Abstract
Edmonston vaccine strains of measles virus (MV) have shown significant antitumor activity in preclinical models of ovarian cancer. We engineered MV to express the marker peptide carcinoembryonic antigen (MVCEA virus) to also permit real-time monitoring of viral gene expression in tumors in the clinical setting. Patients with Taxol and platinum-refractory recurrent ovarian cancer and normal CEA levels were eligible for this phase I trial. Twenty-one patients were treated with MV-CEA i.p. every 4 weeks for up to 6 cycles at seven different dose levels (103–109 TCID50). We observed no dose-limiting toxicity, treatment-induced immunosuppression, development of anti-CEA antibodies, increase in anti-MV antibody titers, or virus shedding in urine or saliva. Dose-dependent CEA elevation in peritoneal fluid and serum was observed. Immunohistochemical analysis of patient tumor specimens revealed overexpression of measles receptor CD46 in 13 of 15 patients. Best objective response was dose-dependent stable disease in 14 of 21 patients with a median duration of 92.5 days (range, 54–277 days). Five patients had significant decreases in CA-125 levels. Median survival of patients on study was 12.15 months (DELnths; range, 1.3–38.4 months), comparing favorably to an expected median survival of 6 months (DELnth) in this patient population. Our findings indicate that i.p. administration of MV-CEA is well tolerated and results in dose-dependent biological activity in a cohort of heavily pretreated recurrent ovarian cancer patients.
Introduction
Ovarian cancer is the second most common malignancy of the female genital tract in the United States, and it accounts for approximately 16,000 deaths a year in the United States (1). Despite debulking surgery and chemotherapy, more than 65% of the patients will relapse (2, 3). At relapse, no curative treatment options are available. Although agents such as topotecan, liposomal doxorubicin, gemcitabine, or paclitaxel can lead to responses in a minority of patients (6–20% of patients with platinum-refractory disease; refs. 4–8), these responses are usually short-lived and at the expense of significant toxicity. There is a pressing need for more effective treatments to improve the outcome of these patients.
Recurrent ovarian cancer remains confined in the peritoneal cavity in more than 80% of the patients, providing an opportunity for locoregional administration of novel therapeutics, including gene and viral therapy approaches (9). Despite promising preclinical work with a variety of virotherapy agents in ovarian cancer models (10), this therapeutic modality remains largely untested in the clinic, with only one clinical virotherapy trial having been reported (11).
Measles virus (MV) is a negative-strand, RNA virus belonging to the family of Paramyxoviridae (12). Our interest in its oncolytic properties was founded on reports of spontaneous regression of malignancy in children following infection with wild-type MV (13–17). Tumor cells infected by MV express viral fusogenic proteins, causing fusion with uninfected neighboring cells, formation of multinuclear cell aggregates (syncytia), and apoptotic death. Although wild-type MV is associated with a potentially serious infectious disease, attenuated strains (vaccine strains) of the virus have an excellent safety record (18). Of equal importance, MV vaccine strains predominantly enter cells via the CD46 receptor (19–21). The latter is overexpressed in tumor cells, including ovarian cancer (22, 23), protecting them from complement mediatedlysis (24, 25).
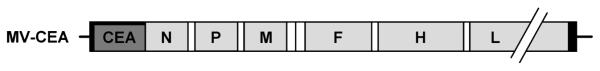
To address one of the challenges in clinical virotherapy trials, that is, the ability to monitor viral gene expression in vivo, we engineered the MV Edmonston vaccine strain by introducing a gene coding for the soluble extracellular domain of human carcinoembryonic antigen (CEA) upstream of the nucleoprotein gene in the MV genome. Production of the maker CEA as the virus replicates allows quantitative monitoring of viral gene expression (ref. 26; Fig. 1). MV-CEA has shown considerable preclinical therapeutic efficacy against primary and established ovarian cancer lines in vitro and against murine subcutaneous and intraperitoneal ovarian cancer xenograft models in vivo (26–28). In contrast, no significant cytopathic effect was observed against nontrans-formed cells such as ovarian surface epithelium, mesothelial cells, and normal dermal fibroblasts (26).
Figure 1.
