Abstract
We report three-dimensional (3D) endoscopic microscopy findings in Barrett’s esophagus, using an endoscopic optical coherence tomography (OCT) system in one patient before and in one patient after radiofrequency ablation (RFA). Findings were compared with those in a normal patient without Barrett’s esophagus. In the normal patient, findings were of regular flat squamous mucosa with small subepithelial vessels and glands. In the Barrett’s esophagus patient, findings were of large, densely packed glands with distortion of mucosal architecture. In the post-RFA case, findings were of a small number of isolated glands buried beneath 300–500 μm of neosquamous epithelium and lamina propria. Neosquamous epithelium is a marker of successful ablative therapy, while buried glands may have potential for dysplastic progression and are difficult to detect using conventional methods. These results indicate a potential role of 3D-OCT endoscopic microscopy for follow-up, including subsurface assessment, of ablative treatments for Barrett’s esophagus.
Introduction
Ablative therapies including photodynamic therapy, argon plasma coagulation, and radiofrequency ablation (RFA) are increasingly performed for Barrett’s esophagus. Residual Barrett’s esophagus from incomplete ablation and buried Barrett’s esophagus glands beneath regenerative neosquamous epithelium are often found, to varying degrees, on follow-up [1]. While neosquamous epithelium indicates successful therapy, detection of residual disease is important for guiding additional treatments. Due to their diminutive or sub-surface nature, detection of residual Barrett’s esophagus, buried glands, and neosquamous epithelium can be challenging using conventional endoscopy alone [1].
Optical coherence tomography (OCT) generates cross-sectional images of internal structure with micrometer resolutions and millimeter imaging depths by measuring the echo time delays of back-scattered light [2]. OCT can be performed with fiberoptic probes introduced through the accessory channel of standard endoscopes. Recently, endoscopic three-dimensional (3D) OCT has become possible due to dramatic increases in imaging speed [3,4], providing an endoscopic microscopy tool with a powerful combination of high resolution, large field of view, and rapid data acquisition.
Two-dimensional OCT has been extensively studied in Barrett’s esophagus, including the detection of specialized intestinal metaplasia, dysplasia, and adenocarcinoma [5,6]. 3D-OCT has recently been used in pilot studies for esophageal [3] and colon imaging [4]. Here we report 3D-OCT findings of Barrett’s esophagus, buried Barrett’s esophagus glands beneath neosquamous epithelium following RFA, and normal squamous mucosa.
Case report
Three white males were recruited between May and December 2008. 3D-OCT was conducted using a fiberoptic probe passed down the accessory channel of a standard esophagogastroduod-noscope (GIF Q180; Olympus, Tokyo, Japan), enabling simultaneous video endoscopy. The endoscope was advanced to a region of interest and the probe was positioned adjacent to the esophageal wall. Biopsies were obtained from the imaged regions and prepared using hematoxylin and eosin stain.
The prototype 3D-OCT system, developed with LightLab Imaging (Westford, Massachusetts, USA), had a 5-μm axial resolution, 14-μm lateral resolution, and tissue imaging depth of 1500 μm. The probe scanned a helical pattern with a 10-mm circumference and 20-mm pullback length. The volumetric data was cylindrically shaped but was converted to rectangular form by unfolding [4] to better visualize en face features. Each acquisition captured a 300 mm3 tissue volume in 20 seconds, providing 3D endoscopic microscopy over a large field of view.
Conventional endoscopy of the mid-esophagus of the 59-year-old control patient showed smooth, pale-pink mucosa. Fig. 1 shows a volumetric rendering of 3D-OCT data from the normal esophagus with orthoplanes marked in red, blue, and green.
Fig. 1.
