Abstract
The synthesis, structural, and photophysical characterization of a series of new fluorescent donor–acceptor and acceptor-acceptor molecules, based on the fluorenyl ring system, with two-photon absorbing properties is described. These new compounds exhibited large Stokes shifts, high fluorescent quantum yields, and, significantly, high two-photon absorption cross sections, making them well suited for two-photon fluorescence microscopy (2PFM) imaging. Confocal and two-photon fluorescence microscopy imaging of COS-7 and HCT 116 cells incubated with probe I showed endosomal selectivity, demonstrating the potential of this class of fluorescent probes in multiphoton fluorescence microscopy.
Keywords: Fluorescent dyes, multiphoton absorption, fluorescence, two-photon, bioimaging
Introduction
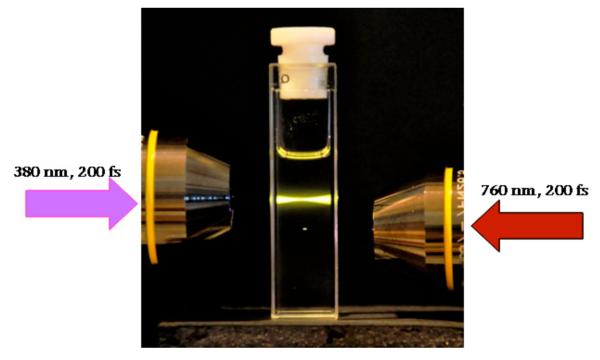
The development of multiphoton absorbing materials has attracted the interest of the scientific community in the last decade due to their potential applications in areas as optical data storage systems, microscopy imaging, optical limiting materials, and photodynamic therapy. In one-photon absorption processes, the absorbed light is directly proportional to the incident light intensity, whereas in two-photon absorption (2PA) processes, the probability of absorbing two photons simultaneously is proportional to the square of the incident light intensity.1 This nonlinear dependence provides several advantages, including highly confined excitation, increased 3D resolution (Figure 1), and the potential increase of penetration depth in tissue, important features in fields such as optical data storage,2-4 3D microfabrication,5-7 and fluorescence microscopy.1,8
Figure 1.
One-photon (left arrow) vs. two-photon (right arrow) excitation, showing the high 3D spatially-localized excitation in 2PA in a solution of dye I.
We previously reported a series of fluorescent dyes and photoacid generators that use fluorene as a core structure.9-14 This core exhibited high thermal and photochemical stability making this class of compounds promising in areas such as lasing applications.15
Recently, a computational quantum chemical study of numerous fluorene derivatives was reported in order to predict and rationalize certain 2PA parameters, such as 2PA cross sections and 2PA wavelength. Though insightful, little comparison and validation of computed results with experimental data was presented, emphasizing the need to accurately measure 2PA spectra and cross sections of new compounds.16
In the interest of developing more efficient 2PA fluorophores, it is essential to improve the key photophysical properties such as quantum yield, absorption maxima, and 2PA cross sections in the tuning range of commercial Ti:sapphire lasers (700-1000 nm). In this work, we present the design, synthesis, and photophysical (linear and nonlinear) characterization of a new series of two-photon absorbing molecules based on a fluorenyl core and demonstration of their use in in vitro confocal and two-photon fluorescence microscopy (2PFM) bioimaging.
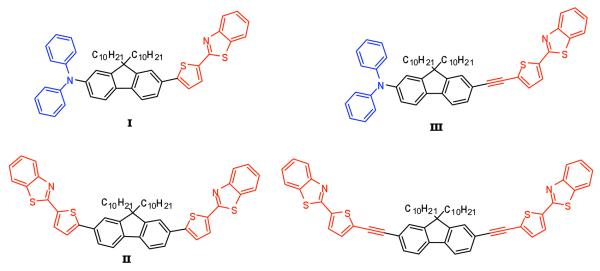
In this approach, a series of dyes were developed with the general structural design donor-pi-acceptor (D-π-A) and acceptor-pi-acceptor (A-π-A) using benzothiazole moieties as electron-acceptor groups and diphenylamino groups as electron-donor moieties. To enhance the solubility of the fluorene core in organic solvents, both protons on the 9-position of fluorene were substituted by alkyl chains. Functionalities including thiophene and alkynes were incorporated in order to increase the length of the π-conjugation and to evaluate the effect of these functionalities on the linear absorption maxima, fluorescence quantum yields, and 2PA cross sections. The chemical structures of the new fluorescent compounds presented in this paper are illustrated in Figure 2.
Figure 2.
Structures of new donor-pi-acceptor (D-π-A) (I and III) and acceptor-pi-acceptor (A-π-A) (II and IV) fluorenyl derivatives. Benzothiazole moieties were used as acceptor groups while diphenylamino groups were used as donor moieties. Thiophene groups and alkynes were incorporated to extend the π-conjugation.
For the work described herein, the thiophene ring was chosen as a electron bridge because it has a higher degree of aromaticity than other five member rings, e.g., furan, it is stable towards oxidation, and its chemical reactivity to allow functionalization is higher than that observed for benzene or furan. Incorporation of these heterocyclic rings in the molecular design of chromophores has been reported to enhance the optical properties, increasing both the maximum of absorption (λabsmax) and the 2PA cross section values, particulary when the thiophene ring is close to the fluorene core.17,18
One of our goals was to compare this new series of compounds with our previously prepared and studied probes that contain benzothiazole groups. Thus, the bezothiazole ring system was used as an electron awithdrawing group. Previous studies suggested that compounds containing this moiety are more robust than analog compounds containing benzoxazole groups. Moreover, benzothiazole groups are relatively strong electron withdrawing groups, and their ability to accept charge transferred from a diphenylamino moiety appears to be higher than that observed for benzoxazolyl containing compounds.19,20
Results and Discussion
Synthesis of new fluorescent probes
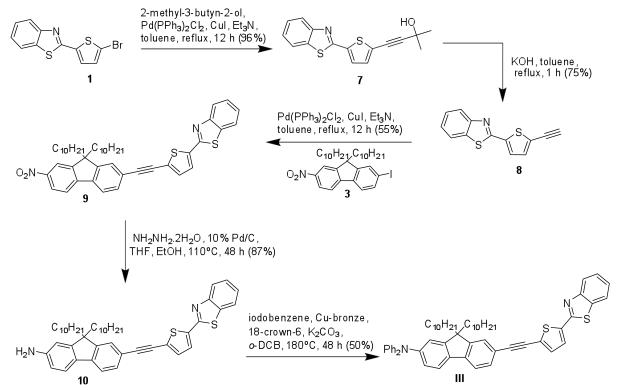
The model 2PA fluorescent probe I was synthesized starting from the transformation of benzothiazole derivative 1 into tin derivative 2, followed by Pd-catalyzed Stille coupling with key fluorenyl intermediate 3 was accomplished, as shown in Scheme 1, to yield nitrofluorenyl 4 in excellent yield.19,21 Reduction of the nitrofluorenyl 4, and subsequent reaction of resulting amine 5 with iodobenzene under Ullman conditions, generated the D-π-A type fluorescent dye I.19,22 Stille coupling between dibromofluorene derivative 6 and the tin thiophene benzothiazole intermediate 2 yielded symmetrical dye II11,19,21 (Scheme 1).
