Abstract
Although p53 is a major cancer preventive factor, under certain extreme stress conditions it may induce severe pathologies. Analyses of animal models indicate that p53 is largely responsible for the toxicity of ionizing radiation or DNA damaging drugs contributing to hematopoietic component of acute radiation syndrome and largely determining severe adverse effects of cancer treatment. p53-mediated damage is strictly tissue specific and occurs in tissues prone to p53-dependent apoptosis (e.g., hematopoietic system and hair follicles); on the contrary, p53 can serve as a survival factor in tissues that respond to p53 activation by cell cycle arrest (e.g., endothelium of small intestine). There are multiple experimental indications that p53 contributes to pathogenicity of acute ischemic diseases. Temporary reversible suppression of p53 by small molecules can be an effective and safe approach to reduce severity of p53-associated pathologies.
Under extreme stress, the p53 tumor suppressor can actually be dangerous: It triggers the hematopoietic cell loss characteristic of acute radiation syndrome and similarly contributes to ischemic injury.
INTRODUCTION: EMERGENCY RESPONSES CAN BE DANGEROUS
p53 is generally considered a protein that is beneficial to the organism. Indeed, its absence has disastrous effects: genomic instability, deregulated metabolism of reactive oxygen species, unleashed acute inflammation, cancer, developmental malformations, etc. Commonly used nicknames for p53 such as “Guardian of the Genome,” “Guardian of Babies,” etc., reflect its importance in protecting organisms and their offspring. p53 plays a critical role in allowing organisms to deal with emergency situations such as genotoxic stress, oncogenic stress, and viral infection, and its multiple specific activities (e.g., induction of DNA repair, growth arrest, and apoptosis) are ideally suited for this role. The activity of p53 in such situations is essential for reducing the risk of accumulation of cells with genetic and epigenetic lesions from which cells with unconstrained growth properties could be selected and form tumors. However, on the other hand, p53 activity can be dangerous to the organism under certain extreme stress conditions. These extreme conditions do not mimic normal environmental or physiological scenarios of stress, and therefore, the potential for unfavorable p53 activity was apparently not eliminated through evolution.
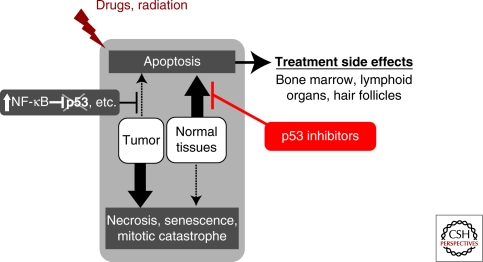
Although most of the information presented and discussed in the other sections of this collection deals with the “useful” functions of p53 and the mechanisms by which p53 exerts these functions, here we will focus on “p53 pathologies”—situations in which p53 activity leads to severe organismal damage. These pathologies have, in many cases, been revealed through analysis of differences between p53-wild-type and isogenic p53-deficient mice in their responses to life-threatening situations. In large part, the specific activity of p53 that produces pathological results is its ability to induce apoptosis. As would be expected, inappropriate or hyper-activation of this activity can lead to death of cells that the organism would be better off retaining.
The fact that p53-dependent pathologies exist raises the possibility of using p53 inhibition as a therapeutic strategy to treat such pathologies. This, of course, is contrary to the prevailing idea of trying to turn p53 “on” as a means to treat cancer, which is solidly based on the fact that p53 deficiency is a bad prognostic factor in cancer. Without questioning this paradigm as a whole, we will present an opposing viewpoint by reviewing cases in which retaining wild-type p53 is useful for the tumor and p53 suppression may have an antitumor effect.
To show the feasibility and potential power of pharmacological p53 suppression, we will review data accumulated using the small molecule p53 inhibitor pifithrin-α (PFTα) and related compounds. PFTs have been used in a variety of experimental models of p53-dependent pathologies and have been successful both in illuminating mechanisms involved in the pathologies and in producing therapeutic effects.
The critical importance of p53 activity for cancer prevention raises the concern that clinical use of p53 inhibitors could be carcinogenic. We will discuss this and other potential safety issues associated with the use of p53 inhibitors and present data aimed at defining which issues, if any, are likely to be real problems.
Finally, we will discuss the evolution of therapeutic approaches which stemmed from the idea of pharmacological suppression of p53 and their progress toward translation into clinical use.
Overall, we expect this article to convey the somewhat radical idea that p53 activity is not always a good thing and that development of p53 inhibitors could lead to significant improvement in the treatment of a variety of human pathologies.
p53 AS A DETERMINANT OF ACUTE RADIOSENSITIVITY
p53 activation in response to DNA damage is clearly an advantageous mechanism for the organism that has been selected through evolution. However, p53 activation resulting from extreme conditions of genotoxic stress can lead to p53-dependent pathologies. Not surprisingly, these pathologies usually occur under conditions created by civilization, and therefore, the mechanisms leading to their development have not been subjected to the process of evolutionary adaptation, which eliminates disadvantageous mechanisms. The genotoxic conditions that we refer to here are those associated with the use of ionizing radiation (IR) and chemotherapeutic drugs that target DNA directly (e.g., doxorubicin, 5-fluorouracil, topoisomerase I and topoisomerase II poisons, methotrexate, etc.) or indirectly by affecting the cell cycle (e.g., vinca alkaloids and taxol). In addition to their desired antitumor effect, all of these agents cause significant adverse side effects that limit the level of therapy that can be safely applied. The adverse toxicity observed in these situations predominantly involves the hematopoietic (HP) system and gastro-intestinal (GI) tract. Traditionally, the radio- and chemo-sensitivity of these tissues has been attributed to their high proliferative index. However, experimental data that became available about 12 years ago called for revision of this, as we now understand, oversimplified concept.
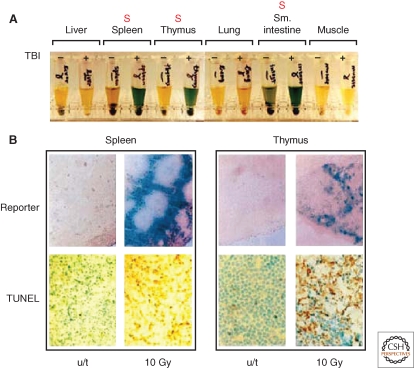
The major breakthrough in our understanding of the mechanisms responsible for the tissue specificity of radio- and chemo-sensitivity followed from generation of p53-deficient mice (Donehower et al. 1992; Jacks et al. 1994) and transgenic mice with p53-responsive reporters (Gottlieb et al. 1997; Komarova et al. 1997). Surprisingly, when mice expressing p53-responsive lacZ were subjected to systemic genotoxic stress, the p53 response was found to be highly tissue-specific. There was a striking coincidence between sites of p53 activity (revealed by in situ detection of the lacZ reporter) and accumulation of apoptotic cells in most radio- and chemo-sensitive tissues (Komarova et al. 2000) (Fig. 1). The observed apoptosis was p53-dependent because it was not seen in p53-null mice. Consistent with these findings, p53-deficient mice were capable of surviving doses of total body γ irradiation (TBI) that were lethal to p53-wild-type mice (Komarov et al. 1999). Interestingly, the rate of cellular proliferation in the tissues of p53-null mice was practically unchanged by TBI (judged by BrdU incorporation). In contrast, DNA replication indicative of blocked proliferation was dramatically reduced in p53-wild-type animals under these conditions (Komarova et al. 2000) (Fig. 2).
Figure 1.
Tissue specificity of p53 activity. (A) Detection of β-galactosidase reporter activity in indicated tissue extracts from transgenic mice carrying p53-responsive lacZ and treated with 10 Gy of total body irradiation. Reporter activity is seen only in radiosensitive organs (marked with red “S”). (B) Massive apoptosis in tissues demonstrating strong response of p53-dependent reporter 8 h after irradiation detected using TUNEL staining for DNA fragmentation in situ. For details, see (Komarova et al. 1997).
Figure 2.
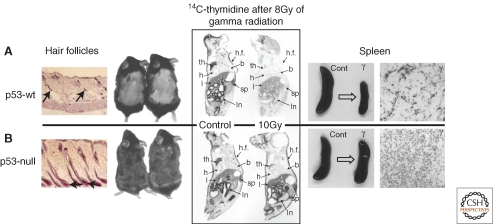
Biological effects driven by p53 in vivo. Left panel: mouse model of chemotherapy-induced alopecia shows resistance of hair follicles of p53-null mice to cyclophosphamide-induced apoptosis accompanied with lack of hair loss (Botchkarev et al. 2001). Middle panel: DNA replication block observed shortly after TBI (figure shows results obtained 24 h postirradiation) is p53-specific and is not seen in p53-null mice (Komarova et al. 2000). Right panel: massive cell loss occurring in the spleen 24 h post TBI (example shown for 10 Gy) because of massive apoptosis is p53-specific and is undetectable in p53-null mice (Komarova et al. 1997).
Taken together, these results showed that (1) p53 plays an important role in the radiation-induced cell death that produces radiation sickness, and (2) the proliferative index of a tissue does not necessarily determine its radiosensitivity. These conclusions had a strong impact on the interpretation of historically accumulated data from radiation biology regarding different pathological components of acute radiation syndrome (ARS). For example, p53 was defined as a critical determinant of the HP component of ARS, which involves massive loss of cells in all HP compartments (bone marrow, thymus, spleen, lymph nodes, etc.) (see Fig. 2) (Cui et al. 1995; Wang et al. 1996). This was based on the finding that p53-null mice were found to be resistant to the range of TBI doses that cause lethal HP syndrome in wild-type animals. Thus, the HP component of ARS is not primarily caused by irreversible damage to HP cells, but by their massive “voluntary” apoptotic death triggered by p53. Interestingly, the involvement of p53 in the other major component of ARS, the GI component, does not follow the same paradigm (discussed in more detail later). However, the radiosensitivity of a number of other tissues was found to be p53-dependent.
As for the HP system, p53 was found to be responsible for the radiosensitivity of hair follicles that leads to radiation- and chemotherapy-associated hair loss. p53-null mice did not develop alopecia after treatments with either radiation or cyclophosphamide (a genotoxic chemotherapeutic drug) that caused hair loss in wild-type animals (Fig. 2) (Song and Lambert 1999; Botchkarev et al. 2001).
In addition, p53 was found to contribute to radiation damage to the spinal cord through p53-dependent apoptosis of oligodendroblasts (Chow et al. 2000). The lack of apoptosis in these cells in p53-deficient mice was accompanied by reduced frequency of paralysis following severe local irradiation of the spinal cord.
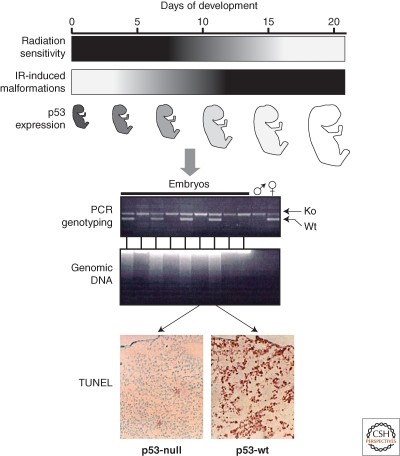
Finally, p53-mediated apoptosis was found to be responsible for the radiosensitivity of early embryos. Decades ago it was shown that there was a dramatic difference in the degree of radiosensitivity of mammalian embryos depending on the stage of embryonic development. For example, in mice, embryos are extremely radiosensitive during the first half of gestation (massive apoptosis is observed following exposure to less than 25% of the minimal lethal dose of TBI determined for adult animals), but highly radioresistant in the second half of gestation (the killing dose is equivalent to or higher than the minimal lethal dose for adults). This phenomenon was attributed to developmental regulation of p53 (MacCallum et al. 1996; Komarova et al. 1997) because (1) p53-null embryos did not show the same degree of stage-dependent differential radiosensitivity seen in wild-type embryos, and (2) strong down-regulation of p53 gene expression at the mRNA level was observed at the time of the switch from high to low radiosensitivity in embryonic development (Komarova et al. 1997) (Fig. 3).
Figure 3.
Radiosensitivity of early embryos is p53-driven (Komarova et al. 1997). Upper panel: schematic description of the correlation among the time stage-dependence of resistance to TBI (black-higher sensitivity), frequency of radiation-induced malformations and reduction of p53 occurring in mid gestation. Lower panel: differential response of embryos to TBI of pregnant female depending on their p53 status. Embryos were either p53-null or p53+/− because they originated from p53-null female and p53+/− male as shown by PCR genotyping. Massive apoptosis detected by TUNEL assay and by electrophoretic assessment of DNA degradation was restricted to those embryos that possess wild-type p53 allele.