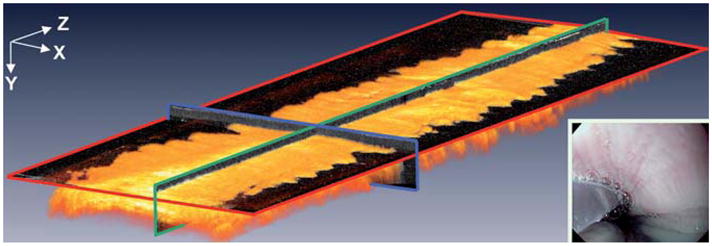
Volumetric rendering of 3D-OCT data from the normal mid-esophagus. Orthoplanes are shown in red, blue, and green. Inset A video endoscopy image with the 3D-OCT probe in position prior to image acquisition.
Fig. 2 shows each orthoplane with colored arrow pairs indicating the locations of the complementary planes.
Fig. 2.
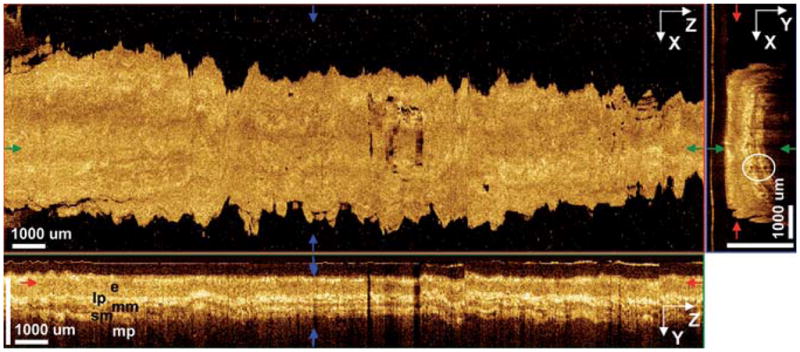
3D-OCT orthoplanes of normal squamous mucosa. The en face XZ orthoplane is located in the epithelial layer at a depth of 175 μm. The colored arrows indicate locations of complementary orthoplanes. The cross-sectional YZ orthoplane shows the epithelium (e), lamina propria (lp), muscularis mucosa (mm), submucosa (sm), and muscularis propria (mp). The cross-sectional XY orthoplane shows subepithelial glands and vessels (circle).
An en face XZ orthoplane at a depth of 175 μm, corresponding to the epithelial layer, was featureless and unremarkable aside from hypointense artifacts from mucus on the probe. Cross-sectional XY and YZ orthoplanes showed well-defined layered architecture. The epithelium, lamina propria, muscularis mucosa, submucosa, and muscularis propria were distinct layers with alternating hypo- and hyperintensity. The epithelium was devoid of glands or vessels, while small (< 100 μm) submucosal structures possibly corresponding to deep esophageal glands were discerned. Complete en face and cross-sectional flythroughs are shown in Video 1.
The patient with nonablated Barrett’s esophagus was 87 years old with Barrett’s esophagus islands near the gastroesophageal junction (GEJ). The patient had been diagnosed with short-segment Barrett’s esophagus in 1991 and had been on proton pump inhibitors (PPIs) for over 15 years. Conventional endoscopy of the distal esophagus showed discrete regions of salmon-pink mucosa consistent with Barrett’s esophagus islands. Fig. 3 shows 3D-OCT orthoplanes from a volumetric dataset spanning the GEJ. At a depth of 345 μm, an en face orthoplane illustrated clear delineation between gastric mucosa on the left (distal) and esophageal mucosa on the right (proximal). Atypical glands with ovular cross-sections and ~150-μm lengths (range 90–500 μm), consistent with Barrett’s esophagus, were observed proximal to the GEJ. Barrett’s esophagus regions were surrounded by normal squamous mucosa, illustrating the difficulty in detecting small glandular islands with 2D-OCT or random biopsy.
Fig. 3.