Scheme 1.
The synthesis of alkyne-containing dyes was achieved by means of successive Sonogashira coupling.23 To prepare the asymmetrical compound III, 2-(5-bromothiophen-2-yl)benzothiazole 1 was converted into protected alkyne 7 which, after hydrolysis in toluene with KOH, generated alkyne 8 (Scheme 2).24,25 Pd-catalyzed Sonogashira coupling between the alkyne 8 and key intermediate 3 yielded nitro derivative 9, which was reduced with hydrazine to produce amine 10 in high yield. Amine 10 was treated with iodobenzene under Ullman conditions to generate the asymmetrical D–π-A III (Scheme 2). 19,22
Scheme 2.
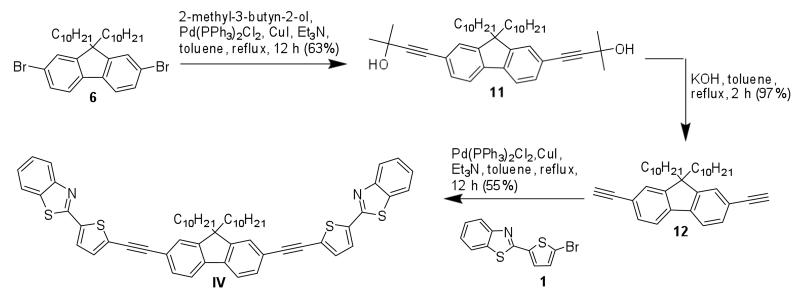
The symmetrical intermediate 11 was prepared using Sonogashira coupling between 9,9-didecyl-2,7-dibromofluorene 6 and 2-methylbut-3-yn-2-ol.24,25 After hydrolysis, the dialkyne 12 was coupled through a second Sonogashira reaction with 1, yielding symmetrical A–π-A fluorenyl dye IV (Scheme 3).
Scheme 3.
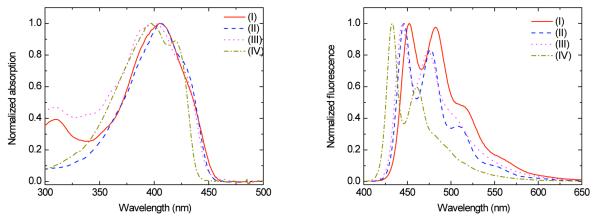
Linear photophysical properties
Table 1 summarizes the photophysical properties of the four new fluorescent dyes (I-IV). As can be seen in Figure 3, all molecules exhibited an intense absorption between 397-405 nm, fluorescent maximum in the range of 433-452 nm, and significant Stokes shift values in hexane (see Table 1). The new compounds also exhibited high fluorescence quantum yields (0.86-0.98), while a slight blue shift in the absorption maximum was observed when alkynyl moieties were incorporated in both symmetrical and asymmetrical derivatives.
Table 1.
Photophysical characterization of new fluorene derivatives I, II, III, and IV in hexane.
| Compound | λabsmax, nm | λemmax, nm | ΔλSt, nm | ϕ 1 | εmax.10−3, M−1.cm−1 |
|---|---|---|---|---|---|
| I | 403±1 | 452±1 | 49±2 | 0.86±0.05 | 53 |
| II | 405±1 | 446±1 | 41±2 | 0.98±0.05 | 31 |
| III | 398±1 | 444±1 | 46±2 | 0.92±0.05 | 50 |
| IV | 397±1 | 433±1 | 36±2 | 0.95±0.05 | 135 |
Fluorescence quantum yield measured relative to 9,10-diphenylanthracene in cyclohexane.
Figure 3.
Normalized absorption (left) and fluorescence (right) spectra for I, II, III, and IV in hexane. Absorption and emission spectra were collected at room temperature using hexanes solutions with optical density of ~0.1 (approximately 10−6 M) without degassing the solutions. Emission was collectad by exciting samples at their λabsmax value.
Nonlinear photophysical properties
Two-photon absorption (2PA) values were determined by the fluorescent method, measuring the two-photon exited fluorescence of the dyes in the range of 730-860 nm in hexane. The 2PA cross section measurements were performed with a tunable Ti:sapphire femtosecond laser system (220 fs pulse width, 76 MHz repetition rate) pumped by a 10 W diode laser (532 nm) as the excitation source and a spectrofluorimeter with PMT detectors (Figure S-1; supp data).
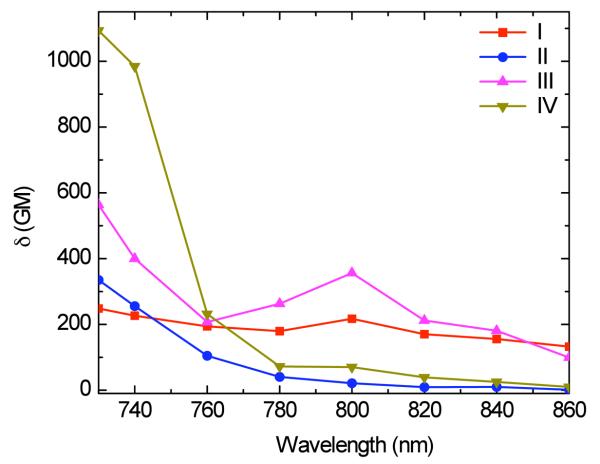
The 2PA spectra for this series of compounds are shown in Figure 4. Compound I exhibited a maximum 2PA cross section of ~248 GM at 730 nm which can be attributed to the tail of the band corresponding to the two-photon allowed S0 → S2 transition; a second maximum of ~217 GM at 800 nm, assigned to S0 → S1 2PA forbidden transition, was also observed. Compound II exhibits a maximum 2PA cross section of ~335 GM at 730 nm. Comparing these results with our previously reported data for similar molecules that did not contain the thiophene moiety a significant increase in the 2PA cross section values was observed when the thiophenyl moiety is introduced in the fluorophore.9-14
Figure 4.
2PA spectra obtained for compounds I-IV in hexane. Incorporation of thiophene and alkynyl moieties resulted in an increase in the 2PA cross section values.
To observe the effect of the presence of alkynyl triple bonds in the new derivatives, comparisons of compound I to compound III (D-π-A) and compound II to compound IV (A-π-A) were conducted. Compound III exhibited a maximum 2PA cross section of ~563 GM at 730 nm while a second maximum of ~356 GM at 800 nm was observed. Comparing these values to those obtained for compound I, it can be inferred that incorporating alkynyl triple bonds in these molecules results in an enhancement in the 2PA cross section for this unsymmetrical compound.
