — USA, cultures CPC 16111 = TN1_2.2F1d = CBS 125227; Davidson County, Tennessee, on fruit surface of apple, Sept. 2005, S.C. Bost, cultures CPC 16110 = KY3_13F1d = CBS 125228; Paducha, Kentucky, on fruit surface of apple, Sept. 2005, J. Hartman.
Notes — Houjia pomigena can be distinguished from H. yanglingensis by having conidia that are 30–47 × 5–8 μm, 4(–5)-septate, while those of H. yanglingensis are shorter (18–30 × 5–8 μm), with 3–6(–10) transverse and 1–4 oblique septa.
Sporidesmajora Batzer & Crous, gen. nov. — MycoBank MB514400
Mycelio ex hyphis ramosis, septatis, brunneis, subtiliter verruculosis, cum chlamydosporis brunneis in fasciculis terminalibus; conidiophoris erectis, subcylindraceis, saepe subclavatis, medio-vel atro-brunneis, subtiliter verruculosis, 1–pluriseptatis, ex celluli hypharum oriundis, pro ramulis lateralibus, vel inflatis et bulbosis; cellulis conidiogenis terminalibus, medio-brunneis, subcylindraceis vel subconicis, holoblasticis; conidiis obclavatis vel longe obclavatis, rectis vel curvatis, medio-brunneis, laevibus vel subtiliter verruculosis, guttulatis, transverse pluriseptatis.
Type species. Sporidesmajora pennsylvaniensis Batzer & Crous, sp. nov.
Etymology. Named after its resemblance to Sporidesmium, but with much larger conidia.
Hyphomycetous. Mycelium consisting of branched, septate, brown, finely verruculose hyphae, frequently constricted at septa, and at times giving rise to terminal clusters of brown chlamydospores. Conidiophores erect, subcylindrical, frequently somewhat clavate, medium to dark brown, finely verruculose, at times with percurrent rejuvenation, 1–multi-septate; base arising as lateral branch directly from hyphal cell, or swollen and bulbous. Conidiogenous cells terminal, medium brown, subcylindrical to subconical; apex neither darkened nor thickened, truncate, holoblastic. Conidia obclavate to long obclavate, straight to curved, medium brown, smooth to finely verruculose, guttulate, transversely multi-euseptate; apex obtuse, base obconical, frequently darker pigmented than the rest of the conidium.
Notes — Even though the Sporidesmium complex was divided into numerous genera by Wu & Zhuang (2005), Sporidesmium remains heterogeneous. Although the type species, S. atrum is not known from culture, morphologically similar species such as S. knawiae, S. macrusum and S. parvum cluster among the Sordariomycetes, distant from the Capnodiales. Sporidesmajora is quite distinct from Sporidesmium in having long, multiseptate conidiophores that frequently have a subconical, darker pigmented apical cell, appearing to give rise to a single holoblastic conidium. Conidia are obclavate, frequently very long, multi-euseptate, and somewhat curved, having a somewhat darker, obconical basal cell.
Sporidesmajora pennsylvaniensis Batzer & Crous, sp. nov. — MycoBank MB514401; Fig. 6
Fig. 6.
Sporidesmajora pennsylvaniensis (CPC 16112). a, b. Colonies on apple surface. c. colony on MEA; d. colony on SNA; e–j. conidiophores; k–n. conidia. — Scale bars = 10 μm.
Mycelio ex hyphis ramosis, septatis, brunnei, subtiliter verruculosis, 3–5 μm latis; conidiophoris erectis, subcylindricaceis, saepe subclavatis, rectis vel unigeniculatis, medio-vel atro-brunneis, subtiliter verruculosis, 1–20-septatis, 35–170 × 4–6 μm; cellulis conidiogenis terminalibus, medio-brunneis (interdum atriore brunneis quam conidiophora), subcylindricaceis vel subconicis, apice distincte attenuato, truncato, 10–15 × 4–6 μm; conidiis obclavatis vel longe obclavatis, rectis vel curvatis, medio-brunneis, laevibus vel subtiliter verruculosis, guttulatis, 45–350 × 5–7 μm, transverse 6–plurieuseptatis.
Etymology. Named after its type locality, Pennsylvania, USA.
On OA (sterile on SNA and PDA). Mycelium consisting of branched, septate, brown, finely verruculose, 3–5 μm wide hyphae, frequently constricted at septa, and at times giving rise to terminal clusters of brown chlamydospores up to 10 μm diam, in chains or in clumps. Conidiophores erect, subcylindrical, frequently somewhat clavate, straight to once geniculate, medium to dark brown, finely verruculose, at times with percurrent rejuvenation, 1–20-septate, 35–170 × 4–6 μm; base arising as lateral branch directly from hyphal cell, or becoming swollen and bulbous. Conidiogenous cells terminal, medium brown (at times darker brown than conidiophores), subcylindrical to subconical, with prominent taper towards truncate apex, 10–15 × 4–6 μm; apex not darkened nor thickened, 2–2.5 μm diam; each conidiogenous cell appearing to give rise to a single conidium. Conidia obclavate to long obclavate, straight to curved, medium brown, smooth to finely verruculose, guttulate, 45–350 × 5–7 μm, transversely 6–multi-euseptate, at times constricted at septa, apex obtuse, tapering from the obconical basal cell to the subtruncate hilum, up to 2 μm wide; widest at basal cell, which is frequently more darkly pigmented than the rest of the conidium.
