Abstract
During the course of research on mammal mycophagy and movement in the Northern Tablelands of New South Wales, Australia, extensive collections of sequestrate fungi were made, including numerous cortinarioid taxa. Historically any novel taxa would have been described in the cortinarioid sequestrate genera Descomyces, Hymenogaster, Protoglossum, Quadrispora, Thaxterogaster or Timgrovea based on broad morphological similarities of the sporocarps and spore ornamentation. However, consistent with other recent analyses of nuclear DNA regions, taxa from sequestrate genera were found to have affinities with Cortinarius and Descolea or Hebeloma, and to be scattered across many sections within Cortinarius. None of the historical sequestrate cortinarioid genera are monophyletic in our analyses. In particular, the gastroid genus Hymenogaster is paraphyletic, with one clade including two species of Protoglossum in Cortinarius, and a second clade sister to Hebeloma. Eight new species of sequestrate Cortinarius are described and illustrated, and discussion of their affinities with various sections provided: C. argyronius, C. caesibulga and C. cinereoroseolus in section Purpurascentes, C. maculobulga in section Rozites, C. sinapivelus in section Splendidi, C. kaputarensis in a mixed section Phlegmacium/Myxacium within a broader section Dermocybe, C. basorapulus in section Percomes and C. nebulobrunneus in section Pseudotriumphantes. Keys to genera of the Bolbitiaceae and Cortinariaceae containing sequestrate taxa and to currently known Australian species of sequestrate Cortinarius and Protoglossum are provided. As with the related agaricoid taxa, macroscopic characters such as colour and texture of basidioma, degree of loculisation of the hymenophore, and stipe-columella development and form remain useful for distinguishing species, but are generally not so useful at the sectional level within Cortinarius. Microscopic characters such as spore shape, size, and ornamentation, and pileipellis structure (simplex vs duplex and size of hyphal elements) are essential for determining species, and also appear to follow sectional boundaries.
Keywords: diversity, systematics, taxonomy, Thaxterogaster
INTRODUCTION
Cortinarius is an important ectomycorrhizal genus, widely distributed in both hemispheres. It is one of the larger, taxonomically diverse genera of basidiomycetes, with approximately 2 000 species recognised worldwide (Kirk et al. 2001). Cortinarius species are highly variable in colour and form, though may be distinguished from other Agaricales by brown, ornamented spores and an inner cobweb (cortina) veil. Several genera of sequestrate fungi have at various times been shown to share these characters, and are known to have affinities to Cortinarius (Dodge & Zeller 1934, Singer 1951, Singer & Smith 1959, Bougher & Castellano 1993). The general characteristics distinguishing sequestrate genera from related agaric taxa are that the hymenophore remains enclosed by the pileus, the spores are not actively discharged, and the sporocarps are hypogeal or emergent. As with many groups of sequestrate fungi, the taxonomy and nomenclature of the cortinarioid fungi is in a state of flux, with many genera proving to be paraphyletic based upon molecular evidence (Peintner et al. 2002a, Hosaka et al. 2006, Lebel & Tonkin 2008). A number of agaricoid and sequestrate genera have been incorporated into Cortinarius, for example the genus Thaxterogaster (Peintner et al. 2002b), however the more gastroid taxa such as Hymenogaster, Quadrispora and Protoglossum have as yet to be transferred.
In Australia 11 genera and 39 species of sequestrate cortinarioid fungi have been fully described (Cunningham 1979, Beaton et al. 1984, Castellano & Trappe 1990, Bougher & Castellano 1993, May et al. 2003, Francis & Bougher 2003, 2004), and numerous DNA sequences made available for another 20–30 undescribed taxa (Francis 2007). During an extensive study of sequestrate fungi and mycophagy in the Northern Tablelands of New South Wales, a large number of new sequestrate taxa were discovered representing some 22 genera. The Cortinariaceae are a dominant component of the mycota, particularly of the sub-alpine zone. We present descriptions and illustrations of eight new species, and a key to currently described Australian sequestrate Cortinarius and Protoglossum species.
MATERIALS AND METHODS
Molecular analyses
Taxon sampling
The regions of nuclear ribosomal DNA data used for examination of species included the ITS1-5.8S-ITS2. Preliminary analyses were conducted on a large dataset (285 taxa; 340 sequences) with several different outgroups and exemplars of main clades suggested by analyses of Peintner et al. (2002a) and Garnica et al. (2005), and additional sequences of Australian sequestrate fungi (Francis 2007). Blast searches were conducted to check for any sequences that matched or were close matches to sequences of novel taxa. Close matches (97–100 %) were included in all alignments. In a series of successive analyses several sections known to be represented solely by Northern hemisphere taxa, i.e. Calochroi, or taxa showing no affinity to the new taxa described here (including numerous undescribed Australian sequestrate taxa), were removed. The final alignment included 178 sequences, representing 156 species, including a range of species within Cortinarius (98), Descolea (2), Hebeloma (3), and sequestrate taxa known to have affinities with these genera. Agaricus bisporus was selected as outgroup in final analyses, and a further 9 taxa from sister clades (Agrocybe, Anamika, Gymnopilus, Inocybe, Laccaria) also included to confirm placement of some Hymenogaster species. Twenty three novel sequences of Australian Cortinariaceae were included in the alignment and analyses (Table 1 lists all taxa with corresponding herbarium and GenBank accession numbers).
Table 1.
Taxa included in DNA analyses. Current nomenclature is given as well as names used in GenBank.
| Current name | Genbank name | voucher | locale | Moser (1986) sections | Garnica et al. (2005) sections | Genbank # |
|---|---|---|---|---|---|---|
| Agaricus bisporus | HAI0235 | UK | AJ884644 | |||
| Agaricus arvensis | ARV1 | USA | AY484691 | |||
| Agrocybe praecox | PMB2310 | USA | AY818348 | |||
| Anamika indica | HK10098 | AY948189 | ||||
| Crepidotus mollis | ubc f16579 | Canada | FJ627025 | |||
| Gymnopilus eucalyptorum | BRV 99/10 | AU | AF501546 | |||
| Gymnopilus penetrans | IB 19980105 | AU | AF325663 | |||
| Inocybe geophylla | OUC97144 | Canada | DQ093854 | |||
| Inocybe rufofusca | 82 | Austria | EU326156 | |||
| Descolea gunnii | NZ2042 | NZ | AF325653 | |||
| Descolea maculata | E4986 | AU | AF325651 | |||
| Descomyces albus | H5339 | AU | DQ328157 | |||
| Descomyces albus | H5372 | AU | DQ328168 | |||
| Descomyces angustisporus | H7216 | AU | DQ328058 | |||
| Descomyces sp. | TL1608 | AU | DQ328188 | |||
| Hebeloma ammophilum | NP122 | Austria | AY948190 | |||
| Hebeloma cavipes | NP121 | Austria | AY948193 | |||
| Hebeloma circinans | dkad638 | Netherlands | AF124699 | |||
| Laccaria ochropurpurea | JMP0038 | USA | EU819479 | |||
| Setchelliogaster australiensis | Claridge 2621 | AU | AF325628 | |||
| Setchelliogaster tenuipes | Trappe 24776 | AU | AF325624 | |||
| Timgrovea ferruginea | H5803 | AU | DQ328128 | |||
| Timgrovea sp. | H4167 | AU | DQ328109 | |||
| Timgrovea sp. | H6171 | AU | DQ328195 | |||
| Cortinarius alboaggregatus | PDD 77472 | NZ | Phlegmacium | Pseudotriumphantes | AY669620 | |
| Cortinarius alboviolaceus | IB 19740181 | Sericeocybe | Telamonia | AF325596 | ||
| Cortinarius allutus | IB 19940224 | Phlegmacium | Alluti | AF325585 | ||
| Cortinarius anomalus | IB 19950138 | Sericeocybe | Anomali | AF325581 | ||
| Cortinarius archeri | PERTH 05506395 | AU | Myxacium | AY669610 | ||
| Cortinarius ardesiacus | HO 970419A0 | AU | Telamonia | AY669650 | ||
| Cortinarius argyrionus sp. nov. | MEL2331641; MD158 | AU | GQ890311 | |||
| Cortinarius argyrionus sp. nov. | MEL2331642; MD163 | AU | GQ890312 | |||
| Cortinarius argyrionus sp. nov. | NE94635; MD162 | AU | GQ890313 | |||
| Cortinarius australiensis | ZT ACT72567 | AU | Phlegmacium | AF389126 | ||
| Cortinarius australis | HO A20420A0 | AU | Phlegmacium | AY669615 | ||
| Cortinarius austrocinnabarinus | MEL2089674 | AU | Dermocybe | Dermocybe | GQ890321 | |
| Cortinarius austrocyanites | PD 70498, CO1034 | NZ | Phlegmacium | AY669626 | ||
| Cortinarius austroduracinus | TUB 011522 | Chile | Renidentes | AY669653 | ||
| Cortinarius austrosaginus | HO 980509A0 | AU | Phlegmacium | AY669619 | ||
| Cortinarius austroturmalis | TUB 011469 | Chile | Phlegmacium | AF539730 | ||
| Cortinarius austrovaginatus | HO 990125A1 | AU | Phlegmacium | AY669635 | ||
| Cortinarius austrovenetus | Dermocybe austroveneta | MEL2089666 | AU | Dermocybe | Dermocybe | GQ890318 |
| Cortinarius balteatus | TUB 011844 | GER | Phlegmacium | Phlegmacioides | AY669526 | |
| Cortinarius basipurpureus | Thaxterogaster basipurpureum | PERTH 04259629 | AU | Myxacium | Myxacium | AY669607 |
| Cortinarius basirubescens | Dermocybe aff. umbonata | MEL2089698 | AU | Dermocybe | Dermocybe | GQ890328 |
| Cortinarius basirubescens | Dermocybe basirubescens | MEL2089702 | AU | Dermocybe | Dermocybe | GQ890319 |
| Cortinarius basorapulus sp. nov. | MEL2331650; KV621 | AU | GQ890309 | |||
| Cortinarius caesibulga sp. nov. | MEL2331651; KV660 | AU | GQ890310 | |||
| Cortinarius caesibulga | TL502A | AU | DQ328070 | |||
| Cortinarius caesibulga | Thaxterogaster ‘fragile’ | Trappe 18313 | AU | Purpurascentes | AF325559 | |
| Cortinarius caesibulga | Thaxterogaster sp. | H7127 | AU | DQ328155 | ||
| Cortinarius caesibulga | Thaxterogaster sp. | H0904 | AU | DQ328146 | ||
| Cortinarius cagei | TUB 011514 | GER | Telamonia | Telamonia | AY669676 | |
| Cortinarius campbellae | Thaxterogaster campbellae | Trappe 19821 | AU | Phlegmacium | Purpurascentes | AF325558 |
| ‘Cortinarius campbellae/levisporus’ | Thaxterogaster campbellae | MEL2032790 | AU | Phlegmacium | DQ328102 | |
| Cortinarius campbellae | Thaxterogaster campbellae | H0727 | AU | Phlegmacium | Purpurascentes | DQ328196 |
| Cortinarius campbellae | Thaxterogaster sp. | TL503 | AU | DQ328071 | ||
| Cortinarius camptoros | TUB 011848 | GER | Phlegmacium | Caerulescentes | AY669540 | |
| Cortinarius canarius | HO A20511C4 | AU | Dermocybe | AY669630 | ||
| Cortinarius canarius | Dermocybe canaria | MEL2089669 | AU | Dermocybe | Dermocybe | GQ890320 |
| Cortinarius caperatus | TUB 011913 | GER | Rozites | Rozites | AY669575 | |
| Cortinarius chalybaeus | PDD77482 | NZ | Phlegmacium | Purpurascentes | AY669613 | |
| Cortinarius cinereobrunneus | IB 19630258 | Myxotelamonia | AF325600 | |||
| Cortinarius cinereoroseolus sp. nov. | KV610 | AU | GQ890314 | |||
| Cortinarius cinereoroseolus sp. nov. | MEL2331646; KV529 | AU | GQ890315 | |||
| Cortinarius clelandii | Dermocybe clelandii | MEL2089677 | AU | Dermocybe | Dermocybe | GQ890322 |
| Cortinarius coelopus | HO 990504A3 | AU | Phlegmacium | Percomes | AY669640 | |
| Cortinarius collinitus | IB 19960061 | Myxacium | Myxacium | AF325573 | ||
| Cortinarius columbinus | TUB 011473 | Chile | Phlegmacium | AF539735 | ||
| Cortinarius cretax | PDD 73148 | NZ | Phlegmacium | AY669622 | ||
| Cortinarius croceus | Dermocybe crocea | JFA9732 | Austria | Dermocybe | Dermocybe | U56038 |
| Cortinarius cystidocatenatus | HO A20518A6 | AU | Telamonia | Obtusi | AY669651 | |
| Cortinarius delaportei | TUB 011853 | GER | Percomes | AY669534 | ||
| Cortinarius delibutus | IB 19860263 | Myxacium | Delibuti | AF325580 | ||
| Cortinarius deminutus | Thaxterogaster redactus | H0726 | AU | Myxacium | DQ328172 | |
| Cortinarius elaphinus | TUB 011474 | Chile | Telamonia | AF539725 | ||
| Cortinarius emodensis | HKAS365-41 | China | Rozites | AY669576 | ||
| Cortinarius erythraeus | PERTH 05506727 | AU | Myxacium | AY669605 | ||
| Cortinarius erythrocephalus | Dermocybe erythrocephala | MEL2089681 | AU | Dermocybe | Dermocybe | GQ890323 |
| Cortinarius favrei | IB 19990627 | Myxacium | Myxacium | AF325575 | ||
| Cortinarius flavofucatus | TUB 011476 | Chile | Icterinula | AF539709 | ||
