Abstract
Pramipexole (PRA) is a preferential D3R agonist that in rats and humans modifies prepulse inhibition (PPI) of the acoustic startle reflex, an operational measure of sensorimotor gating. The ability to use similar PPI measures across species, and the relative ease of genetic manipulations in mice, suggests that molecular studies of the D3R regulation of sensorimotor gating might be best pursued in mice. Here, we evaluate the effects of PRA on PPI and locomotion in C57BL/6J mice, the background strain for many gene knockout mouse models. Male C57BL/6J mice were tested for PPI and locomotor activity after injection of PRA. No significant effects of PRA on PPI were seen at any dose (0.1-10.0 mg/kg), but a significant reduction in startle magnitude was observed after 10 mg/kg PRA. In contrast, the D1/2 agonist, apomorphine (5 mg/kg) significantly reduced PPI in these mice. At doses of PRA that did not alter startle magnitude (0.3, 1.0, 3.0 mg/kg), significant decreases in the amount of locomotor and investigatory behavior were observed. Distinct from findings in rats and humans, it appears that either: 1) PRA does not activate D3Rs in C57BL/6J mice, or 2) D3R agonists are not sufficient to alter PPI in this mouse strain.
Keywords: Prepulse inhibition, locomotor behavior, exploratory behavior, C57BL/6 mouse, pramipexole, dopamine, D3 receptor
Introduction
The study of dopamine (DA) D3 receptors (D3R) may shed new light on the pathogenesis and therapy of several neuropsychiatric disorders, but, due to the homology between D3 and D2 receptors, there are only a small number of well-characterized specific D3 and D2 agonists and antagonists. Pramipexole is a non-ergot dopamine D2/D3 receptor full agonist that has been reported to have a D3:D2 binding preference ranging from 7:1 to 160:1 in vitro (Millan et al., 2002; Piercey et al., 1996; Svensson et al., 1994). In rats, pramipexole is a preferential D3 receptor agonist that reduces prepulse inhibition (PPI) of acoustic startle, an operational measure of sensorimotor gating (Weber et al., 2008, 2009). In humans, pramipexole significantly increases PPI (Talledo et al. 2009), an effect observed with other DA receptor agonists in some populations (Bitsios et al. 2005). Though various in vitro and pharmacological techniques have been employed to assess the receptor specificity of pramipexole, in vivo molecular manipulations would provide definitive evidence of D3 vs. D2 contributions to effects of pramipexole on sensorimotor gating. Such molecular mechanisms might also shed light on the molecular basis for the D3 regulation of PPI, which in humans appears to be linked to the D3 receptor Ser9Gly polymorphism (Roussos et al. 2008).
In mice, dopamine D3 receptor knock-out models have been informative about receptor subtype contributions for the actions of many dopaminergic drugs (Ralph et al., 1999; Carta et al., 2000; Schmauss, 2000; Le Foll et al., 2002; Glickstein and Schmauss, 2004; Siuciak and Fujiwara, 2004; Karasinska et al., 2005; McNamara et al., 2006; Risbrough et al., 2006; Zhou et al., 2007; Doherty et al., 2008; Leggio et al., 2008; Harrison and Nobrega, 2009). Pramipexole has been found to alter nocturnal locomotion, alter operant responding (Lehr, 2002), induce hypothermia (Maj et al., 1997), and decrease the duration of immobility in the forced swim test (Kitagawa et al., 2009; Siuciak and Fujiwara, 2004). The ability to use similar PPI measures across species and the relative ease of genetic manipulations in mice makes them an attractive animal model to further study the role of D3 receptor activation in the regulation of sensorimotor gating. To date, there have not been any published reports of pramipexole effects on PPI in any strain of mice. Previous studies of PRA effects on locomotor activity have had mixed results with different strains of mice. The current report describes the effects of pramipexole on PPI and locomotor activity in C57BL/6J mice, a common background strain for knockout mice.
Methods
Subjects
Male C57BL/6J mice (n = 76) between 7 and 12 weeks of age were obtained from Jackson Labs (Bar Harbor, ME) and housed at a vivarium at the University of California San Diego (UCSD), an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Mice were allowed to acclimate for approximately 1 week after arrival. All mice were housed n = 4 per cage, in a climate-controlled room with a reversed light cycle (lights on at 2000 hours, off at 0800 hours). Food (Harlan Teklad, Madison, WI) and water were provided freely, except during behavioral testing. All testing occurred between 1000 and 1800 hours; animal testing was conducted in accord with the ‘Principles of Laboratory Animal Care’ NIH guidelines and were approved by the UCSD institutional animal care and use committee.