Schematic representation of MV-CEA. The cDNA encoding for the human CEA was inserted upstream of the nucleoprotein (N) gene. P, phosphoprotein gene; M, matrix protein gene; F, fusion protein gene; L, large protein gene (adapted with permission from Peng KW et al., Nat Med 2002;8:527–31).
The goal of this phase I trial was (a) to determine the safety and tolerability of i.p. administration of MV-CEA in patients with recurrent ovarian cancer; (b) to determine the maximum tolerated dose of MV-CEA; (c) to characterize viral gene expression at each dose level as manifested by CEA levels; (d) to assess viremia, viral replication, and MV shedding and persistence; (e) to determine humoral immune response to the injected virus; and (f) to assess in a preliminary fashion the antitumor efficacy of this approach by following CA-125 levels, radiographic response, time to progression, and survival.
Patients and Methods
Patient selection
Eligible patients had persistent, recurrent, or progressive ovarian cancer or primary peritoneal cancer after prior treatment with platinum and Taxol compounds. Histologic confirmation of the original or recurrent tumor was required. Patients had to be older than 18 y with adequate hematologic, liver, and kidney function, as defined by absolute neutrophil count (ANC) ≥1,500/mL; platelets ≥100,000/mL; hemoglobin ≥9 gm/dL; total bilirubin ≤upper limit of normal; aspartate aminotransferase ≤2× upper limit of normal; and creatinine ≤1.5× upper limit of normal. Patients had to be immune to MV as shown by anti-measles IgG levels ≥20 ELISA units/mL, determined by enzyme immunoassay (Diamedix). They also had to have normal serum CEA levels (≤3 ng/mL), both at the time of study entry and in any prior testing. Exclusion criteria included platinum sensitive disease; Eastern Cooperative Oncology Group performance status of 3 or 4; chemotherapy, immunotherapy, or biological therapy ≤4 wk before study entry; or extensive abdominal surgery including enterotomy ≤3 wk before study entry. Patients were also excluded if they had an HIV-positive test or history of other immunodeficiency, organ transplantation, history of chronic hepatitis B or C, intra-abdominal disease >8 cm at the time of registration, intrahepatic disease, or disease beyond the peritoneal cavity.
Treatment
Construction of the MV-CEA virus has been previously described (26). Clinical lots of the virus were produced by the Mayo Clinic Vector Core. All patients under-went either laparoscopy or laparotomy, depending on the presence of ascites and the sites and size of recurrent tumor masses, for placement of the intraperitoneal catheter (Bard Access Systems). Peritoneal adhesions were lysed if technically possible. If ascites was present, it was drained through the peritoneal catheter before the viral administration. Patients received infusion of the assigned dose of the MV-CEA diluted in 500 mL of normal saline over 30 min. Doses ranged from 103 to 109 TCID50 (seven dose levels, dose escalation by 1-log increments). The highest viral dose level administered in the trial was determined based on manufacturing limitations. Patients were observed in the Mayo Clinic Clinical Research Unit for 24 h following the first viral administration. If well tolerated, all subsequent doses were administered on an outpatient basis. Treatment was repeated monthly for up to 6 cycles, provided that toxicity was acceptable and there was no evidence of disease progression.
Statistical design
The standard cohorts-of-three design (29, 30) was applied. Three patients were treated per dose level and observed for 4 wk before accrual to the next higher dose level being initiated. Intra-patient dose escalation was not allowed. Toxicity was assessed using Common Terminology Criteria Version 3.0. Dose limiting toxicity was defined as grade ≥3 hematologic toxicity except for grade 3 ANC lasting <72 h, elevation of serum creatinine ≥2× the baseline, any other nonhematologic toxicity grade ≥3, viremia lasting for ≥6 wk from last viral administration, grade 2 symptomatic bronchospasm or urticaria, and any grade 3 or higher allergic reactions.