| Cortinarius globuliformis | Claridge 2351 | AU | Dermocybe | Splendidi | AF325582 | |
| Cortinarius gracilior | TUB 011857 | GER | Phlegmacium | Caerulescentes | AY669525 | |
| Cortinarius hercynicus | TUB011824 | GER | Cortinarius | Cortinarius | AY669580 | |
| Cortinarius holojanthinus | Thaxterogaster violaceus | Halling 5733 | Argentina | AF325557 | ||
| Cortinarius humidicola | IB 19970396 | France | Telamonia | AF325594 | ||
| Cortinarius iringa | PDD 73135 | NZ | Phlegmacium | AY669624 | ||
| Cortinarius kapaturensis sp. nov. | MEL2331649 KV603 | AU | GQ890308 | |||
| Dermocybe kula | HO 980515A0 | AU | Dermocybe | Splendidi | AY669643 | |
| Dermocybe kula | Dermocybe kula | MEL2089692 | AU | Dermocybe | Dermocybe | GQ890325 |
| Cortinarius lacteus | HO A20504A2 | AU | Phlegmacium | AY669642 | ||
| Cortinarius langei | TUB 011861 | GER | Phlegmacium | Percomes | AY669527 | |
| Cortinarius laniger | IB 19740251 | Telamonia | Telamonia | AF325591 | ||
| Cortinarius lavendulensis | PERTH 05506735 | AU | Phlegmacium | Phlegmacioides | AY669617 | |
| Cortinarius lavendulensis | HO 990304A2 | AU | Phlegmacium | Phlegmacioides | AY669631 | |
| Cortinarius levisporus | Thaxterogaster leucocephalus | MEL2057558 | AU | DQ328103 | ||
| Cortinarius levisporus | Thaxterogaster levisporus | MEL2057536 | AU | DQ328148 | ||
| Cortinarius lignyotus | TUB 011478 | Chile | Telamonia | AF539718 | ||
| Cortinarius lividoochrascens | IB 19960258 | Myxacium | Myxacium | AF325565 | ||
| Cortinarius lividus | TUB 011479 | Chile | Telamonia | AF539734 | ||
| Cortinarius maculobulga sp. nov. | MEL2331647 KV532 | AU | GQ890306 | |||
| Cortinarius mairei | IB 93/619 | Austria | Phlegmacium | Caerulescentes | AY669548 | |
| Cortinarius memoriaannae | HO A20502A0 | AU | Phlegmacium | EU660945 | ||
| Cortinarius minoscaurus | PDD 71005 | NZ | Phlegmacium | AY669628 | ||
| Cortinarius nanceiensis | TUB 011422 | GER | Percomes | AY174856 | ||
| Cortinarius nebulobrunneus sp. nov. | MEL2331648 KV588 | AU | GQ890307 | |||
| Cortinarius nebulobrunneus | Thaxterogaster sp. | Trappe 18741 | AU | AF325587 | ||
| Cortinarius ochraceoazureus | ZT RA6743 | Argentina | Telamonia | AY033122 | ||
| Cortinarius olivaceopictus | Dermocybe olivaceopicta | JFA11110 | USA | Dermocybe | Dermocybe | DOU56050 |
| Cortinarius olivaceopictus | Dermocybe aff. olivaceopicta | MEL2120743 | AU | Dermocybe | Dermocybe | GQ890316 |
| Cortinarius pachynemeus | AH 13475 | Chile | Telamonia | Obtusi | AF539727 | |
| Cortinarius papulosus | TUB 011867 | GER | Phlegmacium | Percomes | AY669555 | |
| Cortinarius parahumilis | TUB 011293 | Renidentes | AF539731 | |||
| Cortinarius pavelekii | Thaxterogaster pavelekii | Trappe 7962 | USA | Myxacium | AF325564 | |
| Cortinarius percomis | TUB 011868 | GER | Percomes | AY669529 | ||
| Cortinarius permagnificus | AH 19524 | Chile | Phlegmacium | AF539722 | ||
| Cortinarius persplendidus | Dermocybe splendida | Horak NZ920 | NZ | Dermocybe | Dermocybe | AF325583 |
| Cortinarius persplendidus | Dermocybe splendida | MEL2089694 | AU | Dermocybe | Dermocybe | GQ890327 |
| Cortinarius pingue | Thaxterogaster pinguis | IB 19951102 | Myxacium | AF325571 | ||
| Cortinarius piriforme | Thaxterogaster piriformis | Trappe 20116 | AU | Myxacium | AF325569 | |
| Cortinarius ‘porphyroideus’ | Thaxterogaster piriformis | MEL2079347 | NZ | Myxacium | DQ328106 | |
| Cortinarius porphyroideus | Thaxterogaster porphyreus | NZ8468 | NZ | Myxacium | AF325577 | |
| Cortinarius porphyropus | IB 19990515 | Phlegmacium | Purpurascentes | AF325560 | ||
| Cortinarius pseudotriumphans | TUB 011873 | Chile | Phlegmacium | Pseudotriumphantes | AY669600 | |
| Cortinarius purpurascens | TUB 011401 | GER | Phlegmacium | Purpurascentes | AY174858 | |
| Cortinarius purpurascens | ||||||
| var. largusoides | TUB011871 | GER | Phlegmacium | Purpurascentes | AY669538 | |
| Cortinarius quaresimalis | HO A20606A5 | AU | Myxacium | AY669616 | ||
| Cortinarius rapaceus var. luridus | TUB 011485 | Chile | Phlegmacium | Pseudotriumphantes | AF539724 | |
| Cortinarius renidens | TUB 011516 | GER | Renidentes | AY669652 | ||
| Cortinarius rotundisporus | NZ8501 | NZ | Myxacium | AF389127 | ||
| Cortinarius salmaster | HO A20528A3 | AU | Phelgmacium | AY669618 | ||
| Cortinarius salor | IB 19940297 | Myxacium | Delibuti | AF325579 | ||
| Cortinarius sarcinochrous | Thaxterogaster albocanus | Halling 5832 | Argentina | Myxotelamonia | AF325599 | |
| Cortinarius scaurus | IB 19940243 | Phlegmacium | Scauri | AF325563 | ||
| Cortinarius sclerophyllarum | HO A20430A6 | AU | Phlegmacium | Anomali | AY669637 | |
| Cortinarius sebosus | H7265 | AU | DQ328060 | |||
| Cortinarius sejunctus | HO 990125A0 | AU | Phlegmacium | Splendidi | AY669636 | |
| Cortinarius similis | HKAS 26154 | China | Rozites | Rozites | AY669577 | |
| Cortinarius sinapicolor | PERTH 05506778 | AU | Myxacium | AY669604 | ||
| Cortinarius sinapivelus sp. nov. | MEL2331645 KV518 | AU | GQ890305 | |||
| Cortinarius spadicellus | O-65723 | Norway | Phlegmacium | Phlegmacioides | AY669539 | |
| Cortinarius subcastanellus | NZ800 | NZ | Rozites | AY033112 | ||
| Cortinarius subcastanellus | PDD 77482 | NZ | Rozites | AY669623 | ||
| Cortinarius submagellanicus | HO A20518A1 | AU | Myxacium | Purpurascentes | AY669614 | |
| Cortinarius submeleagris | HO 990411A1 | AU | Rozites | Rozites | AY669638 | |
| Cortinarius talus | IB 19990590 | Phlegmacium | Alluti | AF325586 | ||
| Cortinarius tenellus | TUB 011489 | Chile | Telamonia | Obtusi | AF539728 | |
| Cortinarius walkeri | HO A20528A0 | AU | Dermocybe | AY669632 | ||
| Cortinarius vinaceolamellatus | PERTH 05506786 | AU | Phlegmacium | Rozites | AY669608 | |
| Cortinarius violaceus | PERTH 05506794 | AU | Cortinarius | AY669578 | ||
| Cortinarius viridibasilis | TUB 011490 | Chile | Telamonia | Renidentes | AF539717 | |
| Cortinarius sp. | Dermocybe austrosanguinea | MEL2089685 | AU | Dermocybe | Dermocybe | GQ890317 |
| Cortinarius sp. | Dermocybe chloroapica | MEL2120747 | AU | Dermocybe | Dermocybe | GQ890324 |
| Cortinarius sp. | Dermocybe magentiannulata | MEL2089705 | AU | Dermocybe | Dermocybe | GQ890326 |
| Cortinarius sp. | PDD 77486 | NZ | Phlegmacium | Percomes | AY669644 | |
| Cortinarius sp. | Thaxterogaster sp. | H5362 | AU | DQ328077 | ||
| Cortinarius sp. | Thaxterogaster sp. | H6585 | AU | DQ328080 | ||
| Cortinarius sp. | Thaxterogaster sp. | H0920 | AU | DQ328090 | ||
| Cortinarius sp. | Thaxterogaster sp. | MEL2059057 | AU | DQ328107 | ||
| Cortinarius sp. | Thaxterogaster sp. | H1194 | AU | DQ328117 | ||
| Cortinarius sp. | Thaxterogaster sp. | H1120 | AU | DQ328122 | ||
| Cortinarius sp. | Thaxterogaster sp. | H1013 | AU | DQ328145 | ||
| Cortinarius sp | Thaxterogaster sp | H6558 | AU | DQ328149 | ||
| Cortinarius sp | Thaxterogaster sp | H4770 | AU | DQ328151 | ||
| Cortinarius sp. | Thaxterogaster sp. | H0910 | AU | DQ328179 | ||
| Cortinarius sp. | Thaxterogaster sp. | H1446 | AU | DQ328216 | ||
| Hymenogaster arenarius | H0790 | AU | DQ328124 | |||
| Hymenogaster australis | H0791 | AU | DQ328132 | |||
| Hymenogaster brunnescens | AHS 68806 | EU084967 | ||||
| Hymenogaster bulliardii | OSC Trappe12842 | Spain | AF325641 | |||
| Hymenogaster citrinus | K(M)136970 | ?Europe | EU784360 | |||
| Hymenogaster subalpinus | Trappe 22752 | USA | AF325640 | |||
| Hymenogaster subolivaceus | AHS 34677 | EU084961 | ||||
| Protoglossum aromaticum | VIDAL 980620-6 | EU084962 | ||||
| Protoglossum violaceum | H6358 | AU | Myxacium | DQ328081 | ||
| Protoglossum viscidum | Rodway 1272a | AU | EU084982 | |||
| Quadrispora oblongispora | Trappe 18111 | AU | Myxacium | AF325566 | ||
| Quadrispora tubercularis | PERTH00960403 | AU | Myxacium | DQ328113 |
Nucleic acid preparation, amplification and sequencing
Genomic DNA was isolated with the QIAGEN DNeasy® Plant Mini Kit, following the manufacturer’s protocol. The targeted regions were amplified from purified DNA using standard fungal primer pairs: ITS1/ITS4B and ITS5/ITS4 (Gardes & Bruns 1993, White et al. 1990).
Reactions were conducted in a volume of 50 μl and contained 1.25 U QIAGEN HotStar Taq DNA Polymerase, 10 pmol of each primer, 1.5 mM MgCl2 and 0.25 mM each dNTP. Amplifications were performed in an Eppendorf Mastercycler Gradient Thermal Cycler. Cycling conditions consisted of a 15 m activation at 95 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 58 °C, and 1 m at 72 °C. These cycles were followed by 5 m of final extension at 72 °C, after which the product was held at 4 °C.
Products of amplification were purified using the Concert Rapid PCR Purification System (Life Technologies). Purified DNA was directly sequenced using the ABI Prism BigDye Terminator Cycle Sequencing Kit with primers for the ITS region ITS1, ITS5, and ITS4 (White et al. 1990). Sequencing was carried out by means of an ABI model Automated 377DNA Sequencer.
Assembly and manual editing of sequences for each region were performed using Sequencher 4.7 (GeneCodes). Sequences were then transferred to BioEdit v7.0.9 (Hall 2007) for alignment. Alignments were automated using ClustalX v2.0 (Thompson et al. 1997), and the alignment then manually edited. Previously unpublished sequence data is deposited in GenBank.
Phylogenetic analysis
Missing and ambiguous regions were removed from the analyses. All transformations were weighted equally. Gaps in the alignment were treated as missing data. All trees were rooted by the outgroup method (Maddison et al. 1984). Maximum parsimony analyses were performed using PAUP* 4.0b10 (Swofford 2002). Heuristic searches of the dataset were conducted with 1 000 replicates of random addition sequence, tree bisection-connection (TBR) branch swapping and MULTREES on. Nodal support was tested by bootstrapping of 200 replicates with the heuristic search option (TBR and MULTREES off), including groups compatible with 50 % majority rule consensus, with 10 random addition sequences.
Morphology
The loss of gross morphological characters in the evolution of sequestrate sporocarp forms has led to a separate descriptive terminology to develop which we feel is confusing and, in the light of affinities shown by analysis of DNA sequences, unnecessary. In this paper we use the agaricoid descriptive terms wherever possible, however determining homology of some tissue types is difficult (i.e. veil and pellis structure). Macroscopic characters were described directly from fresh material. Colours are described in general terms only. Macrochemical tests were not recorded. Fresh material was dried in a food dehydrator at 35 °C for 12 h. Habitat, associated plant communities, and fruiting season are based on field notes.
Hand-cut sections of fresh and dried material were mounted in 5 % aqueous solution of KOH, then stained with Congo Red. To determine the amyloid reaction, dried material was stained with Melzer’s reagent. Measurements were made at × 400 or × 1 000 with a calibrated ocular micrometer. Spore dimensions are given as length range × width range, mean length × width (n = 10 unless specified). The length : width ratio (Q) is presented as the range of Q values and the mean Q. Measurements do not include the apiculus or ornamentation. Basidia and cystidia dimensions are given as length range × width range (n = 10). Material for scanning electron microscopy (SEM) was sputter-coated with gold and photomicrographs taken using a JEOL JSM-5600 machine. Scanning electron microscope photographs were referred to wherever possible to aid interpretation of spore ornamentation patterns, however descriptions are in terms of structures visible by light microscopy and are based on type material (except where noted).
Names of herbaria are abbreviated according to Holmgren et al. (1990).
RESULTS
The alignment of ITS sequences consisted of 774 characters, of which 672 were included in analyses (222 were constant and 450 were parsimony informative). Analyses produced 4 298 trees of 3 716 steps, CI = 0.873, RI = 0.881 (Fig. 1.)
Fig. 1.
Heuristic analysis of ITS sequence data; one of 4 298 trees of length 3 716. Bootstrap support shown above lines. ‘oval’ = sequestrate taxa.