Although the majority of studies of p53 pathologies associated with genotoxic stress have been performed in radiation models, it is likely that their conclusions can be applied to the genotoxicity caused by chemotherapeutic agents with some modifications because of specific pharmacological properties of certain drugs. One study that directly assessed the involvement of p53 in the toxicity of a chemotherapeutic agent focused on cisplatin and its induction of acute kidney injury, which limits its use and efficacy as an anticancer treatment. The finding that cisplatin-induced nephrotoxicity was abrogated in p53-deficient mice (Jiang et al. 2006; Wei et al. 2007) showed that p53 plays a critical role in the sensitivity of the kidney tissue to genotoxic stress produced by drug exposure.
Taken together, the data described earlier (primarily obtained through comparison of p53-null and wild-type mice) show that genotoxic stress-induced p53 activation can lead to apoptotic death of normal cells and produce tissue damage and pathologies that can limit the usefulness of genotoxic treatments as anticancer therapies.
THE ROLE OF p53 AS RADIOSENSITIZER IS STRICTLY TISSUE-SPECIFIC: p53 ACTS AS A SURVIVAL FACTOR IN THE GI TRACT
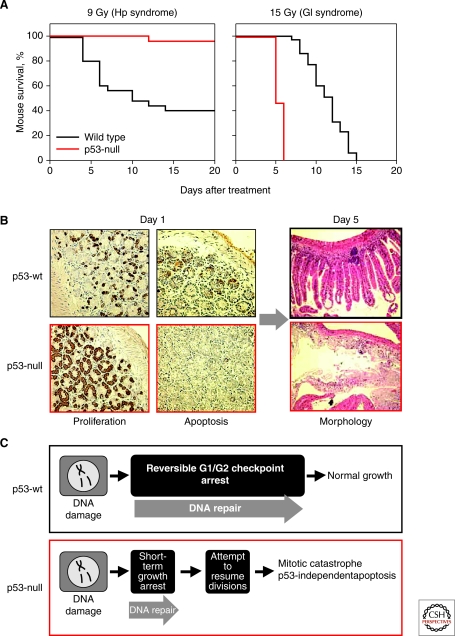
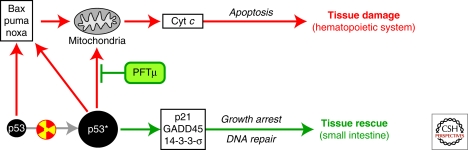
As mentioned earlier, the role of p53 in the radiosensitivity of the GI tract was found to be fundamentally different from the role it plays in the HP system. Although p53 is responsible for the HP component of ARS, it actually functions as a survival factor in the GI tract under conditions of severe irradiation. This conclusion is based on studies performed in p53-knockout mice showing that lack of p53 dramatically increases the sensitivity of the small intestine to γ-radiation (Komarova et al. 2004). Although these mice do not develop HP ARS, they do acquire GI ARS and the symptoms of this syndrome are more severe than those that develop in p53 wild-type mice (Fig. 4A). This observation came as a surprise because p53 had been shown to induce apoptosis in several layers of proliferating cells in the crypts of the small intestine (above the presumed stem cell level) and death of these cells is the most obvious radiation-induced event in this organ (Clarke et al. 1994; Merritt et al. 1994) (Fig. 4B). It is now clear that this rapid p53-mediated apoptosis of intestinal crypt cells does not cause serious organ damage or significantly contribute to the GI component of ARS. Rather, it appears that GI radiosensitivity may be determined by vascular endothelial cells within the tissue. The heightened radiosensitivity of the small intestine in p53-deficient mice was found to be due, at least in part, to increased sensitivity of their vascular endothelial cells to genotoxic stress, which results in their p53-independent apoptosis (Burdelya et al. 2006). Vascular endothelial cells were previously defined through the work of Zvi Fukes and Richard Kolesnick as the primary targets of IR responsible for GI injury within the range of doses that damage the GI tract (Paris et al. 2001; Maj et al. 2003). The mechanisms underlying the protective role of p53 in endothelial cells remain unclear, but may involve the ability of p53 to keep wild-type cells arrested at cell-cycle checkpoints whereas p53-deficient cells undergo lethal mitotic catastrophe (Gudkov and Komarova 2003; Komarova et al. 2004). Another function of p53 that may contribute to its GI rescuing effect is its ability to activate the DNA excision repair machinery (Gudkov and Komarova 2003) (Fig. 4C).
Figure 4.
p53 is a survival factor in irradiated small intestine (Komarova et al. 2004). (A) p53-null mice are resistant to doses of TBI that induce hematopoietic (HP) but are hypersensitive to gastrointestinal (GI) acute radiation syndrome (ARS). (B) Complete destruction of the epithelium of small intestine in p53-null mice by day 5 after 15 Gy of TBI follows continuous proliferation and lack of early apoptosis observed on day 1 (8 h) post TBI. Growth arrest (determined by BrdU incorporation) and massive apoptosis in the crypts of p53 wild-type mice following TBI are associated with better preserved organ structure 5 d later. (C) Schematic interpretation of the earlier described results, which attributes radioresistance of p53 wild type small intestine to growth arrest function of p53.
These observations indicate that the pro-apoptotic function of p53, if exerted in cells critical for tissue function, can determine general radiosensitivity, whereas other p53 functions (cell cycle checkpoint control, DNA repair activation) contribute to radioprotection.
MECHANISMS UNDERLYING TISSUE-SPECIFIC EFFECTS OF p53
Tissue-specific differences in relative expression of distinct p53 functions (apoptosis, cell cycle checkpoint control, and DNA repair activation) determine the fate of specific cell types after exposure to genotoxic stress. Although some cell types are prone to a predominantly apoptotic response almost regardless of the severity of DNA damage (e.g., HP cells), others never undergo p53-dependent apoptosis, but rather respond to p53 activation exclusively with growth arrest (e.g., fibroblasts). The outcome of apoptosis versus growth arrest might be determined by differentiation-specific or stage of development-specific levels of p53 gene expression (see description of the radiosensitivity of embryos in legend to Fig. 3) or by differential activation of p53-regulated genes (e.g., because of differential availability of cofactors that regulate the ability of p53 to bind to specific subsets of target genes (Gudkov and Komarova 2003)). Recently, two newly identified p53 modulators were added to the list of known p53-interacting proteins that direct p53 to different subsets of target genes (Aylon and Oren 2007). Binding of the Hzf zinc finger protein to p53 favors its association with promoters of growth-inhibitory genes and decreases its association with promoters of proapoptotic genes. In contrast, CAS associates with p53 on the promoters of several proapoptotic genes within chromatin and activates methylation within the transcribed regions of the genes. This increases their transcription and promotes apoptosis (Das et al. 2007; Tanaka et al. 2007). Despite these new findings, tissue-specific regulation of p53 remains poorly understood and will likely be a major focus of future studies.
The rapid p53-dependent death of p53-wild-type animals exposed to severe systemic genotoxic stress from HP syndrome does not mimic any likely naturally occurring evolutionary scenario and in itself does not obviously provide any selective advantage to the organism. However, it likely represents an exaggerated performance of the mechanism that normally plays a useful role by protecting the organism from the long-term pathological consequences of mutagenicity associated with sporadically occurring, less extreme, genotoxic stress. There is obvious “engineering sense,” presumably supported by evolution, in having some tissues be more prone to p53-mediated apoptosis than others. The apoptotic response induced by activated p53 is characteristic of those cell populations (such as HP cells) that (1) are technically capable of rapid clonal expansion, (2) can be replenished through proliferation of stem cells (it is noteworthy that there is growing body of evidence indicating that pluripotent self-renewing HP stem cells respond to irradiation by growth arrest rather than apoptosis (Wang et al. 2006)), and (3) do not perform functions essential for tissue structure or integrity. Connective tissue and epithelial cells, which do perform essential tissue structure functions, typically respond to p53 activation with either reversible or permanent growth arrest rather than apoptosis. Growth arrest is an ideal response in these cases because it allows the cells to continue exerting their structural function without propagating possible genetic damage through proliferation.
This paradigm of tissue-specific p53 responsiveness is supported by the experimental data described earlier demonstrating that p53-dependent apoptosis is a critical determinant of the radiosensitivity of HP cells (as well as intestinal crypt cells, hair follicles, and other cell types), whereas p53-dependent growth arrest may be the prevailing response in cells such as vascular endothelial cells (as well as connective tissue and epithelial cells). The rapid development of lethal malignancies in p53-deficient mice leaves no time to appreciate the role of p53 (specifically p53-dependent apoptosis) in prevention of radiation-induced tumors. However, this role is well illustrated by the expedited development of tumors (predominantly lymphomas and sarcomas) in p53+/− mice treated with sublethal doses of γ-radiation (Kemp et al. 1994; French et al. 2001; Mitchel et al. 2003). On the other hand, the predominant involvement of the growth arrest function of p53 in irradiated connective tissue cells is shown by the finding that genotoxic stress-induced fibrosis develops much more quickly and is more severe in the lungs (E.A.K. and A.V.G., in preparation) and livers (Krizhanovsky et al. 2008) of p53-knockout mice than those of wild-type mice. In addition to the lack of growth arrest, deregulated cytokine secretion (because of alterations in nuclear factor κB [NF-κB] responses stemming from the lack of p53) may also contribute to this pathology (Komarova et al. 2005).
p53 AND PATHOLOGIES ASSOCIATED WITH ACUTE ISCHEMIA
p53 is a major regulator of developmental and DNA damage-induced apoptosis during embryogenesis (Armstrong et al. 1995; Sah et al. 1995; Komarova et al. 1997; Frenkel et al. 1999) and in neural precursor cells of the developing central nervous system (CNS) (Akhtar et al. 2006; Geng et al. 2007). In the adult brain, p53 controls self-renewal of adult neural stem cells (Meletis et al. 2006; Gil-Perotin et al. 2006), and loss of p53 leads to expansion of the stem cell/progenitor compartment (Gil-Perotin et al. 2006). Many specific death-inducible signals for neurons, including the excitotoxic agents glutamate and kainic acid (an analog of glutamate) and dopamine (a neurotransmitter) can induce p53-dependent apoptosis (Blum et al. 1997; Hughes et al. 1997; Sakhi et al. 1997; Uberti et al. 1998; Cregan et al. 1999; Daily et al. 1999; Inamura et al. 2000). DNA-damaging factors such as γ-radiation, treatment with anticancer drugs (camptothecin, etoposide), hypoxia, and oxidative stress can also activate p53-dependent apoptosis in neurons (Jordan et al. 1997; Banasiak and Haddad 1998; Johnson et al. 1998; Capranico et al. 1999; Takimoto et al. 1999). Neurons from p53-null mice are resistant to these stresses both in vitro and in vivo, and p53 overexpression induces apoptosis in them (Hughes et al. 1997). p53-associated apoptosis might be a common mechanism of cell loss in several neurological disorders including Alzheimer’s disease (Mattson et al. 1993; de la Monte et al. 1998), Parkinson’s disease (Jenner and Olanow 1998) and stroke (Crumrine et al. 1994; Li et al. 1994; Li et al. 1997; Watanabe et al. 1999).
Ischemia-induced p53-dependent apoptosis of neurons plays an important role in the pathologies of cortical infarction and brain stroke (Covini et al. 1999; Tomasevic et al. 1999a; Tomasevic et al. 1999b; Watanabe et al. 1999; Zhao et al. 2001). The involvement of p53 in ischemic neuron loss is supported by the reduction of infarct volumes measured in p53 knockout mice and the increased neuronal p53 expression that temporally precedes neuronal death in the ischemic brain (Crumrine et al. 1994; Li et al. 1994). Ischemic preconditioning led to decreased p53 levels and resistance of neurons to subsequent ischemia (Tomasevic et al. 1999b; Maulik et al. 2000), presumably via NF-κB-mediated p53 suppression (Culmsee et al. 2003).