Laboratory evaluation
Before treatment, patients had a history and physical exam done, as well as a complete blood count (CBC), prothrombin time (PT) and activated partial thromboplastin time (aPTT), chemistry group, urinalysis, chest X-ray, HIV testing, CA-125 and CEA measurements, and electrocardiogram. CBC, chemistry group, PT, and aPTT were repeated on day 8, day 15, and before re-treatment (cycles 2–6). CEA levels were determined at multiple time points (Supplementary Fig. S1). In addition, peritoneal aspirates (or peritoneal lavage samples if no ascites) were obtained at baseline, day 3, day 8, and before all subsequent cycles. The peritoneal aspirate was tested for the presence of the virus by Vero cell overlay and quantitative reverse transcription-PCR (RT-PCR), CEA levels, and anti-MV IgG antibodies. Patients’ blood, urine, and mouth gargle specimens were tested for the presence of the virus (viremia and shedding) at multiple time points (Supplementary Fig. S1). Patient’s immune competence [CD4, CD8 counts, immunoglobulins, complement, delayed-type hypersensitivity (DTH) reaction to Candida, purified protein derivative, tetanus, and trichophyton], development of anti-CEA antibodies, and humoral immunity against the virus were also tested at multiple time points as outlined in Supplementary Fig. S1.
Assessment of antitumor response
Response Evaluation Criteria in Solid Tumors criteria (31) were applied for response assessment. Computed tomography or magnetic resonance imaging and CA-125 measurements were done at baseline and before re-treatment (cycles 2–6).
Detection and quantitation of MV nucleoprotein mRNA by quantitative RT-PCR in peripheral blood mononuclear cells, mouth gargle, and urine specimens
Total RNA was extracted using either Trizol reagent (Invitrogen) and ethanol precipitation (urine and mouth gargle specimens) or the PAX-gene Blood RNA kit (Qiagen). Blood for isolation of peripheral blood mononuclear cells (PBMC) was collected using the PAX-gene Blood RNA tubes as recommended by the manufacturer. Quantitative RT-PCR was done as previously described (32). Briefly, the quantitative RT-PCR assay was optimized for primers, probe, and magnesium concentration with the Stratagene Brilliant II QRT-PCR Core Reagent One-Step Kit and run on the MX4000 Stratagene machine. A 50-μL quantitative RT-PCR reaction volume was used to amplify a 63-bp MV-N genomic RNA target, in the presence of 0.3 mmol/L each of forward (5’-GAGAAGCCAGGGAGAGCTACAG-3’) and reverse (5’-GGGCAGC-TCTCGCATCAC-3’) primers, 0.2 mmol/L Black Hole Quencher–labeled probe (5’-/56-FAM/ AAACCGGGCCCAGCAGAGCCA/3BHQ_1/-3’), 4 mmol/L MgCl, and 1 μg or a maximum total volume of 10 μL of the RNA isolate. One cycle of reverse transcriptase reaction (30 min at 45°C) was applied, followed by a denaturation step (10 min at 95°C) and 40 cycles of amplification (30 s 95°C and 1 min 55°C), with fluorescence measured during the extension. A standard curve of 10-fold dilutions containing 108 to 103 MV-N gene copies/mL had been generated using a manufactured RNA oligo (Dharmacon) and having the following sequence: 5’-GAAGCCAGGGAGAGCUACAGAGAAACC-GGGCCCAGCAGAGCAAGUGAUGCGAGAGCUGCCC-3’. Quantification and subsequent calculation of copy number were done using the standard curve and the MX4000 Multiplex Quantitative PCR System software.
Vero cell overlay assay for detection of viral replication
Vero cells (American Type Culture Collection) were plated at a concentration of 2 × 105 per well and incubated overnight at 37°C. The next day, 103 patient cells isolated from the peritoneal fluid were added to duplicate wells. SKOV.IP3 cells infected with MV-CEA at a multiplicity of infection of 1.0, and MV-CEA were used as positive controls. Plates were incubated for 5 d and examined daily for syncytia formation.
Assessment of CD46 expression in ovarian tumors by immunohistochemistry
The primary antibody CD46 (H-294; Santa Cruz Biotech., Inc.) was diluted 1:300. Slides were incubated overnight at 4°C in a humidified chamber and then incubated with a donkey anti-rabbit IgG-B secondary antibody (Santa Cruz Biotech.) for 45 min at room temperature, followed by a detection step with Vectastain ABC and peroxidase substrate DAB kit (Vector Laboratories, Inc.), and counterstained using Accustain solution (Sigma).