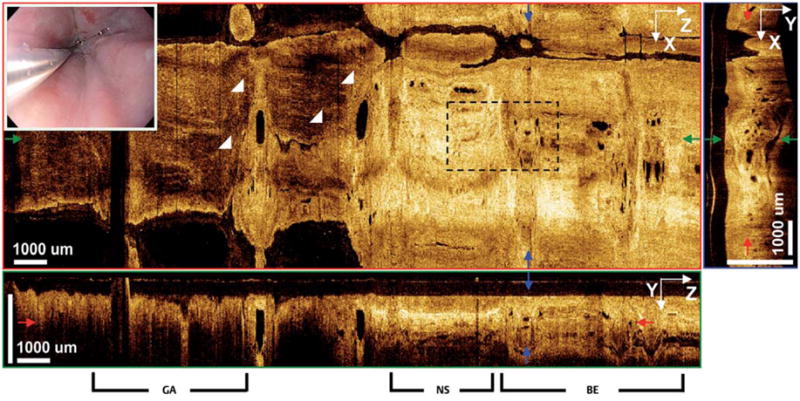
3D-OCT orthoplanes of Barrett’s esophagus glands near the gastroesophageal junction (GEJ) in a patient with no ablation. The en face XZ or-thoplane at 345 μm depth shows the GEJ (triangles) and irregular glandular features. Dashed box indicates the region of interest shown in Fig. 4. The cross-sectional YZ orthoplane shows gastric (GA), normal squamous (NS), and Barrett’s esophagus (BE) regions. The cross-sectional XY orthoplane shows Barrett’s esophagus glands. Inset A video endoscopy image.
Cross-sectional orthoplanes revealed clear differences in layered architecture between gastric, normal squamous, and Barrett’s esophagus regions. A YZ orthoplane showed normal gastric mucosa with a vertical pit pattern and reduced light penetration, consistent with previous 2D-OCT studies [6], as well as regions of normal squamous and Barrett’s esophagus. An XY orthoplane showed distortion of layered architecture and abnormal glandular features in the Barrett’s esophagus regions. Fig. 4 shows magnified orthoplanes in a region spanning normal squamous mucosa and a Barrett’s esophagus island.
Fig. 4.
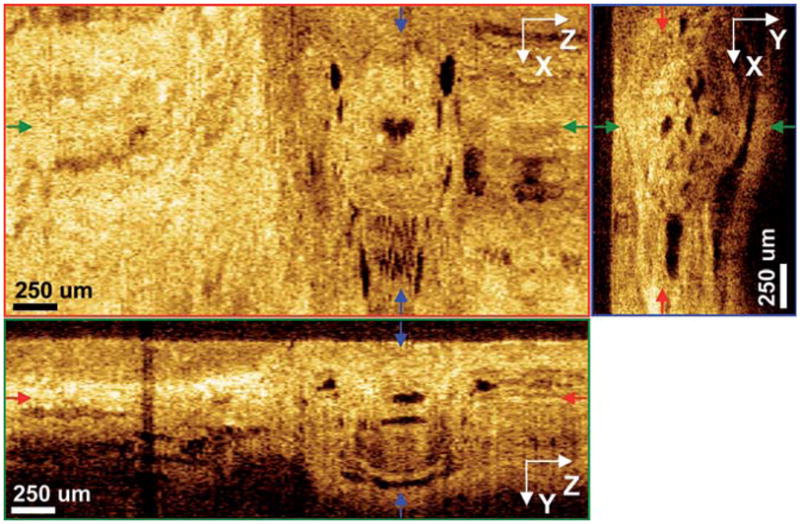
Region of interest shown as dashed box in Fig. 3. Magnified orthoplanes of Barrett’s esophagus glands, showing transition from normal squamous mucosa to Barrett’s esophagus in a patient with no radiofrequency ablation (RFA).
Densely packed Barrett’s esophagus glands beneath 300–500 μm of superficial tissue were discernible. Biopsy findings from the same area (Fig. 5) showed good correlation with 3D-OCT findings, with numerous glands and distorted layered architecture visible.
Fig. 5.
