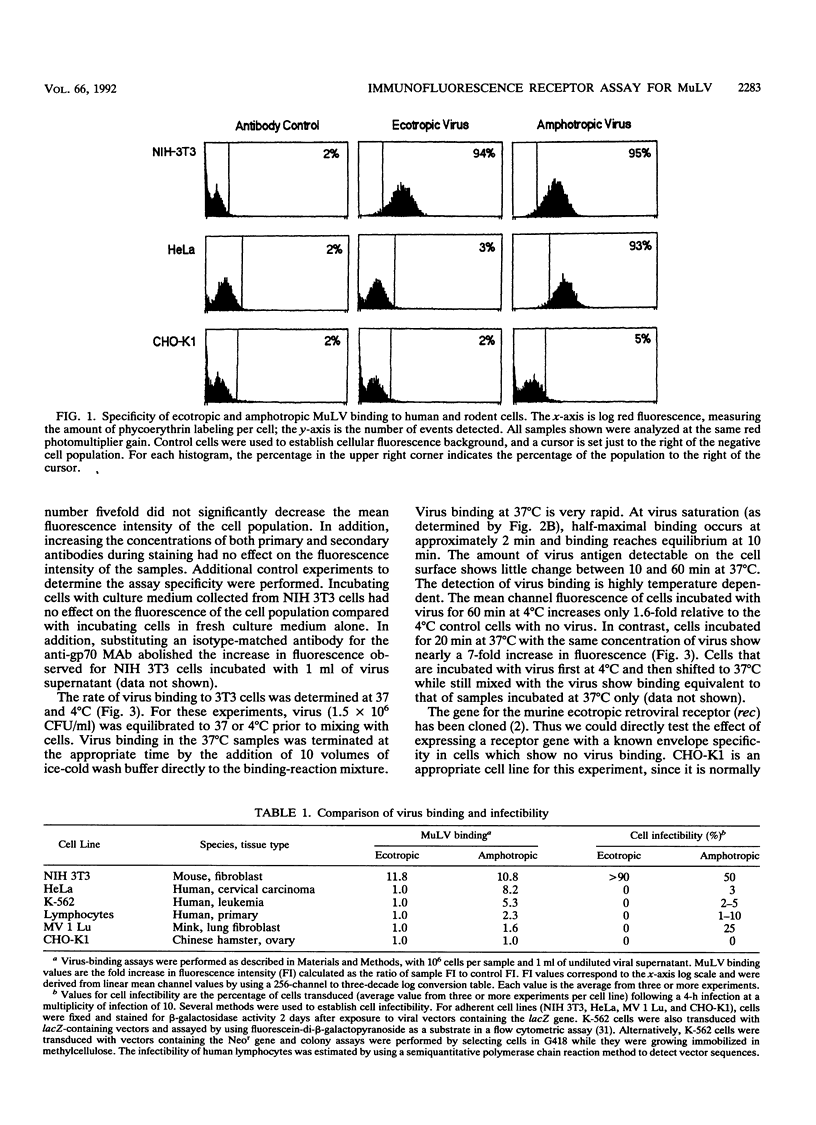
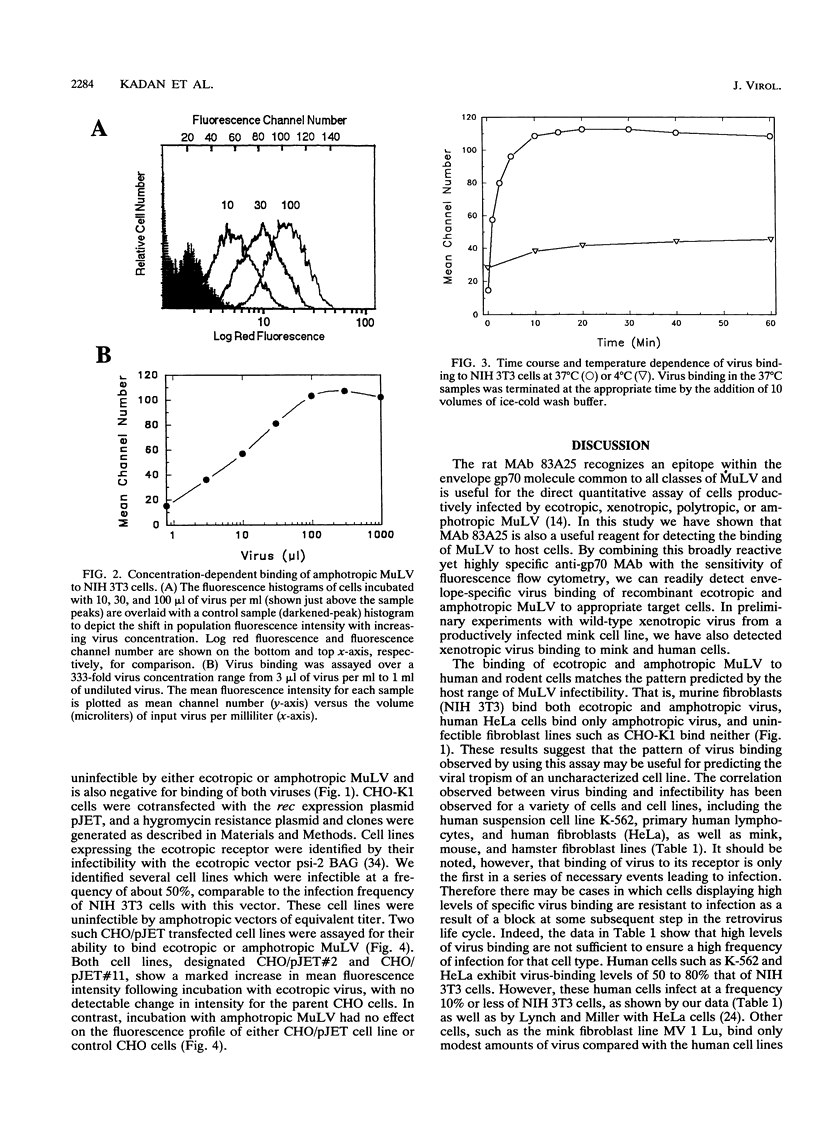
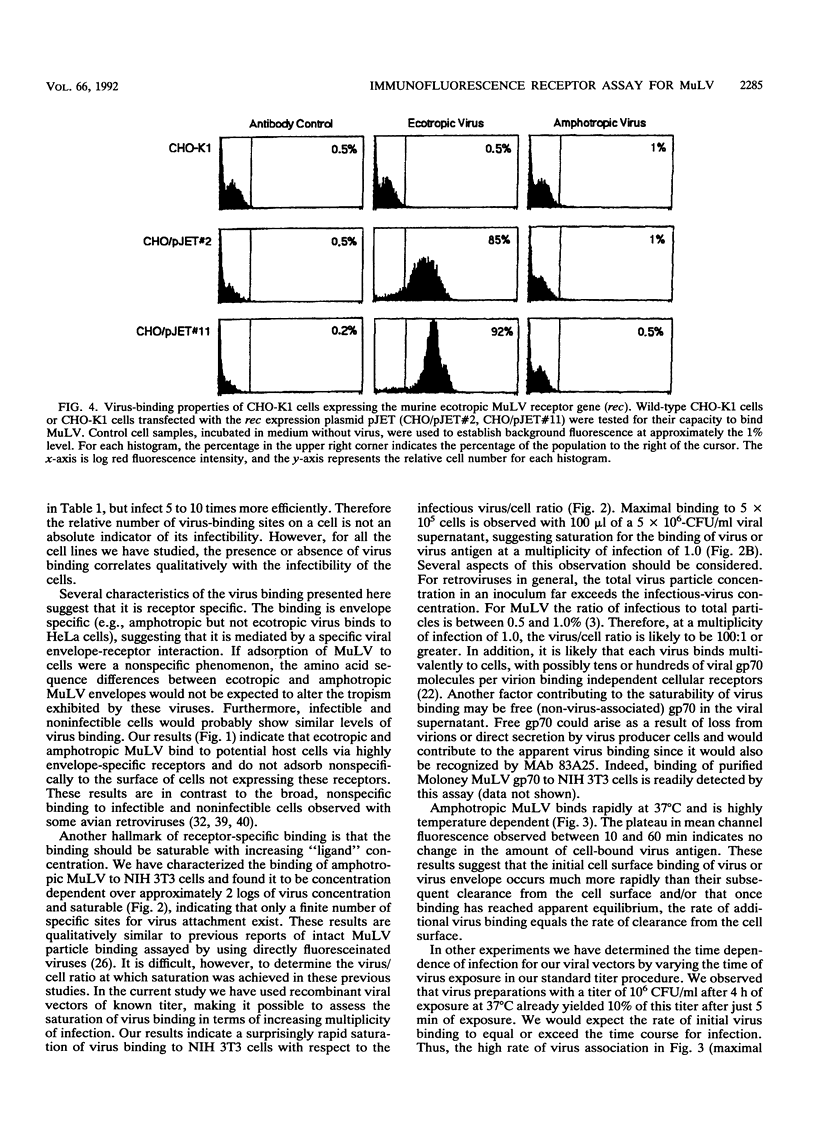
Abstract
Four classes of murine leukemia virus (MuLV) which display distinct cellular tropisms and bind to different retrovirus receptors to initiate virus infection have been described. In the present study, we describe a rapid, sensitive immunofluorescence assay useful for characterizing the initial binding of MuLV to cells. By using the rat monoclonal antibody 83A25 (L. H. Evans, R. P. Morrison, F. G. Malik, J. Portis, and W. J. Britt, J. Virol. 64:6176-6183, 1990), which recognizes an epitope of the envelope gp70 molecule common to the different classes of MuLV, it is possible to analyse the binding of ecotropic, amphotropic, or xenotropic MuLV by using only a single combination of primary and secondary antibodies. The MuLV binding detected by this assay is envelope receptor specific and matches the susceptibility to infection determined for cells from a variety of species. The binding of amphotropic MuLV to NIH 3T3 cells was shown to be rapid, saturable, and temperature dependent. Chinese hamster ovary (CHO-K1) cells normally lack the ability to bind ecotropic virus and are not infectible by ecotropic vectors. Expression of the cloned ecotropic retrovirus receptor gene (Rec) in CHO-K1 cells confers high levels of ecotropic virus-specific binding and confers susceptibility to infection. Characterization of MuLV binding to primary cells may provide insight into the infectibility of cells by retroviruses and aid in the selection of appropriate vectors for gene transfer experiments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Shoor R., Salzberg S. Adsorption, penetration, and uncoating of murine leukemia virus studied by using its reverse transcriptase. J Virol. 1979 Apr;30(1):32–37. doi: 10.1128/jvi.30.1.32-37.