Detection of anti-CEA antibodies
Detection of anti-CEA antibodies was done as previously described (33). A positive anti-CEA antibody response was defined as a posttreatment absorbance ≥2× pretreatment absorbance for the individual patient and >mean + 2SD of 10 normal donor sera assayed at the same dilution.
Determination of CEA levels (in serum and ascites)
The Bayer Advia Centaur assay was used (Bayer) as per manufacturer’s instructions.
Results
Patient characteristics
Twenty-one recurrent ovarian cancer patients were treated in this phase I trial. Table 1 summarizes patient characteristics. All participating patients were platinum refractory and had been heavily pretreated, having received a median of three chemotherapy regimens for recurrent disease.
Table 1.
Patient characteristics (N = 21)
| Age, y | |
| Median (range) | 57.0 (43.0–82.0) |
| Performance score, n (%) | |
| 0 | 8 (38.1) |
| 1 | 10 (47.6) |
| 2 | 3 (14.3) |
| Ascites present, n (%) | |
| Yes | 7 (33.3) |
| No | 14 (66.7) |
| Prior treatments, n (%) | |
| Chemotherapy | 21 (100) |
| No. of prior chemo regimens | median 3.0 (range 1–7) |
| Radiation therapy | 0 (0) |
| Surgery | 21 (100) |
Toxicity
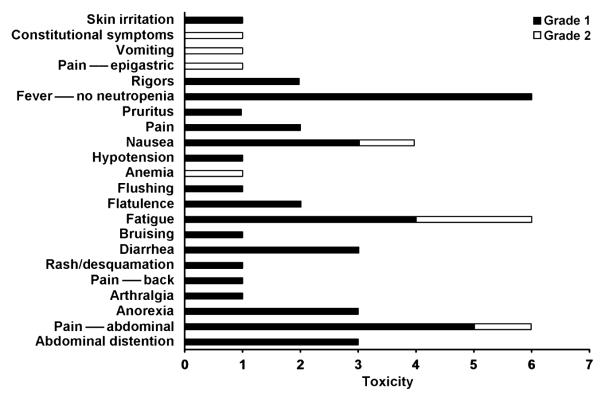
Dose escalation proceeded from 103 to 109 TCID50 as per study design, without dose limiting toxicity being observed. Figure 2 summarizes cycle 1 toxicity for all study patients. All observed toxicities were grade 1 and 2, with most common cycle 1 toxicities being fever (grade 1: 6 patients, 28.5%), fatigue (grade 1: 4 patients, 19%; grade 2: 2 patients, 9.5%), and abdominal pain (grade 1: 5 patients, 23.8%; grade 2: 1 patient, 4.7%). Table 2 summarizes the most common nonhematologic toxicities for all patients and treatment cycles. The only grade 3 toxicity observed in the study was grade 3 arthralgia observed in cycle 4 in one patient; symptoms started a few hours following treatment administration and increased in intensity over the next 24 hours, but responded well to nonsteroidal anti-inflammatory drugs. Arthralgias recurred with subsequent treatments in this patient, despite a decrease in viral dose, although improved in severity (grade 2).
Figure 2.
Treatment-related adverse events in cycle 1. MV-CEA treatment was well tolerated with only mild (grade 1 and 2) toxicity being observed.
Table 2.
