SUMMARY
Insomnia afflicts many individuals, but particularly those in chronic methadone treatment. Studies examining sleep deprivation (SD) have begun to identify sleep restoration processes involving brain bioenergetics. The technique [31]P magnetic resonance spectroscopy (MRS) can measure brain changes in the high-energy phosphates: alpha-, beta-, and gamma-nucleoside triphosphate (NTP). In the present study, 21 methadone-maintained (MM) and 16 control participants underwent baseline (BL), SD (40 wakeful hrs), recovery1 (RE1), and recovery2 (RE2) study nights. Polysomnographic sleep was recorded each night and [31]P MRS brain scanning conducted each morning using a 4T MR scanner (dual-tuned proton/phosphorus headcoil). Interestingly, increases in total sleep time (TST) and sleep efficiency index (SEI) commonly associated with RE sleep were not apparent in MM participants. Analysis of methadone treatment duration revealed that the lack of RE sleep increases in TST and SEI were primarily exhibited by short-term MM participants (methadone<12 months), while RE sleep in long-term MM (methadone>12 months) participants was more comparable to control participants. Slow wave sleep increased during RE1, but there was no difference between MM and control participants. Spectral power analysis revealed that compared to control participants; MM participants had greater delta, theta, and alpha spectral power during BL and RE sleep. [31]P MRS revealed that elevations in brain beta-NTP (a direct measure of ATP) following RE sleep were greater in MM compared to control participants. Results suggest that differences in sleep and brain chemistry during RE in MM participants may be reflective of a disruption in homeostatic sleep function.
Keywords: methadone, sleep deprivation, magnetic resonance spectroscopy, beta-NTP, sleep homeostasis, sleep restoration
1. INTRODUCTION
Due to public health concern surrounding widespread opioid abuse, a strong focus on treatment and rehabilitation has been established. The development of pharmacological therapies to address withdrawal and relapse in those seeking treatment has proven to be of great importance (Connell, 1967; Senay, 1985; Leukefeld and Tims, 1990; Ling and Wesson, 1990; Weddington, 1990; Zweben and Payte, 1990). Methadone-maintenance is widely used and a standard pharmacotherapy for treating opioid-dependent persons as it results in cross-tolerance that attenuates the effects of illicitly administered opioids (Zaks et al., 1971; Volavka et al., 1978; Doverty et al., 2001; Eugenio, 2004; Athanasos et al., 2006). Data suggests that compared to untreated opioid-dependent persons, methadone-maintained (MM) patients have higher nutritional status, fewer emergency room visits, and a reduction in violent crimes (Szpanowska-Wohn et al., 2004; Gossop et al., 2005; Friedmann et al., 2006). Elucidating the effects of methadone early in treatment and through the duration of therapy may be useful in extracting factors leading to failed treatment and relapse. One notable example is that insomnia particularly afflicts methadone patients who regularly self-report difficulties initiating and/or maintaining sleep and poor sleep quality (Oyefeso et al., 1997; Stein et al., 2004; Peles et al., 2006).
Clinical studies have noted that the chronic use of opiates including methadone results in marked disruptions in sleep (Gossop and Bradley, 1984; Oyefeso et al., 1997; Stein et al., 2004). It has been shown that a single administration of an opioid drug can alter normal sleep architecture in healthy adults (Dimsdale et al., 2007). Research examining the effects of opioid medications on sleep architecture has demonstrated some common nocturnal abnormalities with a range of opiate drugs. For example, morphine, heroin, and methadone have been shown to result in a reduction in SWS and a suppression of REM sleep (Roubicek et al., 1969; Kay, 1975; Howe et al., 1980b; Howe et al., 1980a, 1981; Pickworth et al., 1981; Staedt et al., 1996; Dimsdale et al., 2007). Although the identification of diminished SWS and REM sleep during methadone treatment has been reported by several studies, data indicates that sleep architecture abnormalities are variable during periods of methadone treatment initiation, stabilization, and long-term maintenance (Kay, 1975).
Sleep studies have established that sleep deprivation (SD) results in marked behavioral challenge evidenced by increased daytime sleepiness and task-specific functional impairment (Pilcher and Huffcutt, 1996; Van Dongen et al., 2003). Sleep electroencephalogram (EEG) studies have established that the sleep deficit associated with SD results in alterations to the following nights recovery (RE) sleep indicative of sleep homeostatic function (Tilley et al., 1987; Akerstedt et al., 2009). The prolonged wakeful period involved in total SD reliably results in a physiologic challenge to sleep homeostasis and SD has been an effective tool to study homeostatic sleep mechanisms in animal and human studies (Johnson et al., 2004; Everson et al., 2005; Zeitzer et al., 2006). The involvement of sleep restoration processes in sleep homeostasis becomes evident when an individual experiences RE sleep following SD. As expected, RE sleep from an extended wakeful period is commonly associated with reduced WASO, increased TST, and enhanced SEI (De Gennaro et al., 2002). The activity of sleep homeostatic mechanisms during RE sleep is further exemplified by increased non-rapid eye movement (NREM) slow wave sleep (SWS). In addition to the standard visual criteria used for the analysis of SWS, the examination of slow wave activity (SWA) using of spectral power analysis (SPA) allows for a quantitative measure of frequency in the delta band that does not require a specific amplitude criterion be met. SWA in sleep has been shown to be a marker of sleep homeostatic processes, as enhancements in SWA have been strongly correlated to the duration of extended wakefulness (Besset et al., 1998; Finelli et al., 2000). While SWS and SWA correlate in healthy individuals, it has been demonstrated that SWS and SWA may be uncoupled in patients with neuropsychiatric illness (Armitage et al., 1995). Collectively, the study of RE sleep following SD provides objective measures of compensatory sleep homeostatic changes in response to an acute sleep deficit. While there have been no previous studies of sleep homeostasis in MM participants, a novel study examined the effects of partial SD in alcohol-dependent participants in order to probe potential differences in sleep homeostatic function (Irwin et al., 2002). In part, the findings identified abnormal sleep physiology in alcoholic participants, reflected in diminished SWS and SWA during RE sleep when compared to age-matched healthy control participants.
Recent brain imaging studies have begun to identify the brain processes and mechanisms involved in sleep homeostatic processes and the subsequent involvement of sleep restoration function during RE sleep (Zeitzer et al., 2006; Dang-Vu et al., 2008). Examinations of the effects of SD on the brain have demonstrated changes in the function of brain bioenergetics due to increased wakefulness such as a reduction of brain glycogen stores (Kong et al., 2002) as well as a potential interaction with glucocorticoids (Gip et al., 2004). A report from our laboratory demonstrated in healthy participants that RE sleep following SD results in elevations in brain high energy phosphates such as beta–nucleoside triphosphate (beta-NTP) (Dorsey et al., 2003). These brain energy changes after RE sleep support theories that wake time activity is sustained by brain bioenergetic restoration during sleep (Ticho and Radulovacki, 1991).
Based upon consistent reports of sleep disturbances in MM patients and to ascertain a greater understanding of methadone-induced physiological changes, we were particularly interested in how SD may differentially affect MM participants compared to healthy controls. The premise of the current study was that MM participants would exhibit a greater susceptibility to disturbances in sleep architecture and continuity as a result of SD, which would be accompanied by more pronounced enhancements in brain high-energy phosphates compared to control participants. We hypothesized that the enhanced sleep homeostatic response in MM participants would be evidenced by elevated objective sleep measures of sleep efficiency and SWS in MM participants when compared to that of control participants. It was predicted that if greater increases in RE sleep measures of SWS were not evident in MM participants, that the additional examination of SWA may reflect greater delta spectral power during RE sleep in MM participants. Based upon previous brain MRS imaging findings demonstrating that SD results in RE sleep elevations of brain ATP levels, it was hypothesized that subsequent RE sleep elevations in brain ATP would be more pronounced in MM participants when compared to control participants. A critical aspect of methadone maintenance is the relationship between treatment retention/duration and positive treatment outcomes (Condelli and Dunteman, 1993; Gottheil et al., 1993; Cacciola et al., 1998; Peles et al., 2008). Clinical data and practices stress the importance of methadone stabilization early in treatment related to a number of factors, such as physiological adjustment to methadone, stabilizing methadone dosing, and providing appropriate medical and social services (Kay, 1975; Brown et al., 1982; Hoffman and Moolchan, 1994; Eap et al., 2002; King and Brooner, 2008). Based upon a potentially greater physiological instability in patients early in methadone treatment, it was also hypothesized that the effects of methadone maintenance may be a function of treatment duration, in that MM participants who are early in treatment may exhibit a greater impact of SD on sleep homeostasis when compared to MM participants who have been in methadone treatment for more than a year. The present study took a novel approach in investigating the sleep homeostatic response to SD in MM participants by examining objective EEG measures of sleep and utilizing magnetic resonance spectroscopy (MRS) brain imaging to obtain in vivo determinations of high-energy brain phosphates, such as beta-NTP in the mornings following baseline sleep, a night of SD, and two subsequent RE nights.
2. MATERIALS AND METHODS
2.1 Participants
The study participants were between the ages of 21 and 53 and were recruited via newspaper, radio, and web-based advertisements. 16 control participants and 21 MM participants completed the study. See Table 1 for participant demographics. PSG data from 3 control and 5 MM participants was not submitted to power spectral analysis (PSA) due to inadequate data or insufficient calibration. [31]P MRS scan data from 3 MM participants was excluded due to inadequate data resulting from MR scanner malfunction. Participants were excluded during initial telephone screening if they had unstable primary medical or psychiatric illness, any current Axis I psychiatric diagnosis, or history of psychotic disorders. Control participants were excluded if they met any criteria for abuse or any current use of any drugs. MM participants were included in this study if they met the DSM-IV criteria for opioid dependence, and were currently enrolled in a stable methadone maintenance program. MM participants were excluded based upon any of the following criteria: (1) diagnosis of substance dependence (other than opioids or nicotine) (2) current primary medical or psychiatric illness (3) and any contraindications to the MR scanning procedures. All participants were additionally screened for psychiatric, medical, and primary sleep disorders during an onsite-screening visit. Structured Clinical Interviews for DSM-IV (SCID) were conducted to further rule out primary psychiatric disorders. A physician obtained a detailed health and medical history and performed a physical examination and routine laboratory tests prior to selection.
Table 1. Subject Demographics.
Subject Demographics - age, sex, and drug use demographics for control and methadone-maintained subjects. Secondary analysis dichotomized methadone-maintained participants by methadone treatment duration as either short-term methadone-maintained or long-term methadone-maintained. Age, dose, methadone duration, and opiate use values are represented as mean ± SEM.
| Age (years) |
N | Dose (mg) |
Methadone Duration (months) |
Opiate Use (years) |
|
|---|---|---|---|---|---|
| Control | 34.5±2.91 | 16 total 50% male |
-- | -- | -- |
| Methadone Maintained |
40.1±2.19 | 22 total 57% male |
84.2±6.73 | 17.4±2.90 | 14.1±1.16 |
|
Short-Term Methadone |
40.8±3.07 | 11 total 55% male |
94.7±6.93 | 7.7±1.58 | 15.6±3.07 |
|
Long-Term Methadone |
39.3±3.30 | 11 total 55% male |
73.2±12.42 | 28.2±3.91 | 13.3±1.49 |
All participants who provided informed consent participated in a screening night of PSG measures to objectively rule out primary sleep disorders. Three participants were disqualified from the study due to clinically significant obstructive sleep apnea syndrome (>10 respiratory events per hour of sleep) or periodic limb movement disorder (>10 periodic leg movements per hour of sleep). Urine screens were performed during the initial screening visit and on each of the laboratory nights to ensure the absence of drug use in control participants and to verify the absence of drugs other than methadone and its respective metabolites in MM participants. Due to the high occurrence of polydrug use in MM participants, MM participants were not excluded for positive drug screens for tobacco, low-level marijuana, or past cocaine use. Six participants were excluded from the study due to the presence of other drugs in urine screens prior to sleep study nights and three participants for positive drug screens other than methadone during the study procedure.