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee S., Strand M., August J. T. Cellular membrane receptors for oncovirus envelope glycoprotein: properties of the binding reaction and influence of different reagents on the substrate and the receptors. Arch Biochem Biophys. 1978 Jul;189(1):161–171. doi: 10.1016/0003-9861(78)90129-7. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin J., Schaffar-Deshayes L., Debré P., Lévy J. P. Lymphoid cell surface receptor for Moloney leukemia virus envelope glycoprotein gp71. I. Binding characteristics. J Immunol. 1981 Jun;126(6):2347–2351. [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Anderson W. F. Retroviral vectors for introduction of genes into mammalian cells. Biotechniques. 1988 Jul-Aug;6(7):608–614. [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Morrison R. P., Malik F. G., Portis J., Britt W. J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990 Dec;64(12):6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Binding characteristics of Rauscher leukemia virus envelope glycoprotein gp71 to murine lymphoid cells. J Virol. 1977 Dec;24(3):729–735. doi: 10.1128/jvi.24.3.729-735.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hunter K., Housman D., Hopkins N. Isolation and characterization of irradiation fusion hybrids from mouse chromosome 1 for mapping Rmc-1, a gene encoding a cellular receptor for MCF class murine retroviruses. Somat Cell Mol Genet. 1991 Mar;17(2):169–183. doi: 10.1007/BF01232974. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Rosner M. R. Characterization of murine-specific leukemia virus receptor from L cells. J Virol. 1986 Jun;58(3):900–908. doi: 10.1128/jvi.58.3.900-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Characterization of Rauscher murine leukemia virus envelope glycoprotein receptor in membranes from murine fibroblasts. J Virol. 1978 Dec;28(3):686–696. doi: 10.1128/jvi.28.3.686-696.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Albritton L. M., Cunningham J. Genetic mapping of a cloned sequence responsible for susceptibility to ecotropic murine leukemia viruses. J Virol. 