Drugs
Pramipexole (PRA) was purchased from Toronto Research Chemicals (North York, Ontario, Canada), and apomorphine hydrochloride hemihydrates (APO) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Drug doses are based on milligram/kilogram salts. All drugs were injected subcutaneously in a volume of 5 ml/kg body weight. Based on published studies in other mouse strains, PRA (saline vehicle, 0.01, 0.03, or 1.0 mg/kg) was administered 10 minutes before PPI testing (Maj et al., 1997; Siuciak and Fujiwara, 2004) in Experiment 1. Due to lack of any evidence of bioactivity of these doses of PRA in Exp. 1, Exp. 2 assessed whether the correct pretreatment time had been used by measuring effects of PRA (saline vehicle, 0.1, 0.3, 1.0 mg/kg) on PPI in the 60 minutes immediately after drug administration. In Exp. 3 and 4, doses were increased for PRA (Exp. 3: saline vehicle, 0.3, 1.0, 3.0 mg/kg; Exp. 4: saline vehicle, 10 mg/kg) and administered 15 minutes before PPI testing. APO (0.01% ascorbate/saline vehicle, 5 mg/kg) was administered 5 min before PPI testing (Exp. 4). A range of stimulus types was used in Exps. 1-4, described below, to attempt to identify PRA-sensitive parameters. In Exp. 5, PRA (saline vehicle, 0.3, 1.0, 3.0 mg/kg) was administered immediately before locomotion testing.
Startle apparatus
Startle reactivity was measured using four startle chambers (SR-LAB, San Diego Instruments, San Diego, CA). Each chamber consisted of a clear nonrestrictive Plexiglas cylinder (inner diameter = 3.8 cm) resting on a platform inside a ventilated box. A high-frequency loudspeaker inside the chamber produced both a continuous background noise of 65 dB(A) and the various acoustic stimuli. Vibrations of the Plexiglas cylinder caused by the whole-body startle response of the animal were transduced into analog signals by a piezoelectric unit attached to the platform. These signals were then digitized and stored by a computer. Sixty-five readings were taken at 1 ms intervals, starting at stimulus onset, and the average amplitude was used to determine the acoustic startle response (ASR). The SR-LAB calibration unit was used routinely to ensure consistent stabilimeter sensitivity between test chambers and over time, and sound levels in dB SPL (A scale) were measured as described previously (Geyer and Dulawa, 2003).
Startle testing procedure
All PPI test sessions consisted of startle trials (PULSE-ALONE), prepulse trials (PREPULSE + PULSE), and no-stimulus trials (NOSTIM). The PULSE-ALONE trial consisted of a 40 ms, 120 dB(A) pulse of broad-band noise. The NOSTIM trial consisted of background noise only. Parameters of PREPULSE + PULSE trials and the duration of the sessions varied with each Experiment. The test session began and ended with four to six presentations of the PULSE-ALONE trial; in between, each acoustic or NOSTIM trial type was presented in a pseudo-random order. There was an average inter-trial interval (ITI) of 15 s (range: 12-30 s). A background noise level of 65 dB(A) was presented for a 5-min acclimation period and continued throughout the matching and test sessions. 7 days after shipment arrival, mice were exposed to a 11 minute ‘matching’ startle session consisting of (10) PULSE-ALONE trails that were interspersed with (6) PREPULSE + PULSE trials consisting of PULSE-ALONE preceded 100 ms (onset-to-onset) by a 20 ms noise burst that was 81 dB(A) in intensity (i.e., 16 dB above the 65 dB(A) background). One “non-responder” was excluded from further testing due to a mean startle response to PULSE-ALONE trials of < 10 units during the matching session. Mice were assigned to drug treatment groups balanced for baseline PPI and startle magnitude during this matching session, as well as previous drug history. Groups were reassigned for each experiment. Test sessions were at least 1 week apart.
Exp. 1, 4
PREPULSE + PULSE trials consisted of PULSE-ALONE preceded 100 ms by a 20 ms noise burst that was 69, 73, or 81 dB(A) in intensity (i.e., 4, 8, or 16 dB above the 65 dB(A) background). Session duration was approximately 18.5 minutes.