The research protocol was reviewed and approved by the Institutional Review Board of McLean Hospital and written informed consent was obtained from all participants with a complete description of the study procedures. Participants completing segments of the experimental protocol received monetary and/or voucher redeemable at local retail shops as compensation for their voluntary participation.
2.2 Sleep Deprivation Paradigm
Following the initial screening night all participants underwent a night of baseline sleep, which was followed by a night of total sleep deprivation (SD) involving 40 total consecutive wakeful hours. Following SD, participants completed two additional nights of recovery sleep (RE). PSG recordings were collected each night excluding the SD night and between 7:00 and 8:00 each study morning the participants underwent brain phosphorous [31] P MRS scanning.
2.3 Sleep Physiologic Monitoring
Electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), and electrocardiograph (ECG) measurements were collected during screening, baseline, and RE nights. For participant screening full respiratory monitoring was applied including respiratory flow, effort, and oximetry. Electrode placement was done in accordance with standard PSG procedure (Rechtschaffen and Kales, 1968). Objective sleep measures obtained from physiological recording were defined as follows: wakefulness after sleep onset (WASO) - the amount of awake time in bed that occurred after sleep onset, sleep onset latency (SOL)- the total time accumulated from wakefulness to sleep, sleep efficiency index (SEI) - the total time asleep as a proportion of the total time in bed, total sleep time (TST) - total minutes of sleep accumulated after sleep onset, slow wave sleep (SWS) - the total minutes of SWS scored by standard scoring criteria, rapid eye movement (REM) sleep – total minutes of REM sleep scored by standard criteria.
2.4 Spectral Power Analysis (SPA)
Electroencephalogram spectral power analysis (SPA) was calculated from obtain PSG data using Somnologica™ Science (Embla®) software. Thirty-second epochs of non-rapid eye movement (NREM) sleep from stages 2, 3, and 4 obtained with a data sampling rate of 220 Hz and a 70 Hz high frequency filter from the electrode pairs C3-A2, which were submitted to SPA and absolute values were averaged across the night. Specific frequency bands were defined as: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), beta 1 (16–24 Hz), beta 2 (24–32 Hz), gamma (32–48 Hz). PSA data units were obtained for these frequency bands in microVolts2/Hz.
2.5 Brain Magnetic Resonance Spectroscopy Imaging
All MRI/[31]P-MRS imaging experiments were performed on a 4 Tesla whole-body (Varian/Unity-INOVA; Varian, Palo Alto, CA, USA), magnetic resonance scanner operating at 170.31 MHz and 68.95 MHz for proton and phosphorus measurements, respectively. A dual-frequency, transverse electromagnetic (TEM)-design volume head-coil tuned to both proton and phosphorus frequencies was used for all imaging and spectroscopy experiments (Bioengineering Inc, Minneapolis, MN, USA).
2.6 Proton MRI
High-contrast, T1-weighted sagittal images of the entire brain were first acquired using a three-dimensional, magnetization-prepared FLASH imaging sequence (3D-FLASH), allowing for clear differentiation between grey-matter and white-matter, as well as clearly delineating between the different anatomical regions of interest. The acquisition parameters for the sagittal images were: TE/TR=6.2/11.4 ms, field-of-view (FOV)=24 cm × 24 cm, readout-duration=4 ms, receive bandwidth= ±32 kHz, in-plane matrix size=256×256, in-plane resolution=0.94×0.94 mm, readout points=512, axial-plane matrix size=16, axial-plane resolution=2.5 mm sagittal, scan time = 1 minute, 15 seconds. Then, high-resolution T1-weighted images were acquired in the transverse plane, lasting 2 minutes 30 seconds each with the same 3D-FLASH imaging sequence, but instead 32 slices were collected (phase-encodes) of 4 mm nominal thickness (TE/TR=6.2/11.4 ms, field-of-view (FOV)=24 cm × 24 cm, readout-duration=4ms, receive bandwidth= ±32 kHz, in-plane matrix size=256×256, in-plane resolution=0.94×0.94 mm, readout points=512).
2.7 Phosphorus MRS
Phosphorus MRS was performed using the phosphorus channel of the dual tuned proton-phosphorus head coil. Initially, eight control participants were scanned using a three-dimensional (3D) [31]P MRS sequence for the phosphorus acquisition. It was recognized that MM participants were unable to complete the required 1-hour scan duration of the 3D [31]P MRS scan. In order to maintain valuable scan data and to maintain comparability of additional scan data, a two-dimensional (2D) [31]P MRS version of the original 3D [31]P MRS sequence was used for the remaining twenty-nine participants. Limiting the acquisition of voxels from a 2D slab, allowed for a markedly shorter 2D [31]P MRS scan duration of 9 minutes compared to the 46-minute 3D [31]P MRS sequence.
The 2D-MRSI version phase encoded over a 6cm thick excitation slab placed in the exact same mid-sagittal position as the 3 slices involved in the 3D [31]P MRS sequence. Aside from this, all other parameters were identical between the 2D- and 3D-[31]P MRS protocols, including FOV (33×33`cm), TR (500`ms), matrix (16×16, sparse sampling scheme using the same SINC-lobe-modulated, weighted-average k-space filter). Transmit/receive frequency was first centered on the PCr resonance, as measured with a global free-induction decay (FID). The 2D-CSI sequence used a reduced phase-encoding scheme based on prior work (Ponder and Twieg, 1994). This scheme allows for the inclusion of circularily-bound, reduced-point, weighted k-space acquisition, providing approximately 35% more signal-to-noise for a given scan time and effective voxel volume over conventional methods. All viable voxels from three mid-sagittal slices over the entire brain were analyzed from 3D [31]P MRS data. This was essentially equivalent to the 2D axial-plane consisting of 2×2×6 cm voxels (slices are effectively 2cm thick × 3 slices equals 6cm thick slab-of-interest). The 2D-PSF (actual voxel signal distribution) as well as signal-to-noise, were analogous between the 2D and 3D [31]P MRS acquisitions. Also, since the tip-angle (32 degrees) and TR were the same between sequences, the resultant spectra were virtually-identical between sequences when tested back-to-back on a healthy control in terms of metabolite T1-weighting. In addition to matching scan parameters, fundamental differences exist between the 2D- and 3D- [31]P MRS sequences that primarily emanate from diminutive differences in the tip angle between sequences (global square pulse for 3D vs. selective SINC pulse for 2d), leading to minute T1-weighted differences in derived peak areas. There is an inherent chemical-shift displacement artifact using 2D [31]P MRS, which could affect measures of any off-resonance metabolites due to spatial-shifts in slab excitation. To rectify these potential influences, correction-factors for each measured metabolite from an in vivo healthy control participant were derived and applied to the 3D [31]P MRS data. The resultant metabolite measures were extremely in-line with the acquired 2D [31]P MRS data and there were no significant differences for any of the MRS metabolites.
All in vivo CSI/image data was processed and viewed using Varian Nuclear Magnetic Resonance (VNMR) software, Version 6.1b (Varian, Palo Alto, CA, USA) and software designed and written on site. Prior to Fast Fourier Transform (FFT) reconstruction to spatially resolve the CSI spectra, the collected k-space data was centered in a 16 × 16 square matrix. Each time-domain FID was then zero-filled out to 2048 complex points and left-shifted five points to remove residual bone/rigid membrane signal. Using the MRI images, the 2D-CSI data grid was shifted in the x and y dimensions in order to position the sampling grid such that it was centered inside the brain according to anatomical landmarks. The peak areas of the following metabolites: phosphoethanolamine (Pe), phosphocholine (PCh), inorganic phosphate (Pi), glycerophosphoethanolamine (GPE) and glycerophosphocholine (GPC), phosphocreatine (PCr), and three peaks for adenosine triphosphate (alpha-, gamma-, and beta-NTP) (Figure1), were fitted to an in vivo spectral model through the use of a non-linear, iterative (Potwarka et al., 1999; Jensen et al., 2002). The fitting routine is based on a Marquardt-Levenberg algorithm, utilizing prior spectral knowledge for the relative amplitudes, linewidths, lineshapes, peak positions and J-coupling constants to model the in vivo [31]P brain spectrum. Each fitted spectral peak area was expressed as a ratio to the total [31]P signal per voxel. The fitted metabolite amplitudes are not T2-weighted since the fitting algorithm back-extrapolates to time zero.
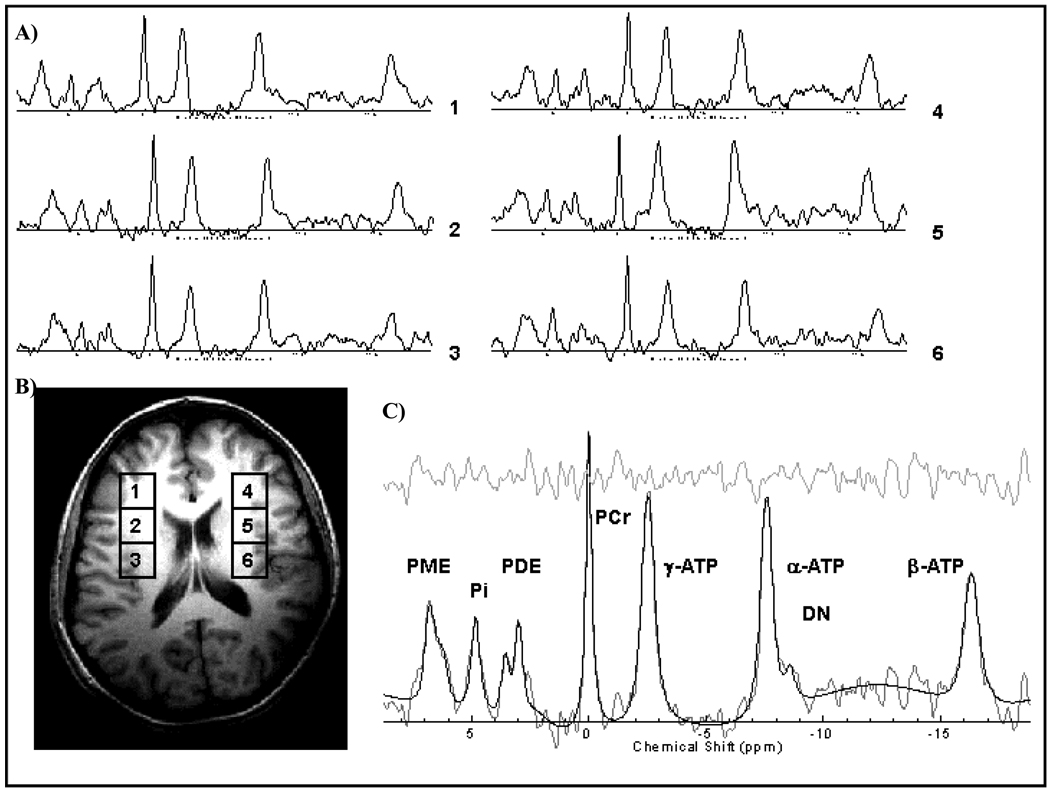
Figure 1.