Heart ischemia is another common pathology associated with hypoxia. It can result in the apoptotic death of cardiomyocytes and is one of the frequent causes of fatal heart failure. Data are accumulating that implicate p53 in the regulation of cardiomyocyte death. The accumulation of p53 protein and occurrence of apoptosis was shown in reperfused ventricular heart tissue after coronary occlusion (Xie et al. 2000). p53-dependent apoptosis plays an important role in cardiac remodeling after myocardial infarction because p53+/− mice have significantly higher survival rate and lower incidence of left ventricular rupture after ligating the left coronary artery (Matsusaka et al. 2006) suggesting that this pathology may be suitable for treatment with p53 inhibitors (Mocanu and Yellon 2003).
p53 has also been shown to play a crucial role in mediating apoptotic cell death in renal ischemia-reperfusion injury (Wada et al. 2005; Wada et al. 2008), a common cause of acute kidney injury that is characterized by widespread tubular and microvascular apoptosis (Dagher 2004; Hochegger et al. 2007; Sutton et al. 2008; Singaravelu et al. 2009; Molitoris et al. 2009). It was suggested that an apoptosis-associated p53 transcriptional target PERP can play a key role in p53-mediated apoptotic pathways (Singaravelu et al. 2009).
In summary, p53 activity can contribute to pathologies originating from a variety of acute ischemia scenarios by strengthening the degree of tissue damage via broadening hypoxia-induced apoptotic zones.
OTHER POTENTIAL AREAS OF p53 PATHOLOGIES: p53 IN THE REGULATION OF INFLAMMATION AND AGING
There is a growing body of evidence indicating that p53 is a negative regulator of inflammation. Manifestations of autoimmune diseases including collagen-induced arthritis (Yamanishi et al. 2002) and experimental autoimmune encephalitis (Okuda et al. 2003) were found to be more severe in p53-deficient mice than wild-type mice. In addition, inflammatory infiltration of the lung and subsequent disruption of alveolar architecture caused by chronic exposure to the DNA damaging agent bleomycin was markedly increased in p53-null mice and transgenic mice expressing mutant p53 in the lung as compared with wild-type mice (Davis et al. 2000; Ghosh et al. 2002). Accelerated growth of atherosclerotic plaques was observed in p53−/−/apoE−/− mice as compared with p53+/+/apoE−/− mice and in LDL receptor-knockout mice that were lethally irradiated and transplanted with bone marrow from p53−/− mice as compared with the same mice transplanted with p53-wild-type bone marrow (Guevara et al. 1999; van Vlijmen et al. 2001; von der Thusen et al. 2002; Merched et al. 2003). The observed acceleration in plaque growth was associated with increased invasion of activated macrophages into the plaques. It was also shown that IR induces faster and stronger invasion of inflammatory cells and fibroblasts into damaged tissues in p53-null mice than in wild-type mice (Komarova et al. 2004). Finally, a significant proportion of p53-null mice (25%) die before tumor development from unresolved infections that result in abscesses, gastroenteritis, or myocarditis (Donehower et al. 1992), suggesting that the innate immune system is defective in these mice.
Consistent with the observations described earlier, we found that p53 is a general inhibitor of inflammation and that this activity is because of its antagonism of NF-κB (Komarova et al. 2005). This was first suggested by our observation of striking similarities in the global gene expression profiles of human LNCaP prostate cancer cells transduced with a p53-inhibitory genetic element or treated with TNF. This data suggested that p53 inhibits transcription of TNF-inducible genes, many of which are regulated by NF-κB. In support of this, ectopically expressed p53 was shown to inhibit transcription from NF-κB-dependent promoters. Furthermore, suppression of inflammatory responses by p53 was observed in vivo by comparing wild-type and p53-null mice at molecular, cellular, and organismal levels. For example, p53-deficient mice were significantly more susceptible to septic shock-inducing treatments than wild-type mice (Komarova et al. 2005). Recently, similar results were described in the work of Liu et al. (Liu et al. 2009). These observations indicate that p53, acting through suppression of NF-κB, plays the role of a general “buffer” of innate immune responses in vivo. This role is consistent with both the tumor suppressor function of p53 (because inflammation is frequently associated with tumorigenesis [Karin et al. 2006]) and the constitutive activation of NF-κB that is commonly observed in tumors, the majority of which are p53-deficient.
It has been well shown that p53 and NF-κB are involved in mutual negative regulation. For example, pro-inflammatory NF-κB-induced cytokines (such as IL-6 and MIF) can suppress p53 transcriptional activity (Yonish-Rouach et al. 1991; Hudson et al. 1999) and drugs suppressing NF-κB cause activation of p53 (Gurova et al. 2005). Nevertheless, many mechanistic questions about the role of p53–NF-κB interaction in regulation of inflammation remain to be resolved.
To date, the immunosuppressive function of p53 has been primarily considered in the context of its positive anti-inflammatory function. However, one can presume that there may be circumstances in which this p53 activity could be disadvantageous to the organism. This could happen, for example, if p53 weakened the immune response to a pathogen. An extreme example of this type of scenario would be complete disarmament of the immune system by p53 following from its activation by systemic genotoxic stress. This could result in death from infections. Such possibilities should be kept in mind when considering possible clinical applications of pharmacological inhibitors of p53, which in addition to their other properties, are expected to act as immunostimulators.
Regulation of inflammation by p53 is conceptually linked to another systemic pathology: aging. Although p53 has been implicated as a potential regulator of longevity and aging (Campisi 2002; Donehower 2002), the data currently available on its role in these processes are somewhat controversial. At the cellular level, p53 has been shown to mediate the irreversible growth arrest that occurs in fibroblasts undergoing replicative senescence (Gottlieb and Oren 1996). Gradual accumulation of senescent cells characterized by the so-called “pathological secretion phenotype” is considered one of the important systemic pathological alterations accompanying and possibly contributing to aging (Coppe et al. 2008). At the same time, negative regulation of AKT signaling by p53, which mechanistically mimics calorie restriction, provides further support for a putative antiaging role of p53 (Feng et al. 2008).
In vivo, a misbalance in p53 activity can result in different effects on longevity. For example, increasing potential p53 activity, by adding an extra copy of the p53 gene in transgenic “super p53” mice significantly protected them from cancer and did not show premature aging (Garcia-Cao et al. 2002). “Super Ink4a/Arf” mice carrying a transgenic copy of the entire Ink4a/Arf locus show higher resistance to cancer than wild-type mice and have normal aging and lifespan as well. Thus, modest increases in the activity of the Ink4a/Arf tumor suppressor result in a beneficial cancer-resistant phenotype without affecting normal viability or aging (Matheu et al. 2004). Also, mice in which Mdm2 expression was genetically reduced had normal lifespan and were resistant to tumor development (Mendrysa et al. 2003; Mendrysa et al. 2006). However, mice in which overexpression of p53 was accompanied by an imbalance in the normal ratios of different p53 isoforms showed an alarming premature aging phenotype (Maier et al. 2004). Also, several knockout and transgenic mouse lines that showed increased p53 activity had premature aging phenotypes (Chin et al. 1999; Lim et al. 2000; Tyner et al. 2002; Cao et al. 2003; Wong et al. 2003; Varela et al. 2005). In some cases, these aging phenotypes were partially rescued by reduction of the p53 dosage (Purdie et al. 1994; Chin et al. 1999; Donehower 2002; Bauer et al. 2005).
In summary, despite some remaining controversies, the existing evidence shows a clear role for p53 in the negative regulation of inflammation and a possible role for p53 in the regulation of aging. Although p53-dependent pathologies related to these functions of the protein have not been described as such, they may arise from conditions of insufficient p53 function, such as the decline in p53 function observed in old organisms (Feng et al. 2007). In addition, as mentioned earlier, both hyper-activation and inactivation of p53 have the potential to alter inflammatory/immune responses in ways that may be unfavorable to the organism.
CAN FUNCTIONAL p53 BE USEFUL FOR TUMORS?
p53 is inactivated (directly or indirectly) in the vast majority of human tumors (Levine 1997; Soussi et al. 2000), and loss of p53 in tumors is associated with an unfavorable prognosis in many forms of cancer. This has led the p53 field to predominantly focus on the possibility of restoring p53 activity as a way to induce cancer cell death (Hupp et al. 1995; Bottger et al. 1997; Selivanova et al. 1997; Foster et al. 1999; Midgley et al. 2000; Bykov et al. 2002; Issaeva et al. 2004; Vassilev et al. 2004). The validity of this approach is supported by numerous demonstrations of the antitumor effect of p53 activated either by genetic manipulation (Ventura et al. 2007; Xue et al. 2007) or by small molecules (Selivanova et al. 1997; Vassilev 2005; Kravchenko et al. 2008) including drugs that inhibit the interaction between p53 and Mdm2, such as RITA (Issaeva et al. 2004) and Nutlin-3 (Vassilev 2005). The potential power of therapeutic activation of wild-type p53 was recently strengthened by works in which mouse tumor models were engineered to conditionally express p53. Three papers (Martins et al. 2006; Ventura et al. 2007; Xue et al. 2007) reported that restoration of p53 function in established tumors (including lymphomas, sarcomas, and hepatocellular carcinomas) caused regression of the tumors in vivo and could, therefore, represent an effective approach to treating cancer. In addition, a recent paper reported that treatment of a tumor in a patient with Li-Fraumeni syndrome with a replication-deficient adenoviral vector containing a human p53 cDNA under the control of the cytomegalovirus promoter (Advexin) was strikingly successful (Senzer et al. 2007).
Despite the demonstrations of p53’s antitumor effect described earlier, there are also indications that p53 activity may not always be a beneficial factor in preventing tumor progression. Clinical studies indicate that death of tumor cells by apoptosis in response to treatment is characteristic of tumors that originate from tissues that are naturally prone to apoptosis (Kemp et al. 2001), such as HP and reproductive tissues. In most other cancers, treatment-induced apoptosis did not correlate with a decrease in clonogenic survival (Brown and Wouters 2001) or with a favorable prognosis (Nieder et al. 2000). Interestingly, the effect of p53 activation on tumor clearance was also shown to be tumor-type dependent. p53-dependent apoptosis was important for clearance of lymphomas (Martins et al. 2006; Ventura et al. 2007), whereas p53-dependent senescence was required for clearance of sarcomas (Ventura et al. 2007) and liver carcinomas (Xue et al. 2007).
p53 is inactivated by mutations in more than half of human malignancies and is not functional in the remainder because of alterations in other components of the p53 pathways (Johnstone et al. 2002). What is the role of p53 in those tumors that lack the p53-dependent apoptotic pathway? Because p53 is responsible for the prolonged cell cycle arrest after IR treatment, it is expected to promote DNA repair in the absence of an apoptotic response. Therefore, tumors that inactivate p53 during progression should be less capable of DNA repair and more sensitive to DNA damage-induced mitotic catastrophe. Hence, in the absence of apoptosis, p53 can act as a survival factor. A remarkable example of the counterintuitive role played by p53 in determining sensitivity of some tumors to anticancer treatment is seen in human glioblastoma tumors (Kim et al. 2009). Glioma cells with wild-type p53 show higher resistance to cytotoxic treatments used in clinical practice than glioma cells with transcriptionally inactive mutant p53 (Hermisson et al. 2006; Batista et al. 2007; Roos et al. 2007). Inhibition of endogenous wild-type p53 augmented apoptosis in glioma cells in response to temozolomide and the chloroethylating nitrosoureas ACNU and BCNU (Batista et al. 2007). It was shown that wild-type p53 activities associated with DNA repair contribute to the overall survival potential and drug resistance of glioma cells. Clearly, in this case, the prosurvival DNA repair activity of p53 succeeds in overruling the apoptosis-inducing activity of p53. The possibility of p53 acting as a survival factor in some tumor cells is supported by a number of studies demonstrating enhanced cytotoxic chemotherapeutic responses in association with p53 inhibition in vitro and in different types of cancer (Pellegata et al. 1996; Peller and Rotter 2003; Sak et al. 2003; Scott et al. 2003).
By comparing tumor models differing in the p53 status of their stromas, we showed that tumors with p53-deficient stroma were significantly more sensitive to experimental chemo- and radio-therapy than tumors with p53-wild-type stroma (Burdelya et al. 2006). A similar effect was achieved when anticancer treatment was combined with pharmacological suppression of p53 by PFTα (see later). Potentiation of the anticancer effect of chemo- and radiotherapy by p53 suppression in the tumor stroma is likely because of increased sensitivity of p53-deficient endothelial cells to genotoxic stress, as shown both in cell culture and in experimental tumors.
In summary, tumors that retain wild-type p53 (which is usually not fully functional because of deficiencies in other components of p53 pathways) can benefit from the prosurvival functions of p53 either directly, by finding “smart” solutions to avoid its proapoptotic functions, or indirectly, through increased resistance of tumor vascular endothelial cells to anticancer treatment. Thus, reversible pharmacological suppression of p53 may be a viable approach to improving anticancer treatment via enhancement of the antiangiogenic effect of chemo- and radiotherapy.