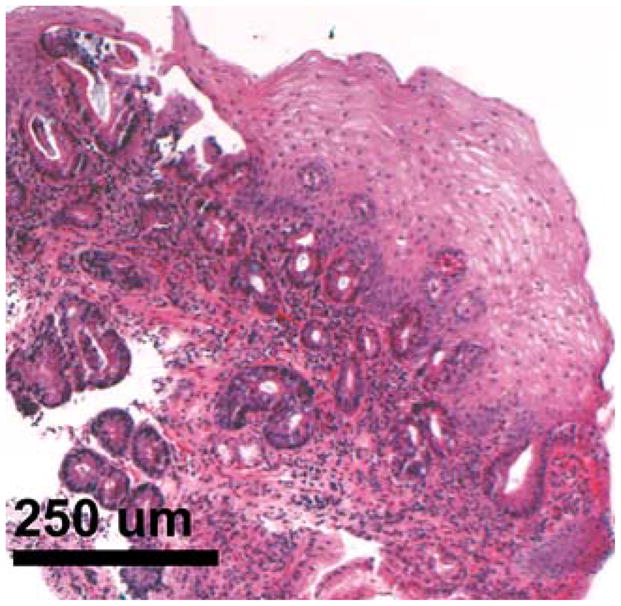
Biopsy specimen from a Barrett’s esophagus island in the same area imaged with 3D-OCT in Fig. 4. A good correlation with cross-sectional 3D-OCT orthoplanes is observed.
Complete en face and cross-sectional flythroughs are shown in Video 2.
The patient with RFA-treated Barrett’s esophagus was 72 years old with long-segment Barrett’s esophagus first diagnosed in 1996. The patient had received three RFA treatments, the most recent being 9 months prior to 3D-OCT imaging. Conventional endoscopy of the distal esophagus showed a normal GEJ with no evidence of residual or buried Barrett’s esophagus. Fig. 6 shows 3D-OCT orthoplanes taken from a volumetric dataset spanning the GEJ
Fig. 6.
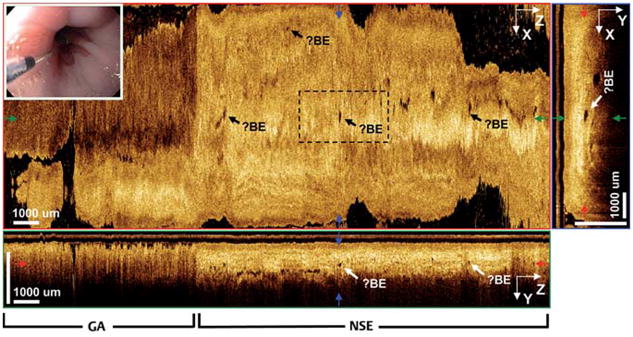
3D-OCT orthoplanes of distal esophagus previously treated with radiofrequency ablation (RFA). The en face XZ orthoplane of the epithelium at 390 μm tissue depth shows scattered, buried glands (?Barrett’s esophagus [?BE]) beneath neo-squamous epithelium. The cross-sectional YZ or-thoplane shows gastric (GA) and neosquamous epithelium (NSE) regions. The cross-sectional XY orthoplane shows scattered glands buried 350 –400 μm beneath the tissue surface. Inset Video endoscopy image.
An en face orthoplane at a depth of 390 μm showed clear delineation between gastric mucosa on the left (distal) and esophageal mucosa on the right (proximal), and was remarkable for scattered glandular structures of a size and shape consistent with Barrett’s esophagus glands. Cross-sectional XY and YZ orthoplanes showed deeply buried glands beneath ~350 μm (range 300–500 μm) of comparatively normal epithelium and lamina propria. Normal epithelium on top of glands suggests that this is neosquamous epithelium which has regenerated following RFA. Biopsy specimens from this area contained gastric mucosa and GEJ tissue with no evidence of buried Barrett’s esophagus glands. This is not surprising given the scattered and sparse nature of the glands visible with 3D-OCT. Complete en face and cross-sectional fly-throughs are shown in Video 3.