A) Numbered 1–6 phosphorus [31]P MRS spectrum from example voxels on the corresponding mid-axial slice. B) Representative mid-axial slice indicating the location of [31]P MRS acquisition with voxel locations 1–6 corresponding to spectral output. C) Representative [31]P MRS spectrum indicating labeled phosphorus containing peaks, modeled fit and residual. All spectra are displayed with 15 Hz exponential filtering for display.
2.8 Statistical Analysis
In order to identify differences under baseline conditions, PSG, SPA, and [31]P MRS data measures were subjected to ANOVA analysis. All obtained data were initially analyzed for effects of age and sex in order to identify potential treatment interactions. Significant interactions with age or sex with methadone treatment were not detected for any obtained dependent measures. Statistical linear mixed model analyses were conducted to determine effects of treatment for PSG and SPA data obtained on baseline, RE1, and RE2 study nights and [31]P MRS data collected the mornings following baseline, SD, RE1, and RE2 study nights. When appropriate, post-hoc analyses of pair-wise comparisons were performed using Fischer’s LSD analysis. Alpha was set to p<0.05 for all statistical testing.
Although the identification of treatment effects between control and MM participant data was of primary interest, additional treatment variables were considered. Based upon the possibility that significant treatment differences between MM and control participants may be further explained by either the duration of methadone treatment, the duration of opioid use, and/or methadone dose were submitted as additional variables for further analysis for all PSG, SPA, and [31]P MRS measures. To determine effects of the duration of methadone treatment, participant data was dichotomized as short-term MM (methadone<12 months) and long-term MM (methadone≥12 months) or the data was examined as a continuous covariate by the number of months enrolled in methadone treatment. Similarly, potential effects of methadone dose were determined with participant data dichotomized as low-dose MM (daily dose < 80 mg methadone) and high-dose MM (daily dose ≥ 80 mg methadone) or the data was examined as a continuous covariate by mg dosage of methadone. The effects of the duration of opioid use were examined as a continuous covariate.
3. RESULTS
3.1 Polysomnogram (PSG) Sleep Measures
3.1.1 Slow Wave Sleep (SWS)
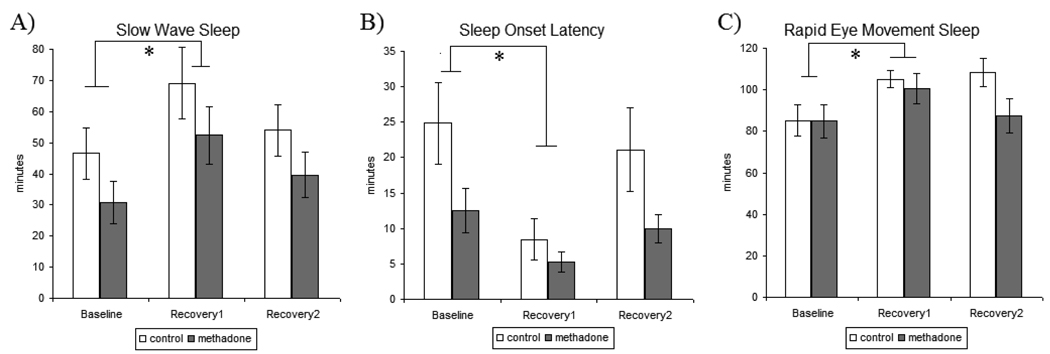
The overall amount of SWS during baseline sleep was not significantly different between treatment groups as both control and MM participants had comparable total SWS time. Statistical analysis across the sleep deprivation paradigm identified strong effect of study day [F[1,36]=15.542, p<0.0001], indicative of a reliable elevation in SWS observed across all participants during RE1 sleep compared to SWS during baseline sleep (Figure 2A). Despite lower mean values of SWS across sleep nights in MM participants compared to control participants, there were no significant treatment differences in SWS with respect to study day. Additionally, there were no significant treatment effects in the secondary analysis that differentiated effects by duration of methadone treatment, methadone treatment dose, or duration of opioid use on SWS.
Figure 2.
Polysomnogram sleep measures recorded for baseline, recovery1, and recovery2 sleep nights in control and methadone maintained participants. A) Slow Wave Sleep (SWS)-participants exhibited a reliable increase in SWS during recovery1 sleep compared to baseline sleep B) Sleep Onset Latency (SOL)- participants exhibited a marked decrease SOL during recovery1 sleep compared to baseline sleep C) Rapid Eye Movement (REM) Sleep- participants exhibited a reliable increase in REM during recovery1 sleep compared to baseline sleep. All values are represented as mean ± SEM with an alpha of p<0.05. * denotes significantly different than baseline sleep data.
3.1.2 Sleep Onset Latency (SOL)
There was a significant main effect of treatment [F[1,36]=4.761, p<0.05], as MM participants exhibited decreased overall amounts of SOL during baseline sleep when compared to that of control participants. Similarly, statistical analysis across the sleep deprivation paradigm identified a statistically significant main effect of treatment [F[1,36]=5.268, p<0.05], as MM participants had lower mean SOL across sleep nights when compared to control participants. Statistical analysis across the sleep deprivation paradigm identified a significant effect of study day [F[1,36]=23.514 p<0.0001], exemplifying a reliable reduction in SOL across all participants during RE1 sleep compared to baseline sleep (Figure2B). Additionally, there were no significant treatment effects in the secondary analysis that differentiated effects by methadone treatment, methadone treatment dose, or duration of opioid use on SOL.
3.1.3 Rapid Eye Movement (REM) Sleep
There were no significant treatment differences in baseline REM sleep and although MM participants had lower mean REM across sleep nights compared to control participants analysis of treatment effects across the sleep deprivation paradigm were not statistically significant. Statistical analysis across the sleep deprivation paradigm identified strong effect of study day [F[1,36]=10.64, p<0.01], exemplifying increases in REM sleep across participants during RE1 sleep compared to baseline sleep (Figure2C). Additionally, there were no significant treatment effects in the secondary analyses that differentiated effects by methadone treatment, methadone treatment dose, or duration of opioid use on REM sleep. Additionally, there were no treatment differences in REM latency across SD study nights.
3.1.4 Total Sleep Time (TST)
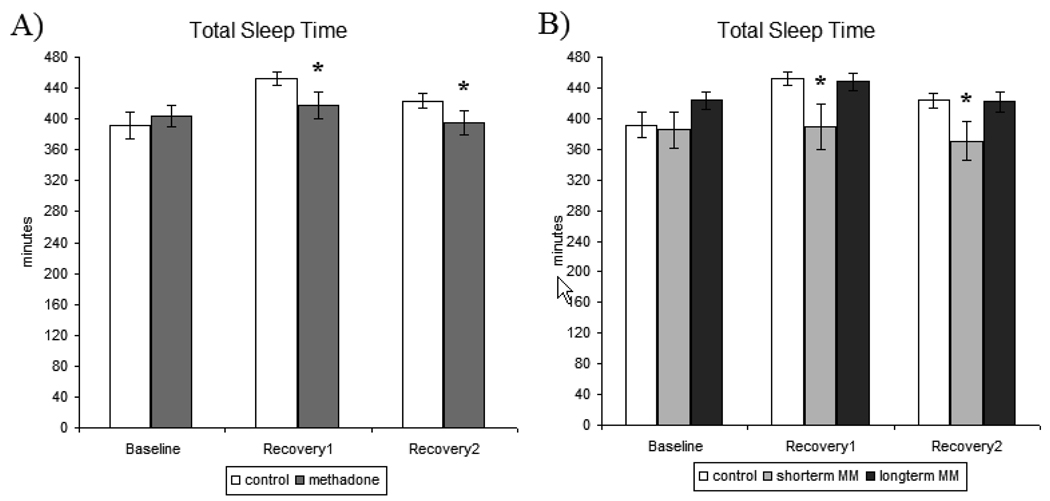
There were no significant treatment effects in baseline sleep TST. Statistical analysis across the sleep deprivation paradigm identified a statistically significant treatment x sleep night interaction [F[1,36]=7.922, p<0.01] (Figure 3A). Post hoc analysis revealed that there was a significant difference in TST between treatment groups during both RE1 [p<0.05] and RE2 [p<0.05] sleep nights, as MM participants exhibited significantly reduced TST compared to control participants.
Figure 3.
A) Total sleep time (TST)- measures recorded for baseline, recovery1, and recovery2 sleep nights in control and methadone-maintained participants. B) Total sleep time (TST)-secondary analysis of methadone treatment duration for baseline, recovery1, and recovery2 for control, short-term methadone-maintained, and long-term methadone-maintained participants. All values are represented as mean ± SEM with an alpha of p<0.05. * denotes significantly different from control participant data.
Secondary analysis of treatment effects specific to the participants’ duration of methadone treatment revealed a significant main effect of treatment duration [F[2,31]=5.937, p<0.05] and a treatment x study day interaction [F[2,35]=3.910, p<0.05] (Figure 3B). Post hoc revealed that the original treatment difference (control vs. MM) was primarily driven by increases in TST in control and long-term MM participants during RE1 night, that were not exhibited by short-term MM participants [p<0.5]. There were no significant treatment effects in the secondary analysis that differentiated effects by methadone treatment dose or duration of opioid use on TST data.
3.1.5 Wake After Sleep Onset (WASO)
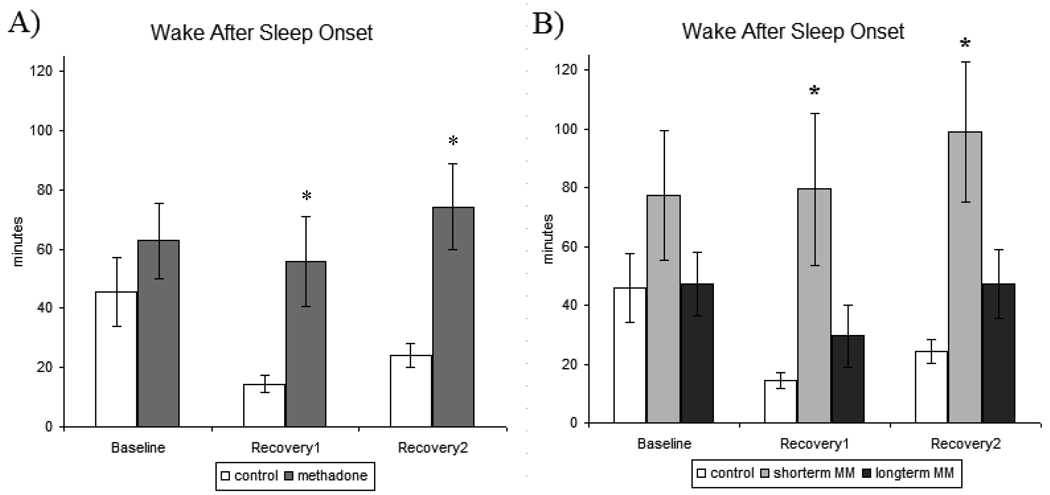
There were no significant effects in WASO during baseline sleep. Statistical analysis across the sleep deprivation paradigm identified a significant main effect of treatment [F[1,34]=4.386, p<0.05], as WASO amounts were greater in MM participants across sleep nights when compared to control participants (Figure 4A). There was a significant treatment x sleep night interaction [F[1,36]=5.239, p<0.05], which emanated from significantly lower WASO in control participants when compared to MM participants on RE1 [p<0.05] and RE2 [p<0.05].