POTENTIAL APPLICATIONS OF p53 INHIBITORS
The experimental evidence described earlier shows that p53-mediated apoptosis can significantly increase the severity of tissue damage under conditions of systemic or local genotoxic or ischemic stresses such as those associated with radio- and chemotherapy of cancer patients or present in patients with heart, brain or renal ischemia. Theoretically, in all of these circumstances, the severity of tissue damage could be reduced by pharmacological suppression of the p53 response. Although this was first described as only a theoretical possibility (Komarova and Gudkov 1998), we soon provided experimental evidence supporting this idea by isolating a small molecule inhibitor of p53 named pifithrin-α(PFTα) with radioprotective properties (Komarov et al. 1999). PFTα undergoes spontaneous circularization in solution forming a circular derivative named PFTβ which has similar properties in terms of p53 inhibition and radioprotection, but is more stable and less toxic than PFTα. Injection of either PFTα or PFTβ shortly before TBI rescued mice from doses of radiation that induce lethal HP ARS, but failed to protect against doses that induce GI ARS. This pattern of radioprotection is exactly as expected for a p53 inhibitor based on the different roles played by p53 in HP and GI radiosensitivity (see earlier).
We isolated small molecule p53 inhibitors with the expectation that they might eventually be developed into radio- and chemotherapy adjuvants capable of reducing the severity of adverse side effects of cancer treatment (Komarov et al. 1999; Komarova and Gudkov 2001; Gudkov and Komarova 2005). We presumed that the cytoprotective effect of p53 inhibitors would be limited to normal tissues (and not alter tumor cell killing), in cases in which the tumor has mutations inactivating the p53 pathway (Fig. 5). In reality, situation appeared to be even better: We were not able to identify any experimental tumor model, regardless of its p53 status, in which treatment with PFTα or PFTβ was beneficial (in terms of tumor escape from irradiation or chemotherapeutic drugs) possibly because p53 activity is universally impaired in tumors, even in those that retain wild-type p53. Furthermore, PFTβ administration was shown to increase the radio- and chemo-sensitivity of tumor vasculature (Burdelya et al. 2006), which is consistent with the earlier-described prosurvival role of p53 in endothelial cells (Gudkov and Komarova 2003).
Figure 5.
Schematic description of the strategy of pharmacological inhibition of p53 to protect mammalian organism from tissue specific toxicities associated with systemic genotoxic stresses.
Since its discovery in 1999, PFTα, as well as PFTβ and several more potent structural analogs of these compounds (Zhu et al. 2002; Pietrancosta et al. 2005; Pietrancosta et al. 2006), has been widely used as a tool to assess possible therapeutic applications of p53 inhibitors in animal models. In addition to studies on prevention of toxicity associated with radio- and chemotherapy (discussed earlier and reviewed in (Gudkov and Komarova 2005)), such studies have been performed in a variety of animal models mimicking different acute stresses that involve induction of p53-dependent apoptosis (e.g., ischemic heart, brain, and kidney injury) (Crumrine et al. 1994; Li et al. 1994; Li et al. 1997; Watanabe et al. 1999) or human disorders in which p53-associated apoptosis is suspected as a mechanism of cell loss (e.g., Alzheimer’s and Parkinson’s diseases) (Mattson et al. 1993; de la Monte et al. 1998; Jenner and Olanow 1998). The accumulated data show a protective effect of PFTα in different apoptosis-dependent pathologies. Here we briefly review these results.
PFTα and Neuropathology
Ischemia-induced p53-dependent apoptosis of neurons plays an important role in the pathologies of cortical infarction and brain stroke (Covini et al. 1999; Tomasevic et al. 1999a; Tomasevic et al. 1999b; Watanabe et al. 1999; Zhao et al. 2001). The involvement of p53 in ischemic neuron loss is supported by observations of reduced infarct volumes in p53 knockout mice and increased expression of p53 in neurons before their death in the ischemic brain (Crumrine et al. 1994; Li et al. 1994). Moreover, ischemic preconditioning led to decreased p53 levels and resistance of neurons to subsequent ischemia (Tomasevic et al. 1999b; Maulik et al. 2000). Using different animal models of transient global cerebral ischemia, it was shown that PFTα had a protective effect on hippocampal neurons as judged by the outcome of histological, motor, and behavioral assays (Culmsee et al. 2001; Leker et al. 2004; Endo et al. 2006; Gupta et al. 2007; Niizuma et al. 2009). Remarkably, even delayed application of PFTα (6–9 d after cerebral artery occlusion) enhanced the survival, proliferation, and migration to the subventricular zone of neural progenitor cells which died after ischemia-induced stroke in control animals (Luo et al. 2009). Thus, delayed treatment with PFTα is able to modify stroke-induced endogenous neurogenesis and improve functional recovery in stroke-affected animals. Because drug administration after the event is the only realistic scenario for use of supportive care drugs in stroke patients, the feasibility of using PFTα as a human treatment will largely depend on how long after stroke the drug can be administered to see protective effects.
In addition to its neuroprotective effects under ischemic conditions, PFTα (and a set of its novel functionally active structural analogs) protected primary hippocampal neurons against death induced by DNA-damaging drugs such as camptothecin and etoposide (Culmsee et al. 2001; Zhu et al. 2002; Martin et al. 2009). Culmsee et al. reported that the mechanism underlying this protective effect involves PFTα-mediated inhibition of the interaction of p53 with the transcriptional activator p300, whereas NF-κB binding to p300 was enhanced. Thus, PFTα suppressed p53 activity and preserved NF-κB activity, with a net effect of protecting neurons against apoptosis. A similar protective mechanism was also observed in neurons following glucose deprivation and in ischemic brain tissue (Culmsee et al. 2003).
In Alzheimer’s disease, an association was proposed between p53 and amyloid β-peptide (ABP)-related neuronal death (LaFerla et al. 1996). PFTα protected against ABP-induced cell death in cortical primary neurons and differentiated neurons (Culmsee et al. 2001; Tamagno et al. 2003; Strosznajder et al. 2005; Uberti et al. 2007). The lysosomal branch of the apoptotic cascade (destabilization of the lysosomal membrane and concomitant increase in cytosolic cathepsin-l activity), which contributes to ABP-mediated neurodegeneration, was abolished by PFTα (Fogarty et al. 2008). Remarkably, in cultured cortical neurons, PFTα prevented cell death mediated by Delta9-THC (the psychoactive ingredient of marijuana) by the same mechanism of decreasing lysosomal permeability (Downer et al. 2007; Gowran and Campbell 2008). Inhibition of caspase activity by PFTα was also detected in neurons overexpressing presenilins, which modulate cell apoptotic responses during early onset Alzheimer’s disease (Alvarez-Salas et al. 1999; Alves da Costa et al. 2003).
In mouse models of Parkinson’s disease, both p53 knockout mice and mice pretreated with PFTα were protected from death of dopamine neurons in the substantia nigra pars compacta region of the brain (Trimmer et al. 1996; Duan et al. 2002; Nakaso et al. 2004; Perier et al. 2007; Karunakaran et al. 2008). PFTα was also shown to protect against neuron degeneration induced by 6-Hydroxydopamine (Biswas et al. 2005; Liang et al. 2007), proteasome inhibitors (Nair et al. 2006), and the herbicide paraquat (a suspected etiologic factor in the development of Parkinson’s disease) (Yang and Tiffany-Castiglioni 2008). Moreover, PFTα inhibited glutamate- and kainite (an analog of glutamate)-induced p53-mediated neuronal death (Morrison et al. 1996; Xiang et al. 1998; Liu and Zhu 1999; Culmsee et al. 2001; Neema et al. 2005) and protected mouse synaptosomes against excitotoxic injuries (Gilman et al. 2003).
A role for p53 was also shown in the mitochondria-associated cellular dysfunction of Huntington’s disease (HD). In neuronal cultures, the mutant huntingtin protein (mHtt) that is associated with HD binds to p53 and up-regulates its levels in the nucleus and its transcriptional activity. Perturbation of p53 function by PFTα, RNA interference, or genetic deletion prevents cytotoxicity in HD cells and mHtt-transgenic mice (Bae et al. 2005).
Finally, use of PFTα has shown a role for p53 in spinal cord cell death. PFTα reduced the toxic effect of FeSO4 applied to organotypic slice cultures of mouse spinal cord as a model of amyotrophic lateral sclerosis associated with spinal cord cell loss (Eve et al. 2007).
Thus, the results of numerous studies using PFTα and its analogs in mouse models of different neuropathologies clearly indicate that p53–mediated apoptosis plays an important role in these diseases and that pharmacological suppression of p53 presents a possible strategy for their treatment.
Prevention of Renal-, Cardio-, Liver-, and Oto-Toxicity by PFTα
p53 response can complicate a variety of renal pathologies. Cisplatin, a widely used chemotherapeutic drug, can induce acute kidney injury, which sets limitations on its clinical use. Cisplatin-induced nephrotoxicity is mediated by p53, as indicated by its absence in p53-deficient mice (Wei et al. 2007; Jiang et al. 2006). In addition, cisplatin-induced apoptosis of renal tubular cells was suppressed by PFTα both in vitro and in vivo (Jiang et al. 2004; Jiang et al. 2006; Wei et al. 2007; Yano et al. 2007). This protective effect of PFTα coincided with inhibition of expression of proapoptotic genes (Seth et al. 2005; Jiang et al. 2006; Tsuruya et al. 2008). Similarly, PFTα abrogated puromycin aminonucleoside-induced apoptosis of kidney cells (podocytes) (Wada et al. 2005; Wada et al. 2008). p53 also plays a role in ischemia-reperfusion injury, a common cause of acute kidney injury that is characterized by widespread tubular and microvascular apoptosis. Short-term inhibition of p53 with PFTα provided significant renal protection in mouse models of kidney ischemia-reperfusion (Dagher 2004; Hochegger et al. 2007; Sutton et al. 2008). In this scenario, PFTα-mediated protection correlated with modulation of HIF-1α and Von Hippel-Lindau protein expression.
Apoptosis was shown to be involved in cardiac damage following myocardium infarction (Xie et al. 2000; Mocanu and Yellon 2003). Consistent with this, PFTα significantly improved cardiac function in animals following myocardial ischemia/reperfusion which induces apoptosis in cardiac myocytes (Mocanu and Yellon 2003; Liu et al. 2006; Liu et al. 2008b). Doxorubicin is also known to cause both acute and long-term cardiotoxicity (in addition to general side effects that are common to all genotoxic drugs) (Horenstein et al. 2000; Kemp et al. 2001). PFTα injection inhibited doxorubicin-induced apoptosis in rat cardiac myoblasts in vitro and in mouse hearts in vivo (Liu et al. 2004; Chua et al. 2006; Liu et al. 2008a; Venkatakrishnan et al. 2008). Thus, PFTα could be a prototype for a novel cardioprotective drug that might be especially useful for treatment of cancer patients with pre-existing heart conditions.
PFTα also protected normal hepatocytes against death induced by the genotoxic agent arsenic trioxide, ammonia, diterpenoids (oridonin), alkaloids (arecoline), mycotoxins, or LPS both in vitro and in vivo (Begum et al. 2002; Schafer et al. 2003; Farah et al. 2007; Bouaziz et al. 2008; Chou et al. 2008; Huang et al. 2008; Panickar et al. 2009). Leukocyte recruitment and microvascular dysfunction in the liver were also reduced in PFTα-pretreated animals exposed to these drugs. PFTα lowered the ratio of nuclear-to-cytoplasmic p53 and reduced activation of both NF-κB and caspase 3 (Schafer et al. 2003). PFTα effectively attenuated spontaneous apoptosis in liver grafts (rat liver transplantation model) as well (El-Gibaly et al. 2004).
In addition to its general genotoxicity and induction of renal toxicity, cisplatin also frequently causes complications in hearing (Rybak et al. 2007). Addition of PFTα to cisplatin-treated cochlear and utricular cultures resulted in an increase in hair cell survival (Zhang et al. 2003). This result suggests that p53 inhibition could be a feasible approach for reducing the ototoxic, vestibulotoxic and neurotoxic side effects of cisplatin, a strategy, which is currently being clinically tested.
PFTα can Rescue Mice from Developmentally Lethal Gene Defects
Pax-3 is essential for brain development and Pax-3 gene knockout is embryonically lethal. The embryonic lethality observed in Pax-3 knockout mice results from a neural tube closure defect and increased apoptosis in neural tissue. This defect is not seen on a p53-null background, suggesting that excessive p53-dependent apoptosis is the cause of embryonic death. Remarkably, injection of pregnant Pax-3-deficient females with PFTα during a critical period of gestation prevented the neural tube defect in the embryos (Pani et al. 2002). Administration of PFTα also prevented the defective cardiac neural crest cell migration and apoptosis observed in Pax3-deficient embryos and restored proper development of cardiac outflow tracts (Morgan et al. 2008). Thus, PFTα provides a rare example of pharmacological rescue of embryonic lethality caused by a congenital defect.