The 2PA cross section value obtained for compound II (~335 GM at 730 nm) can be compared with the large value obtained for compound IV at the same wavelength (~1093 GM). A significant increase in the 2PA cross section was observed when the number of alkynyl triple bonds was doubled.
Since the number of electrons in these molecules is different, it is also useful to compare the ratios of 2PA cross section (δ) over the number of π-electrons (Ne) in the chromophores (normalized cross section).18,26 When comparing I to III, it was observed that the incorporation of a triple bond in the π system resulted in a two-fold increase in the normalized cross section value (7 to 14 GM per π electron). Similarly, a three-fold increase of the normalized cross section was observed when comparing II and IV (9 and 27 GM per π electron, respectively).
In general, a considerable increase in the value of 2PA cross section was obtained when thiophene moieties were introduced in both fluorophore archetypes (D-π-A and A-π-A) studied in this series. In addition, extending the π-conjugation with alkynyl triple bond functionalities also significantly increased the 2PA for this class of molecules.
Fluorescence Imaging
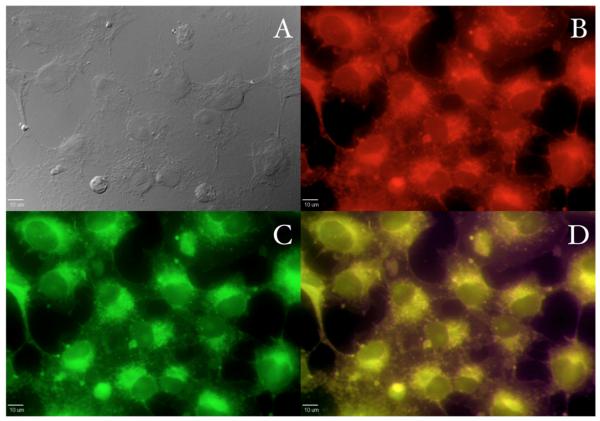
In order to evaluate the efficiency of these archetypes as fluorophores in 2PFM cell imaging, fluorene I was incubated in COS-7 and HCT 116 cell lines at different concentrations and incubation times. Probe I was chosen as model compound due to its relatively constant two-photon absorptivity across a useful NIR wavelength range (750-860 nm for the Ti:sapphire laser, Figure 4) and because it exhibited better solubility in DMSO than the more symmetrical compounds II and IV (important for cell incubation) Fluorescence micrographs indicated the distinct localization the probe in well confined areas within the cytosol in both COS-7 and HCT 116 cell lines (Figures 5 and 6). Probe I was incubated with a commercially available lysosomal selective dye (Lysotracker Red) and incubated for 10 min, 30 min, 50 min, 1 h 10 min, 1 h 30 min and 1 h 50 min, after incubation the cells were fixed, mounted, and imaged. Early colocalization with Lysotracker dye suggests a similar uptake mechanism for both the Lysotracker dye and probe I. However, longer incubation times (50 min and 1 h 50 min) indicates a difference in the progression of the probe from the endosomal vesicles to the lysosomes, where the Lysotracker dye reached the lysosomes more quickly than probe I (see Figure 5). The slower progression of probe I may prove useful for the study of late endosomal processes.
Figure 5.
Confocal fluorescence images of COS-7 cells incubated with probe I (20 μM, 1 h 50 min) and Lysotracker Red (100 nM, 1 h 50 min). (A) DIC, one-photon fluorescence image showing (B) Lysotracker Red (Texas Red filter cube (Ex:562/40; DM: 593; Em:624/40)), (C) probe I (custom made filter cube (Ex:377/50; DM: 409; Em:525/40)), and (D) colocalization (overlay of B and C). 10 μm scale bar.
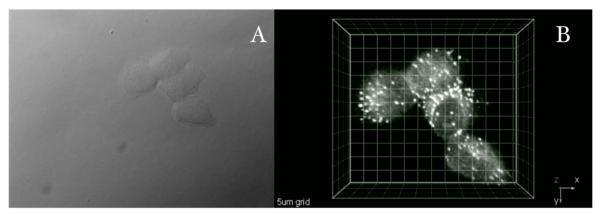
Figure 6.
Two-photon fluorescence micrograph of HCT 116 cells incubated with probe I (20 μM, 1 h 50 min). A) DIC, B) 3D reconstruction from overlaid 2PFM images obtained from a modified laser scanning confocal microscopy system equipped with a broadband, tunable Ti:sapphire laser (220 fs pulse width, 76 MHz repetition rate), pumped by a 10 W frequency doubled Nd:YAG laser. (60x objective, NA= 1.35). Scale: 5 μm grid.
The high contrast and good resolution observed in 2PFM images of HCT 116 cells, incubated with probe I, support use of the fluorenyl core as an integral part of the fluorescent probe's molecular architecture. The excellent linear and nonlinear photophysical properties points towards their potential as efficient 2PA fluorophores. The 2PFM image, shown in Figure 6, where the vesicles (presumably late endosomes) are clearly visualized in both 2D and 3D (see also supporting information), is a testament of the efficacy of the 2PA fluorophore. The potential of these dyes exceeds that of imaging the endosomes, as further functionalization of the dye can be performed to modulate the solubility properties and, more importantly, impart selectivity to other organelles or biomarkers of interest, aspects of current investigation. The new fluorenyl derivatives provide the basis for the development of fluorescent probes for 2PFM.
Conclusions
Efficient synthetic routes were developed to prepare four new fluorescent compounds containing functionalities such as thiophene and alkynyl moieties on a fluorene core, affording systematic variation of molecular symmetry and extent of conjugation. Comprehensive characterization of the new compounds revealed excellent linear and nonlinear photophysical properties, such as high fluorescent quantum yields (0.86-0.98) and high two-photon absorptivity (220-1060 GM). Incorporation of thiophene and alkynyl moieties resulted in an increase in the 2PA cross section values. Probe I proved to have low cytotoxocity when incubated with COS-7 and HCT 116 cells, exhibiting endosomal selectivity, as evidenced by one-photon fluorescence imaging and colocalization studies. The advantageous properties of probe I facilitated 2PFM endosomal imaging, with the 2PFM images providing much better resolution, allowing the visualization of individual endosomes. Due to their linear and nonlinear photophysical properties, these new fluorenyl derivatives should find use in a number of emerging photonics applications such as targeted two-photon fluorescence imaging and two-photon based optical data storage.
Experimental section
Materials and Methods
2-(5-Bromothiophen-2-yl)benzothiazole 1, 9,9-didecyl-2-iodo-7-nitro-9Hfluorene 3, and 2,7-dibromo-9,9-didecyl-9H-fluorene 6 were prepared as described in the literature.19,27,28 All reactions were carried out under N2. All other reagents and solvents were used as received from commercial suppliers. 1H and 13C NMR spectra were recorded in CDCl3 on a NMR spectrometer at 500 and 125 MHz, respectively. MALDI MS analyses were performed at the University of Florida.