A number of clades representative of sections sensu Peintner et al. (2002a) and Garnica et al. (2005) were recovered, though bootstrap support was not strong in the deeper branches (Table 1; Fig. 2). However, there is strong support (100 % bootstrap) for a distinct Cortinarius clade separate from a Descolea/Hebeloma clade. The Hymenogaster A clade, appears to have strong affinities to Descolea/Hebeloma rather than within Cortinarius with Hymenogaster B, including Protoglossum viscidum and P. aromaticum (Fig. 2). Although preliminary results only, some microscopic features such as spore size (generally considerably larger in Hymenogaster A), and ornamentation (more robust in Hymenogaster A), provide some support for distinguishing the two Hymenogaster clades.
Fig. 2.
Sporocarps of new species. a. Cortinarius argyrionus; b. C. basorapulus; c. C. caesibulga; d. C. cinereoroseolus; e. C. kaputarensis; f. C. maculobulga; g. C. nebulobrunneus; h. C. sinapivelus. — Scale bars = 10 mm.
The eight newly described taxa are scattered in different lineages within Cortinarius: C. argyronius, C. caesibulga and C. cinereoroseolus are in section Purpurascentes, C. maculobulga in section Rozites, C. sinapivelus in section Splendidi, C. kaputarensis in a mixed section Phlegmacium/Myxacium within a broader section Dermocybe, C. basorapulus in section Percomes and C. nebulobrunneus in section Pseudotriumphantes. Macroscopic and microscopic characters of the new species provide further support for placement in these various sections (discussed further in notes under each taxon). Historically these taxa would have been ascribed, based on morphology, to the genera Thaxterogaster, Hymenogaster or Protoglossum. However, Thaxterogaster has been synonymised with Cortinarius, and species of Hymenogaster and Protoglossum are scattered in several different clades thus the genera can no longer be considered monophyletic nor distinct from Cortinarius or Descolea/Hebeloma. Nomenclatural changes in the genus Protoglossum will be dealt with in a separate paper (May & Lebel in prep).
Very few clades contain solely sequestrate taxa. This may be partially a consequence of taxon sampling, as relatively few sequences have been available until recently, and also to the great diversity of sequestrate fungi currently undescribed from Australasia. Several sequestrate taxa will require further investigation of type material, for example C. porphyroideus, as sequences appear in radically different clades (Purpurascentes and Myxacium).
Taxonomy
Key to genera of the Bolbitiaceae and Cortinariaceae containing sequestrate taxa
-
1.
Spores with a smooth, rostrate apex, and distinct utricle……………2
-
1.
Spores with a rounded ornamented apex, and lacking a distinct utricle sequestrate……………Cortinarius (and Protoglossum)
-
2.
Basidiomes with distinct stipe; spores prominently asymmetrical……………Setchelliogaster
-
2.
Basidiomes lacking stipe-columella; spores more or less symmetrical……………Descomyces/Timgrovea
Key to Australian sequestrate species of Cortinarius and Protoglossum
-
1.
Stipe-columella lacking, much reduced or as a truncate basal pad……………2
-
1.
Stipe-columella distinct, prominent……………21
-
2.
Basidiomes white, yellow, orange or brown……………9
-
2.
Basidiomes with lilac/violet tints……………3
-
3.
Gleba sublamellate……………3. C. caesibulga
-
3.
Gleba loculate or labyrinthoid……………4
-
4.
Peridium gelatinous, viscid or with a thick layer of slime……………5
-
4.
Peridium not as above……………7
-
5.
Basidiomes initially pale tan becoming reddish/purple brown or grey/violet; stipe-columella white to cream, dry, with a gelatinous purple collar at junction of peridium and stipe……………C. basipurpureus
-
5.
Basidiomes lacking the reddish/brown tones; stipe-columella absent or truncate, white to violet, dry or viscid, lacking a gelatinised purple collar……………6
-
6.
Spores ellipsoid, 12.5–14.5(–16) × 6–8 μm; peridium silvery white with violet tints to violet/lilac overall……………P. niphophilum
-
6.
Spores obovoid to broadly ellipsoid, 8.5–10.5(–12) × 6–7 (–10) μm; peridium violet fading to greyish violet, or greyish brown/orange……………P. violaceum
-
7.
Basidiomes not caespitose, cream to pink-lilac-grey, slightly shiny; spores ornamented with robust irregular nodules pegs and some short broad lines to 1.5 μm tall……………4. C. cinereoroseolus
-
7.
Basidiomes often caespitose, lacking pink tones, may be shiny or not; spore ornamentation lower and less robust or lacking any connecting lines between elements……………8
-
8.
Spores ornamented with irregular crowded nodules to 1.5 μm tall. Basidiomes silvery grey to violet with metallic sheen; stipe-columella white staining violet at margins……………1. C. argyrionus
-
8.
Spores ornamented with low nodules < 0.8 μm tall, connected by scattered short lines. Basidiomes brownish violet, lacking metallic sheen; stipe-columella remaining white……………C. campbellae
-
9.
Peridium overall viscid or covered with a layer of slime……………10
-
9.
Peridium dry or appearing moist……………18
-
10.
Spores retained in tetrads after release……………11
-
10.
Spores not retained in tetrads after release……………13
-
11.
Basidiomes pale violet fading to brown with age……………Quadrispora musispora
-
11.
Basidiomes brown, warm brown to apricot yellow, drying brown or greyish yellow……………12
-
12.
Basidiomes brown; spores subovoid, coarsely ornamented with irregular tubercules and ridges to 2 μm high……………Quadrispora tubercularis
-
12.
Basidiomes warm brown to apricot yellow; spores ellipsoidal to oblong, ornamented with crowded, irregular tubercules and ridges to 1 μm high……………Quadrispora oblongispora
-
13.
Spores 15–18 × 8.5–12 μm, ornamented with crowded fine verrucae; basidiomes dark brown overall……………C. deminutus Peintner
-
13.
Spores almost all < 15 μm in length, ornamented with crowded fine verrucae or short ridges; basidiomes may have brown tints but not dark brown overall……………14
-
14.
Basidiomes creamy tan with brown patches……………6. C. maculobulga
-
14.
Basidiomes brown with yellow, orange or copper tints……………15
-
15.
Basidiomes yellow-orange with orange-red stains; stipe pale yellow with gelatinous red collar at junction of peridium and stipe; spores densely ornamented with irregular rods and short ridges to 1 μm tall……………C. luteirufescens
-
15.
Basidiomes coloured differently and lacking orange-red stains; stipe lacking gelatinous red collar; spores mostly finely verrucose and less than 1 μm tall……………16
-
16.
Basidiomes brownish orange to brown; stipe-columella variable; spores mostly ≤ 8 μm wide……………C. piriforme
-
16.
Basidiomes yellow/orange/copper red to dark brown; small basal pad present or lacking; spores mostly > 8 μm wide……………17
-
17.
Spores 11–13 × 8.5–11 μm; ornamentation to 1.5 μm tall, of irregular rods and short ridges, perisporium conspicuous, closely adhering……………P. luteum
-
17.
Spores 11–15.5 × 7.5–9 μm, more ellipsoid; ornamentation to 0.8 μm tall, finely verrucose, perisporium conspicuous, appearing reticulately wrinkled……………P. viscidum
-
18.
Spores ≤ 10 × 6 μm……………19
-
18.
Spores > 10 × 6 μm……………20
-
19.
Basidiomes whitish, sometimes with cinnamon fibrils; spores golden brown, ellipsoid, ornamented with small warts or rods, 7–10 × 3–5.5 μm……………C. walpolensis
-
19.
Basidiomes white to yellow ochre; spores yellow brown to red brown, ovoid to ellipsoid, finely verrucose, 6–9.5 × 4–5.5 μm……………C. levisporus
-
20.
Spores 12.6–14 × 6.3–7.8 μm, cinnamon brown, ovoid, finely nodulose; basidiomes whitish with brown patches; stipe-columella truncate to percurrent……………6. C. maculobulga
-
20.
Spores 14.5–18.5 × 9–13 μm, dark chestnut brown, amygdal-citriniform, verrucose; basidiomes olive brown; stipe-columella truncate or lacking……………C. scabrosus
-
21.
Basidiomes with distinct yellow persistent partial veil……………22
-
21.
Basidiomes not as above……………25
-
22.
Basidiomes ‘flattened parachute-shaped’, bright yellow; stipe-columella short, somewhat bulbous; spores ellipsoid to subglobose, ornamented with rods and short ridges to 1 μm tall……………C. globuliformis
-
22.
Basidiomes agaricoid, brown with some yellow tints; stipe-columella long; spores shaped differently, ornamentation nodulose to roughly verrucose……………23
-
23.
Spores ellipsoid to ovoid, 11.4–15.2 × 6.8–8.7 μm……………C. flavovelus
-
23.
Spore ovoid to almond-shaped or broadly ovoid, all less than 12 μm long……………24
-
24.
Pileus convex, pale brown with yellow fibrils; spores broadly ovoid, 8.9–10.0 × 6.5–7.5 μm……………8. C. sinapivelus
-
24.
Pileus conical to subglobose often with flattened apex, yellow-brown to orange-brown with brown fibrils, subviscid; spores ovoid to almond-shaped, 9.9–12.0 × 5.5–7.5 μm……………5. C. kaputarensis
-
25.
Basidiomes pigmented with distinct dark brown, purple, orange or green tints; stipe generally lacking marginate base……………29
-
25.
Basidiomes white, cream, grey or pale tan brown; stipe with distinctly marginate bulbous or angular base or barely slightly bulbous (not marginate)……………26
-
26.
Spores 14–21 μm long; basidiomes of variable colour cream, greenish grey……………C. sebosus
-
26.
Spores all < 15 μm long; basidiomes off-white, greyish to pale tan brown……………27
-
27.
Basidiomes off-white to greyish, viscid; spores 12.5–14.5 × 8–11 μm……………C. leucocephalus
-
27.
Basidiomes pale tan, sometimes with whitish bloom overlying, not viscid; spores 9.0–11.6 × 5.5–9.5 μm……………28
-
28.
Stipe with marginate bulbous base; spores broadly ellipsoid, 9.6–11.6 × 7–9.4 μm……………2. C. basorapulus
-
28.
Stipe attenuating towards base; spores ellipsoid, 9–11 × 5.5–6.5 μm……………C. cunninghamii
-
29.
Basidiomes with purple tints……………30
-
29.
Basidiomes lacking purple tints, brown or orange present……………31
-
30.
Spores 7.7–9(–11) × 5–6.5 μm; basidiomes with a silvery sheen……………1. C. argyrionus
-
30.
Spores 14–21 × 9–18 μm; basidiomes dull……………C. sebosus
-
31.
Basidiomes parachute-shaped; stipe-columella short, base marginate; with thick white partial veil……………C. debbiae
-
31.
Basidiomes shaped differently; stipe-columella either longer or not marginate; lacking thick white partial veil……………32
-
32.
Stipe-columella white slightly translucent, robust, extending > 30 mm beyond pileus; spores 9–11.9 × 5.5–6.5 μm……………7. C. nebulobrunneus
-
32.
Stipe-columella brown, slender, extending < 12 mm beyond pileus; spores 8.5–10.5 × 4.7–6 μm……………C. orphinus
DESCRIPTIONS
1. Cortinarius argyrionus Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515235; Fig. 2a, 3, 4
Fig. 3.
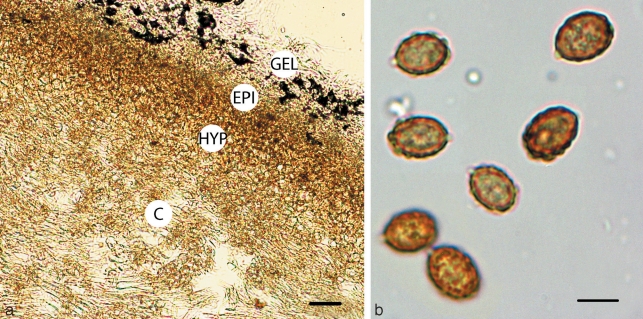
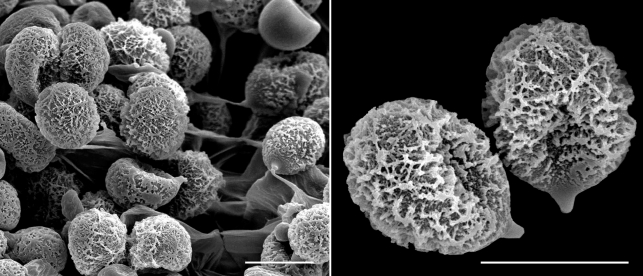
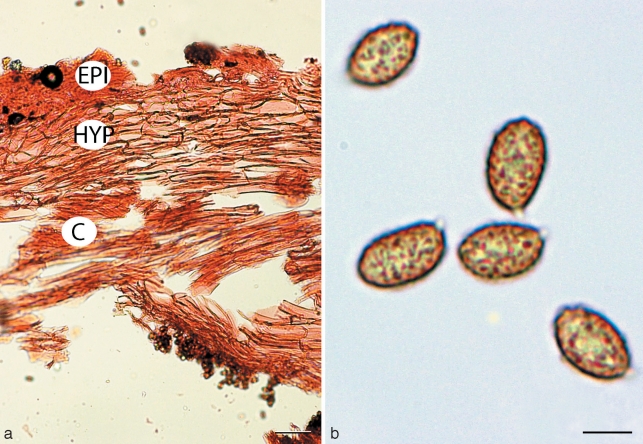
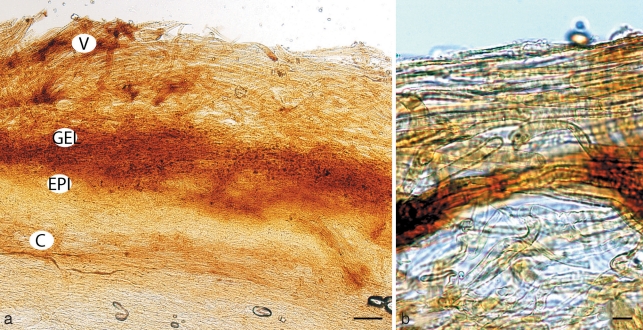
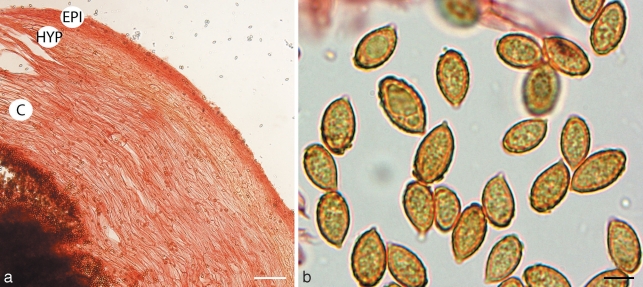
Cortinarius argyrionius. a. Pileipellis; b. spores (GEL = outer layer; EPI = epicutis; HYP = hypocutis; C = context). — Scale bars: a = 50 μm; b = 5 μm.