PFTα and Autophagy
Autophagy is a cellular catabolic process that involves sequestration of portions of the cytoplasm in vesicles that then fuse with lysosomes to allow degradation of their contents. This process is important for normal cell growth and homeostasis and can promote cell survival under some stress conditions, for example by allowing starving cells to reallocate limited nutrients to essential cellular components and processes or to eliminate damaged cytoplasmic organelles. Thus, the increase in autophagy that frequently accompanies cell stress constitutes an attempt to adapt to and survive the stress (Levine and Kroemer 2008). It was shown that physiological levels of p53 inhibit autophagy (Tasdemir et al. 2008a), which may represent another mechanism by which p53 eliminates damaged (and potentially “dangerous”) cells from the organism. The role of p53 as a negative regulator of autophagy is supported by work showing that deletion, depletion or PFTα-mediated inhibition of p53 induced autophagy in human, mouse and nematode cells (Tasdemir et al. 2008b). PFTα triggered autophagy in cytoplasts as well, thereby demonstrating that p53 inhibits autophagy through a cytoplasmic (nonnuclear) effect. Systemic administration of PFTα in mice also induced autophagy in such tissues as the cerebellum and heart. These studies suggest that induction of cytoprotective autophagy by p53 inhibitors could have potential therapeutic applications.
p53-Independent Effects of PFTα
Although most of the effects of pifithrins that have been published are consistent with their p53 inhibitory activity, it is clear that these compounds also have some p53-independent effects. For example, it was shown that PFTα is a potent agonist of the aryl hydrocarbon receptor (AhR) and that this activity of PFTα is not related to its p53 inhibitory properties (Hoagland et al. 2005). Consistent with this, PFTα abolished cardiac and neural crest-derived jaw and brachial cartilage defects induced in zebra fish by the environmental mutagen polychlorinated biphenyl, which exerts its effects through AhR (Grimes et al. 2008). In addition, it was shown that PFTα directly inhibits the catalytic activity of recombinant isoforms of the human cytochrome P450 superfamily of enzymes, CYP1A1, CYP1A2, and CYP1B1. Although it has yet to be examined in vivo, this observation provides a plausible explanation for the protective effects of PFTα against chemical carcinogens (Sparfel et al. 2006). Finally, at least in some cell systems, PFTα is capable of suppressing heat shock response and inhibiting glucocorticoid receptor signaling (Komarova et al. 2003; Murphy et al. 2004). These examples show that it is essential to use appropriate controls (e.g., comparison of PFTα effects in isogenic p53-null and p53-wild-type models) to determine whether experimental data can be interpreted as a p53-specific effect of the compound.
POTENTIAL SAFETY ISSUES ASSOCIATED WITH THE USE OF p53 INHIBITORS
The increased frequency of cancer that is observed in p53 deficient mice and men (Donehower et al. 1992; Jacks et al. 1994; Varley et al. 1997) presents the theoretical risk that use of p53 inhibitors to treat the various p53-dependent pathologies described earlier could have carcinogenic side effects. However, accumulating experimental evidence argues against this. For example, there was no increase in cancer development in wild-type mice rescued from lethal irradiation by PFTα injection (Komarov et al. 1999). We also tested the effect of PFTβ on radiation-induced carcinogenicity in cancer-prone p53+/− mice, 100% of which develop tumors (mostly lymphomas and sarcomas) within 1 yr after sublethal total body irradiation (4 Gy) (Kemp et al. 2001; Mitchel et al. 2003; Perez-Losada et al. 2005). We found that treatment with PFTβ just before irradiation (the optimal regimen for improved survival) does not affect the timing, frequency, and spectrum of tumors that develop in the p53+/− mice (A.V.G. and E.A.K., in preparation). These findings indicate that temporary pharmacological inhibition of p53 does not increase the risk of tumor development.
The most convincing evidence showing that p53 does not need to be at a constantly high level to exert its tumor suppressor function was provided by a recent study from Gerard Evan’s laboratory (Christophorou et al. 2006). They created a transgenic mouse model allowing for pharmacological switching between functional and nonfunctional forms of p53 by substituting the coding regions of p53 with an artificial construct encoding chimeric p53 fused to part of the estrogen receptor. Thus, hydroxytamoxifen treatment was used to switch phenotypically p53-deficient animals into phenotypically p53-wild-type animals. Using this elegant model, it was shown that switching off p53 function immediately after DNA damage protects animals from development of HP ARS but leads to subsequent rapid tumor development, as expected for p53-deficient animals. However, even transient restoration of p53 activity at remote time points after irradiation was sufficient to suppress tumor development, presumably because of elimination (through apoptosis or growth arrest) of cells with damaged DNA and heightened tumorigenic potential. An important conclusion that can be drawn from this work is that some responses to genotoxic stress involving p53-dependent apoptosis, such as widespread apoptosis in lymphoid organs and the intestinal epithelium, are not necessary for tumor suppression by p53 and are detrimental to the organism. Thus, both pharmacological and genetic evidence indicates that temporary and reversible suppression of p53 function does not significantly alter its tumor suppression function. This provides additional support for the potential usefulness of pharmacological suppression of p53 as the way to prevent and treat p53-related pathologies.
Another theoretical risk associated with use of p53 inhibitors stems from the fact that p53 acts as a survival factor in some specific tissues under conditions of genotoxic stress. For example, as mentioned earlier, studies using p53-knockout mice showed that lack of p53 dramatically increases the susceptibility of the small intestine to γ-radiation (Komarova et al. 2004). This property of p53-deficient animals was found to be due, at least in part, to increased sensitivity of their vascular endothelium to genotoxic stress (Burdelya et al. 2006), which was previously defined as the primary target of IR in the GI system (Paris et al. 2001; Maj et al. 2003). The mechanisms underlying the protective role of p53 in endothelial cells are not completely understood, but may involve the ability of p53 to protect cells from mitotic catastrophe by inducing cell cycle arrest (Gudkov and Komarova 2003; Komarova et al. 2004) and/or its ability to activate DNA repair (Gudkov and Komarova 2003).
Thus, the dual role of p53 in defining tissue and organismal survival provides an additional challenge for the use of p53 inhibitors. An ideal solution would be if one could separately target the tissue-damaging (apoptosis induction) and tissue-protecting (cell cycle arrest, DNA repair) functions of p53. We explored this possibility by attempting to identify small molecules capable of suppressing the proapoptotic activity of p53 whereas having no effect on other (mostly prosurvival) p53 functions. Our approach was based on findings from Uta Moll’s lab demonstrating that p53 can induce apoptosis through a mechanism that does not depend on transactivation, but rather involves translocation of p53 to mitochondria (Marchenko et al. 2000; Mihara et al. 2003). In contrast, other p53 functions (growth arrest, DNA repair, etc.) are known to be exerted through the transactivation function of p53 (Prives and Hall 1999). This bifurcation of the p53 signaling pathway provides support for the possibility of pharmacological dissection of p53 functions by a small molecule. Using p53-mediated apoptosis as a cell-based readout, we screened libraries of chemicals and isolated a small molecule named pifithrin-μ (PFTμ). PFTμ inhibits p53 binding to mitochondria by reducing its affinity to the antiapoptotic proteins Bcl-xL and Bcl-2, but has no effect on p53-dependent transactivation. PFTμ rescues primary mouse thymocytes from p53-mediated apoptosis induced by radiation and protects mice from doses of radiation that cause lethal HP syndrome (Strom et al. 2006). These results indicate that selective inhibition of the mitochondrial branch of the p53 pathway is sufficient for radioprotection in vivo (at least for doses of radiation producing HP ARS) and show the feasibility of developing safe tissue-protective p53 inhibitors that preserve the prosurvival functions of p53 (Fig. 6).
Figure 6.
Existence of two branches in the p53 pathway allows pharmacological dissection of different p53 activities. By selective blocking of the mitochondrial branch of the pathway (by PFTμ) can protect from lethal hematopoietic radiation syndrome without affecting transactivation-mediated functions of p53 (growth arrest at cell cycle checkpoints, DNA repair, etc.), thus preserving tissue protective functions of p53. For details, see Strom et al. (2006).
TRANSLATION OF EXPERIMENTAL p53 SUPPRESSION INTO CLINICAL USE
The accumulated information described earlier provides numerous indications of potential clinical use of p53 inhibitors to prevent or treat p53-mediated pathologies. Such potential therapeutic uses include reduction of adverse toxicity associated with genotoxic cancer treatment regimens (radio- or chemotherapy), protection from or mitigation of acute radiation syndrome in biodefense scenarios (nuclear accidents or warfare), reduction of tissue damage in acute ischemic conditions (especially brain and renal ischemia, in which the role of p53 is well established), and treatment of neurodegenerative diseases. However, despite the significant experimental data supporting their therapeutic potential, there are currently no p53 inhibitors approved for any clinical use. Both PFTα-like “general” inhibitors of p53 and PFTμ-like inhibitors specifically targeting the pro-apoptotic branch of p53 function seem to be attractive prototype tissue-protecting agents and will hopefully be developed into drugs by the relevant intellectual property holders (see http://www.quarkpharma.com/qbi-en/newslist/us_patent_ on_p53_inhibitor/).
The most clinically advanced version of a p53-inhibitory approach involves use of siRNA technology for in vivo p53 gene knockdown. The candidate p53-inhibitory drug QPI-1002 (a formulation of anti-p53 siRNA) is being assessed in ongoing Phase I/II clinical trials for prevention of kidney ischemia-reperfusion injury (in renal transplantation and cardiac surgery, see http://www.quarkpharma.com/qbi-en/products/qpi-1002/). The expectation is that p53 inhibition will reduce the frequency of delayed graft function, which is a result of ischemia-reperfusion injury and is one of the most common complications during the immediate postoperative period in renal transplantation.
CONCLUDING REMARKS
We have reviewed here a series of situations in which the activity of wild-type p53 creates the risk of development of a serious—sometimes life threatening—pathology. Most of these situations are related to stresses associated with medical treatment of diseases (radiotherapy and chemotherapy of cancer, various types of surgery), whereas some reflect naturally occurring, acute, typically age-related pathologies (ischemias, neurodegenerative diseases). Research demonstrating that p53 inhibitors can reduce the severity of these pathologies underscores the practical importance of understanding the role played by p53 in these situations and presents a new strategy for treatment of conditions that, in many cases, are currently poorly treatable. Although development of these inhibitors into drugs has been delayed (predominantly because of theoretical safety concerns), recent advances in our understanding of the mechanisms underlying the cancer preventive role of p53 and experiments directly testing the carcinogenicity of p53 inhibitors have practically resolved these concerns. Based on the accumulated data illustrating both the efficacy and safety of prototype small molecule p53 inhibitors, we expect that development of clinically useful inhibitors will now be expedited.
Although the existence of p53-mediated pathologies initially came as a surprise, there is a clear conceptual similarity between this situation and another essential emergency response mechanism—the immune response. Similar to p53, the activity of the immune system is critical for protection of the organism from a number of extrinsic and intrinsic stresses, in this case through recognition and elimination of “foreign” components of endogenous (mutant cells) and exogenous (infectious agents) origin using a combination of innate and adaptive responses. And, as in the case of p53, overreaction of the immune system, can lead to disastrous outcomes (e.g., autoimmune disease, septic shock, etc.). Indeed, the potential danger associated with mechanisms responsible for emergency responses can be seen in virtually any social system, regardless of whether it is a society of cells or organisms.
Exploration of the concept of using temporary pharmacological suppression of p53 for tissue protection led us to consider the possibility of achieving similar effects through pharmacological activation of another stress response factor, NF-κB. Constitutive activation of NF-κB, like inactivation of p53, is frequently acquired by tumor cells as part of their survival strategy. Thus, we predicted that compounds capable of activating NF-κB in normal tissues would promote cell survival under stress conditions. Consistent with this prediction, we recently showed that bacterial flagellin, the natural agonist of Toll-like receptor 5 which activates NF-κB signaling in TLR5-expressing tissues, protects such tissues from radiation damage. Importantly, TLR5 is expressed in the critical cell types affected in both of the major components of ARS (HP and GI). Moreover, unlike p53 inhibitors which were found to increase the radiosensitivity of the GI tract, flagellin-based NF-κB activators were found to protect against radiation doses producing both GI and HP toxicity (Burdelya et al. 2006). This indicates that NF-κB-activating agents will be superior as compared with p53 inhibitors for tissue protection in the context of radiation toxicity; however, this will not necessarily be applicable to other pathologies and by no means downplays the potential clinical importance of p53 inhibitors.