Synthesis of 2-(5-(tributylstannyl)thiophen-2-yl)benzothiazole (2)
At −78 °C 2-(5-bromothiophen-2-yl)benzothiazole 1 (200 mg, 0.67 mmol) was dissolved in dry THF (5 mL). A solution of n-BuLi in hexanes (0.27 mL, 2.5 M, 0.68 mmol) was added dropwise into the reaction mixture. After stirring for 1 h, Sn(Bu)3Cl (360 mg, 1.10 mmol) was added and the mixture was allowed to reach room temperature, and stirred overnight. The mixture was added to water, extracted with Et2O twice, dried over Mg2SO4, and purified by column chromatography using a mixture of hexanes:EtOAc (9:1) as an eluent to yield 240 mg (70%) of viscous oil. 1H NMR (500 MHz, CDCl3) δ 8.02(d, J= 8.2 Hz, 1H, Ph-H), 7.82 (d, J= 8.0 Hz, 1H, Ph-H), 7.74 (d, J= 3.5 Hz, 1H, Thy-H), 7.43-7.47 (m, 1H, Ph-H), 7.31-7.35 (m, 1H, Ph-H), 7.18 (d, J= 3.5 Hz, 1H, Thy-H), 1.55-1.63 (m, 6H, CH2), 1.31-1.39 (m, 6H, CH2), 1.12-1.19 (m, 6H, CH2), 0.90 (t, J= 7.34 Hz, 9H, CH3). 13C NMR (125 MHz, CDCl3) δ 161.4, 153.9, 143.5, 142.4, 136.2, 134.7, 129.6, 126.2, 125.1, 122.9, 121.4, 28.9, 27.3, 13.7, 11.0. Anal. Calcd for C23H33NS2Sn: C, 54.56; H, 6.57; N, 2.77. Found: C, 54.85; H, 6.67; N, 2.86.
Synthesis of 2-(5-(9,9-didecyl-7-nitro-9H-fluoren-2-yl)thiophen-2-yl)benzothiazole (4)
9,9-Didecyl-2-iodo-7-nitro-9H-fluorene 3 (200 mg, 0.32 mmol), 2-(5-(tributylstannyl)thiophen-2-yl)benzothiazole 2 (191 mg, 0.38 mmol), and Pd(PPh3)2Cl2 (6 mg, 0.008 mmol) were dissolved in toluene (4 mL). The mixture was heated under reflux for 5 h. The solvent was removed under reduced pressure and the crude was purified by column chromatography using a mixture of hexanes:EtOAc (9.5:0.5) as an eluent to yield 222 mg (98%) of yellow oil that solidified upon standing (m.p. 73.2-74.9 °C). 1H NMR (500 MHz, CDCl3) δ 8.28 (dd, J= 8.3 Hz, J= 2.0 Hz, 1H, Ph-H), 8.22 (d, J= 2.0 Hz, 1H, Ph-H), 8.05 (d, J= 8.0 Hz, 1H, Ph-H), 7.88 (d, J= 7.8 Hz, 1H, Ph-H), 7.79-7.84 (m, 2H, Ph-H), 7.72-7.76 (m, 1H, Ph-H), 7.69 (d, J= 1.4 Hz, 1H, Ph-H), 7.66 (d, J= 3.7 Hz, 1H, Thy-H), 7.48-7.53 (m, 1H, Ph-H), 7.46 (d, J= 3.7 Hz, 1H, Thy-H), 7.37-7.42 (m, 1H, Ph-H), 2.01-2.09 (m, 4H, CH2), 0.99-1.24 (m, 28H, CH2), 0.82 (t, J= 7.0 Hz, 6H, CH3), 0.54-0.69 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 161.0, 153.7, 153.4, 152.2, 147.9, 147.2, 146.8, 139.0, 136.6, 134.7, 134.4, 129.5, 126.6, 125.3, 124.40, 124.36, 123.5, 122.0, 121.9, 121.5, 120.4, 120.0, 118.3, 55.9, 40.1, 31.8, 29.8, 29.49, 29.47, 29.24, 29.19, 23.8, 22.6, 14.1. Anal. Calcd for C44H54N2O2S2: C, 74.74; H, 7.70; N, 3.96. Found: C, 74.97; H, 7.85; N, 3.97.
Synthesis of 7-(5-(benzothiazol-2-yl)thiophen-2-yl)-9,9-didecyl-9H-fluoren-2-amine (5)
2-(5-(9,9-Didecyl-7-nitro-9H-fluoren-2-yl)thiophen-2-yl)benzothiazole 4 (160 mg, 0.23 mmol) and 10% Pd/C (16 mg) were dissolved in a mixture 1:1 of THF:EtOH (8 mL). NH2NH2.2H2O (136 mg, 2.7 mmol) was added to the mixture slowly at room temperature, and then heated to 70 °C for 20 h. The mixture was filtered though a silica plug with CH2Cl2, and, after removing the solvent under reduced pressure, the material was purified by column chromatography using as a solvent a mixture of hexanes:EtOAc (9:1), to yield 130 mg (84%) of dark yellow oil that was used directly since the primary amine is prone to oxidation. 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J= 8.0 Hz, 1H, Ph-H), 7.85 (d, J= 8.0 Hz, 1H, Ph-H), 7.59-7.64 (m, 2H, Ph-H, Thy-H), 7.54-7.58 (m, 2H, Ph-H), 7.45-7.51 (m, 2H, Ph-H), 7.34-7.39 (m, 2H, Ph-H, Thy-H), 6.65-6.69 (m, 2H, Ph-H), 3.80 (s, 2H, NH2), 1.83-2.00 (m, 4H, CH2), 0.99-1.28 (m, 28H, CH2), 0.83 (t, J= 7.0 Hz, 6H, CH3), 0.62-0.73 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) 161.4, 153.8, 153.1, 150.7, 149.6, 146.4, 142.4, 135.0, 134.6, 131.6, 130.5, 129.5, 126.4, 125.1, 124.7, 123.0, 122.8, 121.4, 120.8, 120.1, 118.8, 114.1, 109.7, 54.9, 40.6, 31.9, 30.1, 29.6, 29.5, 29.29, 29.28, 23.8, 22.7, 14.1.