Fig. 4.
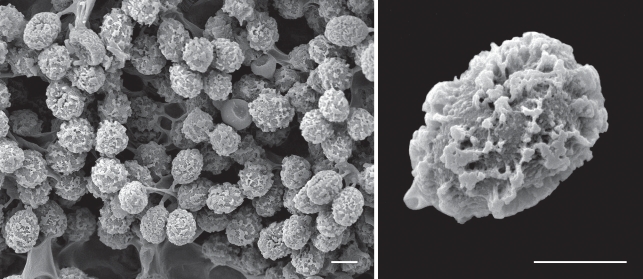
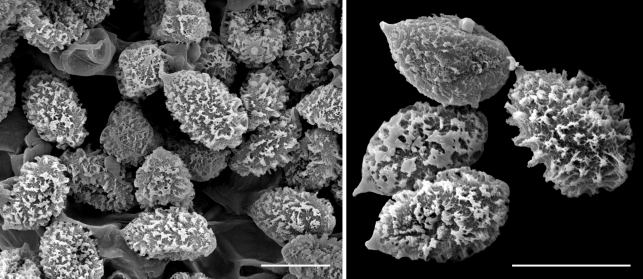
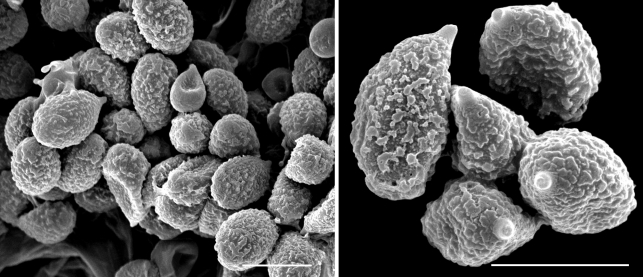
Scanning electron micrographs of Cortinarius argyrionus spores. — Scale bars = 10 μm.
Sporocarpia hypogaea vel emergentia, caespitosa, 6–20 mm lata, subglobosa vel turbinata, grosse rugosa; pileus argenticinereus vel pallidoviolaceus, non viscidus. Velum violaceum, crassum, persistens. Hymenophorum loculatum initio sordido brunneum vel cinnamomeum, maturitate fuscobrunneum; trama violascens suffusa retinens. Stipes 9–30 × 2–7 mm, percurrens, solidus, albus vel argentiviolaceus, contextus albus dein centrum luteolum et margine violaceo. Pileipellis bistrata, extus hyphis tenuibus hyalinis gelatinosis; interne hyphis latis luteobrunneis non-gelatinosis. Basidiosporae asymmetricae, late ovatae, 7.7–9(–11) × (4.5–)5–6.5 μm, in KOH pallide luteobrunnaea, nodulosis irregularibus inconspictis vel robustis, < 1.5 μm altis. — Typus: M. Danks K. Vernes T. Cooper & S. Steinhart MD163 (MEL2331642) (holotypus hic designatus), Australia, New South Wales, Armidale, Newholme Field Station, Plot PA4, 1 July 2008.
Etymology. Name refers to the metallic sheen and silvery-violet colour of the sporocarps (Gk.: argyrionus = silvery violet).
Sporocarps hypogeous to emergent under leaf litter, fruiting in large clusters, often caespitose (multi-bodied). Pileus 7–38 × 6–20 mm diam, irregularly subglobose to pyriform or turbinate, coarsely wrinkled with plicate margin, attached to stipe by a persistent, cottony violet partial veil becoming paler with age. Pellis pale violet to silvery-grey with a metallic sheen, radiate-fibrillose, dry to moist when fresh but not viscid, sometimes with adhering debris, not hygrophanous, not bruising, with overlying remnant silvery-grey, fibrillose-silky veil, easily rubbed off with handling. Context 0.5–1.5 mm thick, white to cream, generally thicker at apex. Hymenophore dull brown to cinnamon brown initially becoming rich dark brown, trama initially pale violet, becoming white to grey retaining some violet tints in older specimens; loculate, chambers empty, regular, rounded to elongate and radially arranged. Stipe-columella generally percurrent in immature sporocarps and occasionally percurrent, more often truncate, in mature sporocarps, 9–30 × 2–7 mm diam, white to silvery violet, context white gradually becoming pale yellow tinted in centre and violet at margins, solid, central, slender, convoluted and equal or slightly bulbous to base or tapering to somewhat inserted base; partial veil inconspicuous but present between inrolled margin and stipe-columella, cortinoid to cottony, concolorous silvery-grey to violet (more obvious in younger specimens). Basal mycelium inconspicuous. Odour strong earthy fungoid, not unpleasant; taste not distinctive.
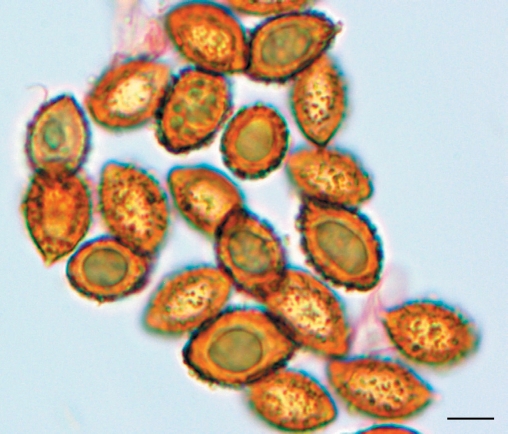
Spores 7.7–9(–11) × (4.5–)5–6.5 μm, mean (30 spores) = 8.8 × 5.6 μm, Q = 1.2–2.0, mean Q = 1.62, cinnamon brown (KOH), broadly ovoid, slightly asymmetrical, ornamented with irregular crowded nodules, nodules inconspicuous or robust to 1.5 μm tall, usually angular, often taller and more robust towards apex; hilar appendage to 1 μm, hyaline, conspicuous, tapering, truncate, entire; spores inamyloid non-dextrinoid. Basidia 20–40 × 5–7 μm, hyaline, clavate to cylindrical, thin-walled, with 4 sterigmata. Cystidia 12–32 × 3–9 μm, hyaline, clavate, thin-walled, scattered and never protruding beyond hymenium. Hymenophoral trama 65–140 μm wide, of interwoven, hyaline, gelatinised, thin-walled, narrow hyphae, 3–6 × 20–50 μm; subhymenium 20–35 μm undifferentiated from trama. Pileipellis duplex. Outer gelatinised layer 35–50 μm wide, of loosely interwoven, hyaline, partially gelatinised, thin-walled, narrow hyphae, 4–6 μm diam; epicutis 30–65 μm wide, of more densely compacted, hyaline to pale yellowish in KOH, narrow filamentous hyphae, 3–6 μm diam; hypocutis 65–110 μm wide, of densely packed, yellow-brown tinted, non-gelatinised, thick-walled (to 1 μm), ovoid, ellipsoid to subglobose or rectangular inflated hyphae 8–18 μm diam × 8–22 μm long; context 250–300 μm wide, of parallel to somewhat interwoven, hyaline, non-gelatinised, hyphae 8–12 μm diam. Partial veil of subparallel to somewhat interwoven, thin-walled, hyaline hyphae 2–5 μm broad. Clamp connections present in the pileus and hymenial tissues.
Habitat & Distribution — In New South Wales, found in low hills and plains near Mt Duval on the New England Tableland, among paddock shelterbelt plantings of Acacia filicifolia, Eucalyptus nova-anglica, E. stellulata, E. viminalis, Hakea microcarpa, H. salicifolia and Leptospermum flavescens. Fruiting: June and July.
Specimens examined. Australia, New South Wales, Armidale, Newholme Field Station, Plot PA4, 1 July 2008, M. Danks K. Vernes T. Cooper & S. Steinhart MD158 (MEL2331641); Armidale, Newholme Field Station, Plot PA4, 1 July 2008, M. Danks K. Vernes T. Cooper & S. Steinhart MD 162 (NE94635); Armidale, Newholme Field Station, Plot PA6, 2 June 2009, M. Danks & S. Steinhart MD213 (MEL2331643 & NE94636); Armidale, Newholme Field Station, Plot PA4, 2 June 2009, S. Steinhart MD222 (MEL2331644).
Notes — Numerous violet-lilac tinted species of Cortinarius occur in Australia, including several that lack a thick gelatinous pileipellis. Cortinarius argyrionus differs macroscopically in the often caespitose sporocarps, with loculate hymenophore, and the initially white stipe context which gradually becomes pale yellow tinted in the centre and violet at the margins. Microscopically, the robust nodulose spore ornamentation (to 1.5 μm), and structure of the pileus are distinct from other violet-lilac tinted sequestrate Cortinarius species.
Based on analyses of ITS sequence data, Cortinarius argyrionus belongs in a well-supported (bootstrap 64 %) section Purpurascentes, with two other new species C. caesibulga and C. cinereoroseolus (Fig. 1). Section Purpurascentes also includes a strongly supported subclade with northern hemisphere species C. porphyropus, C. purpurascens and C. purpurascens var. largusoides (bootstrap 77 %), the southern hemisphere species C. australis, C. chalybaeus, C. submagellanicus and C. campbellae, and several undescribed Australian sequestrate taxa. Section Scauri is a strongly supported sister clade (bootstrap 96 %). All taxa within section Purpurascentes have lilac/purple-tinted sporocarps with varying degrees of gelatinisation of pellis hyphae, minute to robust spore ornamentation, and a pileipellis duplex.
Cortinarius argyrionus is strongly supported (bootstrap 96 %) in a subclade as distinct from two undescribed sequestrate taxa C. sp. H1120 and C. sp. H0910 & H1013 (bootstrap 80 %). Both of these undescribed taxa have a pileus that is pale matt brownish purple rather than pale violet to silvery-grey with metallic sheen, and a stipe that lacks the violet margin staining reaction of C. argyrionus. The structure of the pileus, and spore size and ornamentation also differ from C. argyrionus.
The Australian sequestrate fungus, Cortinarius campbellae (boot-strap 82 %), may have some purple tints to the sporocarp, however it is much darker ‘brownish violet or madiera’ than either C. argyrionus or C. caesibulga, has slightly smaller spores with less robust ornamentation, and hypocutis hyphae that are broader (Beaton et al. 1984). A third sequence labelled ‘C. campbellae’ (MEL2032790) appears distant in a well-supported subclade (bootstrap 79 %) with C. levisporus, and sister to a section Obtusi subclade (62 %). On examination, this collection matches reasonably with C. levisporus with a very pale tan pileus with concolorous veil rather than brownish violet or madiera, the hymenophore is loculate rather than lamellate, the stipe reduced, the pileipellis hyphae predominantly ellipsoid, and the spores slightly narrower (Beaton et al. 1984). Further investigation, including examination of type material is required in order to determine the appropriate name for other sequestrate taxa in the section Purpurascentes clade.
2. Cortinarius basorapulus Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515236; Fig. 2b, 5, 6
Fig. 5.
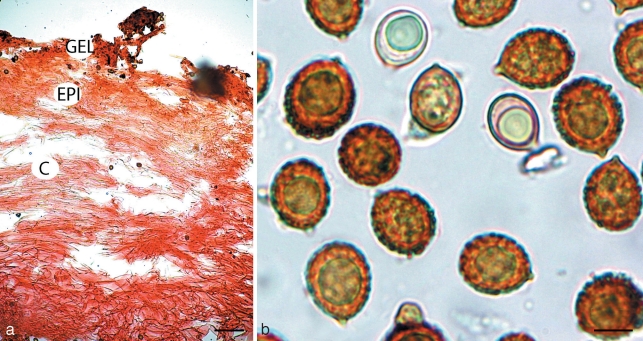
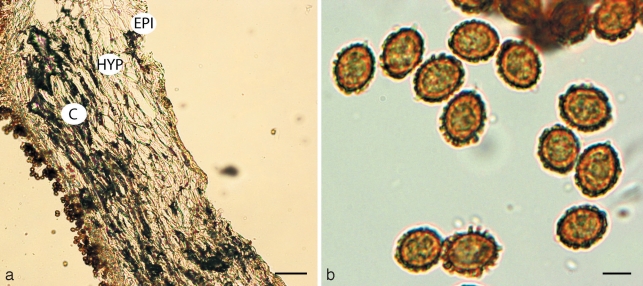
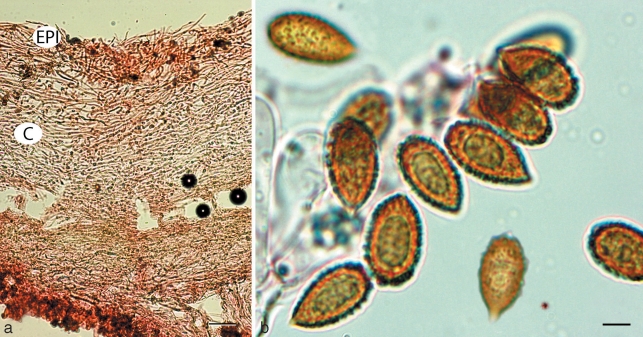
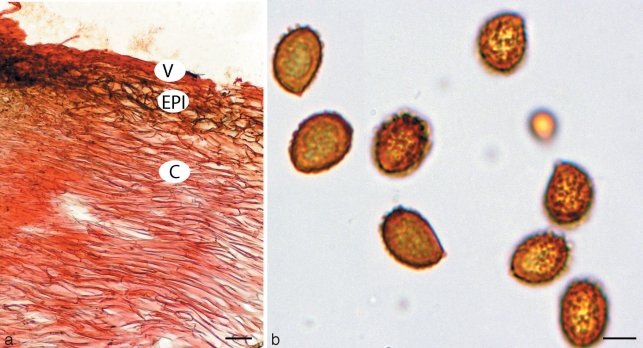
Cortinarius basorapulus. a. Pileipellis; b. spores. — Scale bars: a = 50 μm; b = 5 μm.