Figure 4.
A) Wake after sleep onset (WASO)- measures recorded for baseline, recovery1, and recovery2 sleep nights in control and methadone-maintained participants. B) Wake after sleep onset (WASO)- secondary analysis of methadone treatment duration for baseline, recovery1, and recovery2 for control, short-term methadone-maintained, and long-term methadone-maintained participants. All values are represented as mean ± SEM with an alpha of p<0.05. * denotes significantly different from control participant data.
Secondary analysis of treatment effects specific to the participants’ duration of methadone treatment revealed marked differences in WASO between short-term and long-term MM participants (Figure 4B). When treatment groups were specified as control vs. short-term MM vs. long-term MM, analysis revealed a significant main effect of treatment [F[2,32]=5.122, p<0.05] as well as a significant treatment x study day interaction [F[2,36=6.983, p<0.01]. Post hoc analysis determined that these effects were driven by significantly lower WASO in both control and long-term MM participants in comparison to WASO from short-term MM participants on both RE1 [p<0.05] and RE2 [p<0.05] nights. There were no significant treatment effects in the secondary analysis that differentiated effects by methadone treatment dose or duration of opioid use on WASO data.
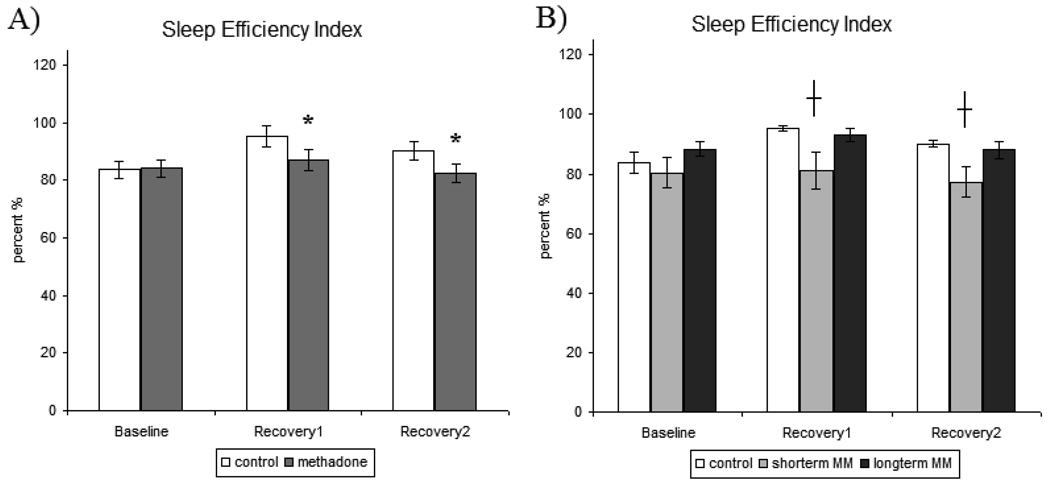
3.1.6 Sleep Efficiency Index (SEI)
There were no significant effects of treatment in baseline sleep SEI. There was a significant treatment x sleep night interaction [F[1,32]=7.390, p<0.01]. Post hoc analysis revealed that there was a significant difference in SEI between treatment groups during RE1 [p<0.05] and RE2 [p<0.05] sleep nights, as MM participants exhibited significantly reduced SEI compared to control participants (Figure 5A).
Figure 5.
A) Sleep efficiency Index (SEI)- measures recorded for baseline, recovery1, and recovery2 sleep nights in control and methadone-maintained participants. B) Sleep efficiency index (SEI)-secondary analysis of methadone treatment duration for baseline, recovery1, and recovery2 for control, short-term methadone-maintained, and long-term methadone-maintained participants. All values are represented as mean ± SEM with an alpha of p<0.05. * denotes significantly different from control participant data and denotes significantly different from control and long-term MM participant data.
Secondary analysis of treatment effects specific to the participants’ duration of methadone treatment identified a significant main effect of treatment [F[2,32]=4.166, p<0.05] and a treatment x study day interaction [F[2,36]=5.941, p<0.05]. Post hoc analyses identified statistically significant increases in SEI during RE1 and RE2 nights in control [p<0.05] and long-term MM [p<0.05] participants were not exhibited by short-term MM participants (Figure 5B). There were no significant treatment effects in the secondary analysis that differentiated effects by methadone treatment dose or duration of opioid use on SEI data.
3.2 Spectral Power Analysis (SPA)
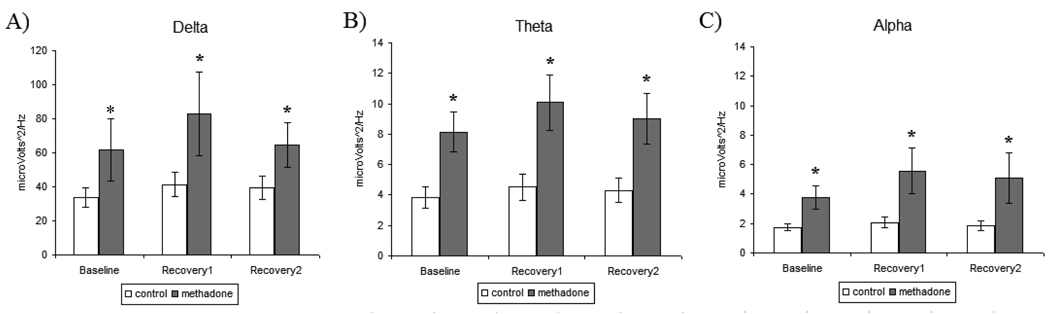
3.2.1 Delta
Analysis of Power during baseline sleep revealed a significant main effect of treatment [F[1,26]=5.61, p<0.05], as MM participants exhibited elevated delta power during baseline sleep when compared to control participants (Figure 6A). Statistical analysis across the sleep deprivation paradigm identified a significant main effect of treatment [F[1,28]=7.28, p<0.01], illustrating that Delta power levels were elevated in MM participants at baseline [p<0.05] and continuing during recovery sleep [p<0.05], when compared to control participants. There were no significant treatment effects in the secondary analysis that differentiated effects by duration of methadone treatment, methadone treatment dose, or duration of opioid use on delta power.
Figure 6.
Spectral power analysis (SPA) of polysomnogram sleep measures for baseline, recovery1, and recovery2 sleep nights in control and methadone maintained participants. A) Delta (0.5–4Hz) B) Theta (4–8Hz) C) Alpha (8–12Hz). All values are represented as mean ± SEM with an alpha of p<0.05. * denotes significantly different than control participant SPA data.
3.2.2 Theta
Analysis of theta during baseline sleep revealed a statistically significant main effect of treatment [F[1,26]=11.66, p<0.01], as MM participants exhibited elevated theta during baseline sleep when compared to control participants (Figure 6B). Statistical analysis across the sleep deprivation paradigm identified a significant main effect of treatment [F[1,28]=12.24, p<0.01], illustrating that theta levels were elevated in MM participants at baseline [p<0.01] and continuing during recovery sleep [p<0.01], when compared to control participants. There were no significant treatment effects in the secondary analysis that differentiated effects by duration of methadone treatment, methadone treatment dose, or duration of opioid use on theta measures.
3.2.3 Alpha
Analysis of alpha power during baseline sleep revealed a statistically significant main effect of treatment [F[1,26]=9.38, p<0.01], as MM participants exhibited elevated alpha during baseline sleep when compared to control participants (Figure 6C). Statistical analysis across the sleep deprivation paradigm identified a significant main effect of treatment [F[1,28]=7.61, p<0.05], illustrating that alpha levels were elevated in MM participants at baseline [p<0.05] and continuing during recovery sleep [p<0.05], compared to control participants. There were no significant treatment effects in the secondary analysis that differentiated effects by duration of methadone treatment, methadone treatment dose, or duration of opioid use on alpha power.
3.3 Brain Phosphorus [31]P Magnetic Resonance Spectroscopy
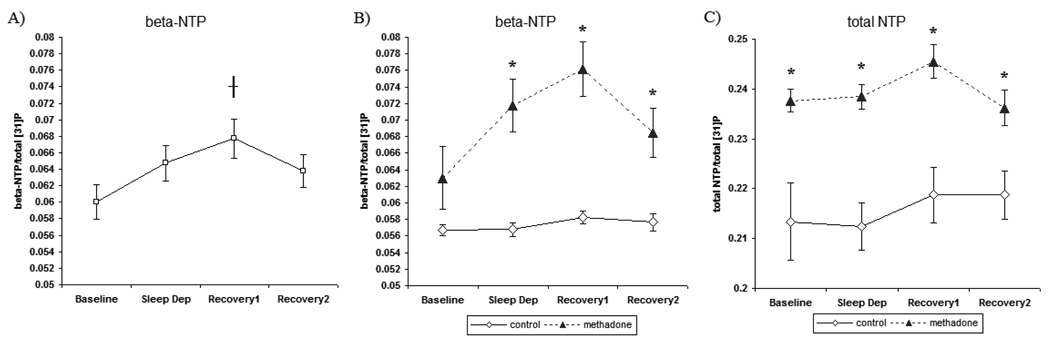
3.3.1 beta-nucleoside triphosphate (beta-NTP)
There were no treatment differences in baseline levels of brain beta-NTP between MM and control participants. Analysis of changes in beta-NTP over the course of the sleep deprivation paradigm revealed notable differences with respect to treatment. There was a significant main effect of treatment [F[1, 32]=26.19, p<0.001], as MM participants had greater levels of beta-NTP compared to levels in control participants when collapsed over the study days. There was a significant effect of study day [F[1, 68]=11.872, p<0.01] (Figure 7A). Similar to previous findings (Dorsey et al., 2003), total sleep deprivation enhanced brain beta-NTP levels which was most evident in [31]P MRS following a night of recovery sleep. Post hoc analysis verified significantly greater levels of beta-NTP following recovery sleep when compared to baseline beta-NTP levels [p<0.05]. These results provide confirmation of a build up of brain beta-NTP following recovery sleep from sleep deprivation, indicating marked changes in brain ATP as a result of sleep deprivation.
Figure 7.
Global brain phosphorus [31]P MRS measurements recorded the mornings following baseline, sleep deprivation (Sleep Dep), recovery1, and recovery2 sleep nights. A) Brain beta-NTP across study nights in all participants. B) beta-NTP- in control and methadone-maintained subjects C) total-NTP- in control and methadone-maintained subjects. Values are represented as mean ± SEM with an alpha of p<0.05. *denotes significant difference between beta-NTP levels following recovery1 when compared to baseline. * denotes significantly different than controls subjects.
Most notably, there was a significant interaction of treatment x study day [F[1,68]=27.925, p<0.001], identifying significant differences in brain beta-NTP levels between control and MM participants over the course of the sleep deprivation paradigm (Figure 7B). Post hoc analyses determined that brain beta-NTP levels following SD [p<0.05], RE1 [p<0.05], and RE2 [p<0.05] nights were significantly greater in MM participants than in control participants. These data demonstrate that elevations in beta-NTP levels were greatest in MM participants following RE1 sleep, indicating a differential influence of sleep deprivation on metabolic processes involving the regulation and/or restoration of brain ATP in these drug-dependent participants.