Most frequent adverse events possibly, probably, or definitely related to treatment for all treatment cycles and dose levels
| Toxicity | Grade |
|
|---|---|---|
| 1 |
2 |
|
| n (%) | n (%) | |
| Pain—abdominal | 4 (19.0) | 4 (19.0) |
| Fatigue | 7 (33.3) | 2 (9.5) |
| Fever—no neutropenia | 8 (38.1) | 0 (0) |
| Abdominal distention | 5 (23.8) | 0 (0) |
| Anorexia | 6 (28.6) | 1 (4.8) |
| Nausea | 4 (19.0) | 2 (9.5) |
Assessment of immune response
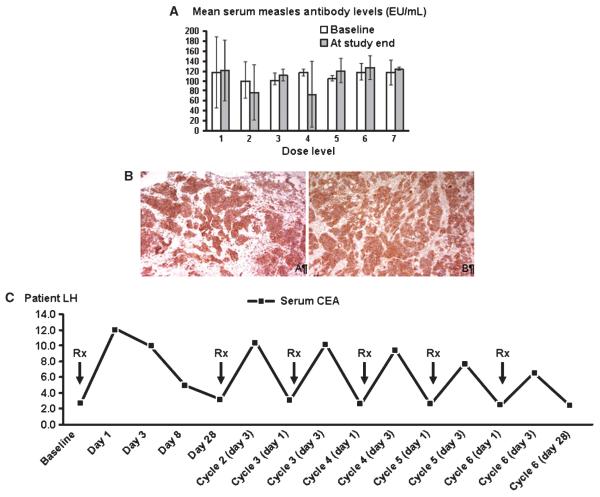
Figure 3A depicts mean serum anti-measles antibody levels at baseline and on study completion according to dose levels. Antibody titers remained stable both in blood and in peritoneal fluid as compared with baseline, indicating a lack of significant boost to the humoral immune response. Furthermore, no development of anti-CEA antibodies was observed.
Figure 3.
A, mean serum anti-MV antibody titers at baseline and prior to the patients going off-study, presented per dose level. No significant difference was observed between pre- and post-treatment values. B, strong expression of MV receptor CD46 in the tumors of two study patients (A and B). C, serum CEA kinetics in a patient treated at the 109 TCID50 dose level. CEA elevation was observed even following repeat dosing although at gradually decreasing levels.
Immunosuppression has been observed following wild-type MV infection and can be associated with DTH suppression, bacterial infections, and reactivation of tuberculosis (34). It is, however, infrequent and transient following measles vaccination (35). In our study, no evidence of treatment-induced immunosuppression was observed. Specifically, there were no treatment-related infections and no significant change in CD4, CD8, immunoglobulin, or complement levels (Supplementary Figs. S1–S5). In addition, in no patient did the treatment result in suppression of an initially positive DTH reaction.
Viral dissemination
There was no evidence of shedding as tested by quantitative RT-PCR in mouth gargle and urine specimen for any of the study patients. Viral genomes were detected at low levels in the PBMCs of four patients (Supplementary Table S1). All patients were asymptomatic at the time of viral genome detection.
Expression of the MV-CEA receptor CD46 in tumor specimens
Immunohistochemical analysis of the tumor samples from the patients showed high expression of the MV-CEA receptor CD46 in 13 of 15 patients for whom tissue was available (Fig. 3B). The strong diffuse expression of MV receptor CD46 in ovarian tumors underscores the potential of CD46-targeted therapeutics such as MV derivatives in the treatment of ovarian cancer. There was no association, as determined by immunohistochemistry, between CD46 levels and disease stabilization (P = 0.5692, Fisher’s Exact test) or CA-125 response to treatment (P = 0.4573, Fisher’s exact test). The small number of patients in this trial (n =21), the even fewer patients in whom tissue samples for CD46 analysis were available (n = 15), the fact that the majority of these patients (13 of 15) were CD46 positive, and that many CD46 positive patients were treated with lower—less effective—viral doses preclude definitive conclusions, however.
CEA detection
Increased CEA in the peritoneal fluid was observed in three patients: one patient at the 108 TCID50 dose level and two patients at the 109 TCID50 dose level. Modest increases of CEA levels in the serum (12–16 ng/mL) were observed in all three patients treated at the 109 TCID50 dose level. Figure 3C illustrates serum CEA kinetics in relation to treatment administration in a patient at the 109 TCID50 dose level, who received six viral doses.
Efficacy
Best objective response was stable disease in 14 of 21 evaluable patients, with median duration of 92.5 days (range, 54–277 days). Outcome was dose dependent, with 9 of 9 patients with stable disease in dose levels 5 to 7, versus 5 of 12 patients in dose levels 1 to 4. Median overall survival of the study patients was 12.15 months (range, 1.3–38.4 months; Supplementary Table S2), which compares favorably with the expected median survival of 6 months in this patient population (36).