Exp. 2
The test session was divided into 6 10-minute blocks after the 5 minute acclimation period. The initial 5 min of each block included four trial types: PULSE-ALONE, PREPULSE+PULSE (i.e. PULSE-ALONE preceded 100 ms by a 20 ms noise burst 8 dB above background), PREPULSE-ALONE, and NOSTIM.
Exp. 3 and 4
PREPULSE + PULSE trials consisted of PULSE-ALONE preceded 10, 20, 30, 60, 120 ms (onset-to-onset) by a 20 ms noise burst at 73 dB(A) (8 dB above background). Session duration was approximately 15.5 minutes.
Analysis of PPI Data
PPI was defined as 100-[(startle amplitude on PREPULSE trials/startle amplitude on PULSE-ALONE trials) × 100], and was analyzed by mixed design ANOVAs. All data were inspected for the presence of “non-responders”, defined by a mean startle response to PULSE-ALONE trials of < 10 units; aside from one mouse excluded after the matching session, none met this criteria. Other ANOVAs were used to assess PULSE-ALONE magnitude, or NOSTIM trials. Post-hoc comparisons were conducted using Fisher's protected least significance difference, with the threshold for α set at 0.05. Data were collapsed across prepulse intensities and PPI blocks.
Locomotion apparatus
Investigatory behavior and locomotor activity were measured in 10 mouse Behavior Pattern Monitor (BPM) chambers (San Diego Instruments, San Diego, CA). The design of the mouse BPM system was based on the rat BPM (for a detailed description, see Geyer et al, 1986). The mouse BPM chamber was a clear Plexiglas box containing a 30 × 60 cm holeboard floor. Each chamber was enclosed in a ventilated outer box to protect it from light and ambient noise from outside the chambers. The chamber contained 11 1.4-cm holes (3 in the floor and 8 in the walls), each provided with an infrared photobeam to detect investigatory nose pokes (hole pokes). The location of the mouse was obtained from a grid of 12 × 24 photobeams 1 cm above the floor. Rearing was detected by an array of 16 photobeams placed 2.5 cm above the floor and aligned with the long axis of the chamber. The status of photobeams was sampled every 55 ms. A change in the status of photobeams triggered the storage of the information in a binary data file, together with the duration of the photobeam status. Subsequently, the raw data files were transformed into (x, y, t, event) ASCII data files comprised of the (x, y) location of the animal in the mouse BPM chamber with a resolution of 1.25 cm, the duration of each event (t), and whether a hole poke or rearing event occurred.
Locomotor/exploratory activity testing procedure
Exp. 5
Mice were tested in the dark and during the dark phase of their light cycle. The animals were brought into the testing room under black cloth 1 h before testing. During testing, a white noise generator produced background noise at 65 dB. Pretreatment and test injections were made under red lights in the testing room. Data were collected for 90 min. The chambers were cleaned thoroughly between testing sessions.
Analysis of locomotor activity
Horizontal locomotor activity was quantified as distance traveled. The number of hole pokes and rearings were calculated as measures of exploratory behavior. Data were examined using mixed-model ANOVAs with treatment as between-subject factors and time as a repeated measure. Specific post hoc comparisons between the selected groups in each time block were done using Tukey's studentized range method. Statistical significance was assessed using an α-level of 0.05.