Discussion
3D-OCT may enhance the assessment of Barrett’s esophagus patients prior to and following ablative therapies such as RFA, by enabling comprehensive imaging of glandular microstructure over a surface area of 200 mm2 with a 1.5-mm imaging depth. One pinch biopsy specimen, by comparison, samples a 4 mm2 surface area and penetrates < 1 mm. The increased analysis volume of 3D-OCT may be used to guide re-ablation or re-biopsy, with improved detection of buried glands or neosquamous epithelium following therapy and with reduced histology processing loads. Datasets can be acquired in 20 seconds, suggesting the possibility of volumetric assessment without histology processing. With 3D-OCT endomicroscopy, en face tissue sections can be visualized at arbitrary depths to determine the density and location of untreated, residual, or buried Barrett’s esophagus glands and neosquamous epithelium. Cross-sectional image slices can be precisely aligned to en face features or anatomic landmarks such as the GEJ, enabling quantification of gland size and location for possible use in treatment planning. In comparison with confocal endoscopic microscopy, 3D-OCT provides fields of view that are orders of magnitude larger, with increased imaging depths at the expense of decreased transverse resolution. In the future, 3D-OCT may be used to guide biopsy for enhanced detection of buried Barrett’s esophagus glands or, ultimately, to assist in directing re-treatment. This work was supported by NIH grant R01-CA75289-13 (JGF) and AFOSR contracts FA-9550-(JGF)07-1-0014 (JGF) and FA 9550-07-1-0101.
Supplementary Material
En face and cross-sectional flythroughs corresponding to Fig. 2: three-dimensional optical coherence tomography (3D-OCT) findings in normal mid-esophagus.
En face and cross-sectional flythroughs corresponding to Fig. 4: three-dimensional optical coherence tomography (3D-OCT) findings in Barrett’s esophagus with no radiofrequency ablation (RFA).
En face and cross-sectional flythroughs corresponding to Fig. 6: 3D-OCT findings in a patient with Barrett’s esophagus, after treatment with radiofrequency ablation (RFA).
Footnotes
Competing interests: JGF receives royalties from intellectual property owned by MIT and licensed to Carl Zeiss Meditech, Inc. and Lightlab Imaging. JGF is a scientific advisor to and has stock options in Optovue.
References
- 1.Odze RD, Lauwers GY. Histopathology of Barrett’s esophagus after ablation and endoscopic mucosal resection therapy. Endoscopy. 2008;40:1008–1015. doi: 10.1055/s-0028-1103416. [DOI] [PubMed] [Google Scholar]
- 2.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suter MJ, Vakoc BJ, Yachimski PS, et al. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc. 2008;68:745–753. doi: 10.1016/j.gie.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler DC, Zhou C, Tsai T-H, et al. Three-dimensional endomicroscopy of the human colon using optical coherence tomography. Optics Express. 2009;17:784–796. doi: 10.1364/oe.17.000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isenberg G, Sivak MV, Chak A, et al. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: a prospective, double-blinded study. Gastrointest Endosc. 2005;62:825–831. doi: 10.1016/j.gie.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Aguirre AD, Hsiung PL, et al. Ultrahigh resolution optical coherence tomography of Barrett’s esophagus: preliminary descriptive clinical study correlating images with histology. Endoscopy. 2007;39:599–605. doi: 10.1055/s-2007-966648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
En face and cross-sectional flythroughs corresponding to Fig. 2: three-dimensional optical coherence tomography (3D-OCT) findings in normal mid-esophagus.
En face and cross-sectional flythroughs corresponding to Fig. 4: three-dimensional optical coherence tomography (3D-OCT) findings in Barrett’s esophagus with no radiofrequency ablation (RFA).
En face and cross-sectional flythroughs corresponding to Fig. 6: 3D-OCT findings in a patient with Barrett’s esophagus, after treatment with radiofrequency ablation (RFA).