Synthesis of 7-(5-(benzothiazol-2-yl)thiophen-2-yl)-9,9-didecyl-N,N-diphenyl-9H-fluoren-2-amine (I)
7-(5-(Benzothiazol-2-yl)thiophen-2-yl)-9,9-didecyl-9H-fluoren-2-amine 5 (130 mg, 0.19 mmol), iodobenzene (157 mg, 0.77 mmol), Cu-bronze (61 mg, 0.96 mmol), 18-crown-6 (15 mg, 0.058 mmol), and K2CO3 (212 mg, 1.54 mmol) were combined with 1,2-dichlorobenzene (3 mL). The mixture was heated to 180 °C for 48 h. The crude product was passed through a silica plug with CH2Cl2. The solvent was removed under reduced pressure and the crude was purified by column chromatography on silica gel using as a solvent a mixture of hexanes:EtOAc (9:1) to yield 150 mg (94%) of yellow solid (m.p. 107.5-109.5 °C). 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J= 8.0 Hz, 1H, Ph-H), 7.84 (d, J= 8.0 Hz, 1H, Ph-H), 7.58-7.65 (m, 4H, Ph-H, Thy-H), 7.56 (d, J= 8.0 Hz, 1H, Ph-H), 7.45-7.50 (m, 1H, Ph-H), 7.33-7.39 (m, 2H, Ph-H, Thy-H), 7.22-7.30 (m, 5H, Ph-H), 7.11-7.16 (m, 5H, Ph-H), 7.00-7.05 (m, 2H, Ph-H), 1.82-1.95 (m, 4H, CH2), 1.01-1.23 (m, 28H, CH2), 0.85 (t, J= 7.0 Hz, 6H, CH3), 0.66-0.75 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 161.3, 153.8, 152.5, 151.6, 149.2, 147.9, 147.5, 141.6, 135.4, 134.6, 131.4, 129.6, 129.5, 129.24, 129.16, 125.2, 125.0, 124.0, 123.9, 123.4, 123.3, 122.8, 122.6, 121.5, 121.4, 120.1, 105.0, 55.2, 40.2, 31.9, 30.0, 29.6, 29.6, 29.3, 23.9, 22.7, 14.1. Anal. Calcd for C56H64N2S2: C, 81.11; H, 7.78; N, 3.38. Found: C, 80.81; H, 7.74; N, 3.30.
Synthesis of 2,2′-(5,5′-(9,9-didecyl-9H-fluorene-2,7-diyl)bis(thiophene-5,2-diyl))dibenzothiazole (II)
2-(5-(Tributylstannyl)thiophen-2-yl)benzothiazole 2 (460 mg, 0.90 mmol), 2,7-dibromo-9,9-didecyl-9H-fluorene 6 (250 mg, 0.41 mmol), and Pd(PPh3)2Cl2 (7.2 mg, 0.01mmol) were dissolved in toluene. The mixture was heated under reflux for 24 h. The solvent was removed under reduced pressure and the crude was purified by column chromatography using a mixture of hexanes:EtOAc (9.9:0.1) to yield 126 mg (35%) of yellow oil that solidified upon standing (m.p. 125.2-126.5 °C). 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J= 7.76 Hz, 2H, Ph-H), 7.87 (d, J= 7.82 Hz, 2H, Ph-H), 7.74 (d, J= 7.63 Hz, 2H, Ph-H), 7.68-7.71 (m, 2H, Ph-H), 7.64-7.67 (m, 4H, Ph-H, Thy-H), 7.47-7.52 (m, 2H, PhH), 7.43 (d, J= 4.10 Hz, 2H, Thy-H), 7.36-7.40 (m, 2H, Ph-H), 2.00-2.06 (m, 4H, CH2), 1.04-1.23 (m, 28H, CH2), 0.80 (t, J= 7.04 Hz, 6H, CH3), 0.67-0.74 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 161.2, 153.7, 152.1, 148.8, 140.9, 135.8, 134.7, 132.6, 129.5, 126.5, 125.2, 125.0, 123.7, 122.9, 121.5, 120.5, 120.3, 55.4, 40.4, 31.9, 30.0, 29.6, 29.5, 29.3, 29.2, 23.8, 22.6, 14.1. HRMS (MALDI) m/z calcd 877.3712 [M+H]+, m/z found 877.3702 [M+H]+.
Synthesis of 4-(5-(benzothiazol-2-yl)thiophen-2-yl)-2-methylbut-3-yn-2-ol (7)
2-(5-Bromothiophen-2-yl)benzothiazole 1 (200 mg, 0.67 mmol), 2-methyl-3-butyn-2-ol (170 mg, 2.02 mmol), Pd(PPh3)2Cl2 (19 mg, 0.027 mmol), and CuI (5 mg, 0.027 mmol) were dissolved in a 1:4 mixture of Et3N:toluene (5 mL). The mixture was heated under reflux for 12 h. After cooling to room temperature, the mixture was filtered through a celite plug, and purified by column chromatography using a mixture of hexanes:EtOAc (1:1) to yield 192 mg (96%) of pale yellow solid (m.p. 163.9-164.6 °C). 1H NMR (500 MHz, CDCl3) 8.02 (d, J= 8.04 Hz, 1H, Ph-H), 7.85 (d, J= 7.87 Hz, 1H, Ph-H), 7.50 (d, J= 3.95 Hz, 1H, Thy-H), 7.46-7.49 (m, 1H, Ph-H), 7.35-7.40 (m, 1H, Ph-H), 7.18 (d, J= 3.95 Hz, 1H, Thy-H), 2.11 (s, 1H, -OH), 1.64 (s, 6H, CH3 ). 13C NMR (125 MHz, CDCl3) δ160.4, 153.6, 137.9, 134.7, 132.9, 128.3, 126.4, 125.4, 123.1, 121.5, 121.4, 100.2, 75.2, 65.8, 31.3. . Anal. Calcd for C16H13NOS2: C, 64.18; H, 4.38; N, 4.68. Found: C, 64.10; H, 4.33; N, 4.64.
Synthesis of 2-(5-ethynylthiophen-2-yl)benzothiazole (8)
4-(5-(Benzothiazol-2-yl)thiophen-2-yl)-2-methylbut-3-yn-2-ol 7 (300 mg, 1.0 mmol) and KOH (300 mg, 5.3 mmol) were heated under reflux in toluene for 1 h. The mixture was filtered, then purified by column chromatography eluting with hexanes to yield 180 mg (75%) of a white solid (m.p. 117.3-118.3 °C). 1H NMR (500 MHz, CDCl3δ 8.03 (d, J= 8.21Hz, 1H, Ph-H), 7.86 (d, J= 8.21Hz, 1H, Ph-H), 7.51 (d, J= 3.94 Hz, 1H, Thy-H), 7.47-7.50 (m, 1H, Ph-H), 7.37-7.42 (m, 1H, Ph-H), 7.28 (d, J= 3.94 Hz, 1H, Thy-H), 3.50 (s, 1H, C≡C-H). 13C NMR (125 MHz, CDCl3) δ 160.2, 153.6, 138.5, 134.8, 133.9, 133.7, 127.8, 126.5, 125.4, 123.2, 121.6, 84.0, 76.5. Anal. Calcd for C13H7NS2: C, 64.70; H, 2.92; N, 5.80. Found: C, 64.64; H, 2.91; N, 5.75.