There were significant treatment and a treatment x study day interaction effects in the secondary analysis of duration of methadone treatment on baseline beta-NTP levels and over the course of the sleep deprivation paradigm. Despite these effects, post hoc analysis revealed that beta-NTP levels in short-term MM and long-term MM participants were different than control participants, but there were no differences between beta-NTP levels from short-term and long-term MM participants. Similarly, secondary examination of treatment effects on beta-NTP levels emanating from participant differences in methadone dose or the duration of opioid dependence did not reveal informative significant effects when each was considered a continuous covariate within the MM group or when methadone dose was dichotomized as high or low dose MM.
3.3.2 alpha-, gamma-, and total nucleoside triphosphate (NTP)
Although, changes in brain beta-NTP were of primary interest in reflecting changes in brain ATP with sleep deprivation and recovery sleep, results are reported for alpha-NTP, gamma-NTP, total-NTP, PCr, and Pi. There were no significant treatment differences in brain alpha- or gamma-NTP levels between MM and control participants following a normal baseline night of sleep. Examination of baseline levels of total-NTP revealed that brain total NTP levels were elevated in MM participants when compared to controls participants [F[1,34]=10.31, p<0.01].
Analysis over the course of the sleep deprivation paradigm revealed notable differences with respect to gamma- and total NTP levels. There were no significant differences detected in brain alpha-NTP levels over the course of the sleep deprivation paradigm (Table 2). There was a main effect of treatment [F[1,32]=8.12, p<0.01], as brain gamma-NTP levels were significantly greater in MM participants compared to control participants collapsed over sleep nights. Analysis of brain total NTP levels revealed a significant main effect of treatment [F[1,32]=30.81, p<0.0001], as total NTP levels were significantly greater in MM participants compared to control participants collapsed over sleep nights (Figure 7C).
Table 2. Global Brain levels of alpha-NTP, gamma-NTP, Pi, and PCr.
Results summary of alpha-nucleoside triphosphate (NTP), gamma-NTP, inorganic phosphate (Pi), and phosphocreatine (PCr), obtained from global brain phosphorus [31]P MRS measurements. The data was recorded the mornings following baseline, sleep deprivation, recovery1, and recovery2 study nights in control (n=16) and methadone-maintained (n=19) participants. All values are a mean metabolite ratio × 10−2 of the respective metabolite/total 31P ± SEM with an alpha of p<0.05.
| Baseline | Sleep Deprivation |
Recovery1 | Recovery2 | ||
|---|---|---|---|---|---|
| alpha-NTP | control | 10.14 ± 3.2 | 6.88 ± 0.1 | 6.86 ± 0.1 | 6.96 ± 0.1 |
| methadone | 7.57 ± 0.2 | 10.9 ± 3.0 | 7.97 ± 0.1 | 7.71 ± 0.1 | |
| gamma-NTP | control | 8.70 ± 0.1 | 8.78 ± 0.1 | 8.98 ± 0.1 | 8.93 ± 0.1 |
| methadone | 9.59 ± 0.4 | 8.95 ± 0.1 | 9.12 ± 0.1 | 9.11 ± 0.1 | |
| Pi | control | 7.84 ± 0.1 | 7.67 ± 0.1 | 7.66 ± 0.1 | 7.63 ± 0.1 |
| methadone | 7.40 ± 0.2 | 7.01 ± 0.2 | 6.77 ± 0.2 | 7.21 ± 0.2 | |
| PCr | control | 15.75± 0.2 | 15.92 ± 0.3 | 15.87 ± 0.2 | 15.71 ± 0.3 |
| methadone | 16.29± 0.4 | 14.97 ± 0.2* | 14.98 ± 0.2* | 15.54 ± 0.4 | |
denotes significantly different than controls participants.
Additionally, there were no significant effects in the secondary analysis of duration of methadone treatment, methadone treatment dose, or duration of opioid use on alpha-NTP levels. There were statistically significant treatment and/or treatment x study day interaction effects on gamma- and total NTP in the secondary analyses of duration of methadone treatment, methadone dose, or the duration of opioid use, but these treatment effects did not differentiate effects between either short-term MM vs. long-term MM or high vs. low dose MM. There were no significant effects when treatment variables were considered a continuous covariate within the MM group.
3.3.3 Phosphocreatine (PCr) and inorganic phosphate (Pi)
There were no treatment differences in baseline levels of brain phosphocreatine (PCr) or inorganic phosphate (Pi) between MM and control participants. There was significantly reduced baseline Pi levels [F[1,34]=4.39, p<0.05]in MM participants compared to that of control participants.
Brain PCr levels markedly increased over the course of the sleep deprivation, which was reflected in a significant main effect of study visit [F[1,68]=5.18, p<0.05]. Post hoc analysis revealed that brain PCr levels were significantly elevated following recovery1 sleep night when compared to after baseline sleep night [p<0.05]. There was a significant interaction of treatment x study day [F[1,68]=6.62, p<0.01] as brain PCr levels following recovery1 sleep in MM participants were greater than that of control participants [p<0.05] (Table 2). In contrast, brain Pi levels markedly decreased over the course of the sleep deprivation and there was a significant main effect of study visit [F[1,68]=7.98, p<0.01]. Post hoc analysis revealed that brain Pi levels following recovery sleep in MM participants were greater than that of control participants [p<0.05] (Table 2). There was a main effect of treatment across study days [F[1,32]=17.52, p<0.001] as MM participants had lower Pi levels when compared to control participants.
There were statistically significant treatment and/or treatment x study day interaction effects on Pi and PCR in the secondary analyses of duration of methadone treatment, methadone dose, or the duration of opioid use, but these treatment effects did not differentiate effects between either short-term MM vs. long-term MM or high vs. low dose MM. There were no significant effects when treatment variables were considered a continuous covariate within the MM group.
4. DISCUSSION
The current study aimed to further understand sleep disturbances in MM participants by examining the response to 40 hours of total SD followed by two RE sleep nights while measuring neurophysiologic PSG and [31]P MRS neuroimaging. The present investigation is the first study of SD in MM participants to investigate the integrity of sleep homeostatic and brain restoration processes. We presently hypothesized that MM-participants would exhibit greater reductions in WASO and enhancements of TST and SEI during RE sleep when compared to control participants. Contrary to our predictions, although control participants exhibited the typical enhancements in RE sleep, MM participants not only did not appear to experience the expected increases in TST and SEI and reductions in WASO during RE sleep. Analyses examining potential effects of the duration of methadone treatment provided greater insight into the mediation of the treatment effect, furthermore indicating that short-term MM participants did not display the expected changes in RE sleep such as decreased WASO and greater TST and SEI, while long-term MM participants were more similar to controls. These data indicate that RE sleep is altered in MM participants, but particularly in short-term MM participants who have been enrolled in methadone treatment for less than 12 months. The RE sleep differences observed in short-term MM participants may be reflective of a greater disruptive impact of SD to RE-related sleep restoration processes when methadone patients are not yet stabilized to methadone treatment. Overall, the reduced RE sleep efficiency in short-term MM participants may result in a partial loss of the expected restorative qualities of sleep, require a longer duration of RE sleep, and contribute to greater and/or prolonged consequences to behavioral performance.
Additionally, based upon the notion that SD would potentially have a greater impact in MM participants and the strong relationship of homeostatic control and measures of SWS and SWA, it was hypothesized that MM participants would have greater levels of SWS and/or SWA during RE sleep when compared to that of control participants. Although we did not detect treatment effects in SWS across sleep nights, SWS did increase during RE sleep compared to baseline with a subsequent return to baseline becoming evident during RE2 sleep in both MM and control participants. The lack of an effect of MM on SWS is interesting considering the consistent treatment differences presently obtained and in lieu of reports implicating a primary role of SWS in sleep homeostatic and restoration processes (Horne and Staff, 1983; Dorsey et al., 1996; Dorsey et al., 2003). One interpretation may be that although a strong relationship exists between SWS and sleep restoration; other sleep processes may also contribute to restorative sleep. Alternatively, it is also plausible that examination of SWA as determined by delta SPA may better characterize differences in MM participants, otherwise unidentifiable using standard SWS criteria, due to a potential uncoupling of SWS and SWA measures in MM participants. In order to further investigate changes in restorative processes in MM participants that were not observed in SWS measures during RE sleep, we conducted power spectral analysis (PSA) to characterize delta spectral power related SWA for all study sleep nights. PSA findings demonstrated that MM participants had significantly greater delta spectral power during RE1 and RE2 sleep compared to control participants, supporting the notion that RE sleep restoration processes are elevated during methadone treatment.
In contrast to the findings of Irwin et al., which demonstrated that alcoholics have diminished plasticity in the sleep homeostatic regulation of SWS and SWA (2002), the current study found that MM participants did not display a loss in sleep homeostatic plasticity. Alternatively, MM participants exhibited RE sleep increases in SWS that were comparable to control participants and intriguingly, a hyper-responsive sleep homeostatic response in SWA. The directional disparity between alcoholic and MM participants in sleep homeostatic function may in part be driven by underlying changes in sleep related aspects of central nervous system physiology. The acute effects of alcohol have been shown to result in increases in SWS and reductions in REM sleep (Salamy and Williams, 1973; Prinz et al., 1980; Roehrs et al., 1999). Similar to sleep findings with MM, alcohol dependence has been shown to result in sleep difficulties and disruptions in sleep architecture, with the most notable findings identifying a reduction in SWS and REM sleep (Wagman et al., 1978; Gillin et al., 1990; Nicholas et al., 2002). Another comparison exists from findings of congruent alterations in sleep measures of SWS in MM participants and those observed in depression patients. A strong relationship between sleep regulation and depression has been established and interestingly, acute SD has been shown to have therapeutic effects on depressive symptomology (van den Burg and van den Hoofdakker, 1975; Reyero and Muller, 1977; Duncan et al., 1980). The therapeutic effect of SD on depressive symptoms has been shown to have a direct relationship to the occurrence of SWS recovery or plasticity during subsequent RE sleep (Reynolds et al., 1987; Nissen et al., 2001). Despite similar sleep architecture findings such as reduced SWS between alcohol dependence, depression, and methadone treatment, there are some clear differences when comparing the response to SD. The diminished SWS plasticity in response to SD in alcoholics is in contrast to the elevated SWS in response to SD observed in depressed patients. In comparison, the present findings demonstrate a differential profile of SWS/SWA plasticity in response to SD, as MM participants exhibited RE sleep plasticity in SWS comparable to that of control subjects and additionally displayed markedly enhanced SWA.