Culture characteristics — Colonies after 1 mo at 25 °C in the dark spreading on MEA, with sparse aerial mycelium; surface folded, margin even, lobate, surface leaden-grey, reverse leaden-black, reaching up to 25 mm diam. On PDA similar, but margins more feathery, reaching 25 mm diam. On OA similar, reaching 20 mm diam.
Appearance on apple — Fuliginous mycelia type; lacking sclerotium-like bodies; irregularly shaped pale brown colonies with uneven margins reaching 5 mm diam.
Specimen examined. USA, Adams County, Pennsylvania, on fruit surface of apple, Sept. 2005, J.W. Travis, CBS H-20326 holotype, cultures ex-type CPC 16112 = PA1_9F1a = CBS 125229.
DISCUSSION
All five of these newly identified fungi have been proven to cause SBFS signs on apple, based on a modified Koch’s postulates (Batzer et al. 2005). These findings are among the first evidence that the same SBFS species occur in both Asia and North America. The fact that several isolates of Houjia yanglingensis were isolated from two provinces in China and two states in the USA suggest it is a widely distributed member of the SBFS complex. There is only one previous report of a SBFS fungus, Zygophiala cryptogama, occurring in both continents (Zhai et al. 2008). Intercontinental distribution of SBFS species in Europe and North America was also documented (Wrona 2004, Ivanović et al. In press). Difficulty in isolating SBFS species from apple has impeded our understanding of the distribution of these slow-growing fungi. For example, recent use of specific primer pairs ITS1/Myc1R (Duttweiler et al. 2008) to amplify SBFS fungi directly from the thalli on apple peels has revealed that Phaeothecoidiella species are widely distributed throughout Iowa (Sisson 2009), despite the fact that we obtained only four pure cultures of this genus from a 30-orchard survey of the eastern USA (Batzer et al. 2005, Díaz Arias et al. In press).
The genus Phaeothecoidiella resembles Hyphospora, Phaeotheca and Phaeothecoidea, which also have endoconidia, and are placed in the Dothideomycetes. Morphologically they are distinct from Phaeothecoidiella in that the latter tends to have more phragmospores, and hyphae that are covered in a mucoid sheath, as found in typical sooty moulds, but lacking in the former genera. Phylogenetically these genera also cluster apart.
The recent treatment of the Sporidesmium complex by Wu & Zhuang (2005) reiterated the numerous, as yet undefined genera present within the Sporidesmium complex. A complete revision of this polyphyletic complex has largely been hampered by the unavailability of cultures (Shenoy et al. 2006). Two genera in this complex are newly introduced in the present study, namely Houjia and Sporidesmajora. An important ecological feature of these genera lies in their growth habit, namely growing as epiphytes (Capnodiales) on apple surfaces. Using the key provided by Wu & Zhuang (2005), Houjia, which is morphologically similar to Stanjehughesia (Chaetosphaeriaceae), can be distinguished based on its solitary conidiogenous cells. Sporidesmajora, on the other hand, is distinguished from Sporidesmium (Sordariomycetes) based on its multiseptate conidiophores with darkly pigmented, subconical apical conidiogenous cells, and long, multi-euseptate conidia.
Altogether we described three new genera and five new species in the SBFS complex. Although this finding is quite significant, the Dothideomycetes, and the Capnodiales in particular, still host numerous undescribed families and genera (Schoch et al. 2006, Crous et al. 2007, 2009b), suggesting that these novelties reported here represent but the tip of the iceberg. What is extremely interesting is that apple fruit surfaces appear to represent yet another unique epiphytic niche, similar to that reported by Ruibal et al. (2008) from rock surfaces. By employing morphological and DNA comparisons, we were able to resolve the status of several previously collected genera and species which would otherwise have escaped recognition.
Acknowledgments
This work was supported by Program for Changjiang Scholars and Innovative Research Team in University (IRT0748), the 111 Project from Education Ministry of China (B07049) and Top Talent Project of Northwest A&F University.