Synthesis of 2-(5-((9,9-didecyl-7-nitro-9H-fluoren-2-yl)ethynyl)thiophen-2-yl)benzothiazole (9)
2-(5-Ethynylthiophen-2-yl)benzothiazole 8 (180 mg, 0.75 mmol), 9,9-didecyl-2-iodo-7-nitro-9Hfluorene 3 (460 mg, 0.75 mmol), Pd(PPh3)2Cl2 (20 mg, 0.03 mmol), and CuI (5.6 mg, 0.03 mmol),were dissolved in a 1:4 mixture of Et3N:toluene (5 mL). The mixture was heated under reflux for 12 h. After cooling to room temperature, the mixture was filtered through a celite plug and purified by column chromatography eluting with a mixture of hexanes:EtOAc (9:1), and then recrystallized from hexane to yield 300 mg (55%) of yellow solid (m.p. 142.3-143.6 °C). 1H NMR (500 MHz, CDCl3) 8.28 (dd, J= 8.40 Hz, J= 2.18 Hz, 1H, Ph-H), 8.21 (d, J= 2.05 Hz, 1H, Ph-H), 8.05 (d, J= 7.74 Hz, 1H, Ph-H), 7.88 (d, J= 7.74 Hz, 1H, Ph-H), 7.77-7.82 (m, 2H, Ph-H), 7.55-7.61 (m, 3H, Ph-H, Thy-H), 7.48-7.52 (m, 1H, Ph-H), 7.39-7.42 (m, 1H, Ph-H), 7.33 (d, J= 4.08 Hz, 1H, Thy-H), 2.01-2.08 (m, 4H, CH2), 0.99-1.29 (m, 28H, CH2), 0.84 (t, J= 6.93 Hz, 6H, CH3), 0.50-0.64 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 160.3, 153.7, 152.5, 152.3, 147.4, 146.6, 139.3, 138.4, 134.8, 132.8, 131.2, 128.5, 128.3, 126.7, 126.2, 126.0, 123.2, 123.1, 121.6, 121.5, 120.2, 118.3, 105.0, 96.3, 83.8, 55.9, 40.0, 31.9, 29.9, 29.51, 29.48, 29.2, 23.8, 22.6, 14.14, 14.06. Anal. Calcd for C46H54N2O2S2: C, 75.57; H, 7.45; N, 3.83. Found: C, 75.69; H, 7.47; N, 3.85.
Synthesis of 7-((5-(benzothiazol-2-yl)thiophen-2-yl)ethynyl)-9,9-didecyl-9H-fluoren-2-amine (10)
2-(5-((9,9-Didecyl-7-nitro-9H-fluoren-2-yl)ethynyl)thiophen-2-yl)benzothiazole 9 (180 mg, 0.24 mmol) and 10% Pd/C (18 mg) were dissolved in a 1:1 mixture of THF:EtOH (4 mL). NH2NH2.2H2O (146 mg, 2.8 mmol) was added to the mixture dropwise at room temperature, followed by heating to 110 °C for 48 h. The mixture was filtered though a silica plug with CH2Cl2, and, after removing the solvent under reduced pressure, the material was purified by column chromatography using a mixture of hexanes:EtOAc (9:1) as eluent to yield 150 mg (87%) of dark yellow oil that was used immediately due to the oxidative lability of the primary amine. 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J= 7.45 Hz, 1H, Ph-H), 7.86 (d, J= 7.67 Hz, 1H, Ph-H), 7.56 (d, J= 3.99 Hz, 1H, Thy-H), 7.52 (d, J= 7.75 Hz, 1H, PhH), 7.46-7.50 (m, 3H, Ph-H), 7.43-7.44 (m, 1H, Ph-H), 7.36-7.40 (m, 1H, Ph-H), 7.27 (d, J= 3.99 Hz, 1H, Thy-H), 6.65-6.68 (m, 2H, Ph-H), 3.83 (s, 2H, NH2), 1.84-1.97 (m, 4H, CH2), 1.03-1.27 (m, 28H, CH2), 0.85 (t, J= 7.16 Hz, 6H, CH3), 0.56-0.68 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 161.0, 160.6, 153.8, 153.1, 150.2, 149.9, 146.6, 146.0, 144.4, 142.7, 141.6, 136.1, 134.6, 133.7, 132.7, 132.2, 131.5, 128.4, 122.9, 121.6, 118.5, 114.0, 109.8, 97.7, 81.9, 54.9, 40.5, 31.9, 30.2, 29.7, 29.6, 29.4, 29.3, 23.9, 22.7, 14.2.
Synthesis of 7-((5-(benzothiazol-2-yl)thiophen-2-yl)ethynyl)-9,9-didecyl-N,N-diphenyl-9Hfluoren-2-amine (III)
7-((5-(Benzothiazol-2-yl)thiophen-2-yl)ethynyl)-9,9-didecyl-9H-fluoren-2-amine 10, (0.135 mg, 0.19 mmol), iodobenzene (157 mg, 0.77 mmol), Cu-bronze (61 mg, 0.96 mmol), 18-crown-6 (15 mg, 0.058 mmol), and K2CO3 (212 mg, 1.54 mmol) were combined with, 1,2-dichlorobenzene (3 mL). The mixture was heated at 180 °C for 48 h. The crude product was passed through a silica plug with CH2Cl2. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel using a mixture of hexanes:EtOAc (9.5:0.5) as eluent to yield 81 mg (50%) of yellow viscous oil. 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J= 8.11 Hz, 1H, Ph-H), 7.85 (d, J= 8.11 Hz, 1H, Ph-H), 7.59 (d, J= 7.90 Hz, 1H, Ph-H), 7.56 (d, , J= 3.72 Hz, 1H, Thy-H), 7.45-7.52 (m, 4H, Ph-H), 7.35-7.39 (m, 1H, Ph-H), 7.24-7.30 (m, 5H, Ph-H), 7.10-7.15 (m, 5H, Phe-H), 7.00-7.05 (m, 3H, Phe-H), 1.80-1.92 (m, 4H, CH2), 1.03-1.28 (m, 28H, CH2), 0.85 (t, J= 7.14 Hz, 6H, CH3), 0.62-0.69 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 160.5, 153.7, 152.6, 150.8, 147.8, 147.8, 141.9, 137.7, 135.2, 134.7, 132.4, 130.8, 129.2, 128.4, 127.4, 126.6, 125.7, 125.4, 124.0, 123.3, 123.01 122.7, 121.5, 120.9, 119.6, 119.1, 118.9, 97.4, 82.2, 55.2, 40.2, 31.9, 30.0, 29.6, 29.6, 29.4, 29.3, 23.9, 22.7, 14.1. Anal. Calcd for C58H64N2S2: C, 81.64; H, 7.56; N, 3.28. Found: C, 81.63; H, 7.62; N, 3.05.