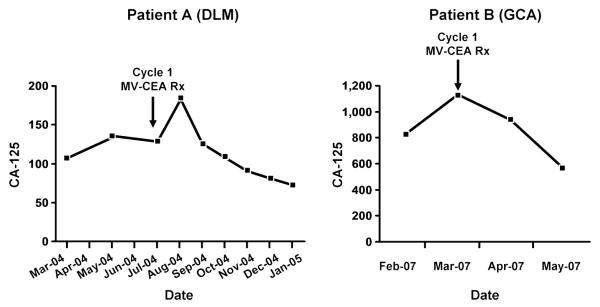
Five patients (including two of three patients at the 108 TCID50 dose level) had >30% decrease in the levels of the tumor marker CA-125 (32%, 34%, 44%, 72%, and 78%, respectively; Fig. 4). There was no significant association between baseline anti-measles antibody titers and likelihood of CA-125 response or disease stability on study (P = 0.148 and P = 0.189, respectively, Wilcoxon rank sum test).
Figure 4.
CA-125 response curves in two study patients who received six (patient A) and three (patient B) treatment cycles. Continued CA-125 response was observed in response to repeat viral dosing. Patient A maintained disease stability for 8 mo (i.e., 2 mo following treatment completion), whereas patient B had extra-abdominal (central nervous system) disease progression, while on treatment.
Discussion
This trial represents the first in-human testing of an oncolytic engineered MV strain as an anticancer agent. We chose recurrent ovarian cancer as our first target because of high levels of expression of the MV CD46 receptor, the possibility of delivering the viral therapy in a “confined” compartment, and the high mortality of this disease with immediate need for development of novel therapeutics. We showed excellent safety of this oncolytic virus following i.p. administration. No DLT was observed in doses up to 109 TCID50 and no immunosuppression. Most common toxicities were mild (grade 2) abdominal pain and fatigue and grade 1 fever at the absence of neutropenia. Two additional trials of engineered MV strain, a trial of intratumoral administration of MV-CEA in patients with recurrent glioblastoma multiforme and a trial of i.v. administration of the measles derivative MV-NIS in patients with multiple myeloma, have since been activated. No DLT has been observed in doses up to 107 TCID50 in the GBM trial and 109 TCID50 in the myeloma study, further highlighting the safety of MV as an oncolytic platform.
Our study represents the second reported clinical trial of a replicating oncolytic virus in recurrent ovarian cancer patients. In an earlier study, Vasey and colleagues (11) administered i.p. the conditionally replicating adenovirus ONYX-015. Although safe, there was no evidence of antitumor activity (11). The low or variable expression of the adenoviral receptor CAR in primary ovarian cancer cells can possibly explain this lack of efficacy (37, 38). In contrast, in our trial, despite the accrual of heavily pretreated patients (median number of three prior chemotherapy regimens for recurrent disease) and the fact that a very low starting dose was man-dated by the regulatory authorities (the first dose level was 10-fold lower than the dose of infectious viruses used for measles vaccination), the observed, dose-dependent disease stabilization with median duration of 92 days (54–277) days, tumor marker CA-125 responses in 5 patients, and the doubling of median survival in this phase I trial, as compared with the expected survival in this study population (36), points toward the promising potential of oncolytic measles therapy in recurrent ovarian cancer patients. Furthermore, these data in conjunction with the observed overexpression of the MV-CEA receptor CD46 in the majority of the study patients underscore the potential importance of CD46 targeting in ovarian cancer therapeutics.
Patients in our trial were required to be measles immune to increase safety in this first human testing of the virus. Of note, however, there was no significant change in the titers of anti-MV antibody following treatment initiation, despite repeat dosing, this likely being the result of high serum antibody levels at baseline in study patients. Furthermore, in CA-125 responders, continuous CA-125 decrease following repeat dosing was observed (Fig. 4) and points toward the value of repeat viral administration even in the setting of pre-existing immunity. One of the trial end points was detection of marker CEA as a correlate of viral gene expression. As expected, detection of CEA was dose dependent. CEA was detected in the peritoneal fluid of patients treated with a viral dose of 108 TCID50 or higher and in the serum of patients treated at the highest viral dose of 109 TCID50. Of note, eligible patients were required to have normal CEA levels so that CEA elevation observed in this study could only represent a reflection of viral replication. In general, elevation of CEA in the serum of study patients was modest (12–18 ng/mL) and recurred, although at lower levels following repeat viral administration. Given the lack of detection of anti-CEA antibodies in the study patients, this observation could be indicative of decreased viral spread associated with repeat administration.