1990 Jun;64(6):3119–3121. doi: 10.1128/jvi.64.6.3119-3121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Lynch C. M., Miller A. D. Production of high-titer helper virus-free retroviral vectors by cocultivation of packaging cells with different host ranges. J Virol. 1991 Jul;65(7):3887–3890. doi: 10.1128/jvi.65.7.3887-3890.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Decléve A., Lieberman M., Kaplan H. S., Weissman I. L. Specificity of cell surface virus receptors on radiation leukemia virus and radiation-induced thymic lymphomas. J Virol. 1978 Dec;28(3):819–827. doi: 10.1128/jvi.28.3.819-827.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- McLachlin J. R., Cornetta K., Eglitis M. A., Anderson W. F. Retroviral-mediated gene transfer. Prog Nucleic Acid Res Mol Biol. 1990;38:91–135. doi: 10.1016/s0079-6603(08)60709-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., ROBINSON A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958 Dec 1;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Evans L. H. Infectious entry of murine retroviruses into mouse cells: evidence of a postadsorption step inhibited by acidic pH. J Virol. 1985 Sep;55(3):806–812. doi: 10.1128/jvi.55.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERER W. F., SYVERTON J. T., GEY G. O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953 May;97(5):695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology. 1966 Aug;29(4):628–641. doi: 10.1016/0042-6822(66)90287-x. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- The ADA human gene therapy clinical protocol. Hum Gene Ther. 1990 Fall;1(3):327–362. doi: 10.1089/hum.1990.1.3-327. [DOI] [PubMed] [Google Scholar]
- The N2-TIL human gene transfer clinical protocol. Hum Gene Ther. 1990 Spring;1(1):73–92. doi: 10.1089/hum.1990.1.1-73. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]