Synthesis of 4,4′-(9,9-didecyl-9H-fluorene-2,7-diyl)bis(2-methylbut-3-yn-2-ol) (11)
2,7-Dibromo-9,9-didecyl-9H-fluorene 6 (1.0 g, 1.65 mmol), 2-methyl-3-butyn-2-ol (0.83 g, 9.92 mmol), Pd(PPh3)2Cl2 (95 mg, 0.135 mmol), and CuI (25 mg, 0.13 mmol) were dissolved in a 1:4 mixture of Et3N:toluene (15 mL). The mixture was heated under reflux for 12 h. After it was cooled down, the mixture was filtered through a celite plug and purified by column chromatography using hexanes as a eluent, followed by a mixture of hexanes:EtOAc (10:1) to yield 0.64 g (63%) of white solid (m.p. 89.0-89.9 °C). 1H NMR (500 MHz, CDCl3) δ 7.58-7.61 (m, 2H, Ph-H), 7.35-7.41 (m, 4H, Ph-H), 2.05 (s, 2H, -OH), 1.89-1.96 (m, 4H, CH2), 1.63-1.68 (s, 12H, CH3), 0.98-1.27 (m, 28H, CH2), 0.85 (t, J= 6.49 Hz, 6H, CH3), 0.50-0.58 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 150.9, 140.6, 130.7, 126.0, 121.4, 119.8, 93.9, 83.0, 65.7, 55.2, 40.4, 31.9, 31.6, 30.0, 29.6, 29.5, 29.3, 29.2, 23.7, 22.6, 14.1. Anal. Calcd for C43H62O2: C, 84.53; H, 10.23. Found: C, 84.46; H, 10.25.
Synthesis of 9,9-didecyl-2,7-diethynyl-9H-fluorene (12)
4,4′-(9,9-Didecyl-9H-fluorene-2,7-diyl)bis(2-methylbut-3-yn-2-ol) 11 (450 mg, 0.736 mmol) and KOH (206 mg, 3.68 mmol) were heated under reflux in toluene for 2 h. The mixture was filtered, then purified by column chromatography using hexanes to yield 350 mg (97%) of light yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.63 (dd, J= 7.73 Hz, J= 0.65 Hz, 2H, Ph-H), 7.48 (dd, J= 7.73 Hz, J= 1.42 Hz, 2H, Ph-H), 7.45-7.46 (m, 2H, Ph-H), 3.15 (s, 2H, C≡C-H), 1.90-1.96 (m, 4H, CH2), 0.99-1.25 (m, 28H, CH2), 0.85 (t, J= 7.0 Hz, 6H, CH3), 0.52-0.58 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 151.0, 141.0, 131.2, 126.5, 120.8, 119.95, 84.5, 77.3, 55.2, 40.2, 31.9, 29.9, 29.5, 29.5, 29.2, 23.7, 22.7, 14.1. Anal. Calcd for C37H50: C, 89.81; H, 10.19. Found: C, 89.79; H, 10.24.
Synthesis of 2,2′-(5,5′-(9,9-didecyl-9H-fluorene-2,7-diyl)bis(ethyne-2,1-diyl)bis(thiophene-5,2-diyl))dibenzothiazole (IV)
9,9-Didecyl-2,7-diethynyl-9H-fluorene 12 (300 mg, 0.61 mmol), 2-(5-bromothiophen-2-yl)benzothiazole 1 (377 mg, 1.27 mmol), Pd(PPh3)2Cl2 (34 mg, 0.05 mmol), and CuI (10 mg, 0.05 mmol) were dissolved in a 1:4 mixture of Et3N:toluene (5 mL). The mixture was heated under reflux for 12 h. After it was cooled down, the mixture was filtered through a celite plug, and purified by column chromatography using a mixture of hexanes:EtOAc (10:1) as eluent to yield 310 mg (55%) of yellow solid (m.p. 74.0-75.5 °C). 1H NMR (500 MHz, CDCl3) δ 8.04 (dd, J= 8.09 Hz, J= 0.54 Hz, 2H, Ph-H), 7.87 (dd, J= 8.04 Hz, J= 0.53 Hz, 2H, Ph-H), 7.70 (d, J= 7.83 Hz, 2H, Ph-H), 7.57 (d, J= 3.89 Hz, 2H, Thy-H), 7.54-7.56 (m, 2H, Ph-H), 7.52-7.53 (m, 2H, Ph-H), 7.48-7.51 (m, 2H, Ph-H), 7.37-7.41 (m, 2H, Ph-H), 7.31 (d, J= 3.89 Hz, 2H, Thy-H), 1.97-2.04 (m, 4H, CH2), 1.04-1.23 (m, 28H, CH2), 0.84 (t, J= 6.96 Hz, 6H, CH3), 0.58-0.65 (m, 4H, CH2). 13C NMR (125 MHz, CDCl3) δ 160.5, 153.7, 151.3, 141.1, 138.0, 134.7, 132.6, 130.8, 128.4, 127.1, 126.6, 125.9, 125.5, 123.1, 121.5, 121.3, 120.2, 97.0, 82.9, 55.4, 40.3, 31.9, 30.0, 29.6, 29.5, 29.3, 29.3, 23.8, 22.7, 14.1. Anal. Calcd for C59H60N2S4: C, 76.58; H, 6.54; N, 3.03. Found: C, 76.79; H, 6.75; N, 2.79.
Measurements
Absorption spectra were recorded with an UV–visible spectrophotometer. Steady-state fluorescence spectra were measured with a spectrofluorimeter in the photon counting regime of the PMT using an L-format configuration. The fluorescence spectra were corrected for the spectral dependence of the PMT. All measurements were performed in hexane at room temperature in 1 cm quartz cuvettes, with dye concentrations on the order of 10−6 M. Fluorescence quantum yields were determined relative to 9, 10-diphenylanthracene in cyclohexane as a standard.29
Two-photon absorption (2PA) cross sections of the final compounds were determined by the two-photon induced fluorescence method.30 In this method, the sample is excited simultaneously by two photons of low energy, resulting in fluorescence emission. The number of photons emitted can be measured by a PMT, and the latter related to the numeric value of the 2PA cross section relative to a reference.
Cell culture and incubation
COS-7 and HCT 116 cell were cultured in DMEM, supplemented with 10% FBS, and 1% penicillin, and 1% streptomycin at 37 °C, under 5% CO2 environment. N° 1 round 12 mm coverslips were treated with poly-D-lysine to improve cell adhesion, and washed (3x) with PBS buffer solution. The treated cover slips were placed in 24-well plates, seeded with 80,000 cells/well, and incubated at the same conditions as indicated above until 75-85% confluency was reached on the coverslips. From a 4.10 × 10−4 M stock solution of dye I a series of 0.1, 1, 10, 20, and 50 μM solutions in DMSO were prepared with all solutions also containing 75 nM of Lysotracker Red. These solutions were used to incubate the cells for 10 min to 3h. The dye solutions were extracted and the coverslipped cells were washed abundantly with PBS (4x).
Cell fixing and mounting
Cells were fixed with 3.7% solution of paraformaldehyde in pH=7.4 PBS buffer for 10 min. The fixing agent was extracted and washed (2x) with PBS. To reduce autofluorescence, a fresh solution of NaBH4 (1 mg/mL) in pH=8 PBS buffer was used to treat the fixed cells (2x). The coverslipped cells were then washed with buffer PBS (2x) and mounted on microscope slides using an antifade mounting media.