Fig. 6.
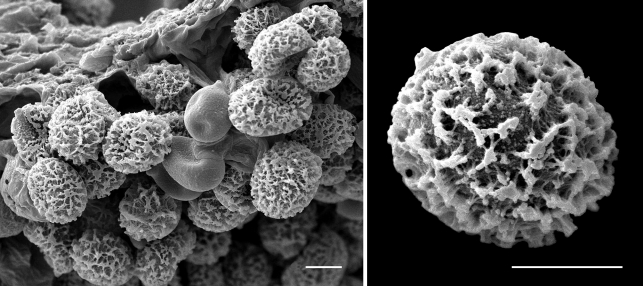
Scanning electron micrographs of Cortinarius basorapulus spores. — Scale bars = 10 μm.
Sporocarpia hypogaea vel emergentia, 11–29 mm lata, convexa vel subglobulosa; pileus eburneus vel pallidobrunneolus, non viscidus. Velum album crassum, persistens. Hymenophorum sublammellatum contortum et plicatum vel loculatum labyrinthiforme, juventute pallidobrunneolum, maturitate leviter fuscum. Stipes 14–36 × 5–8 mm, percurrens, solidus, robustus, basi bulbosus marginatus angustus ad apicem, albus vel eburneus, non-viscidus; contextus albus maculates, basi brunneus. Pileipellis monostrata, hyphis tenuibus luteis non-gelatinosis. Basidiosporae asymmetricae, late ellipsoidae, 9.6–11.6(–12.1) × 7–9.4 μm, in KOH auranteobrunneum, verrucis irregularibus tenuibus 0.3–0.5 μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV621 (holo MEL2331650 (holotypus hic designatus); iso NE94642), Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot DS3, 18 July 2007.
Etymology. Name refers to the turnip-like shape of the bulbous base of the sporocarps (L.: rapulum = a little turnip; baso = base).
Sporocarps hypogeous to emergent under leaf litter, in a small group. Pileus 8–24 × 11–29 mm diam, convex to subglobose, occasionally with a flattened apex, and slightly plicate margin attached to stipe by a persistent white, cottony partial veil. Pellis off-white to pale tan brown, finely fibrillose to fealty, dry, not hygrophanous, not bruising, with overlying remnant pale tan fibrillose veil, giving a white to tan ‘bloom’, easily rubbed off with handling. Context 0.5–3.0 mm thick, translucent white becoming pale yellow-brown at apex of stipe. Hymenophore dull tan brown initially darkening slightly as spores mature, sublamellate to labyrinthine loculate, compact, contorted/wrinkled and intervenose, forming irregular labyrinthine chambers 0.5–2 mm diam. Stipe-columella percurrent, 14–36 × 5–8 mm diam, white staining slightly tan brown, densely fibrillose, white in section with brown stains at base, central to slightly asymmetric, solid, robust, equal then expanding into marginate bulbous base that tapers slightly to a point, dry; veil remnants apparent as brown fibrils on margin of base, and partial veil as a dense white cortina between pellis margin and stipe. Basal mycelium not conspicuous. Odour mild, not distinctive; taste not distinctive.
Spores 9.6–11.6(–12.1) × 7–9.4 μm, mean (11 spores) = 10.8 × 7.9 μm, Q = 1.3–1.6, mean Q = 1.56, golden yellow brown (KOH), broadly ellipsoid, ornamented with irregular, scattered warts, 0.3–0.5 μm high, warts flat topped or rounded; hilar appendage to 1 μm, conspicuous, tapering, truncate; spores inamyloid non-dextrinoid; apex ornamented. Basidia 32.0–36.5 × 6.7–10.2 μm, hyaline, narrowly clavate, 4-spored. Cystidia 18.0–32.0 × 7.0–9.5 μm, elongate to cylindrical or narrowly clavate, with yellowish oily contents in KOH, rare. Hymenophoral trama 30.0–74.5 μm wide, of interwoven to subparallel, hyaline, inflated hyphae, 5–13 μm diam. Subhymenium undifferentiated from trama. Pileipellis simplex. Narrow overlying gelatinised layer, 6–15 μm wide, of narrow, parallel hyaline hyphae 2–4 μm diam; epicutis well developed, 40–75 μm wide, of interwoven light golden yellow hyphae, 2–6 μm diam with scattered ellipsoid to elongate elements 6–13 × 4–9 μm; context 220–450 μm wide, of interwoven, light golden yellow (KOH), septate hyphae, mostly 2–5 μm diam, intermixed with scattered inflated elements 6–14 μm diam, becoming slightly more inflated towards the hymenium, 18–24 μm diam. Clamp connections present in the pileus.
Habitat & Distribution — In New South Wales, occurring in dry sclerophyll forest on the high slopes of the Kaputar Plateau among Brachychiton populneus, Eucalyptus albens, E. elliptica, E. laevopinea and Exocarpus cupressiformis. Fruiting: July.
Specimens examined. Known only from type collection.
Notes — Cortinarius basorapulus may be distinguished by the combination of sporocarps with pale tan brown pileus, distinctively marginate bulbous base that tapers slightly, and pileipellis simplex. Macroscopically this species resembles descriptions of the sequestrate New Zealand taxon C. leucocephalus and sequestrate Australian taxon C. cunninghamii, with pale pileus and loculate hymenophore with elongate cells. However, few collections have been made of either species and the notes available for macroscopic characters are limited (Horak 1973, Beaton et al. 1984, Grgurinovic 1997). Examination of type and other material of C. cunninghamii in the State Herbarium Adelaide (AD) confirmed the presence of a slender attenuated stipe, subgelatinised hyphae forming the cutis, and spores in the range 9–11 × 5–6.5 μm. Cortinarius basorapulus sporocarps appear to be slightly darker brown, with a strongly marginate rather than slender attentuated stipe base, and the spores are more broadly ellipsoid than either C. cunninghamii or C. leucocephalus.
Analyses of ITS sequences places C. basorapulus in a poorly supported section Percomes (bootstrap 61 %), in a subclade (bootstrap 58 %) with the European taxa C. langei, C. nanciencis and C. percomis. A sister subclade with strong support (bootstrap 93 %) includes the Australian taxon C. coelopus, European taxon C. papulosus, and the New Zealand taxon C. sp PDD77486 (Fig. 1). Cortinarius delaportei is nearby but not included in the Percomes clade in this analysis.
All species in this clade have a basic pileus colour of a ‘variation on brown’, a pileipellis simplex, and ellipsoid to elongate spores. However, this group of species does vary in the stipe shape and degree of violet coloration present. The stipe shape is cylindrical in C. nanciencis and C. percomis, and bulbous in all other taxa; and in C. coelopus violet blue coloration is restricted to the pileus margin, in C. delaportei and C. sp PDD77486 violet blue is exclusively in the lamellae and stipe apex, in C. nanciencis violet-blue occurs only in the veil at the stipe base, and C. basorapulus, C. langei, C. papulosus and C. percomis lack any violet-blue coloration.
3. Cortinarius caesibulga Vernes, Danks & T. Lebel, sp. nov. — MycoBank MB515237; Fig. 2c, 7, 8
Fig. 7.
Cortinarius caesibulga. a. Pileipellis; b. spores. — Scale bars: a = 50 μm; b = 5 μm.
Fig. 8.
Scanning electron micrographs of Cortinarius caesibulga spores. — Scale bars = 10 μm.
Sporocarpia hypogaea vel emergentia, 4–27 mm lata, subglobosa vel irregulariter turbinata, margine plicata; pileus griseocaesius decoloratus ad brunneocaesius, non-viscidus. Velum argentigriseum, tenue, sericeum, persistens. Hymenophorum sublamellatum vel lamellatum contortum et plicatum, initio pallidocinnamomeum, maturitate leviter fuscum. Stipes 9–25 × 2–3 mm, percurrens, solidus, protrudens argenticaesius, sericeus. Pileipellis bistrata, extus hyphis tenuibus hyalinis vel pallide luteobrunneis gelatinosis, interne hyphis hyalinis inflatis non-gelatinosis. Basidiosporae asymmetricae, ovatae, 8.7–11 × 4.8–6.2 μm, in KOH pallidocinnamomeae, nodulosis tenuibus 0.5(–0.8) μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV660 (MEL2331651) (holotypus hic designatus), Australia, New South Wales, off Waterfall Way, near junction with Point Lookout Rd, Plot DS5, 19 July 2007.
Etymology. Name refers to the appearance of the sporocarps as ‘little bags or dumplings’ (L.: caesius = lavender pale blue with grey tinge; bulga = purse or bag).
Sporocarps hypogeous to emergent under leaf litter, singly or in small groups. Pileus 2–18 × 4–27 mm diam, irregularly subglobose to turbinate with a flattened apex, and irregularly folded margin which may be lacerate and seceding slightly in mature specimens, attached to stipe by a persistent cobweb veil. Pellis lavender fading to tan-lavender with a silky, silvery sheen, finely fibrillose, dry to moist when fresh but not viscid, not hygrophanous, not bruising, with overlying remnant of a silvery-grey, fibrillose-silky universal veil, easily rubbed off with handling. Context 0.3–0.8 mm thick, white to cream. Hymenophore pale cinnamon brown initially darkening slightly as spores mature, trama, if noticeable, white to translucent grey; sublamellate to lamellate, compressed, distorted/wrinkled, and intervenose, especially near the apex and stipe. Stipe-columella percurrent, 9–25 × 2–3 mm diam, silvery lavender, in section white to translucent in younger specimens and very pale greyish lilac in old specimens, central, solid, slender, convoluted, equal or slightly bulbous to base, dry, silky; partial veil inconspicuous but present between inrolled margin of pileus and stipe-columella, cortinoid to cottony, concolorous silvery-grey with slight lilac tint (more obvious in younger specimens). Basal mycelium inconspicuous. Odour mild, not distinctive, though in older specimens becoming more pungent and unpleasant; taste slightly farinaceous.
Spores 8.7–11 × 4.8–6.5 μm, mean (20 spores) = 9.6 × 5.6 μm, Q = 1.6–2.0, mean Q = 1.82, pale cinnamon brown (KOH), ovoid to ellipsoid, slightly asymmetrical, densely ornamented with isolated nodules to 0.5(–0.8) μm; hilar appendage to 1 μm, conspicuous, tapering, truncate; spores inamyloid non-dextrinoid; apex ornamented. Basidia 26–39 × 5–8 μm, hyaline, clavate to cylindrical, thin-walled, with 4 sterigmata. Cystidia 23–57 × 6–11 μm, hyaline, narrowly clavate, thin-walled, scattered and never protruding beyond hymenium. Hymenophoral trama 20–45 μm wide, of loosely interwoven to subparallel, hyaline inflated hyphae, 4–11 μm diam; subhymenium undifferentiated from trama. Pileipellis duplex. Overlying partially gelatinised layer, 9–38 μm wide, of narrow parallel, hyaline to pale yellow hyphae, 2–4 μm diam; epicutis narrow, 18–30 μm wide, integrating with overlying gelatinised layer in parts, of subparallel, hyaline to pale yellow hyphae, 2–6 μm diam; hypocutis 25–60 μm wide, of interwoven to subparallel, hyaline, inflated hyphae, 4–12 μm diam intermixed with irregular, hyaline, inflated isodiametric elements, 14–39 × 6–22 μm; context 65–225 μm wide, of loosely interwoven to subparallel, inflated, septate, elongate, hyaline hyphae 30–80 × 4–11 μm diam. Clamp connections present in the pileus.
Habitat & Distribution — In northern New South Wales, occurring in dry sclerophyll forest on the high eastern slopes of the New England Plateau among Allocasuarina littoralis, Eucalyptus caliginosa, E. dalrympleana subsp. heptantha and E. radiata subsp. sejuncta; and in southern New South Wales, occurring in mixed forest of E. cypellocarpa and E. sieberii near Mt Imlay. In Victoria, occurring in wet sclerophyll forest among E. regnans. Fruiting: May–July.
Specimens examined. Australia, New South Wales, off Waterfall Way, near Serpentine Nature Reserve, Plot DS6, 20 July 2007, M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV715 (NE94638); Off Nungatta Rd, 3.35 km from junction with Imlay Rd, on western side of rd, 31 May 2001, T. Lebel & S. Lewis TL502A (MEL2310527); Off Laings Rd west, near corner with Reef Rd west, 3 June 2001, T. Lebel & J. Zdravevski TL621 (MEL2310487); Off Laings Rd west, 1.2 km from junction with Imlay Rd, on eastern side of rd, 3 June 2001, J. Zdravevski TL661 (MEL2314440); Off Imlay Rd to south, 1.05 km west of junction with Brushtail Rd, 30 May 2001, J. Zdravevski TL430 (MEL2310509). Victoria, Acheron Way, Acheron Gap between Narbethong and Warburton, 29 June 2005, G.M. Mueller 7232 (MEL2293662); Dom Dom Saddle, Maroondah Hwy, 23 May 2004, K. Syme 1303/04 (MEL2292312); Mt Baw Baw National Park, Mt Erica, Mt Monarch Walk, 80 m from trailhead, 17 May 2003, A. Francis & T. Lebel H0904 (PERTH); Nunniong State Forest, Bentleys Plain Rd, Claridge site 104, 26 May 1996, A. Jumpponen T18313 (MEL, CANB, OSC130729); Yambulla State Forest, Falkner Rd 3.1 km west of Kallack Rd, 9 July 1996, A.W. Claridge H7127 (PERTH).