REFERENCES
- Batzer JC, Díaz Arias MM, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. 2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 246 – 258 . [DOI] [PubMed] [Google Scholar]
- Batzer JC, Gleason ML, Harrington TC, Tiffany LH. 2005. Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia 97: 1268 – 1286 . [DOI] [PubMed] [Google Scholar]
- Colby AS. 1920. Sooty blotch of pomaceous fruits. Transactions Illinois State Academic Science 13: 139 – 175 . [Google Scholar]
- Crous PW. 1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1 – 170 . [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 . [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, Hoog GS de, Groenewald JZ . 2009a. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17 – 47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ. 2009b. Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99 – 118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkleij GJM, Groenewald JZ, Samson RA. (eds). 2009c. Fungal Biodiversity. CBS Laboratory Manual Series Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: . [Google Scholar]
- Díaz Arias MM, Batzer JC, Harrington TC, Wong AW, Bost SC, Cooley DR, Ellis MA, Hartman JR, Rosenberger DA, Sundin GW, Sutton TB, Travis JW, Wheeler MJ, Yoder KS, Gleason ML . In press. Diversity and biogeography of sooty blotch and flyspeck fungi on apple in the Eastern and Midwestern United States. Phytopathology . [DOI] [PubMed] [Google Scholar]
- Duttweiler KB, Sun GY, Batzer JC, Harrington TC, Gleason ML. 2008. An RFLP-based technique for identifying fungi in the sooty blotch and flyspeck complex on apple. Plant Disease 92: 794 – 799 . [DOI] [PubMed] [Google Scholar]
- Hickey KD. 1960. The sooty blotch and flyspeck diseases of apple with emphasis on variation within Gloeodes pomigena (SCW.) Colby. PhD thesis Department of Plant Pathology, The Pennsylvania State University, USA: . [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183 – 189 . [DOI] [PubMed] [Google Scholar]
- Hughes SJ. 1976. Sooty moulds. Mycologia 68: 693 – 820 . [Google Scholar]
- Hughes SJ. 1983. New Zealand fungi 32. Janetia capnophila sp. nov. and some allies. New Zealand Journal of Botany 21: 177 – 182 . [Google Scholar]
- Hughes SJ, Illman WI. 1974. Sporidesmium hormiscioides. Fungi Canadensis No. 58 National Mycological Herbarium, Biosystematics Research Institute, Agriculture; Canada, Ottawa, Ontario, Canada: . [Google Scholar]
- Ivanović MM, Ivanović MS, Batzer JC, Tatalović N, Oertel B, Latinović J, Latinović N, Gleason ML . In press. Fungi in the apple sooty blotch and flyspeck complex from Serbia and Montenegro. Journal of Plant Pathology . [Google Scholar]
- Johnson EM, Sutton TB, Hodges CS. 1996. Peltaster fructicola: a new species in the complex of fungi causing apple sooty blotch. Mycologia 88: 114 – 120 . [Google Scholar]
- Johnson EM, Sutton TB, Hodges CS. 1997. Etiology of apple sooty blotch disease in North Carolina. Phytopathology 87: 88 – 95 . [DOI] [PubMed] [Google Scholar]
- Li HY, Zhang R, Sun GY, Batzer JC, Gleason ML . In press. New species and record of Zygophiala on apple fruit from China. Mycological Progress . [Google Scholar]
- Ma YQ, Zhang R, Sun GY, Zhu HX, Tang M, Batzer JC, Gleason ML . In press. A new species of Zygophiala associated with the flyspeck complex on apple from China. Mycological Progress . [Google Scholar]
- Rayner RW. 1970. A mycological colour chart British Mycological Society. Commonwealth Mycological Institute, Kew, Surry: . [Google Scholar]
- Réblová M, Winka K. 2001. Generic concepts and correlations in ascomycetes based on molecular and morphological data: Lecythothecium duriligni gen. et sp. nov. with a Sporidesmium anamorph, and Ascolacicola austriaca sp. nov. Mycologia 93: 478 – 493 . [Google Scholar]
- Rehner SA, Samuels GJ. 1994. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625 – 634 . [Google Scholar]
- Ruibal C, Platas G, Bills GF. 2008. High diversity and morphological convergence among melanised fungi from rock formations in the central mountain system of Spain. Persoonia 21: 93 – 110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1043 – 1054 . [DOI] [PubMed] [Google Scholar]
- Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD. 2006. Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycological Research 110: 916 – 928 . [DOI] [PubMed] [Google Scholar]
- Sisson AJ. 2009. Assessing new methods of integrated pest management for apple orchards in the midwest and phenology of sooty blotch and flyspeck fungi on apples in Iowa. Masters thesis Department of Plant Pathology, Iowa State University, USA: . [Google Scholar]
- Sun GY, Zhang R, Li H, Gleason ML. 2008. Diversity of fungi causing flyspeck-like signs on apple in China. Phytopathology 98: S153 . [Google Scholar]
- Sun GY, Zhang R, Zhang Z, Zhang M. 2003. Isolation of sooty blotch and flyspeck fungi from apple surface by picking up the thalli. Acta Phytopathology Sinica 33: 479 – 480[in Chinese] . [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238 – 4246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, USA: . [Google Scholar]
- Wrona BR. 2004. Etiology of apple sooty blotch in Poland. Journal of Plant Protection Research 44: 289 – 292 . [Google Scholar]
- Wu W, Zhuang W. 2005. Sporidesmium, Endophragmiella and related genera from China. Fungal Diversity Research Series 15: 1 – 351 . [Google Scholar]
- Zhai X, Li H, Zhang R, Sun G, Tang M, Batzer JC, Gleason ML. 2008. Zygophiala (hyphomycetes) – a genus newly recorded from China. Mycotaxon 105: 325 – 330 . [Google Scholar]