ACKNOWLEDGMENTS
We thank Patricia Stanhope Baker for her help with manuscript preparation. The work of the authors on p53 inhibitors and p53-associated pathologies was supported by grants from National Institutes of Health (CA75179) and from Cleveland BioLabs, Inc., to A.V.G.
Footnotes
Editors: Arnold J. Levine and David P. Lane
Additional Perspectives on The p53 Family available at www.cshperspectives.org
REFERENCES
- Akhtar RS, Geng Y, Klocke BJ, Roth KA 2006. Neural precursor cells possess multiple p53-dependent apoptotic pathways. Cell Death Differentiation 13:1727–1739 [DOI] [PubMed] [Google Scholar]
- Alvarez-Salas LM, Arpawong TE, DiPaolo JA 1999. Growth inhibition of cervical tumor cells by antisense oligodeoxynucleotides directed to the human papillomavirus type 16 E6 gene. Antisense Nucleic Acid Drug Dev 9:441–450 [DOI] [PubMed] [Google Scholar]
- Alves da Costa C, Mattson MP, Ancolio K, Checler F 2003. The C-terminal fragment of presenilin 2 triggers p53-mediated staurosporine-induced apoptosis, a function independent of the presenilinase-derived N-terminal counterpart. J Biol Chem 278:12064–12069 [DOI] [PubMed] [Google Scholar]
- Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR 1995. High-frequency developmental abnormalities in p53-deficient mice. CurrBiol 5:931–936 [DOI] [PubMed] [Google Scholar]
- Aylon Y, Oren M 2007. Living with p53, dying of p53. Cell 130:597–600 [DOI] [PubMed] [Google Scholar]
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, et al. 2005. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron 47:29–41 [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Haddad GG 1998. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res 797:295–304 [DOI] [PubMed] [Google Scholar]
- Batista LF, Roos WP, Christmann M, Menck CF, Kaina B 2007. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer research 67:11886–11895 [DOI] [PubMed] [Google Scholar]
- Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL 2005. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr Biol 15:2063–2068 [DOI] [PubMed] [Google Scholar]
- Begum RA, Farah IO, Ishaque AB 2002. Pifithrin-α(PFT-α) caused differential protection of rat liver cells and HepG2 cell line in response to the selective cytotoxicity of arsenic and cadmium. Biomed Sci Instrumentation 38:41–46 [PubMed] [Google Scholar]
- Biswas SC, Ryu E, Park C, Malagelada C, Greene LA 2005. Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochem Res 30:839–845 [DOI] [PubMed] [Google Scholar]
- Blum D, Wu Y, Nissou MF, Arnaud S, Alim Louis B, Verna JM 1997. p53 and Bax activation in 6-hydroxydopamine-induced apoptosis in PC12 cells. Brain Res 751:139–142 [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Komarova EA, Siebenhaar F, Botchkareva NV, Sharov AA, Komarov PG, Maurer M, Gudkov AV, Gilchrest BA 2001. p53 Involvement in the control of murine hair follicle regression. AmJPathol 158:1913–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottger A, Bottger V, Sparks A, Liu WL, Howard SF, Lane DP 1997. Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. CurrBiol 7:860–869 [DOI] [PubMed] [Google Scholar]
- Bouaziz C, Sharaf El Dein O, El Golli E, Abid-Essefi S, Brenner C, Lemaire C, Bacha H 2008. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 254:19–28 [DOI] [PubMed] [Google Scholar]
- Brown JM, Wouters BG 2001. Apoptosis: mediator or mode of cell killing by anticancer agents? Drug Resist Updat 4:135–136 [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Komarova EA, Hill JE, Browder T, Tararova ND, Mavrakis L, Dicorleto PE, Folkman J, Gudkov AV 2006. Inhibition of p53 response in tumor stroma Improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res 66:9356–9361 [DOI] [PubMed] [Google Scholar]
- Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G 2002. Restoration of the tumor suppressor function to mutant p53 by a low- molecular-weight compound. Nat Med 8:282–288 [DOI] [PubMed] [Google Scholar]
- Campisi J 2002. Cancer and aging: yin, yang, p53. Sci Aging Knowledge Environ 2002:pe1. [DOI] [PubMed] [Google Scholar]
- Cao L, Li W, Kim S, Brodie SG, Deng CX 2003. Senescence, aging, malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Develop 17:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capranico G, Giaccone G, D’Incalci M 1999. DNA topoisomerase II poisons and inhibitors. Cancer Chemother Biol Response Modif 18:125–143 [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA 1999. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527–538 [DOI] [PubMed] [Google Scholar]
- Chou WW, Guh JY, Tsai JF, Hwang CC, Chen HC, Huang JS, Yang YL, Hung WC, Chuang LY 2008. Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology 243:1–10 [DOI] [PubMed] [Google Scholar]
- Chow BM, Li YQ, Wong CS 2000. Radiation-induced apoptosis in the adult central nervous system is p53- dependent. Cell DeathDiffer 7:712–720 [DOI] [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI 2006. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443:214–217 [DOI] [PubMed] [Google Scholar]
- Chua CC, Liu X, Gao J, Hamdy RC, Chua BH 2006. Multiple actions of pifithrin-α on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol 290:H2606–2613 [DOI] [PubMed] [Google Scholar]
- Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH 1994. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following γ-irradiation. Oncogene 9:1767–1773 [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. Plos Biol 6:2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covini N, Tamburin M, Consalez G, Salvati P, Benatti L 1999. ZFM1/SF1 mRNA in rat and gerbil brain after global ischaemia. Eur J Neurosci 11:781–787 [DOI] [PubMed] [Google Scholar]
- Cregan SP, MacLaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS 1999. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci 19:7860–7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumrine RC, Thomas AL, Morgan PF 1994. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab 14:887–891 [DOI] [PubMed] [Google Scholar]
- Cui YF, Zhou PK, Woolford LB, Lord BI, Hendry JH, Wang DW 1995. Apoptosis in bone marrow cells of mice with different p53 genotypes after gamma-rays irradiation in vitro. J Environ Pathol Toxicol Oncol 14:159–163 [PubMed] [Google Scholar]
- Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, El-Metainy S, Behnke H, Mattson MP, Krieglstein J 2003. Reciprocal inhibition of p53 and nuclear factor-κ transcriptional activities determines cell survival or death in neurons. J Neurosci 23:8586–8595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP 2001. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, amyloid β-peptide. J Neurochem 77:220–228 [DOI] [PubMed] [Google Scholar]
- Dagher PC 2004. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int 66:506–509 [DOI] [PubMed] [Google Scholar]
- Daily D, Barzilai A, Offen D, Kamsler A, Melamed E, Ziv I 1999. The involvement of p53 in dopamine-induced apoptosis of cerebellar granule neurons and leukemic cells overexpressing p53. Cell Mol Neurobiol 19:261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Raj L, Zhao B, Kimura Y, Bernstein A, Aaronson SA, Lee SW 2007. Hzf Determines cell survival upon genotoxic stress by modulating p53 transactivation. Cell 130:624–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DW, Weidner DA, Holian A, McConkey DJ 2000. Nitric oxide-dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J Exp Med 192:857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Sohn YK, Ganju N, Wands JR 1998. P53- and CD95-associated apoptosis in neurodegenerative diseases. Lab Invest 78:401–411 [PubMed] [Google Scholar]
- Donehower LA 2002. Does p53 affect organismal aging? J Cell Physiol 192:23–33 [DOI] [PubMed] [Google Scholar]
- Downer EJ, Gowran A, Murphy AC, Campbell VA 2007. The tumour suppressor protein, p53, is involved in the activation of the apoptotic cascade by Delta9-tetrahydrocannabinol in cultured cortical neurons. Eur J Pharmacol 564:57–65 [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215–221 [DOI] [PubMed] [Google Scholar]
- Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP 2002. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol 52:597–606 [DOI] [PubMed] [Google Scholar]
- El-Gibaly AM, Scheuer C, Menger MD, Vollmar B 2004. Improvement of rat liver graft quality by pifithrin-alpha-mediated inhibition of hepatocyte necrapoptosis. Hepatology 39:1553–1562 [DOI] [PubMed] [Google Scholar]
- Endo H, Saito A, Chan PH 2006. Mitochondrial translocation of p53 underlies the selective death of hippocampal CA1 neurons after global cerebral ischaemia. Biochem Soc Trans 34:1283–1286 [DOI] [PubMed] [Google Scholar]
- Eve DJ, Dennis JS, Citron BA 2007. Transcription factor p53 in degenerating spinal cords. Brain Res 1150:174–181 [DOI] [PubMed] [Google Scholar]
- Farah IO, Begum RA, Ishaque AB 2007. Differential protection and transactivation of P53, P21, Bcl2, PCNA, cyclin G, and MDM2 genes in rat liver and the HepG2 cell line upon exposure to pifithrin. Biomed Sci Inst 43:116–121 [PubMed] [Google Scholar]
- Feng Z, Hu W, Rajagopal G, Levine AJ 2008. The tumor suppressor p53: cancer and aging. Cell Cycle 7:842–847 [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ 2007. Declining p53 function in the aging process: A possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci 104:16633–16638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MP, McCormack RM, Noonan J, Murphy D, Gowran A, Campbell VA 2008. A role for p53 in the beta-amyloid-mediated regulation of the lysosomal system. Neurobiol Aging [DOI] [PubMed] [Google Scholar]
- Foster BA, Coffey HA, Morin MJ, Rastinejad F 1999. Pharmacological rescue of mutant p53 conformation and function. Science 286:2507–2510 [DOI] [PubMed] [Google Scholar]
- French J, Storer RD, Donehower LA 2001. The nature of the heterozygous Trp53 knockout model for identification of mutagenic carcinogens. Toxicol Pathol 29Suppl: 24–29 [DOI] [PubMed] [Google Scholar]
- Frenkel J, Sherman D, Fein A, Schwartz D, Almog N, Kapon A, Goldfinger N, Rotter V 1999. Accentuated apoptosis in normally developing p53 knockout mouse embryos following genotoxic stress. Oncogene 18:2901–2907 [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M 2002. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 21:6225–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Akhtar RS, Shacka JJ, Klocke BJ, Zhang J, Chen X, Roth KA 2007. p53 transcription-dependent and -independent regulation of cerebellar neural precursor cell apoptosis. J Neuropathol Exp Neurol 66:66–74 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Mendoza T, Ortiz LA, Hoyle GW, Fermin CD, Brody AR, Friedman M, Morris GF 2002. Bleomycin sensitivity of mice expressing dominant-negative p53 in the lung epithelium. Am J Respir Crit Care Med 166:890–897 [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P 2006. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci 26:1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman CP, Chan SL, Guo Z, Zhu X, Greig N, Mattson MP 2003. p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, oxidative and excitotoxic insults. Neuromol Med 3:159–172 [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Oren M 1996. p53 in growth control and neoplasia. Biochim Biophys Acta 1287:77–102 [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Haffner R, King A, Asher G, Gruss P, Lonai P, Oren M 1997. Transgenic mouse model for studying the transcriptional activity of the p53 protein: Age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J 16:1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowran A, Campbell VA 2008. A role for p53 in the regulation of lysosomal permeability by delta 9-tetrahydrocannabinol in rat cortical neurones: implications for neurodegeneration. J Neurochem 105:1513–1524 [DOI] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML 2008. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sci 106:193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA 2003. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 3:117–129 [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA 2005. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Comm 331:726–736 [DOI] [PubMed] [Google Scholar]
- Guevara NV, Kim HS, Antonova EI, Chan L 1999. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med 5:335–339 [DOI] [PubMed] [Google Scholar]
- Gupta S, Gupta YK, Sharma SS 2007. Protective effect of pifithrin-alpha on brain ischemic reperfusion injury induced by bilateral common carotid arteries occlusion in gerbils. Ind. J Physiol Pharmacol 51:62–68 [PubMed] [Google Scholar]
- Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, et al. 2005. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-κB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci 102:17448–17453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermisson M, Klumpp A, Wick W, Wischhusen J, Nagel G, Roos W, Kaina B, Weller M 2006. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem 96:766–776 [DOI] [PubMed] [Google Scholar]
- Hoagland MS, Hoagland EM, Swanson HI 2005. The p53 inhibitor pifithrin-alpha is a potent agonist of the aryl hydrocarbon receptor. J Pharmacol Exp Therap 314:603–610 [DOI] [PubMed] [Google Scholar]