Notes — Cortinarius caesibulga is distinguished by the silvery-lavender sporocarps with slender stipe-columella, which has a white context, contorted sublamellate to lamellate hymenophore, and the fine spore ornamentation. Based on analyses of ITS sequence data, C. caesibulga belongs in section Purpurascentes (Fig. 1.) This species is in a well-supported clade (bootstrap 84 %) with the agaric C. submagellanicus (Tasmania), and several misidentified sequences of sequestrate taxa. All of these taxa have some lilac-purple tints to the pileus, though to varying degrees.
The Australian sequence labelled ‘C. fragilis T18313’ (AF325559) is unlikely to be the same as the taxon originally described from Chile, instead is here included in the new species C. caesibulga. Cortinarius caesibulga may be differentiated from C. fragilis (Type) by the smaller spores, and sublamellate to lamellate vs elongate labyrinthine hymenophore, and lilac pellis vs whitish with scant lilac tints. The two C. porphyroideus collections (NZ8468 and MEL2079347) included in our analyses, require further examination as the sequences appear in quite different clades, in sections Myxacium and Purpurascentes respectively. The MEL collection appears to conform to the published description of C. porphyroideus (Cunningham 1979), however pileus texture and structure and spore ornamentation make placement of this taxon in Myxacium rather than Purpurascentes more likely. As such we suggest that this sequence (DQ328106) should not be included in analyses for this taxon. The NZ collection (Myxacium) has not been examined by the authors. Cortinarius caesibulga may be differentiated from C. porphyroideus and C. submagellanicus by the much less robust stipe, and silvery lilac pellis vs deep purple or purple-brown pellis.
The two Victorian collections of C. caesibulga, MEL2293662 and MEL2292312, both have slightly more robust spore ornamentation than the New South Wales collections, however are similar in all other characters.
4. Cortinarius cinereoroseolus Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515238; Fig. 2d, 9, 10
Fig. 9.
Cortinarius cinereoroseolus. a. Pileipellis; b. spores. — Scale bars: a = 50 μm; b = 5 μm.
Fig. 10.
Scanning electron micrographs of Cortinarius cinereoroseolus spores. — Scale bars = 10 μm.
Sporocarpia hypogaea, 11–24 mm lata, subglobosa vel irregulariter pyriformia, margine plicata; pileus eburneus vel leviter nitens pallide roseolilacinus-cineraceus, non visdicus. Velum pallidocineraceum tenue, sericeum. Hymenophorum loculatum, initio pallidobrunneum, maturitate fuscocinnamomeum. Stipes 5–11 × 3–6 mm, truncatus vel percurrens, basi bulbosa protrudens, albus, sericeus. Pileipellis tenuis, hyphis latis hyalinis non-gelatinosis. Basidiosporae asymmetricae, late ovatae, 7–8.9 × 5.1–6.4 μm, in KOH cinnamomeae, nodulosis irregularibus et lineis brevis robustis, < 1.5 μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV529 (MEL2331646) (holotypus hic designatus), Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot GW2, 17 July 2007.
Etymology. Name refers to the shiny-pale pink colour of the sporocarps (L.: cinereo = greyish; roseolus = pale pink).
Sporocarps hypogeous under leaf litter, fruiting in large groups. Pileus 11–27 × 11–24 mm diam, irregularly subglobose to pyriform, slightly plicate margin, attached to stipe by a persistent, inconspicuous, white to silvery-grey veil. Pellis cream with pale pink-lilac-grey, slightly shiny, finely fibrillose, smooth, dry to moist when fresh but not viscid, not hygrophanous, not bruising, with overlying remnant pale grey, silky universal veil, easily rubbed off with handling. Context 0.3–0.8 mm thick, white to cream. Hymenophore pale brown initially, becoming dark cinnamon brown, trama, if noticeable, pale brown to dark grey-brown; loculate, chambers empty, regular, rounded to slightly elongate. Stipe-columella a truncate to percurrent columella tapering slightly from a bulbous inserted base towards the apex, 5–11 × 3–6 mm diam, white to translucent in section, central, more or less terete, white, dry, silky, solid, fibrous, base bulbous protruding up to 3 mm below pileus; partial veil inconspicuous but present between inrolled pileus margin and bulbous base, cortinoid, concolorous pale grey. Basal mycelium inconspicuous, white. Odour faintly floral or of chlorine; taste not distinctive.
Spores 7–8.9 × 5.1–6.4 μm, mean (20 spores) = 8.0 × 5.7 μm, Q = 1.3–1.6, mean Q = 1.45, cinnamon brown (KOH), broadly ovoid, slightly asymmetrical, ornamented with irregular nodules pegs and some short broad lines, nodules robust to 1.5 μm tall, usually angular; hilar appendage to 1 μm, conspicuous, tapering, truncate; spores inamyloid non-dextrinoid; apex ornamented. Basidia 28–40 × 7–9 μm, hyaline, clavate to cylindrical, thin-walled, with 4 sterigmata. Cystidia 20–26 × 7–11 μm, hyaline, clavate, thin-walled, scattered and never protruding beyond hymenium. Hymenophoral trama 30–110 μm wide, of scattered inflated round and elongated, hyaline, gelatinised, thick-walled hyphae, 8–22 × 55–70 μm; subhymenium 20–35 μm undifferentiated from trama. Pileipellis duplex. Epicutis a very thin layer, 5–11 μm wide, of gelatinised, parallel, hyaline to pale yellow narrow hyphae, 3–5 μm diam; hypocutis 30–90 wide, difficult to distinguish from the underlying context, of non-gelatinised, subglobose to ellipsoid, hyaline hyphae, 8–26 μm diam × 6–30 μm long; context up to 350 μm wide, of non-gelatinised, subparallel, inflated hyaline hyphae, 10–30 μm wide. Clamp connections present in the pileus and hymenial tissues.
Habitat & Distribution — In New South Wales, found in the sub-alpine and high slopes areas of the Kaputar Plateau, in a grassy woodland community dominated by Eucalyptus dalrympleana, E. pauciflora and Poa sieberiana with scattered Acacia melanoxylon, Acacia sp., Hibbertia obtusifolia, Lomatia arborescens, Monotoca scaparia, Olearia rosemanifolia and Pultanea satulosa. Also found in wet sclerophyll forest dominated by E. dalrympleana, E. laevopinea and E. viminalis with an understorey dominated by Acacia melanoxylon, Blechnum cartilagineum, Coprosma quadrifida, Cyathea australis, Lomandra multiflora, Lomatia arborescens and Poa sieberiana. Fruiting: July.
Specimen examined. Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot WS3, 17 July 2007, M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV610 (NE94637).
Notes — Cortinarius cinereoroseolus may be differentiated from other Australian sequestrate Cortinarius species by the definite pinkish tint to the sporocarp when fresh (note lilac tones are present) and slightly more robust and irregular spore ornamentation. This species also belongs in section Purpurascentes.
Analysis of ITS data places C. cinereoroseolus in a subclade (bootstrap 69 %) with two undescribed sequestrate taxa, sister to a well-supported subclade including C. caesibulga and C. submagellanicus (bootstrap 84 %) and an unsupported subclade with C. australis, C. chalybaeus, C. porphyropus, C. purpurascens and C. purpurascens var. largusoides (Fig. 1). While the colour, texture and form of the sporocarps vary, all of the taxa in the C. cinereoroseolus subclade share a similar pileus structure, of a thin epicutis of subgelatinised, narrow hyaline to pale yellow hyphae overlying a hypocutis and context of inflated hyphae, and robust spore ornamentation. Cortinarius sp. H0920 & H4770 and Cortinarius sp. H1194 share a smooth silky pileus, labyrinthine loculate and yellowish to cinnamon hymenophore, and ellipsoid spores with robust warts and nodules up to 0.8–1 μm high. They differ in the colour of the pileus and form of the stipe-columella: pale greyish silky violet to purplish brown and narrow percurrent in Cortinarius sp. H0920 & H4770, and dull, pale greyish brown and narrow percurrent with bulbous base in Cortinarius sp. H1194.
5. Cortinarius kaputarensis Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515239; Fig. 2e, 11, 12, 13
Fig. 11.
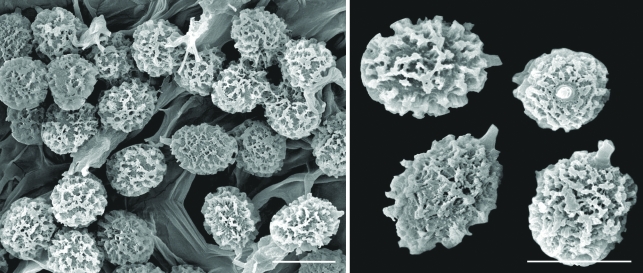
Cortinarius kaputarensis. a. Pileipellis; b. undulating hyphae of outer pellis. — Scale bars: a = 50 μm; b = 10 μm.
Fig. 12.
Cortinarius kaputarensis spores. — Scale bar = 5 μm.
Fig. 13.
Scanning electron micrographs of Cortinarius kaputarensis spores. — Scale bars = 10 μm.
Sporocarpia hypogaea vel emergentia, 15–25 mm lata, conica vel subglobulosa, margine involuta; pileus luteobrunneus vel auranteobrunneus subviscidis. Velum partiale luteum, crassum, persistens et veli universalis vestigium fuscobrunneum fibrillosum persistens. Hymenophorum sublamellatum vel labyrinthiforme loculatum contortum, juventute vivide cinnamomeus, maturitate cinnamomeum. Stipes 15–50 × 3–8 mm, percurrens, protrudens, solidus, gracilis, equalis, pallide luteus, glabrus, non-viscidus; contextus eburneus vel pallide luteus, centro fibrillosus. Pileipellis bistrata, externe hyphis auranteobrunneis, interne hyphis fusco-auranteobrunneis. Basidiosporae asymmetricae, ovatae vel amygdalina, 9.9–12.1 × 5.4–7.4 μm, in KOH cinnamomeae, nodulosis irregularibus robustis < 1.5 μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV603 (holo MEL2331649 (holotypus hic designatus); iso NE94644), Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot WS3, 17 July 2007.
Etymology. Name refers to the type locality, Mt Kaputar.
Sporocarps hypogeous to emergent under leaf litter, in a small group. Pileus 12–30 × 15–25 mm diam, conical to subglobose, occasionally with a slightly flattened apex, and inrolled margin. Pellis yellow-brown to orange-brown, smooth, subviscid when fresh, not hygrophanous, not bruising, with scattered universal veil remnants of darker brown fibrils overlying most of the surface, not easily rubbed off with handling. Context 0.3–2.0 mm thick, rapidly thinning from disc to margin, translucent cream. Hymenophore bright cinnamon brown initially, darkening slightly as spores mature to drab cinnamon brown, sublamellate to loculate, compact, forming irregular, contorted labyrinthine chambers 0.5–1 mm diam, remaining completely enclosed. Stipe-columella percurrent, 15–50 × 3–8 mm diam, pale yellow, smooth, moist but not viscid when fresh, cream to pale yellow in section, with central core of translucent less fibrillose tissue, central, slender, equal; partial veil cottony, yellow, persistent; universal veil remnants present as scattered dark brown fibrils at base and lower half of stipe. Basal mycelium yellow. Odour mild, not distinctive; taste not distinctive.
Spores 9.9–12.1 × 5.4–7.4 μm, mean = 11.2 × 6.1 μm, Q = 1.5– 2.2, mean Q = 1.91, cinnamon brown (KOH), ovoid to almond-shaped, asymmetrical, ornamented with isolated, irregular, rounded nodules, to 1.5 μm tall; hilar appendage to 1.5 μm, conspicuous, tapering, truncate; spores inamyloid non-dextrinoid; apex rostrate, apex ornamentation less conspicuous. Basidia 19–37(–40) × 6–9 μm, cylindrical to clavate, hyaline, mostly 4-, rarely 2-spored. Cystidia 18.5–26.5 × (7–)10–11 μm, clavate, hyaline, abundant. Hymenophoral trama 26–57 μm wide, of interwoven, hyaline, irregularly inflated hyphae, 4–9 μm diam, with scattered to abundant inflated elements, to 12–21 μm diam. Subhymenium undifferentiated from trama. Pileipellis simplex. Veil 110–220 μm wide, of orange-brown pigmented (KOH), finely to zebra encrusted hyphae, 4–11 μm diam, appearing undulating in outline; overlying a gelatinised layer 30–70 μm wide, of darker orange-brown hyphae, 2–7 μm diam; epicutis 40–85 μm wide, of interwoven to parallel, hyaline to pale yellow hyphae, 3–5 μm diam; context 140–370 μm wide, of densely compacted interwoven to sub-parallel, hyaline hyphae, 3–7 μm diam with rare sinuous dark orange-brown hyphae 2–3.5 μm diam, and scattered inflated elements, 9–17 μm diam becoming more common towards the hymenium. Clamp connections present and obvious in the pileus.
Habitat & Distribution — In New South Wales, occurring in wet sclerophyll forest on the high slopes of the Kaputar Plateau among Eucalyptus dalrympleana, E. laevopinea and E. viminalis. Fruiting: July.
Specimens examined. Known only from type.
Notes — No other species of Australian sequestrate Cortinarius has the combination of yellow-brown to orange-brown sub-viscid pileus, persistent bright yellow cottony cortina, pale yellow stipe, and bright cinnamon brown sublamellate to loculate hymenophore. The yellowish brown pigmented veil hyphae, with striped to crustose encrustations are also distinctive, though they do occur in other Cortinarius species.
The bright pigments of sporocarp, veil and basal mycelium are characteristic of section Dermocybe. However, analyses of ITS sequences suggests placement in a poorly supported (bootstrap 62 %) section Phlegmacium subclade, with the Australian species C. austrovaginatus and C. sinapicolor and a sister taxon of the European C. croceus, in a poorly supported (bootstrap 50 %) broader section Dermocybe clade (Fig. 1). Cortinarius austrovaginatus and C. sinapicolor share a glutinous pileus which C. kapaturensis and C. croceus lack, but all species vary considerably in pileus colour and stipe shape. Cortinarius austrovaginatus has a vinaceous-brown pileus, marginate bulbous stipe base, and thick white universal veil, C. sinapicolor has a bright yellow pileus and veil and marginate bulbous stipe base, and C. croceus has a yellowish brown pileus, and a yellowish with olive brown barely bulbous stipe (Moser & Horak 1975, Garnica et al. 2003). Cortinarius croceus is a pine associate and C. austrovaginatus a Nothofagus associate whilst C. kapaturensis and C. sinapicolor are wet Eucalyptus associates.