Another factor negatively affecting the likelihood of significant CEA elevation in the serum of patients treated with MV-CEA i.p. is the dilution that occurs when CEA produced in the peritoneal cavity equilibrates into the bloodstream or extracellular fluid. In this context, a different marker gene that remains localized following expression in infected cells could represent a better correlate of viral gene expression. We are currently conducting a phase I trial of i.p. administration of MV-NIS (39), an MV derivative encoding the sodium-iodine symporter gene (NIS, an iodine transporter), in recurrent ovarian cancer patients. NIS allows the use of iodine or technetium isotopes for imaging, using computed tomography single-photon emission computed tomography or positron emission tomography scan, and radioactive iodine isotopes for therapy.
In addition to the ongoing phase I trial of i.p. administration of MV-NIS virus, which sets the stage for the use of a measles virus-encoded therapeutic transgene, i.e., the NIS gene, we are developing technologies that can lead to further improvement of MV delivery and viral spread in ovarian tumors, including retargeting (40), use of infected cell carriers (41), and combination with cyclophosphamide, an immunomodulatory agent able to suppress innate immune response. The ongoing phase I MV-NIS study and the additional preclinical work currently ongoing will allow us to determine the most promising follow-up clinical step.
In summary, in this first human trial of an oncolytic MV strain in the treatment of recurrent ovarian cancer, we have shown both safety and early, promising biological activity. This oncolytic virus platform warrants further investigation in the treatment of recurrent ovarian cancer.
Supplementary Material
Acknowledgments
We thank Janet Lensing for coordinating patient care; Dolores Nordquist for patient care; Linda Gregory, Ph.D., and the Mayo Clinic Vector Production Laboratory; and Raquel Ostby for her help in manuscript preparation.
Grant Support Goodwin Foundation, Siebens Foundation, NIH grant CA 103276, NIH grant CA 136393 (Mayo Clinic Specialized Program of Research Excellence in Ovarian Cancer), NCCR CTSA grant U54RR 24150, NIH grant CA 15083, the Minnesota Ovarian Cancer Alliance (MOCA), and Andersen Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest H.J. Long: ownership interest, Amgen, Novartis Pfizer, Sanofi-Aventis, AstraZeneca, Eli Lilly, Genentech, GlaxoSmithKline, Merck, and BristolMyers Squibb. The other authors disclosed no potential conflicts of interest.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 4.Gordon EM, Chen ZH, Liu L, et al. Systemic administration of a matrix-targeted retroviral vector is efficacious for cancer gene therapy in mice. Hum Gene Ther. 2001;12:193–204. doi: 10.1089/104303401750061258. [DOI] [PubMed] [Google Scholar]
- 5.Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1748–53. doi: 10.1200/JCO.1994.12.9.1748. [DOI] [PubMed] [Google Scholar]
- 6.McGuire WP, Blessing JA, Bookman MA, Lentz SS, Dunton CJ. Topotecan has substantial antitumor activity as first-line salvage therapy in platinum-sensitive epithelial ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:1062–7. doi: 10.1200/JCO.2000.18.5.1062. [DOI] [PubMed] [Google Scholar]
- 7.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33:S3–11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–70. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- 9.Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD. Gene therapy for ovarian cancer. Curr Oncol Rep. 2006;8:441–7. doi: 10.1007/s11912-006-0073-x. [DOI] [PubMed] [Google Scholar]
- 10.Raki M, Rein DT, Kanerva A, Hemminki A. Gene transfer approaches for gynecological diseases. Mol Ther. 2006;14:154–63. doi: 10.1016/j.ymthe.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Vasey PA, Shulman LN, Campos S, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–9. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 12.Lamb RA, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 4th ed Lippincott-Raven Publishers; Philadelphia (PA): 2001. [Google Scholar]
- 13.Bluming AZ, Ziegler JL. Regression of Burkitt’s lymphoma in association with measles infection. Lancet. 1971;2:105–6. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 14.Gross S. Measles and leukaemia. Lancet. 1971;1:397–8. doi: 10.1016/s0140-6736(71)92232-x. [DOI] [PubMed] [Google Scholar]