- Hochegger K, Koppelstaetter C, Tagwerker A, Huber JM, Heininger D, Mayer G, Rosenkranz AR 2007. p21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 292:F762–768 [DOI] [PubMed] [Google Scholar]
- Horenstein MS, Vander Heide RS, L’Ecuyer TJ 2000. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab 71:436–444 [DOI] [PubMed] [Google Scholar]
- Huang J, Wu L, Tashiro S, Onodera S, Ikejima T 2008. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, mitochondrial signaling pathways. J Pharmacol Sci 107:370–379 [DOI] [PubMed] [Google Scholar]
- Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH 1999. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 190:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Alexi T, Schreiber SS 1997. A role for the tumour suppressor gene p53 in regulating neuronal apoptosis. Neuroreport 8:v–xii [PubMed] [Google Scholar]
- Hupp TR, Sparks A, Lane DP 1995. Small peptides activate the latent sequence-specific DNA binding function of p53. Cell 83:237–245 [DOI] [PubMed] [Google Scholar]
- Inamura N, Araki T, Enokido Y, Nishio C, Aizawa S, Hatanaka H 2000. Role of p53 in DNA strand break-induced apoptosis in organotypic slice culture from the mouse cerebellum. J Neurosci Res 60:450–457 [DOI] [PubMed] [Google Scholar]
- Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G 2004. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 10:1321–1328 [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA 1994. Tumor spectrum analysis in p53-mutant mice. CurrBiol 4:1–7 [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW 1998. Understanding cell death in Parkinson’s disease. Ann Neurol 44:S72–84 [DOI] [PubMed] [Google Scholar]
- Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z 2006. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25:4056–4066 [DOI] [PubMed] [Google Scholar]
- Jiang M, Yi X, Hsu S, Wang CY, Dong Z 2004. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287:F1140–1147 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Xiang H, London S, Kinoshita Y, Knudson M, Mayberg M, Korsmeyer SJ, Morrison RS 1998. Evidence for involvement of Bax and p53, but not caspases, in radiation-induced cell death of cultured postnatal hippocampal neurons. J Neurosci Res 54:721–733 [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW 2002. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153–164 [DOI] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Prehn JH, Weichselbaum RR, Beckett M, Ghadge GD, Roos RP, Leiden JM, Miller RJ 1997. p53 expression induces apoptosis in hippocampal pyramidal neuron cultures. J Neurosci 17:1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V 2006. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124:823–835 [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Saeed U, Mishra M, Valli RK, Joshi SD, Meka DP, Seth P, Ravindranath V 2008. Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. J Neurosci 28:12500–12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp CJ, Sun S, Gurley KE 2001. p53 induction and apoptosis in response to radio- and chemotherapy in vivo is tumor-type-dependent. Cancer Res 61:327–332 [PubMed] [Google Scholar]
- Kemp CJ, Wheldon T, Balmain A 1994. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 8:66–69 [DOI] [PubMed] [Google Scholar]
- Kim E, Giese A, Deppert W 2009. Wild-type p53 in cancer cells: when a guardian turns into a blackguard. Biochem Pharmacol 77:11–20 [DOI] [PubMed] [Google Scholar]
- Komarova EA, Gudkov AV 1998. Could p53 be a target for therapeutic suppression? Semin Cancer Biol 8:389–400 [DOI] [PubMed] [Google Scholar]
- Komarova EA, Gudkov AV 2001. Chemoprotection from p53-dependent apoptosis: potential clinical applications of the p53 inhibitors. Biochem Pharmacol 62:657–667 [DOI] [PubMed] [Google Scholar]
- Komarova EA, Chernov MV, Franks R, Wang K, Armin G, Zelnick CR, Chin DM, Bacus SS, Stark GR, Gudkov AV 1997. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J 16:1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EA, Christov K, Faerman AI, Gudkov AV 2000. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene 19:3791–3798 [DOI] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV 1999. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 85:1733–1737 [DOI] [PubMed] [Google Scholar]
- Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, Gudkov AV 2004. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 23:3265–3271 [DOI] [PubMed] [Google Scholar]
- Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin O, Kuprash DV, Nedospasov SA, et al. 2005. p53 is a suppressor of inflammatory response in mice. FASEB J 19:1030–1040 [DOI] [PubMed] [Google Scholar]
- Komarova EA, Neznanov N, Komarov PG, Gudkov A 2003. p53 inhibitor PFT-alpha can suppress heat shock and glucocorticoid signaling pathways. J Biol Chem 278:15465–15468 [DOI] [PubMed] [Google Scholar]
- Kravchenko JE, Ilyinskaya GV, Komarov PG, Agapova LS, Kochetkov DV, Strom E, Frolova EI, Kovriga I, Gudkov AV, Feinstein E, Chumakov PM 2008. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci 105:6302–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E, Lowe SW 2008. Implications of cellular senescence in tissue damage response, tumor suppression, stem cell biology. Cold Spring Harbor Symposia on Quantitative Biology 73:513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Hall CK, Ngo L, Jay G 1996. Extracellular deposition of beta-amyloid upon p53-dependent neuronal cell death in transgenic mice. J Clin Invest 98:1626–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Aharonowiz M, Greig NH, Ovadia H 2004. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol 187:478–486 [DOI] [PubMed] [Google Scholar]
- Levine AJ 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323–331 [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G 2008. Autophagy in the pathogenesis of disease. Cell 132:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chopp M, Powers C, Jiang N 1997. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res 765:301–312 [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Zhang ZG, Zaloga C, Niewenhuis L, Gautam S 1994. p53-immunoreactive protein and p53 mRNA expression after transient middle cerebral artery occlusion in rats. Stroke; J Cerebral Circ 25:849–855 [DOI] [PubMed] [Google Scholar]
- Liang ZQ, Li YL, Zhao XL, Han R, Wang XX, Wang Y, Chase TN, Bennett MC, Qin ZH 2007. NF-kappaB contributes to 6-hydroxydopamine-induced apoptosis of nigral dopaminergic neurons through p53. Brain Res 1145:190–203 [DOI] [PubMed] [Google Scholar]
- Lim DS, Vogel H, Willerford DM, Sands AT, Platt KA, Hasty P 2000. Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol Cell Biol 20:3772–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XZ 1999. Roles of p53, c-Myc, Bcl-2, Bax and caspases in glutamate-induced neuronal apoptosis and the possible neuroprotective mechanism of basic fibroblast growth factor. Brain Res Mol Brain Res 71:210–216 [DOI] [PubMed] [Google Scholar]
- Liu X, Chua CC, Gao J, Chen Z, Landy CL, Hamdy R, Chua BH 2004. Pifithrin-alpha protects against doxorubicin-induced apoptosis and acute cardiotoxicity in mice. Am J Physiol 286:H933–939 [DOI] [PubMed] [Google Scholar]
- Liu J, Mao W, Ding B, Liang CS 2008a. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol 295:H1956–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E 2009. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J Immunol 182:5063–5071 [DOI] [PubMed] [Google Scholar]
- Liu P, Xu B, Cavalieri TA, Hock CE 2006. Pifithrin-alpha attenuates p53-mediated apoptosis and improves cardiac function in response to myocardial ischemia/reperfusion in aged rats. Shock 26:608–614 [DOI] [PubMed] [Google Scholar]
- Liu P, Xu B, Cavalieri TA, Hock CE 2008b. Inhibition of p53 by pifithrin-alpha reduces myocyte apoptosis and leukocyte transmigration in aged rat hearts following 24 hours of reperfusion. Shock 30:545–551 [DOI] [PubMed] [Google Scholar]
- Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y 2009. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol 65:520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum DE, Hupp TR, Midgley CA, Stuart D, Campbell SJ, Harper A, Walsh FS, Wright EG, Balmain A, Lane DP, Hall PA 1996. The p53 response to ionising radiation in adult and developing murine tissues. Oncogene 13:2575–2587 [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H 2004. Modulation of mammalian life span by the short isoform of p53. Genes Develop 18:306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj JG, Paris F, Haimovitz-Friedman A, Venkatraman E, Kolesnick R, Fuks Z 2003. Microvascular function regulates intestinal crypt response to radiation. Cancer Res 63:4338–4341 [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM 2000. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem 275:16202–16212 [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA 2009. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb Cortex 19:1273–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins CP, Brown-Swigart L, Evan GI 2006. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127:1323–1334 [DOI] [PubMed] [Google Scholar]
- Matheu A, Pantoja C, Efeyan A, Criado LM, Martin-Caballero J, Flores JM, Klatt P, Serrano M 2004. Increased gene dosage of Ink4a/Arf results in cancer resistance and normal aging. Genes Develop 18:2736–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H 2006. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovasc Res 70:457–465 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE 1993. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 10:243–254 [DOI] [PubMed] [Google Scholar]
- Maulik N, Sasaki H, Addya S, Das DK 2000. Regulation of cardiomyocyte apoptosis by redox-sensitive transcription factors. FEBS Lett 485:7–12 [DOI] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J 2006. p53 suppresses the self-renewal of adult neural stem cells. Development 133:363–369 [DOI] [PubMed] [Google Scholar]
- Mendrysa SM, McElwee MK, Michalowski J, O’Leary KA, Young KM, Perry ME 2003. mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol 23:462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrysa SM, O’Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME 2006. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Develop 20:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Williams E, Chan L 2003. Macrophage-specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler Thromb Vasc Biol 23:1608–1614 [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA 1994. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 54:614–617 [PubMed] [Google Scholar]
- Midgley CA, Desterro JM, Saville MK, Howard S, Sparks A, Hay RT, Lane DP 2000. An N-terminal p14ARF peptide blocks Mdm2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene 19:2312–2323 [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM 2003. p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11:577–590 [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, Morrison DP, Carlisle SM 2003. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiation Res 159:320–327 [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Yellon DM 2003. p53 down-regulation: a new molecular mechanism involved in ischaemic preconditioning. FEBS Lett 555:302–306 [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, et al. 2009. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20:1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR 2008. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Develop 125:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA 1996. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci 16:1337–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PJ, Galigniana MD, Morishima Y, Harrell JM, Kwok RP, Ljungman M, Pratt WB 2004. Pifithrin-alpha inhibits p53 signaling after interaction of the tumor suppressor protein with hsp90 and its nuclear translocation. J Biol Chem 279:30195–30201 [DOI] [PubMed] [Google Scholar]
- Nair VD, McNaught KS, Gonzalez-Maeso J, Sealfon SC, Olanow CW 2006. p53 mediates nontranscriptional cell death in dopaminergic cells in response to proteasome inhibition. J Biol Chem 281:39550–39560 [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yoshimoto Y, Yano H, Takeshima T, Nakashima K 2004. p53-mediated mitochondrial dysfunction by proteasome inhibition in dopaminergic SH-SY5Y cells. Neurosci Lett 354:213–216 [DOI] [PubMed] [Google Scholar]
- Neema M, Navarro-Quiroga I, Chechlacz M, Gilliams-Francis K, Liu J, Lamonica K, Lin SL, Naegele JR 2005. DNA damage and nonhomologous end joining in excitotoxicity: neuroprotective role of DNA-PKcs in kainic acid-induced seizures. Hippocampus 15:1057–1071 [DOI] [PubMed] [Google Scholar]
- Nieder C, Petersen S, Petersen C, Thames HD 2000. The challenge of p53 as prognostic and predictive factor in gliomas. Cancer TreatRev 26:67–73 [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Chan PH 2009. Potential role of PUMA in delayed death of hippocampal CA1 neurons after transient global cerebral ischemia. Stroke 40:618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Okuda M, Bernard CC 2003. Regulatory role of p53 in experimental autoimmune encephalomyelitis. J Neuroimmunol 135:29–37 [DOI] [PubMed] [Google Scholar]