Confocal one-photon fluorescence imaging
One-photon (conventional) fluorescence microscopy images were recorded on an inverted confocal microscope equipped with a EM-CCD digital camera. One-photon confocal fluorescence images of the fixed cells were taken using a custom made filter cube (Ex:377/50; DM: 409; Em:525/40) and a Texas Red filter cube (Ex:562/40; DM: 593; Em:624/40) for probe I and Lysotracker Red, respectively.
Two-photon upconverted fluorescence imaging
Two-photon fluorescence microscopy (2PFM) imaging was performed on a modified laser scanning confocal microscopy system equipped with a broadband, tunable Ti:sapphire laser (220 fs pulse width, 76 MHz repetition rate), pumped by a 10 W frequency doubled Nd:YAG laser. The Ti:sapphire laser, tuned 700 nm and modelocked, was used as the two-photon excitation source. Two-photon induced fluorescence was collected by a 60x microscope objective (UPLANSAPO 60x, N.A.=1.35). A high transmittance (>95%) short-pass filter (cutoff 685 nm) was placed in front of the PMT detector within the scanhead in order to filter off background radiation from the laser source (700 nm).
Supplementary Material
Acknowledgment
The authors wish to acknowledge the National Science Foundation (CHE-0832622 and CHE-0840431) and the National Institutes of Health (1 R15 EB008858-01) for support of this work.
Footnotes
Supporting Information Available: Additional experimental descriptions, NMR spectra, and 3D cell images from layer-by-layer reconstruction of two-photon fluorescence micrographs are included. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Denk W, Strickler JH, Webb WW. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 2.Parthenopoulos DA, Rentzepis PM. Science. 1989;245:843–845. doi: 10.1126/science.245.4920.843. [DOI] [PubMed] [Google Scholar]
- 3.Belfield KD, Schafer KJ. Chem. Mater. 2002;14:3656–3662. [Google Scholar]
- 4.Yanez CO, Andrade CD, Yao S, Luchita G, Bondar MV, Belfield KD. ACS Appl. Mater. Interfaces. 2009;1:2219–2229. doi: 10.1021/am900587u. [DOI] [PubMed] [Google Scholar]
- 5.Belfield KD, Schafer KJ, Liu YU, Liu J, Ren XB, Van Stryland EW. J. Phys. Org. Chem. 2000;13:837–849. [Google Scholar]
- 6.Maruo S, Nakamura O, Kawata S. Opt. Lett. 1997;22:132–134. doi: 10.1364/ol.22.000132. [DOI] [PubMed] [Google Scholar]
- 7.Belfield KD, Ren X, Hagan DJ, Van Stryland EW, Dubikovsky V, Miesak EJ. J. Am. Chem. Soc. 2000;122:1217–1218. [Google Scholar]
- 8.Schafer-Hales KJ, Belfield KD, Yao S, Frederiksen PK, Hales JM, Kolattukudy PE. J. Biomed. Opt. 2005;10:015402-1–8. doi: 10.1117/1.2104528. [DOI] [PubMed] [Google Scholar]
- 9.Belfield KD, Bondar MV, Morales AR, Yavuz O, Przhonska OV. J. Phys. Org. Chem. 2003;16:194–201. [Google Scholar]
- 10.Belfield KD, Morales AR, Hales JM, Hagan DJ, Van Stryland EW, Chapela VM, Percino J. Chem. Mater. 2004;16:2267–2273. [Google Scholar]
- 11.Belfield KD, Morales AR, Kang BS, Hales JM, Hagan DJ, Van Stryland EW, Chapela VM, Percino J. Chem. Mater. 2004;16:4634–4641. [Google Scholar]
- 12.Yao S, Belfield KD. J. Org. Chem. 2005;70:5126–5132. doi: 10.1021/jo0503512. [DOI] [PubMed] [Google Scholar]
- 13.Belfield KD, Yao S, Bondar MV. Adv. Polym. Sci. 2008;213:97–156. [Google Scholar]
- 14.Yanez CO, Andrade CD, Belfield KD. Chem. Commun. 2009:827–829. doi: 10.1039/b815831b. [DOI] [PubMed] [Google Scholar]
- 15.Belfield KD, Bondar MV, Yanez CO, Hernandez FE, Przhonska OV. J. Mater. Chem. 2009;19:7498–7502. doi: 10.1021/jp902060m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moura GLC, Simas AM. J. Phys. Chem. C. 2010;114:6106–6116. [Google Scholar]
- 17.Reinhardt BA, Brott LL, Clarson SJ, Dillard AG, Bhatt JC, Kannan R, Yuan LX, He GS, Prasad PN. Chem. Mater. 1998;10:1863–1874. [Google Scholar]
- 18.Mongin O, Porres L, Charlot M, Katan C, Blanchard-Desce M. Chem. Eur. J. 2007;13:1481–1498. doi: 10.1002/chem.200600689. [DOI] [PubMed] [Google Scholar]
- 19.Belfield KD, Schafer KJ, Mourad W, Reinhardt BA. J. Org. Chem. 2000;65:4475–4481. doi: 10.1021/jo991950+. [DOI] [PubMed] [Google Scholar]
- 20.Kannan R, He GS, Yuan LX, Xu FM, Prasad PN, Dombroskie AG, Reinhardt BA, Baur JW, Vaia RA, Tan LS. Chem. Mater. 2001;13:1896–1904. [Google Scholar]
- 21.Kosugi M, Koshiba M, Atoh A, Sano H, Migita T. Bull. Chem. Soc. Jpn. 1986;59:677–679. [Google Scholar]
- 22.Yang CP, Lin JH. J. Polym. Sci., Part A-Polym. Chem. 1994;32:369–382. [Google Scholar]
- 23.Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975:4467–4470. [Google Scholar]
- 24.Umezawa H, Okada S, Oikawa H, Matsuda H, Nakanishi H. J. Phys. Org. Chem. 2005;18:468–472. [Google Scholar]
- 25.Mongin O, Krishna TR, Werts MHV, Caminade AM, Majoral JP, Blanchard-Desce M. Chem. Commun. 2006:915–917. doi: 10.1039/b517270e. [DOI] [PubMed] [Google Scholar]
- 26.Pawlicki M, Collins HA, Denning RG, Anderson HL. Angew. Chem. Int. Edit. 2009;48:3244–3266. doi: 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]
- 27.Zeng DX, Chen Y. J. Photochem. Photobiol. A. 2007;186:121–124. [Google Scholar]
- 28.Belfield KD, Yao S, Morales AR, Hales JM, Hagan DJ, Van Stryland EW, Chapela VM, Percino J. Polym. Adv. Technol. 2005;16:150–155. [Google Scholar]
- 29.Belfield KD, Bondar MV, Przhonska OV, Schafer KJ. J. Fluores. 2002;12:445–450. [Google Scholar]
- 30.Belfield KD, Bondar MV, Yanez CO, Hernandez FE, Przhonska OV. J. Phys. Chem. B. 2009;113:7101–7106. doi: 10.1021/jp902060m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.