- 15.Mota HC. Infantile Hodgkin’s disease: remission after measles. Br Med J. 1973;2:421. doi: 10.1136/bmj.2.5863.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquinucci G. Possible effect of measles on leukaemia. Lancet. 1971;1:136. doi: 10.1016/s0140-6736(71)90869-5. [DOI] [PubMed] [Google Scholar]
- 17.Zygiert Z. Hodgkin’s disease: remissions after measles. Lancet. 1971;1:593. doi: 10.1016/s0140-6736(71)91186-x. [DOI] [PubMed] [Google Scholar]
- 18.Cutts FT, Markowitz LE. Successes and failures in measles control. J Infect Dis. 1994;170:S32–41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 19.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 20.Schneider H, Bullough F, Vongpunsawad S, Russell SJ, Cattaneo R. Recombinant measles viruses efficiently entering cells through targeted receptors. J Virol. 2000;74:9928–36. doi: 10.1128/jvi.74.21.9928-9936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagi Y. [The cellular receptor for measles virus] Uirusu. 2001;51:201–8. [PubMed] [Google Scholar]
- 22.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Surowiak P, Materna V, Maciejczyk A, et al. CD46 expression is indicative of shorter revival-free survival for ovarian cancer patients. Anticancer Res. 2006;26:4943–8. [PubMed] [Google Scholar]
- 24.Adams EM, Brown MC, Nunge M, Krych M, Atkinson JP. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991;147:3005–11. [PubMed] [Google Scholar]
- 25.Oglesby TJ, White D, Tedja I, et al. Protection of mammalian cells from complement-mediated lysis by transfection of human membrane cofactor protein and decay-accelerating factor. Trans Assoc Am Physicians. 1991;104:164–72. [PubMed] [Google Scholar]
- 26.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–31. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 27.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–62. [PubMed] [Google Scholar]
- 28.Peng KW, Hadac EM, Anderson BD, et al. Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 2006;13:732–8. doi: 10.1038/sj.cgt.7700948. [DOI] [PubMed] [Google Scholar]
- 29.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–47. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 30.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–37. [PubMed] [Google Scholar]
- 31.Therasse P, Arbuck SG, Eisenhauer EA, et al. European Organization for Research and Treatment of CancerNational Cancer Institute of the United StatesNational Cancer Institute of Canada New guide-lines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19:690–8. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conry RM, Curiel DT, Strong TV, et al. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clin Cancer Res. 2002;8:2782–7. [PubMed] [Google Scholar]
- 34.Griffin D, Bellini W. Measles virus. In: Fields B, Knipe D, Howley P, editors. Fields virology. Vol. 3. Lippincott-Raven Publishers; 1996. pp. 1267–312. [Google Scholar]
- 35.Okada H, Sato TA, Katayama A, et al. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch Virol. 2001;146:859–74. doi: 10.1007/s007050170121. [DOI] [PubMed] [Google Scholar]
- 36.Markman M, Webster K, Zanotti K, Peterson G, Kulp B, Belinson J. Survival following the documentation of platinum and taxane resistance in ovarian cancer: a single institution experience involving multiple phase 2 clinical trials. Gynecol Oncol. 2004;93:699–701. doi: 10.1016/j.ygyno.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Rocconi RP, Numnum TM, Stoff-Khalili M, Makhija S, Alvarez RD, Curiel DT. Targeted gene therapy for ovarian cancer. Curr Gene Ther. 2005;5:643–53. doi: 10.2174/156652305774964668. [DOI] [PubMed] [Google Scholar]
- 38.Hemminki A, Zinn KR, Liu B, et al. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst. 2002;94:741–9. doi: 10.1093/jnci/94.10.741. [DOI] [PubMed] [Google Scholar]
- 39.Dingli D, Peng KW, Harvey ME, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–6. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa K, Nakamura T, Harvey M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–8. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 41.Iankov ID, Blechacz B, Liu C, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–22. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.