- Pani L, Horal M, Loeken MR 2002. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Develop 16:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panickar KS, Jayakumar AR, Rao KV, Norenberg MD 2009. Ammonia-induced activation of p53 in cultured astrocytes: role in cell swelling and glutamate uptake. Neurochem Int 55:98–105 [DOI] [PubMed] [Google Scholar]
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R 2001. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293:293–297 [DOI] [PubMed] [Google Scholar]
- Pellegata NS, Antoniono RJ, Redpath JL, Stanbridge EJ 1996. DNA damage and p53-mediated cell cycle arrest: a reevaluation. Proc Natl Acad Sci 93:15209–15214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peller S, Rotter V 2003. TP53 in hematological cancer: low incidence of mutations with significant clinical relevance. Hum Mutat 21:277–284 [DOI] [PubMed] [Google Scholar]
- Perez-Losada J, Wu D, DelRosario R, Balmain A, Mao JH 2005. p63 and p73 do not contribute to p53-mediated lymphoma suppressor activity in vivo. Oncogene 24:5521–5524 [DOI] [PubMed] [Google Scholar]
- Perier C, Bove J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V, Rathke-Hartlieb S, Bouillet P, Strasser A, Schulz JB, et al. 2007. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc Natl Acad Sci 104:8161–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrancosta N, Maina F, Dono R, Moumen A, Garino C, Laras Y, Burlet S, Quelever G, Kraus JL 2005. Novel cyclized Pifithrin-alpha p53 inactivators: synthesis and biological studies. Bioorg Med Chem Lett 15:1561–1564 [DOI] [PubMed] [Google Scholar]
- Pietrancosta N, Moumen A, Dono R, Lingor P, Planchamp V, Lamballe F, Bahr M, Kraus JL, Maina F 2006. Imino-tetrahydro-benzothiazole derivatives as p53 inhibitors: discovery of a highly potent in vivo inhibitor and its action mechanism. J Med Chem 49:3645–3652 [DOI] [PubMed] [Google Scholar]
- Prives C, Hall PA 1999. The p53 pathway. J Pathol 187:112–126 [DOI] [PubMed] [Google Scholar]
- Purdie CA, Harrison DJ, Peter A, Dobbie L, White S, Howie SE, Salter DM, Bird CC, Wyllie AH, Hooper ML, et al. 1994. Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene 9:603–609 [PubMed] [Google Scholar]
- Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B 2007. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene 26:186–197 [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V 2007. Mechanisms of cisplatin-induced ototoxicity and prevention. Hearing Res 226:157–167 [DOI] [PubMed] [Google Scholar]
- Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T 1995. A subset of p53-deficient embryos exhibit exencephaly. Nat Gen 10:175–180 [DOI] [PubMed] [Google Scholar]
- Sak A, Wurm R, Elo B, Grehl S, Pottgen C, Stuben G, Sinn B, Wolf G, Budach V, Stuschke M 2003. Increased radiation-induced apoptosis and altered cell cycle progression of human lung cancer cell lines by antisense oligodeoxynucleotides targeting p53 and p21(WAF1/CIP1). Cancer Gene Ther 10:926–934 [DOI] [PubMed] [Google Scholar]
- Sakhi S, Bruce A, Sun N, Tocco G, Baudry M, Schreiber SS 1997. Induction of tumor suppressor p53 and DNA fragmentation in organotypic hippocampal cultures following excitotoxin treatment. Exp Neurol 145:81–88 [DOI] [PubMed] [Google Scholar]
- Schafer T, Scheuer C, Roemer K, Menger MD, Vollmar B 2003. Inhibition of p53 protects liver tissue against endotoxin-induced apoptotic and necrotic cell death. FASEB J 17:660–667 [DOI] [PubMed] [Google Scholar]
- Scott SL, Earle JD, Gumerlock PH 2003. Functional p53 increases prostate cancer cell survival after exposure to fractionated doses of ionizing radiation. Cancer Res 63:7190–7196 [PubMed] [Google Scholar]
- Selivanova G, Iotsova V, Okan I, Fritsche M, Strom M, Groner B, Grafstrom RC, Wiman KG 1997. Restoration of the growth suppression function of mutant p53 by a synthetic peptide derived from the p53 C-terminal domain. Nat Med 3:632–638 [DOI] [PubMed] [Google Scholar]
- Senzer N, Nemunaitis J, Nemunaitis M, Lamont J, Gore M, Gabra H, Eeles R, Sodha N, Lynch FJ, Zumstein LA, Menander KB, et al. 2007. p53 therapy in a patient with Li-Fraumeni syndrome. Mol Cancer Therap 6:1478–1482 [DOI] [PubMed] [Google Scholar]
- Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP 2005. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem 280:31230–31239 [DOI] [PubMed] [Google Scholar]
- Singaravelu K, Devalaraja-Narashimha K, Lastovica B, Padanilam BJ 2009. PERP, a p53 proapoptotic target, mediates apoptotic cell death in renal ischemia. Am J Physiol Renal Physiol 296:F847–858 [DOI] [PubMed] [Google Scholar]
- Song S, Lambert PF 1999. Different responses of epidermal and hair follicular cells to radiation correlate with distinct patterns of p53 and p21 induction. Am J Pathol 155:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T, Dehouche K, Beroud C 2000. p53 website and analysis of p53 gene mutations in human cancer: forging a link between epidemiology and carcinogenesis. Hum Mutat 15:105–113 [DOI] [PubMed] [Google Scholar]
- Sparfel L, van Grevenynghe J, Le Vee M, Aninat C, Fardel O 2006. Potent inhibition of carcinogen-bioactivating cytochrome P450 1B1 by the p53 inhibitor pifithrin {α}. Carcinogenesis 27:656–663 [DOI] [PubMed] [Google Scholar]
- Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, et al. 2006. Small-molecule inhibitor of p53 binding to mitochondria protects mice from γ radiation. Nat Chem Biol 2:474–479 [DOI] [PubMed] [Google Scholar]
- Strosznajder RP, Jesko H, Banasik M, Tanaka S 2005. Effects of p53 inhibitor on survival and death of cells subjected to oxidative stress. J Physiol Pharmacol 56 Suppl 4:215–221 [PubMed] [Google Scholar]
- Sutton TA, Wilkinson J, Mang HE, Knipe NL, Plotkin Z, Hosein M, Zak K, Wittenborn J, Dagher PC 2008. p53 regulates renal expression of HIF-1{alpha} and pVHL under physiological conditions and after ischemia-reperfusion injury. Am J Physiol Renal Physiol 295:F1666–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto CH, Kieffer LV, Kieffer ME, Arbuck SG, Wright J 1999. DNA topoisomerase I poisons. Cancer Chemother Biol Response Modif 18:81–124 [PubMed] [Google Scholar]
- Tamagno E, Parola M, Guglielmotto M, Santoro G, Bardini P, Marra L, Tabaton M, Danni O 2003. Multiple signaling events in amyloid beta-induced, oxidative stress-dependent neuronal apoptosis. Free Radic Biol Med 35:45–58 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ohkubo S, Tatsuno I, Prives C 2007. hCAS/CSE1L associates with chromatin and regulates expression of select p53 target genes. Cell 130:638–650 [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Chiara Maiuri M, Morselli E, Criollo A, D’Amelio M, Djavaheri-Mergny M, Cecconi F, Tavernarakis N, Kroemer G 2008a. A dual role of p53 in the control of autophagy. Autophagy 4:810–814 [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, et al. 2008b. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10:676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasevic G, Kamme F, Stubberod P, Wieloch M, Wieloch T 1999a. The tumor suppressor p53 and its response gene p21WAF1/Cip1 are not markers of neuronal death following transient global cerebral ischemia. Neuroscience 90:781–792 [DOI] [PubMed] [Google Scholar]
- Tomasevic G, Shamloo M, Israeli D, Wieloch T 1999b. Activation of p53 and its target genes p21(WAF1/Cip1) and PAG608/Wig-1 in ischemic preconditioning. Brain Res Mol Brain Res 70:304–313 [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Smith TS, Jung AB, Bennett JP Jr, 1996. Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 5:233–239 [DOI] [PubMed] [Google Scholar]
- Tsuruya K, Yotsueda H, Ikeda H, Taniguchi M, Masutani K, Hayashida H, Hirakata H, Iida M 2008. Involvement of p53-transactivated Puma in cisplatin-induced renal tubular cell death. Life Sci 83:550–556 [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, et al. 2002. p53 mutant mice that display early ageing-associated phenotypes. Nature 415:45–53 [DOI] [PubMed] [Google Scholar]
- Uberti D, Belloni M, Grilli M, Spano P, Memo M 1998. Induction of tumour-suppressor phosphoprotein p53 in the apoptosis of cultured rat cerebellar neurones triggered by excitatory amino acids. Eur J Neurosci 10:246–254 [DOI] [PubMed] [Google Scholar]
- Uberti D, Ferrari-Toninelli G, Bonini SA, Sarnico I, Benarese M, Pizzi M, Benussi L, Ghidoni R, Binetti G, Spano P, et al. 2007. Blockade of the tumor necrosis factor-related apoptosis inducing ligand death receptor DR5 prevents beta-amyloid neurotoxicity. Neuropsychopharmacology 32:872–880 [DOI] [PubMed] [Google Scholar]
- van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, et al. 2001. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ Res 88:780–786 [DOI] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, et al. 2005. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 anti ing activation. Nature 437:564–568 [DOI] [PubMed] [Google Scholar]
- Varley JM, McGown G, Thorncroft M, Santibanez-Koref MF, Kelsey AM, Tricker KJ, Evans DG, Birch JM 1997. Germ-line mutations of TP53 in Li-Fraumeni families: an extended study of 39 families. Cancer Res 7:3245–3252 [PubMed] [Google Scholar]
- Vassilev LT 2005. p53 Activation by small molecules: application in oncology. J Med Chem 48:4491–4499 [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, et al. 2004. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303:844–848 [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan CD, Dunsmore K, Wong H, Roy S, Sen CK, Wani A, Zweier JL, Ilangovan G 2008. HSP27 regulates p53 transcriptional activity in doxorubicin-treated fibroblasts and cardiac H9c2 cells: p21 upregulation and G2/M phase cell cycle arrest. Am J Physiol 294:H1736–1744 [DOI] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T 2007. Restoration of p53 function leads to tumour regression in vivo. Nature 445:661–665 [DOI] [PubMed] [Google Scholar]
- von der Thusen JH, van Vlijmen BJ, Hoeben RC, Kockx MM, Havekes LM, van Berkel TJ, Biessen EA 2002. Induction of atherosclerotic plaque rupture in apolipoprotein E-/- mice after adenovirus-mediated transfer of p53. Circulation 105:2064–2070 [DOI] [PubMed] [Google Scholar]
- Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ 2005. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16:2615–2625 [DOI] [PubMed] [Google Scholar]
- Wada T, Pippin JW, Nangaku M, Shankland SJ 2008. Dexamethasone’s prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron 109:e8–19 [DOI] [PubMed] [Google Scholar]
- Wang L, Cui Y, Lord BI, Roberts SA, Potten CS, Hendry JH, Scott D 1996. Gamma-ray-induced cell killing and chromosome abnormalities in the bone marrow of p53-deficient mice. Radiat Res 146:259–266 [PubMed] [Google Scholar]
- Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D 2006. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 107:358–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ohta S, Kumon Y, Sakaki S, Sakanaka M 1999. Increase in p53 protein expression following cortical infarction in the spontaneously hypertensive rat. Brain Res 837:38–45 [DOI] [PubMed] [Google Scholar]
- Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z 2007. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293:F1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA 2003. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421:643–648 [DOI] [PubMed] [Google Scholar]
- Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS 1998. Bax involvement in p53-mediated neuronal cell death. J Neurosci 18:1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Koyama T, Abe K, Fuji Y, Sawa H, Nagtashima K 2000. Upregulation of P53 protein in rat heart subjected to a transient occlusion of the coronary artery followed by reperfusion. Jap J Physiol 50:159–162 [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, Zvaifler NJ, Green DR, Firestein GS 2002. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol 160:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Tiffany-Castiglioni E 2008. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: involvement of p53 and mitochondria. J Toxicol Environ Health 71:289–299 [DOI] [PubMed] [Google Scholar]
- Yano T, Itoh Y, Matsuo M, Kawashiri T, Egashira N, Oishi R 2007. Involvement of both tumor necrosis factor-alpha-induced necrosis and p53-mediated caspase-dependent apoptosis in nephrotoxicity of cisplatin. Apoptosis 12:1901–1909 [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M 1991. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352:345–347 [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R 2003. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience 120:191–205 [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Velez DA, Wang NP, Hewan-Lowe KO, Nakamura M, Guyton RA, Vinten-Johansen J 2001. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis 6:279–290 [DOI] [PubMed] [Google Scholar]
- Zhu X, Yu QS, Cutler RG, Culmsee CW, Holloway HW, Lahiri DK, Mattson MP, Greig NH 2002. Novel p53 inactivators with neuroprotective action: syntheses and pharmacological evaluation of 2-imino-2,3,4,5,6,7-hexahydrobenzothiazole and 2-imino-2,3,4,5,6,7-hexahydrobenzoxazole derivatives. J Med Chem 45:5090–5097 [DOI] [PubMed] [Google Scholar]