Several studies have demonstrated marked fluctuations in brain bioenergetics as a result of SD and provide support to theories that fluctuations in brain bioenergetics are likely attributable to the prolonged metabolic demands encountered with extended wakefulness (Adam and Oswald, 1977; Benington and Heller, 1995; Frank, 2006). For example, the noted depletion of brain glycogen energy stores resulting from SD may reflect prolonged neuronal energy demands depleting glycogen fuel sources (Kong et al., 2002; Gip et al., 2004). Furthermore, evidence of SD-induced elevations in brain adenosine may reflect the wakeful metabolism of brain ATP into adenosine diphosphate (ADP) and adenosine byproducts, which may underlie an enhancement of sleep pressure experienced with prolonged wakefulness (Radulovacki, 1985; Porkka-Heiskanen et al., 1997; Zeitzer et al., 2006). Collectively, these data support the notion that under normal conditions, brain glycogen driven by metabolic activity may be utilized as ATP and following ATP utilization adenosine is derived promoting sleep drive. Both a depletion of brain glycogen stores and elevated brain adenosine levels with SD implicate corresponding fluctuations in brain ATP. Subsequently, Dorsey et al., provided confirmation of SD-induced changes in brain ATP levels, specifically demonstrating a build up of brain ATP levels the morning following RE sleep (2003).
Given the previous [31]P MRS findings of SD-induced build up of brain beta-NTP following RE (Dorsey et al., 2003), the present study sought to examine changes in brain bioenergetics following SD, but aimed to investigate how the build up of brain ATP following RE sleep may be altered in MM participants. [31]P MRS is the most studied method to examine brain high-energy phosphate metabolites and it provides a direct measure of NTP levels (Renshaw et al., 2001). Beta-NTP is of primary interest as beta-NTP is unique to ATP, whereas alpha- and gamma-NTP also contribute to nucleoside diphosphates (NDP). The present study did not identify methadone maintenance-related differences in baseline levels of brain beta-NTP, which is consistent with previous findings of no differences in baseline brain NTP levels between early treatment MM participants and control participants (Silveri et al., 2004). The present study in accordance with the findings of Dorsey et al., participants of both treatment groups exhibited a significant increase in brain beta-NTP, providing confirmation that brain ATP levels fluctuate over the course of a SD paradigm, with peak ATP levels occurring following a first night of RE sleep (2003). Furthermore, the present global brain [31]P MRS results identified a significant difference in the RE1 associated elevation in brain beta-NTP in MM participants. Specifically, it was shown that the increases in beta-NTP levels following RE1 sleep were significantly larger in MM participants than those in control participants. This greater change in beta-NTP in MM participants was also significant during active SD and following the RE2 sleep night, but the largest change in beta-NTP was following RE1 sleep. Additionally, the [31]P MRS imaging results demonstrate that fluctuations in brain ATP (beta-NTP) were associated with fluctuations in brain Pi and PCr levels with SD and RE sleep. These data are consistent with previous findings that PCr is an energy carrier between sites of ATP production and utilization (Chance et al., 1988; Nishijima et al., 1989; Yoshizaki et al., 1989). Additionally, the larger brain beta-NTP increases in MM participants over the SD paradigm were inversely linked to a trend for reductions in brain Pi levels and significant reductions in PCr levels in MM participants over the course of the SD paradigm when compared to Pi and PCr levels in control participants. The reductions in PCr and Pi levels in MM participants were most evident during active SD and following RE sleep.
The current findings may result in several interpretations. These data may suggest that metabolic sleep restoration processes which occur during RE sleep are characterized by a build up of brain ATP levels in compensation for greater metabolic demands required during the prolonged wake period of SD. One perspective may be that the prolonged metabolic demands necessary to sustain wake-time function with SD initiates a sleep homeostatic process involving enhanced restoration of brain ATP following RE sleep. Following this view, the present findings of greater enhancements in brain ATP following RE in MM participants when compared control participants may reflect a greater impact of SD to sleep homeostasis. A greater impact of SD may imply a greater metabolic challenge in MM participants when compared to healthy individuals, such that the physiological demands experienced with extended wakefulness may be larger in these drug-dependent individuals. It may also be the case that the disruption to sleep homeostasis induced by SD results in sleep restoration processes which may be unable to properly regulate the brain phospholipid balance and maintain brain high-energy equilibrium.
An alternative view may be that the homeostatic response to SD is characterized by a compensatory slowing of bioenergetic metabolism. It is plausible that during SD metabolic processes are slowed down resulting in a build up of unutilized brain ATP. This interpretation corresponds with numerous reports of region specific reductions in metabolic activity during active SD (Wu et al., 1991; Braun et al., 1997; Thomas et al., 2000), possibly indicating that one aspect of the homeostatic response to SD is to initiate a compensatory mode of metabolic conservation. Additional support for this view is provided by the present [31]P MRS brain imaging results demonstrating that in MM participants that while the largest elevation in brain ATP levels was observed following RE sleep, brain ATP levels appear to begin to rise during active SD. Interestingly, the findings of Dorsey et al., in conjunction with the present results illustrate that the elevations in brain ATP in control participants were most evident following RE sleep and there were no apparent changes in brain ATP levels during active SD. Under these conditions it is conceivable that the homeostatic response to SD in MM participants may be altered in that the level of metabolic conservation may be larger in methadone maintained individuals. In contrast, the present results demonstrating corresponding increases in brain PCr levels with increases in brain ATP levels over the course of the SD paradigm indicate that with prolonged wakefulness there is an enhancement in the utilization of ATP. Collectively, it may be the case that both conditions take place, such that with SD a decreased metabolic state is initiated, but the prolonged energy demands still require greater ATP utilization.
In summary, it was hypothesized that short-term MM participants may exhibit the greatest differences over the course of the SD paradigm, as stabilization in methadone treatment is associated with numerous complications, such as alterations in sleep architecture and neuroendocrine function, as well as psychiatric dysfunction (Judson and Goldstein, 1982; Woody et al., 1984; Willenbring et al., 1989; Neshin, 1993). Sleep PSG results identified notable differences in RE sleep in short-term MM participants compared to control participants, highlighting consequences of sleep loss with SD and the importance of monitoring sleep early in treatment. Although long-term MM participants did not exhibit the robust changes in PSG measures observed in short-term MM participants, similar to short-term MM participants, long-term MM participants did exhibit greater elevations in beta-NTP measures of brain ATP levels following SD than control participants. The data highlights the notion that although individuals who have been stabilized to methadone maintenance may not exhibit differences in sleep PSG measures in response to SD, long-term MM individuals may have persistent underlying changes in brain metabolic and/or sleep restorative processes.
Collectively, The present study identified a differential response to SD in MM participants compared to control participants, as measured by PSG and [31]P MRS brain imaging. The lack of increases in TST and SEI during RE sleep in MM participants (particularly short-term MM) indicate disrupted RE sleep, which may result in a prolonging the RE sleep restoration processes into subsequent RE sleep nights. It is likely that MM participants may require more sleep nights to recover from the impact of sleep loss. This theory may be congruent with the notion that SD results in a greater impact to sleep homeostasis in MM individuals. The results identifying differences in brain ATP levels in MM participants may reflect an inability to properly regulate brain bioenergetics in response to sleep loss. Particularly with methadone maintenance, the SD challenge may result in an over compensation in the restoration of brain ATP, indicative of an increase in brain ATP production or a reduction in ATP metabolism. A relationship may exist between SD-induced metabolic conservation and SD-induced cognitive performance deficits, potentially leading to greater cognitive performance deficits in MM individuals. Previous brain imaging studies examining the effects of SD have implicated cognitive task-specific changes in frontal brain activations (Drummond et al., 2005; Zeitzer et al., 2006) and regional decreases in frontal brain metabolism, which were also observed after RE sleep (Wu et al., 2006). The alterations in sleep homeostatic and sleep restoration processes in MM participants in response to SD may have consequences in other physiological processes that require a high metabolic demand. Sleep loss in MM participants may disrupt restorative sleep processes, subsequent sleep patterns, and impact daytime wake function. Sleep in MM patients is important as insomnia is associated with psychiatric disorders, depression, and a decline in executive function (Chuah et al., 2006).
In consideration of neurophysiological mechanisms underlying the present objective sleep and brain MRS imaging findings, one a plausible factor may be the function of the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is a primary component of the neuroendocrine system that regulates physiological functions such as immunity, mood, and energy metabolism and the response to stress (Hauger and Datzenberg, 2000). The HPA axis also function to maintain alertness and in sleep regulation. Dysfunction in the HPA axis such as alterations to corticotrophin-releasing hormone (CRH) or adrenocorticotropic hormone (ACTH) can disrupt sleep (Lesch et al., 1988; Garcia-Borreguero et al., 2000; Rodenbeck et al., 2002; Buckley and Schatzberg, 2005). Cortisol is secreted during the stress response and stress tends to worsen sleep leading to theories that HPA axis abnormalities may directly contribute to some sleep disorders, such as insomnia (Buckley and Schatzberg, 2005). A study demonstrated that SD results in a significant reduction of cortisol secretion which is under the control of increase of slow wave sleep during the recovery night (Vgontzas et al., 1999). One intriguing example is the relationship of HPA axis function and depression, as depression is often associated with sleep disturbances and hypercortisolemia (Arborelius et al., 1999). Investigations seeking to elucidate the neurobiological mechanisms associated with the therapeutic effects of SD in depressed patients have demonstrated that the response to SD in depressed patients results in decreased cortisol levels highlighting decreased HPA axis activity during RE sleep (Arborelius et al., 1999; Vgontzas et al., 1999; Murck et al., 2006). Similarly, opioid addiction and methadone treatment have been shown to result in alterations to noradrenergic regulation and the function of the stress-responsive HPA axis (Stine et al., 2001). For example, findings suggest that chronic opioid dependence results in reduced HPA axis function, while opioid withdrawal may decrease pituitary and increase adrenal responses (Zhang et al., 2008). Although it was initially thought that perturbations in HPA axis function become normalized with methadone treatment stabilization (Kreek et al., 1983; Kreek et al., 1984), more recent studies have observed HPA axis abnormalities, which persist into stable long-term methadone maintenance (Stine et al., 2002; Schluger et al., 2003). It has been postulated that hypoadrenalism in methadone addicts might explain some of the negative symptoms frequently associated with methadone treatment, including fatigue, weakness, and depression (Dackis et al., 1982; Pullan et al., 1983). It has also been shown that alcohol consumption results in the activation of the hypothalamus-pituitary-adrenal (HPA) axis, and with more persistent alterations of the HPA axis existing in heavy drinkers (Badrick et al., 2008; Wong et al., 2008). In contrast to the hypoadrenalism noted with methadone maintenance, data has shown that chronic heavy drinking results in hypercortisolism (Beresford et al., 2006; Badrick et al., 2008; Wong et al., 2008). Although the precise neurobiology remains uncertain, based upon the relationship of the HPA axis to sleep and energy metabolism and the identification of methadone-induced hypoadrenalism, it is conceivable that HPA axis dysfunction in MM participants is a primary mechanism driving the current objective sleep measures and MRS brain imaging findings of a differential RE sleep homeostatic response to SD.