In all preliminary analyses with larger datasets and the analysis presented here, C. sinapicolor remained one of the closest taxa to C. kaputarensis. Cortinarius kaputarenis differs from C. sinapicolor in the yellow-brown to orange-brown instead of bright yellow pileus, and lacks the thick glutinous epicutis over both pileus and stipe of the latter species.
6. Cortinarius maculobulga Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515240; Fig. 2f, 14, 15
Fig. 14.
Cortinarius maculobulga. a. Pileipellis; b. spores. — Scale bars: a = 50 μm; b = 5 μm.
Fig. 15.
Scanning electron micrographs of Cortinarius maculobulga spores. — Scale bars = 10 μm.
Sporocarpia hypogaea, 10–42 mm lata, subglobosa vel irregulariter turbinate; pileus albus vel eburneus maculatus brunneolus, variabilis viscidus. Velum album vel pallidocineraceum, tenue, persistens. Hymenophorum loculatum, juventute pallidocinnamomeum, maturitate fuscocinnamomeum. Stipes 8–35 × 2–6 mm, truncatus vel percurrens, solidus, basi bulbosus, albus vel eburneus, viscidus; contextus albus vel flavescens. Pileipellis monostrata, hyphis tenuibus hyalinis gelatinosis. Basidiosporae asymmetricae, ovatae, 12.6–14 × 6.3–7.8 μm, in KOH cinnamomeae, verrucis tenuibus 0.3–0.5 μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV532 (MEL2331647) (holotypus hic designatus), Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot GW2, 16 July 2007.
Etymology. Name refers to mottled white-brown colour of the sporocarps (L: maculata = mottled; bulga = purse or bag).
Sporocarps hypogeous under leaf litter, fruiting singly or in large groups. Pileus 10–23 × 10–42 mm diam, irregularly subglobose to pyriform or turbinate with flattened or convex apex, completely enclosing hymenophore. Pellis white to cream mottled with brown patches, smooth, moist to viscid overall or in patches near base, not hygrophanous, not bruising, with patchy overlying remnant veil, white to pale grey, easily rubbed off with handling. Context 0.5–1.5 mm thick, translucent white to cream. Hymenophore pale cinnamon brown initially becoming dark cinnamon brown, trama, if noticeable, pale brown to dark brown; loculate, chambers empty, irregular, slightly elongate to labyrinthine. Stipe-columella a truncate to percurrent columella tapering slightly from a bulbous exserted base towards the apex, 8–35 × 2–6 mm diam, white to translucent yellow in section, central or slightly eccentric, white to cream, viscid, solid, base bulbous, somewhat marginate, 5–11 mm diam, protruding up to 4 mm below pileus; universal veil remnants apparent as patchy white to pale grey, viscid fibrils; partial veil inconspicuous, thin membranous, connecting inrolled margin and stipe base, white to cream coloured, dry. Basal mycelium inconspicuous, white. Odour faintly spicy-sweet; taste not distinctive.
Spores 12.6–14 × 6.3–7.8 μm, mean = 13.5 × 7.2 μm, Q = 1.8–2.0, mean Q = 1.94, cinnamon brown (KOH), ovoid, ornamented with fine, rounded, isolated warts, 0.3–0.5 × 0.2–0.3 μm; hilar appendage to 1 μm, conspicuous, tapering, truncate; spores inamyloid non-dextrinoid; apex ornamented. Basidia 37–40 × 9–12 μm, hyaline, clavate to cylindrical, thin-walled, with 4 sterigmata. Cystidia 15–38 × 4–8 μm, hyaline, clavate, thin-walled, scattered and never protruding beyond hymenium. Hymenophoral trama 45–195 μm wide, of interwoven, hyaline, somewhat gelatinised hyphae 2–5(–8) μm diam, with inflated elements 4–16 × 5–18 μm. Subhymenium undifferentiated from trama. Pileipellis simplex. Epicutis narrow, 15–45 μm wide, of patchy upright hyphal tips, becoming interwoven and subparallel below, hyphae 3–6 μm diam; context, 70–320 μm broad, of gelatinised, hyaline hyphae, 2–8 μm diam. Clamp connections present in the pileus.
Habitat & Distribution — In New South Wales, occurring in sub-alpine grassy woodland on the Kaputar Plateau among Eucalyptus dalrympleana, E. pauciflora and E. viminalis. Fruiting: July.
Specimens examined. Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot GW1, 16 July 2007, M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV510 (NE94640); Mt Kaputar, Kaputar Rd, Plot GW1, 16 July 2007, M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV511 (NE94641).
Notes — Cortinarius maculobulga may be distinguished from other Australian sequestrate Cortinarius species by the subglobose to pyriform white to cream sporocarps mottled with brown patches, and largish spores with minute warts. Analyses of ITS sequences places this species in a poorly supported (bootstrap 52 %) Rozites ‘A’ clade with three other southern hemisphere species, Australian C. vinaceolamellatus, New Zealand C. subcastanellus and sequestrate Argentinean taxon C. holojanthinus. The Australian species C. submeleagris is not included in this clade in the analysis presented, however in earlier analyses it did group with these taxa. A second clade of Rozites ‘B’ with strong support (bootstrap 97 %), including the European and Asian species C. caperatus, C. emodensis and C. similis is apparently distinct to this first group (Fig. 1).
Species of Rozites have velar remnants or scales on the pileus (which may disappear in older specimens), a slightly viscid to glutinous pileus, and a membranous partial veil. Cortinarius maculobulga appears to lack the distinct velar remnants apparent on the pileus of many other Rozites taxa, though the veil is somewhat membranous. This species also differs from other Rozites taxa in having a pileipellis simplex rather than duplex. Species in the Rozites ‘A’ clade have either glutinous to viscid brownish (C. subcastanellus, C. submeleagris, C. maculobulga) or silver-greyish purple (C. holojanthinus and C. vinaceolamellatus) pilei. Cortinarius maculobulga and C. subcastanellus appear to lack any purple tints to the lamellae/hymenophore, whereas C. holojanthinus, C. submeleagris and C. vinaceolamellatus all have at least some purple tints when young.
There is no apparent pattern in plant associates, as C. holojanthinus, C. subcastanellus and C. submeleagris are all Nothofagus associates, and C. maculobulga and C. vinaceolamellatus are eucalypt associates.
7. Cortinarius nebulobrunneus Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515241; Fig. 2g, 16, 17
Fig. 16.
Cortinarius nebulobrunneus. a. Pileipellis; b. spores. — Scale bars: a = 50 μm; b = 5 μm.
Fig. 17.
Scanning electron micrographs of Cortinarius nebulobrunneus spores. — Scale bars = 10 μm.
Sporocarpia hypogaea vel emergentia, 22–45 mm lata, convexa apicibus complanata, margine laevia; pileus ferrugineus vel brunneus non-viscidis. Velum album, crassum, persistens. Hymenophorum sublamellatum vel labyrinthiforme loculatum contortum, vivide cinnamomeum. Stipes 40–55 × 5–12 mm, percurrens, protrudens, solidus, robusts, basi leviter bulbosus, albus, glaber, non-viscidus; contextus albus. Pileipellis bistrata, extus hyphis tenuibus hyalinis, interne hyphis luteobrunneis non gelatinosis. Basidiosporae asymmetricae, elongatae ellipsoidea, 9.0–11.9 × 5.5–6.5 μm, in KOH pallide luteae, nodulosis irregularibus tenuibus 0.3–0.5 μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV588 (holo MEL2331648 (holotypus hic designatus); iso NE94643), Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot GW3, 17 July 2007.
Etymology. Name refers to the white ‘bloom’ universal veil overlying the brown pileus (L.: nebulosus = foggy or misty; brunnea = brown).
Sporocarps hypogeous to emergent under leaf litter, in a small group. Pileus 13–27 × 22–45 mm diam, strongly convex, occasionally with a flattened apex, and smooth margin. Pellis light brown-orange to brown, finely fibrillose, viscid, not hygrophanous, not bruising, sometimes with a white remnant of veil on the disc appearing as a white ‘bloom’, easily rubbed off with handling. Context 0.8–3.0 mm thick, rapidly thinning from disc to margin, translucent yellow-brown, slightly waxy texture. Hymenophore bright cinnamon brown at all stages, sublamellate to loculate, compact, forming irregular, contorted labyrinthine chambers 0.3–1 mm diam. Stipe-columella percurrent, 40–55 × 5–12 mm, white slightly translucent, somewhat waxy texture, smooth, moist but not viscid when fresh, white in section, central, solid, robust, equal or sometimes expanding into slightly bulbous base; partial veil remnants inconspicuous, as fine white cottony cortina between pellis margin and stipe. Basal mycelium not conspicuous. Odour mild, not distinctive; taste not distinctive.
Spores 9.0–11.9 × 5.5–6.5 μm, mean (15 spores) = 9.9 × 5.9 μm, Q = 1.6–1.8, mean Q = 1.74, pale yellow (KOH), elongate ellipsoid, asymmetric, ornamented with fine, scattered, irregular, flat-topped or rounded warts, 0.3–0.5 μm high; hilar appendage to 1 μm, inconspicuous, tapering; spores inamyloid non-dextrinoid; apex ornamented. Basidia 25–28(–30) × 5–8 μm, elongate cylindrical to narrowly clavate, hyaline, 4-spored. Cystidia not observed. Hymenophoral trama 20–45 μm wide, of interwoven hyaline hyphae 2–3 μm diam, with occasional inflated elements 10–17 μm diam; subhymenium undifferentiated from trama. Pileipellis duplex. Epicutis narrow, 15–25 μm wide, of interwoven, gelatinised hyaline hyphae 2–3 μm diam; hypocutis 25–75 μm wide, of light golden brown, subglobose to ellipsoid inflated hyphae mostly 6–13 μm diam × 8–48 μm long; context 100–300 μm wide, of mostly hyaline hyphae 4–6 μm diam, subparallel with patches of inflated elements up to 12 μm diam × 30–45 μm long. Clamp connections present in the pileus and hymenophoral trama.
Habitat & Distribution — In New South Wales, occurring in sub-alpine grassy woodland among Eucalyptus dalrympleana, E. pauciflora and E. viminalis. Fruiting: June–July.
Specimen examined. Australia, New South Wales, Coolangubra NP, Waratah Rd 2.1 km NE of junction with Coolangubra Forest Way, A.W. Claridge Trappe 18741, 2 June 1996 (CANB, MEL, OSC130731).
Notes — Cortinarius nebulobrunneus is distinguished by the combination of brown pellis with white bloom of universal veil, sublamellate to loculate hymenophore and robust stipe-columella. The texture of the sporocarp is also distinctive, being slightly waxy. Analysis of ITS sequences places C. nebulobrunneus in a well-supported clade (bootstrap 100 %) of species in section Pseudotriumphantes, with C. iringa, C. rapaceus var. luridus and an undescribed sequestrate Cortinarius sp. H6558 (Fig. 1). In this current analysis, although lacking bootstrap support, other section Pseudotriumphantes species are sister taxa, C. alboaggregatus and C. pseudotriumphans. Cortinarius austrocyanites is also in this clade, though currently placed in section Phlegmacium. All of these taxa are from the southern hemisphere, and except for C. nebulobrunneus and C. sp. H6558, are associates of Nothofagus. All taxa have brownish sporocarps, a pileipellis duplex, and spores with sparse minute ornamentation.
8. Cortinarius sinapivelus Danks, T. Lebel & Vernes, sp. nov. — MycoBank MB515242; Fig. 2h, 18a, b, 19
Fig. 18.
Cortinarius sinapivelus. a. Pileipellis; b. spores. — Scale bars: a = 100 μm; b = 5 μm.
Fig. 19.
Scanning electron micrographs of Cortinarius sinapivelus spores. — Scale bars = 10 μm.
Sporocarpia hypogaea vel emergentia, 12–21 mm lata, convexa vel subglobosa apicibus complanata, margine leviter plicata. Pileus brunneolus non viscidus. Velum luteum, crassum, persistens. Hymenophorum sublamellatum vel lamellatum contortum et plicatum, juventute pallidocinnamomea, maturitate leviter fuscum. Stipes 18–35 × 6–8 mm, percurrens, protrudens, solidus, robustus, vivide luteus, fibrillosus; contextus margine luteus et in centro aurantiacus. Pileipellis monostrata, hyphis tenuibus pallide luteis non-gelatinosis. Basidiosporae asymmetricae, latae ovatae, 8.9–10.2 × 6.5–7.4 μm, in KOH cinnamomeae, nodulosis irregularibus tenuibus < 0.5 μm altis. — Typus: M. Danks, J.M. Trappe, T. Lebel & K. Vernes KV518 (holo MEL2331645 (holotypus hic designatus); iso NE94639), Australia, New South Wales, Mt Kaputar, Kaputar Rd, Plot GW2, 16 July 2007.
Etymology. Name refers to the mustard yellow colour of the veil (L.: sinapis = mustard; velus = veil).
Sporocarps hypogeous to emergent under leaf litter, in a small group. Pileus 6–15 × 12–21 mm diam, convex to subglobose with a flattened apex, and slightly plicate margin attached to stipe by a thick cobweb yellow veil, which pulls away in patches to expose the hymenophore. Pellis pale tan brown, finely fibrillose, dry to moist when fresh but not viscid, not hygrophanous, not bruising, with overlying remnant yellow, fibrillose universal veil, easily rubbed off with handling. Context 0.5–3.0 mm thick, translucent yellow-tan. Hymenophore pale cinnamon brown initially, darkening slightly as spores mature, elongated labyrinthine to sublamellate, compressed, contorted/wrinkled and intervenose, especially near the apex and stipe, locules 0.5–2 mm diam. Stipe-columella percurrent, 18–35 × 6–8 mm diam, bright yellow, fibrillose, in section with yellow edges and bright orange centre, central to slightly asymmetric, solid, slender, equal or slightly contorted, dry; universal veil remnants apparent as scattered slightly darker yellow-orange fibrils on pileus surface and stipe; partial veil a thick mustard yellow cortina between pileus margin and stipe. Basal mycelium bright yellow. Odour mild, not distinctive; taste not distinctive.