While the current findings highlight the importance of long-term treatment stabilization in methadone maintenance, another important and related aspect is that potential functional disruptions such as reduced cognitive and/or executive function(s) due to drug abuse-related insomnia may directly contribute to an increase drug relapse potential (Ford and Kamerow, 1989; Staedt et al., 1996; Beswick et al., 2003). For example, a greater impact of acute sleep loss early in methadone treatment may directly increase the propensity for opioid relapse furthermore impeding the patients’ ability to achieve prolonged participation in methadone maintenance. In other words, MM patients who are more susceptible to certain conditions such as acute sleep loss and are unable to become stabilized to methadone maintenance may become unable to reach long-term MM status. Limitations of this study include the limited sample size, which may contribute to less than ideal statistical power in examining treatment x sex differences, and potential differences in participant drug use history. While the present study excluded MM participants based upon dependence to drugs other than opiates or nicotine and for primary psychiatric disorders, a common feature of methadone maintenance is polydrug abuse, drug chipping, and the occurrence of depression. Studies have shown that methadone maintenance results in a greater occurrence of central sleep apnea (Teichtahl et al., 2004; Teichtahl and Wang, 2007; Webster et al., 2008). Additionally, while participants exceeding criteria for respiratory events and periodic leg movements (PLMs) during study screening were excluded from the study, a potential limitation may exist from the contribution of fluctuations in respiratory and/or PLMs over the course of the study sleep nights. The contribution of polydrug abuse, depressive symptomology, or central sleep apnea to the effects of SD during methadone maintenance treatment are not examined by the current study and the potential interaction of these factors may be important considerations with MM patients. In lieu of the current findings, methadone maintenance remains an effective and affordable treatment for opioid-dependence. Overall, the results highlight the biological sleep homeostatic response to SD in a drug-dependent population, underscoring the need for close monitoring of sleep loss or disturbances in MM patients, and support the notion that SD-induced cognitive deficits may be more prevalent or of a larger magnitude in drug-dependent individuals. Further characterization is necessary to fully understand the impact of sleep disturbances in MM patients on sleep behavior and cognition as well as on the propensity for drug relapse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adam K, Oswald I. Sleep is for tissue restoration. Journal of the Royal College of Physicians of London. 1977;11:376–388. [PMC free article] [PubMed] [Google Scholar]
- Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–222. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotrophin-releasing factor in depression and anxiety disorders. The Journal of endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann R, Fitch T, Morel C, Bonato R. A comparison of period amplitude and power spectral analysis of sleep EEG in normal adults and depressed outpatients. Psychiatry Res. 1995;56:245–256. doi: 10.1016/0165-1781(95)02615-4. [DOI] [PubMed] [Google Scholar]
- Athanasos P, Smith CS, White JM, Somogyi AA, Bochner F, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120:267–275. doi: 10.1016/j.pain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, Kumari M. The relationship between alcohol consumption and cortisol secretion in an aging cohort. J Clin Endocrinol Metab. 2008;93:750–757. doi: 10.1210/jc.2007-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Progress in neurobiology. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Beresford HF, Du Y, Liu D, Shen D, Davatzikos C, Laudenslager ML. Hypercortisolism in alcohol dependence and its relation to hippocampal volume loss. J Stud Alcohol. 2006;67:861–867. doi: 10.15288/jsa.2006.67.861. [DOI] [PubMed] [Google Scholar]
- Besset A, Villemin E, Tafti M, Billiard M. Homeostatic process and sleep spindles in patients with sleep-maintenance insomnia: effect of partial (21 h) sleep deprivation. Electroencephalography and clinical neurophysiology. 1998;107:122–132. doi: 10.1016/s0013-4694(98)00048-0. [DOI] [PubMed] [Google Scholar]
- Beswick T, Best D, Rees S, Bearn J, Gossop M, Strang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: differences between methadone and lofexidine detoxification treatments. Addict Biol. 2003;8:49–57. doi: 10.1080/1355621031000069882. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Brown BS, Watters JK, Iglehart AS. Methadone maintenance dosage levels and program retention. The American journal of drug and alcohol abuse. 1982;9:129–139. doi: 10.3109/00952998209002617. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. The Journal of clinical endocrinology and metabolism. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, McLellan AT. The early course of change in methadone maintenance. Addiction (Abingdon, England) 1998;93:41–49. doi: 10.1046/j.1360-0443.1998.931415.x. [DOI] [PubMed] [Google Scholar]
- Chance B, Waterland RA, Tanaka A, Poyton RO. Mitochondrial function in normal and genetically altered cells and tissues. Ann N Y Acad Sci. 1988;550:360–373. doi: 10.1111/j.1749-6632.1988.tb35350.x. [DOI] [PubMed] [Google Scholar]
- Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condelli WS, Dunteman GH. Exposure to methadone programs and heroin use. The American journal of drug and alcohol abuse. 1993;19:65–78. doi: 10.3109/00952999309002666. [DOI] [PubMed] [Google Scholar]
- Connell PH. Centres for treatment of drug addiction. Importance of research. Br Med J. 1967;2:499–500. doi: 10.1136/bmj.2.5550.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Gurpegui M, Pottash AL, Gold MS. Methadone induced hypoadrenalism. Lancet. 1982;2:1167. doi: 10.1016/s0140-6736(82)92830-6. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P. Spontaneous neural activity during human slow wave sleep. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Spadini V, Curcio G, Cristiani R, Bertini M. The cyclic alternating pattern decreases as a consequence of total sleep deprivation and correlates with EEG arousals. Neuropsychobiology. 2002;45:95–98. doi: 10.1159/000048683. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–36. [PubMed] [Google Scholar]
- Dorsey CM, Lukas SE, Teicher MH, Harper D, Winkelman JW, Cunningham SL, Satlin A. Effects of passive body heating on the sleep of older female insomniacs. J Geriatr Psychiatry Neurol. 1996;9:83–90. doi: 10.1177/089198879600900203. [DOI] [PubMed] [Google Scholar]
- Dorsey CM, Lukas SE, Moore CM, Tartarini WL, Parow AM, Villafuerte RA, Renshaw PF. Phosphorous31 magnetic resonance spectroscopy after total sleep deprivation in healthy adult men. Sleep. 2003;26:573–577. doi: 10.1093/sleep/26.5.573. [DOI] [PubMed] [Google Scholar]
- Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–163. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–223. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Duncan WC, Jr, Gillin JC, Post RM, Gerner RH, Wehr TA. Relationship between EEG sleep patterns and clinical improvement in depressed patients treated with sleep deprivation. Biological psychiatry. 1980;15:879–889. [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- Eugenio KR. Profound morphine tolerance following high-dose methadone therapy. J Pain Palliat Care Pharmacother. 2004;18:47–54. doi: 10.1300/j354v18n04_05. [DOI] [PubMed] [Google Scholar]
- Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2005;288:R374–R383. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Frank MG. The mystery of sleep function: current perspectives and future directions. Reviews in the neurosciences. 2006;17:375–392. doi: 10.1515/revneuro.2006.17.4.375. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hendrickson JC, Gerstein DR, Zhang Z, Stein MD. Do mechanisms that link addiction treatment patients to primary care influence subsequent utilization of emergency and hospital care? Med Care. 2006;44:8–15. doi: 10.1097/01.mlr.0000188913.50489.77. [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Wehr TA, Larrosa O, Granizo JJ, Hardwick D, Chrousos GP, Friedman TC. Glucocorticoid replacement is permissive for rapid eye movement sleep and sleep consolidation in patients with adrenal insufficiency. The Journal of clinical endocrinology and metabolism. 2000;85:4201–4206. doi: 10.1210/jcem.85.11.6965. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Kripke DF, Schuckit M. EEG sleep studies in "pure" primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol Psychiatry. 1990;27:477–488. doi: 10.1016/0006-3223(90)90439-9. [DOI] [PubMed] [Google Scholar]
- Gip P, Hagiwara G, Sapolsky RM, Cao VH, Heller HC, Ruby NF. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1057–R1062. doi: 10.1152/ajpregu.00528.2003. [DOI] [PubMed] [Google Scholar]
- Gossop M, Bradley B. Insomnia among addicts during supervised withdrawal from opiates: a comparison of oral methadone and electrostimulation. Drug Alcoho Depend. 1984;13:191–198. doi: 10.1016/0376-8716(84)90058-9. [DOI] [PubMed] [Google Scholar]
- Gossop M, Trakada K, Stewart D, Witton J. Reductions in criminal convictions after addiction treatment: 5-year follow-up. Drug Alcohol Depend. 2005;79:295–302. doi: 10.1016/j.drugalcdep.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Gottheil E, Sterling RC, Weinstein SP. Diminished illicit drug use as a consequence of long-term methadone maintenance. Journal of addictive diseases. 1993;12:45–57. doi: 10.1300/J069v12n04_04. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Datzenberg FM. Regulation of the stress response by corticotropin-releasing factor receptors. In: Conn PM, Freeman ME, editors. Neuroendocrinology in physiology and medicine. Totowa: Humana Press; 2000. [Google Scholar]
- Hoffman JA, Moolchan ET. The phases-of-treatment model for methadone maintenance: implementation and evaluation. Journal of psychoactive drugs. 1994;26:181–197. doi: 10.1080/02791072.1994.10472266. [DOI] [PubMed] [Google Scholar]
- Horne JA, Staff LH. Exercise and sleep: body-heating effects. Sleep. 1983;6:36–46. doi: 10.1093/sleep/6.1.36. [DOI] [PubMed] [Google Scholar]
- Howe RC, Phillips JL, Hegge FW. Acute heroin abstinence in man. III. Effect upon waking and slow wave sleep. Drug and alcohol dependence. 1980a;6:247–262. doi: 10.1016/0376-8716(80)90329-4. [DOI] [PubMed] [Google Scholar]
- Howe RC, Hegge FW, Phillips JL. Acute heroin abstinence in man: I. Changes in behavior and sleep. Drug and alcohol dependence. 1980b;5:341–356. doi: 10.1016/0376-8716(80)90160-x. [DOI] [PubMed] [Google Scholar]
- Howe RC, Phillips JL, Hegge FW. Acute heroin abstinence in man: IV. Sleep--waking state contingencies. Drug and alcohol dependence. 1981;7:163–176. doi: 10.1016/0376-8716(81)90031-4. [DOI] [PubMed] [Google Scholar]
- Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Drost DJ, Menon RS, Williamson PC. In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed. 2002;15:338–347. doi: 10.1002/nbm.776. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Belenky G, Redmond DP, Thorne DR, Williams JD, Hursh SR, Balkin TJ. Modulating the homeostatic process to predict performance during chronic sleep restriction. Aviat Space Environ Med. 2004;75:A141–A146. [PubMed] [Google Scholar]
- Judson BA, Goldstein A. Symptom complaints of patients maintained on methadone, LAAM (methadyl acetate), and naltrexone at different times in their addiction careers. Drug Alcohol Depend. 1982;10:269–282. doi: 10.1016/0376-8716(82)90021-7. [DOI] [PubMed] [Google Scholar]
- Kay DC. Human sleep and EEG through a cycle of methadone dependence. Electroencephalography and clinical neurophysiology. 1975;38:35–43. doi: 10.1016/0013-4694(75)90208-4. [DOI] [PubMed] [Google Scholar]
- King VL, Brooner RK. Improving treatment engagement in opioid-dependent outpatients with a motivated stepped-care adaptive treatment model. Jt Comm J Qual Patient Saf. 2008;34:209–213. doi: 10.1016/s1553-7250(08)34027-6. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Ragunath J, Plevy S, Hamer D, Schneider B, Hartman N. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5:277–278. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Wardlaw SL, Hartman N, Raghunath J, Friedman J, Schneider B, Frantz AG. Circadian rhythms and levels of beta-endorphin, ACTH, and cortisol during chronic methadone maintenance treatment in humans. Life sciences. 1983;33(Suppl 1):409–411. doi: 10.1016/0024-3205(83)90529-5. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Widerlov E, Ekman R, Laux G, Schulte HM, Pfuller H, Beckmann H. Delta sleep-inducing peptide response to human corticotropin-releasing hormone (CRH) in major depressive disorder. Comparison with CRH-induced corticotropin and cortisol secretion. Biological psychiatry. 1988;24:162–172. doi: 10.1016/0006-3223(88)90271-5. [DOI] [PubMed] [Google Scholar]
- Leukefeld CG, Tims FM. Compulsory treatment for drug abuse. Int J Addict. 1990;25:621–640. doi: 10.3109/10826089009061324. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR. Drugs of abuse--opiates. West J Med. 1990;152:565–572. [PMC free article] [PubMed] [Google Scholar]
- Murck H, Uhr M, Ziegenbein M, Kunzel H, Held K, Antonijevic IA, Schussler P, Steiger A. Renin-angiotensin-aldosterone system, HPA-axis and sleep-EEG changes in unmedicated patients with depression after total sleep deprivation. Pharmacopsychiatry. 2006;39:23–29. doi: 10.1055/s-2006-931476. [DOI] [PubMed] [Google Scholar]
- Neshin SF. Methadone maintenance treatment: dispelling myths and recovering truths. N J Med. 1993;90:850–852. [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. J Sleep Res. 2002;11:247–253. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Nishijima MK, Koehler RC, Hurn PD, Eleff SM, Norris S, Jacobus WE, Traystman RJ. Postischemic recovery rate of cerebral ATP, phosphocreatine, pH, and evoked potentials. Am J Physiol. 1989;257:H1860–H1870. doi: 10.1152/ajpheart.1989.257.6.H1860. [DOI] [PubMed] [Google Scholar]
- Nissen C, Feige B, Konig A, Voderholzer U, Berger M, Riemann D. Delta sleep ratio as a predictor of sleep deprivation response in major depression. J Psychiatr Res. 2001;35:155–163. doi: 10.1016/s0022-3956(01)00021-8. [DOI] [PubMed] [Google Scholar]
- Oyefeso A, Sedgwick P, Ghodse H. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug Alcohol Depend. 1997;48:9–16. doi: 10.1016/s0376-8716(97)00097-5. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend. 2006;82:103–110. doi: 10.1016/j.drugalcdep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Peles E, Linzy S, Kreek M, Adelson M. One-year and cumulative retention as predictors of success in methadone maintenance treatment: a comparison of two clinics in the United States and Israel. Journal of addictive diseases. 2008;27:11–25. doi: 10.1080/10550880802324382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Neidert GL, Kay DC. Morphinelike arousal by methadone during sleep. Clin Pharmacol Ther. 1981;30:796–804. doi: 10.1038/clpt.1981.240. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Ponder SL, Twieg DB. A novel sampling method for 31P spectroscopic imaging with improved sensitivity, resolution, and sidelobe suppression. J Magn Reson B. 1994;104:85–88. doi: 10.1006/jmrb.1994.1058. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science (New York, NY. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potwarka JJ, Drost DJ, Williamson PC. Quantifying 1H decoupled in vivo 31P brain spectra. NMR Biomed. 1999;12:8–14. [PubMed] [Google Scholar]
- Prinz PN, Roehrs TA, Vitaliano PP, Linnoila M, Weitzman ED. Effect of alcohol on sleep and nighttime plasma growth hormone and cortisol concentrations. The Journal of clinical endocrinology and metabolism. 1980;51:759–764. doi: 10.1210/jcem-51-4-759. [DOI] [PubMed] [Google Scholar]
- Pullan PT, Watson FE, Seow SS, Rappeport W. Methadone-induced hypoadrenalism. Lancet. 1983;1:714. doi: 10.1016/s0140-6736(83)92011-1. [DOI] [PubMed] [Google Scholar]
- Radulovacki M. Role of adenosine in sleep in rats. Reviews in clinical & basic pharmacology. 1985;5:327–339. [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. University of California at Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, Frederick Bde B, Fava M, Hennen J, Cohen BM. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–2055. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- Reyero F, Muller C. [Repeated sessions of sleep deprivation in the treatment of depression] L'Encephale. 1977;3:755–761. [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Kupfer DJ, Hoch CC, Stack JA, Houck PA, Berman SR. Sleep deprivation effects in older endogenous depressed patients. Psychiatry Res. 1987;21:95–109. doi: 10.1016/0165-1781(87)90068-0. [DOI] [PubMed] [Google Scholar]
- Rodenbeck A, Huether G, Ruther E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neuroscience letters. 2002;324:159–163. doi: 10.1016/s0304-3940(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20:279–286. doi: 10.1016/S0893-133X(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Roubicek J, Zaks A, Freedman AM. EEG changes produced by heroin and methadone. Electroencephalogr Clin Neurophysiol. 1969;27:667–668. doi: 10.1016/0013-4694(69)91251-6. [DOI] [PubMed] [Google Scholar]
- Salamy A, Williams HL. The effects of alcohol on sensory evoked and spontaneous cerebral potentials in man. Electroencephalography and clinical neurophysiology. 1973;35:3–11. doi: 10.1016/0013-4694(73)90126-0. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Bart G, Green M, Ho A, Kreek MJ. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology. 2003;28:985–994. doi: 10.1038/sj.npp.1300156. [DOI] [PubMed] [Google Scholar]
- Senay EC. Methadone maintenance treatment. Int J Addict. 1985;20:803–821. doi: 10.3109/10826088509047754. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Pollack MH, Diaz CI, Nassar LE, Mendelson JH, Yurgelun-Todd DA, Renshaw PF, Kaufman MJ. Cerebral phosphorus metabolite and transverse relaxation time abnormalities in heroin-dependent subjects at onset of methadone maintenance treatment. Psychiatry Res. 2004;131:217–226. doi: 10.1016/j.pscychresns.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Staedt J, Wassmuth F, Stoppe G, Hajak G, Rodenbeck A, Poser W, Ruther E. Effects of chronic treatment with methadone and naltrexone on sleep in addicts. Eur Arch Psychiatry Clin Neurosci. 1996;246:305–309. doi: 10.1007/BF02189023. [DOI] [PubMed] [Google Scholar]
- Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, Anderson BJ. Sleep disturbances among methadone maintained patients. J Subst Abuse Treat. 2004;26:175–180. doi: 10.1016/S0740-5472(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Stine SM, Grillon CG, Morgan CA, 3rd, Kosten TR, Charney DS, Krystal JH. Methadone patients exhibit increased startle and cortisol response after intravenous yohimbine. Psychopharmacology. 2001;154:274–281. doi: 10.1007/s002130000644. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biological psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Szpanowska-Wohn A, Kolarzyk E, Pach D, Targosz D. [Nutritional status of opiate-dependent persons before and during methadone maintenance therapy] Przegl Lek. 2004;61:339–344. [PubMed] [Google Scholar]
- Teichtahl H, Wang D. Sleep-disordered breathing with chronic opioid use. Expert opinion on drug safety. 2007;6:641–649. doi: 10.1517/14740338.6.6.641. [DOI] [PubMed] [Google Scholar]
- Teichtahl H, Wang D, Cunnington D, Kronborg I, Goodman C, Prodromidis A, Drummer O. Cardiorespiratory function in stable methadone maintenance treatment (MMT) patients. Addiction biology. 2004;9:247–253. doi: 10.1080/13556210412331292578. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. Journal of sleep research. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacol Biochem Behav. 1991;40:33–40. doi: 10.1016/0091-3057(91)90317-u. [DOI] [PubMed] [Google Scholar]
- Tilley A, Donohoe F, Hensby S. Homeostatic changes in slow wave sleep during recovery sleep following restricted nocturnal sleep and partial slow wave sleep recovery during an afternoon nap. Sleep. 1987;10:600–605. [PubMed] [Google Scholar]
- van den Burg W, van den Hoofdakker RH. Total sleep deprivation on endogenous depression. Archives of general psychiatry. 1975;32:1121–1125. doi: 10.1001/archpsyc.1975.01760270053005. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 1999;51:205–215. doi: 10.1046/j.1365-2265.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- Volavka J, Verebey K, Resnick R, Mule S. Methadone dose, plasma level, and cross-tolerance to heroin in man. J Nerv Ment Dis. 1978;166:104–109. doi: 10.1097/00005053-197802000-00004. [DOI] [PubMed] [Google Scholar]
- Wagman AM, Allen RP, Funderburk F, Upright D. EEG measures of functional tolerance to alcohol. Biol Psychiatry. 1978;13:719–728. [PubMed] [Google Scholar]
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain medicine (Malden, Mass. 2008;9:425–432. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Weddington WW. Towards a rehabilitation of methadone maintenance: integration of relapse prevention and aftercare. Int J Addict. 1990;25:1201–1224. doi: 10.3109/10826089109081042. [DOI] [PubMed] [Google Scholar]
- Willenbring ML, Morley JE, Krahn DD, Carlson GA, Levine AS, Shafer RB. Psychoneuroendocrine effects of methadone maintenance. Psychoneuroendocrinology. 1989;14:371–391. doi: 10.1016/0306-4530(89)90007-3. [DOI] [PubMed] [Google Scholar]
- Wong WM, Hasemann S, Schwarz M, Zill P, Koller G, Soyka M, Preuss UW. Citalopram neuropharmacological challenge in alcohol-dependent patients and controls: pharmacogenetic, endocrine and psychobehavioral results. Pharmacopsychiatry. 2008;41:72–78. doi: 10.1055/s-2007-1004595. [DOI] [PubMed] [Google Scholar]
- Woody GE, McLellan AT, O'Brien CP. Treatment of behavioral and psychiatric problems associated with opiate dependence. NIDA Res Monogr. 1984;46:23–35. [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Hazlett E, Sicotte N, Bunney WE., Jr The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–162. [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Darnall LA, Fallon JH, Bunney WE. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Takanashi Y, Motonaga T, Naruse S, Nishikawa H, Hirakawa K. Effect of cerebral ischemia and hypercapnia on cerebral pH studied with 31P-NMR and electrical activity in rat brain. Jpn J Physiol. 1989;39:155–167. doi: 10.2170/jjphysiol.39.155. [DOI] [PubMed] [Google Scholar]
- Zaks A, Fink M, Freedman AM. Duration of methadone induced cross-tolerance to heroin. Br J Addict Alcohol Other Drugs. 1971;66:205–208. doi: 10.1111/j.1360-0443.1971.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Morales-Villagran A, Maidment NT, Behnke EJ, Ackerson LC, Lopez-Rodriguez F, Fried I, Engel J, Jr, Wilson CL. Extracellular adenosine in the human brain during sleep and sleep deprivation: an in vivo microdialysis study. Sleep. 2006;29:455–461. doi: 10.1093/sleep/29.4.455. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Ren YP, Sheng LX, Chi Y, Du WJ, Guo S, Jiang ZN, Xiao L, Luo XN, Tang YL, Smith AK, Liu ZQ, Zhang HX. Dysfunction of the hypothalamic-pituitary-adrenal axis in opioid dependent subjects: effects of acute and protracted abstinence. The American journal of drug and alcohol abuse. 2008;34:760–768. doi: 10.1080/00952990802385781. [DOI] [PubMed] [Google Scholar]
- Zweben JE, Payte JT. Methadone maintenance in the treatment of opioid dependence. A current perspective. West J Med. 1990;152:588–599. [PMC free article] [PubMed] [Google Scholar]