Spores 8.9–10.2 × 6.5–7.4 μm, mean (13 spores) = 9.4 × 7.0 μm, Q = 1.2–1.4, mean Q = 1.40, cinnamon brown (KOH), broadly ovoid, slightly asymmetric, ornamented with nodules, irregular, flat topped or rounded, < 0.5 μm tall; hilar appendage to 1 μm, conspicuous, tapering, truncate; spores inamyloid non-dextrinoid; apex ornamented. Basidia 26–29 × 7–8 μm, hyaline, clavate to cylindrical, thin-walled, with 4 sterigmata. Cystidia (13.5–)16.5–26.5 × (5.5–)8–12.5(–17) μm, hyaline, clavate, thin-walled, abundant, never protruding beyond hymenium. Hymenophoral trama 15–30 μm wide, of interwoven hyaline hyphae 2.5–4 μm diam and irregularly inflated elements 7.5–14 μm diam; subhymenium undifferentiated from trama. Pileipellis simplex. Veil a patchy outer thin layer, 12–17 μm wide, of hyaline subgelatinised hyphae 2.5–3 μm diam; epicutis narrow, 26–55 μm wide, of pale brown pigmented inflated ± isodiametric cells 10–22 × 8–19 μm, merging with the context, 570–1250 μm wide, of subparallel, irregularly inflated, hyaline hyphae, mostly 7.5–12.5 μm diam, with scattered elements 25–30 μm diam. Clamp connections present in the pileus.
Habitat & Distribution — In New South Wales, occurring in sub-alpine grassy woodland on the Kaputar Plateau among Eucalyptus dalrympleana and E. pauciflora. Fruiting: July.
Specimens examined. Known only from type.
Notes — Although our preference is to not describe taxa from single collections, there were multiple sporocarps, all with varying degrees of elongate labyrinthine to sublamellate hymenophore, and a thick partial veil that remained attached to the stipe and pileus margin, even in mature sporocarps. Cortinarius sinapivelus may be distinguished from other Australian Cortinarius species with a yellow cortina by the distinctly sublamellate hymenophore, broadly ovoid spores, and pileus context of inflated ± isodiametric cells. The sequestrate taxon C. flavovelus also has a brownish pileus and yellow veil, similarly structured pileus and robustly ornamented spores. However, C. flavovelus has a cinnamon brown rather than pale tan brown pileus, a distinctly loculate rather than sublamellate hymenophore, and larger spores 13–15 × 6.5–8.5 μm vs 8.9–10.2 × 6.5–7.4 μm. The bright pigments of the universal and partial veils, stipe, and basal mycelium are consistent with close affinities with section Dermocybe or Splendidi for both C. flavovelus and C. sinapivelus.
Analyses of ITS sequences confirms placement of C. sinapivelus in a well supported section Splendidi (bootstrap 76 %), close to a New Zealand sequence of C. persplendidus in a subclade (bootstrap 52 %) with the Australian species C. basirubscens and C. erythrocephalus (Fig. 1). Cortinarius basirubscens, C. erythrocephalus and C. persplendidus have rich red or red and yellow sporocarps with bright yellow basal mycelium. Another Australian red-pigmented species, Dermocybe kula, is also in this broader Splendidi clade, though it has been shown to have unique red pigments. Cortinarius sinapivelus lacks bright red pigments, having a brown pileus, however does resemble C. persplendidus in the bright yellow stipe, bright yellow cortina, and bright basal mycelium. Cortinarius clelandii, which is in a well supported (bootstrap 94 %) subclade with the sequestrate taxa C. globuliformis and C. sejunctus, has duller sporocarps and the spore ornamentation is less robust and more citriniform than C. sinapivelus (Jones 2007).
DISCUSSION
Several clades representing sections within Cortinarius sensu Peintner et al. (2002a) and Garnica et al. (2005) were recovered in our analyses of ITS sequences, with varying support (Fig. 1). Not all subgenera were included, such as solely northern hemisphere section Calochroi (Garnica et al. 2009) in final analyses. Poor bootstrap support for the deeper branches is typical for such a large dataset, based upon a single region. Inclusion of nLSU data could perhaps help to clarify some of these deeper relationships, however in this paper we were more concerned in placing our new taxa in a general sectional framework.
As has been shown by several workers, the separation of a large and diverse genus Cortinarius from Hebeloma and Gymnopilus is strongly supported (Peintner et al. 2002a, 2004, Garnica et al. 2005, Francis 2007). Sequestrate sporocarp forms are scattered throughout many different lineages within Cortinarius, Descolea and Hebeloma, thus sequestrate cortinarioid genera (based on historical morphological characters) are not monophyletic. The sequestrate genus Hymenogaster is again shown to be paraphyletic, with Hymenogaster A (including specimen from Spain of H. buillardii) having affinities to Hebeloma (73 % bootstrap, Fig. 1) and Hymenogaster B (bootstrap 85 %), along with two species of Protoglossum, within Cortinarius. The related sequestrate genera Descomyces and Timgrovea, with affinities to Descolea, are diverse in Australia, with some 35 undescribed species (Francis 2007, Trappe pers. comm.). Further investigation of the affinities of Hymenogaster should include type studies as well as greater incorporation of these southern taxa.
Broader geographic patterns, of subclades of southern hemisphere taxa within larger sectional clades are apparent (Fig. 2). However this is partly due to taxon selection, with an emphasis on southern hemisphere taxa for the present analyses. Host tree association has been considered a driving force in the evolution of the genus Cortinarius (Horak 1973, Garnica et al. 2009). In Australia species in the genera Nothofagus and Eucalyptus are the main tree associates of native ectomycorrhizal fungi. Although we currently lack extensive geographic data for most cortinarioid species, the broader pattern for Australian ectomycorrhizal fungi appears to be a lack of host tree species fidelity, i.e. ‘any eucalypt will do’ (May 2002). In several clades in our analyses, Pseudotriumphantes, Rozites ‘A’, a mixture of closely related taxa with associations with Nothofagus and Eucalyptus occur (Fig. 2). A host-shift from Nothofagus to Eucalyptus has occurred at least once in the Western Australian species C. symea (Bougher et al. 1994). However, at this early stage in species delimitation it is not possible to evaluate radiation of taxa or centres of origin for particular sections within Cortinarius in Australia.
The loss of gross morphological characters in sequestrate sporocarp forms can make placement of taxa within broader sectional groupings difficult. The use of molecular data as additional characters, has helped considerably in this goal. However, for most of the novel species presented here, morphological characters were also found to support their placement with agaric taxa in the same clades. Although no totally sequestrate fungi clades occur in our analyses, the great diversification of the sequestrate form in Australia in many agaric families, means that some of these groupings may become apparent in future analyses. The appearance of sequences from a single ‘species’ in several different lineages, (i.e. C. campbellae), highlights the need for type studies and clarification of cryptic taxa within Australian sequestrate Cortinarius species, particularly section Purpurascentes.
Acknowledgments
This research was supported by an ARC Discovery Grant (DP0557022) to KV and a Hermon Slade Foundation grant awarded to the authors. M. Danks was supported by an Australian Postgraduate Award. The authors would like to thank RBG and UNE for use of facilities; J.M. Trappe, S. Steinhart, F. Bowie, T. Cooper and J. Ford for field assistance and J. Ehrman (Digital Microscopy Facility, Mt Allison University, New Brunswick, Canada) for the SEM images. Thanks also to N. Walsh and W. Gebert for help with Latin. R.H. Jones for provision of Australian Dermocybe sequences. F. Udovicic and T. May for comments on the manuscript.
REFERENCES
- Beaton G, Pegler DN, Young TWK. 1984. Gasteroid Basidiomycota of Victoria State, Australia: 3. Cortinariales. Kew Bulletin 40: 167 – 204 . [Google Scholar]
- Bougher NL, Castellano MA. 1993. Delimitation of Hymenogaster sensu stricto and four new segregate genera. Mycologia 85: 273 – 293 . [Google Scholar]
- Bougher NL, Fuhrer BA, Horak E. 1994. Taxonomy and biogeography of Australian Rozites species mycorrhizal with Nothofagus and Myrtaceae. Australian Systematic Botany 7: 353 – 375 . [Google Scholar]
- Castellano MA, Trappe JM. 1990. Australasian truffle-like fungi. I. Nomenclatural bibliography of type descriptions of Basidiomycotina. Australian Journal of Systematic Botany 5: 631 – 638 . [Google Scholar]
- Cunningham GH. The Gasteromycetes of Australia and New Zealand. 1979 (Reprint published by the author.) [Google Scholar]
- Dodge CW, Zeller SM. 1934. Hymenogaster dan related genera. Annals of the Missouri Botanical Garden 21: 625 – 708 . [Google Scholar]
- Francis AA. 2007. The utility of morphological, ITS molecular and combined datasets in estimating the phylogeny of the cortinarioid sequestrate fungi. PhD thesis, Murdoch University, WA, Australia: (Unpubl.) [Google Scholar]
- Francis AA, Bougher NL. 2003. Historical and current perspectives in the systematics of Australian cortinarioid sequestrate (truffle-like) fungi. Australasian Mycologist 21: 81 – 93 . [Google Scholar]
- Francis AA, Bougher NL. 2004. Cortinaroid sequestrate (truffle-like) fungi of Western Australia. Australasian Mycologist 23: 1 – 26 . [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity of basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113 – 118 . [DOI] [PubMed] [Google Scholar]
- Garnica S, Weib M, Oberwinkler F. 2003. Morphological and molecular phylogenetic studies in South American Cortinarius species. Mycological Research 107: 1143 – 1156 . [DOI] [PubMed] [Google Scholar]
- Garnica S, Weib M, Oertel B, Ammirati J, Oberwinkler F. 2009. Phylogenetic relationships in Cortinarius, section Calochroi, inferred from nuclear DNA sequences. BMC Evolutionary Biology 9: doi: 10.1186/1471-2148-9-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica S, Weib M, Oertel B, Oberwinkler F. 2005. A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Canadian Journal of Botany 83: 1457 – 1477 . [Google Scholar]
- Grgurinovic CA. 1997. Larger fungi of South Australia. The Botanic Gardens of Adelaide and State Herbarium and the Flora and Fauna of South Australia Handbooks Committee, Adelaide: . [Google Scholar]
- Hall T . 1999–2007. Bioedit version 7.0.9 A biological sequence alignment editor Ibis Biosciences, Carlsbad, CA: . [Google Scholar]
- Holmgren PK, Holmgren NH, Barnett LC. 1990. Index herbariorum. Part 1: the herbaria of the world Edn. 8 New York Botanical Garden Publication, New York: . [Google Scholar]
- Horak E. 1973. Fungi Agaricini Novazelandiae I–V. Beihefte zur Nova Hedwigia 43: 1 – 200 . [Google Scholar]
- Hosaka K, Bates ST, Beever RE, Castellano MA, Colgan WIII, Dominguez LS, Nouhra ER, Geml J, Giacchini AJ, Kenney SR, Simpson NB, Spatafora JW, Trappe JM. 2006. Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders. Mycologia 98, 6: 949 – 959 . [DOI] [PubMed] [Google Scholar]
- Jones R. 2007. A systematic study of Australian Dermocybe (Fungi: Cortinariaceae) PhD thesis Botany Department, The University of Melbourne, Victoria, Australia: . [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers J. 2001. Ainsworth & Bisby’s dictionary of the fungi 9th ed CAB International, Oxon, United Kingdom: . [Google Scholar]
- Lebel T, Tonkin JE. 2008. Australasian species of Macowanites are sequestrate species of Russula (Russulaceae, Basidiomycota). Australian Systematic Botany 20, 4: 355 – 381 . [Google Scholar]
- Maddison WP, Donoghue MJ, Maddison DR. 1984. Outgroup analysis and parsimony. Systematic Zoology 33: 83 – 103 . [Google Scholar]
- May TW. 2002. Where are the short range endemics among Western Australian macrofungi? Australian Systematic Botany 15: 501 – 511 . [Google Scholar]
- May TW, Lebel T . In prep. New combinations in Cortinarius for species of Protoglossum and Quadrispora. Mycotaxon . [Google Scholar]
- May TW, Milne J, Shingles S, Jones RH. 2003. Catalogue and Bibliography of Australian Fungi 2. Basidiomycota p.p. & Myxomycota p.p. Fungi of Australia Volume 2B ABRS/CSIRO Publishing, Melbourne: . [Google Scholar]
- Moser M, Horak E. 1975. Cortinarius Fr. und nahe verwandte Gattungen in Südamerika. Beihefte zur Nova Hedwigia 52: 1 – 628 . [Google Scholar]
- Peintner U, Bougher NL, Castellano MA, Moncalvo J-M, Moser MM, Trappe JM, Vilgalys R . 2002a. Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). American Journal of Botany 88, 12: 2168 – 2179 . [PubMed] [Google Scholar]
- Peintner U, Moncalvo J-M, Vilgalys R. 2004. Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota). Mycologia 96, 5: 1042 – 1058 . [PubMed] [Google Scholar]
- Peintner U, Moser MM, Vilgalys R . 2002b. Thaxterogaster is a taxonomic synonym of Cortinarius: new combinations and new names. Mycotaxon 81: 177 – 184 . [Google Scholar]
- Singer R. 1951. Thaxterogaster – a new link between Gasteromycetes and Agaricales. Mycologia 43, 1–6: 215 – 224 . [Google Scholar]
- Singer R, Smith AH. 1959. Studies on secotiaceous fungi VI. Setchelliogaster Pouzar. Madrono 15, 3: 73 – 78 . [Google Scholar]
- Swofford DL. 2002. ‘PAUP*. Phylogenetic analysis using parsimony (*and other methods) Sinauer Associates, Sunderland, Massachusetts, USA: . [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTALX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876 – 4882 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ. (eds), PDR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, CA, USA: . [Google Scholar]