Results
PPI
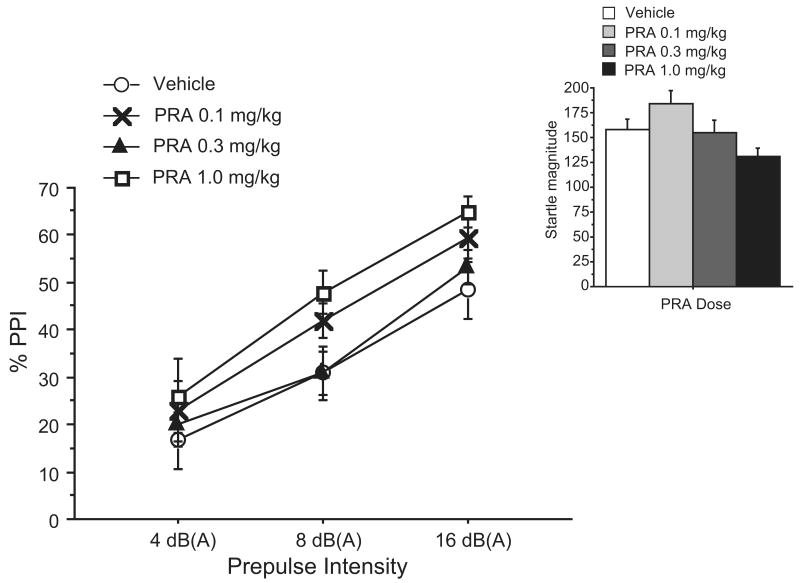
ANOVA of %PPI failed to reveal significant main effects of PRA dose in Exp. 1 (F=1.87, df 3, 35; NS) (Fig. 1). There were also no significant effects of PRA on startle magnitude (F=2.32, df 3, 35; NS) (Fig. 1, inset) or activity during NOSTIM trials (F=3.85, df 3, 35; NS). As expected, there was a significant effect of prepulse intensity (F=116.07, df 6, 35; p < 0.001), but no significant dose × intensity interaction (F < 1). There was a significant block effect on startle magnitude (F=8.82, df 3,35; p < 0.01), reflecting normal reflex habituation, but there was no significant dose × block interaction (F < 1) (data not shown). The lack of effect of PRA on PPI was hypothesized to be due to one of three factors: 1) incorrect pretreatment time, missing the time period of maximal bioactivity, 2) incorrect prepulse to pulse time interval, or 3) inadequate dose of the drug. These three possibilities were addressed in Experiments 2-4. The total sample of mice was split between Experiments 2 and 3.
Figure 1. Effects of 0.1-1.0 mg/kg PRA on PPI and startle magnitude (inset) across varying prepulse intensities in C57BL/6J mice (Exp. 1).
Startle magnitude and prepulse inhibition with prepulses 4, 8, and 16 dB(A) above background were tested 10 minutes after administration of 0.1, 0.3, and 1.0 mg/kg of PRA. Data are mean ± SEM. (n = 9-10 mice per dose group)
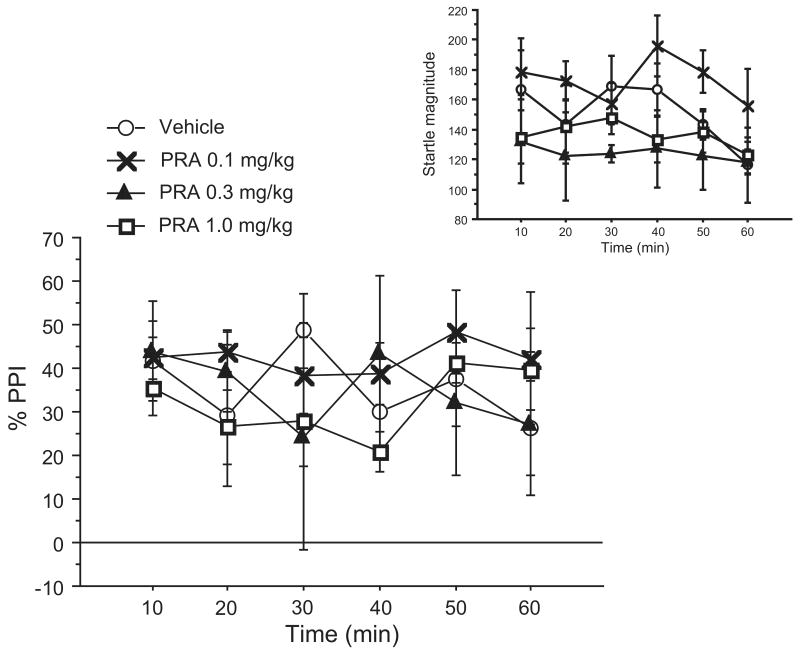
In Experiment 2, PPI was measured in six 10-minute blocks, starting 5 minutes after administration of PRA. Again, there were no significant main effects of PRA (F < 1) (Fig. 2), and there was no significant dose × block interaction (F < 1). As in Exp. 1, there was no significant effect of PRA dose on startle magnitude (F=2.31, df 3,16; NS); there was also no signifciant effect of block (F=1.54, df 5,16; NS), or dose × block interaction (F < 1) (Fig. 2, inset).
Figure 2. Effects of 0.1-1.0 mg/kg PRA on PPI and startle magnitude (inset) across 60 minutes of testing in C57BL/6J mice (Exp. 2).
To assess the time course for maximal bioactivity, PPI was tested in 6 10-minute time blocks after administration of 0.1, 0.3, and 1.0 mg/kg PRA. As in Exp. 1, there was no evidence of bioactivity of PRA at these doses. Data are mean ± SEM. (n = 5 mice per dose group)
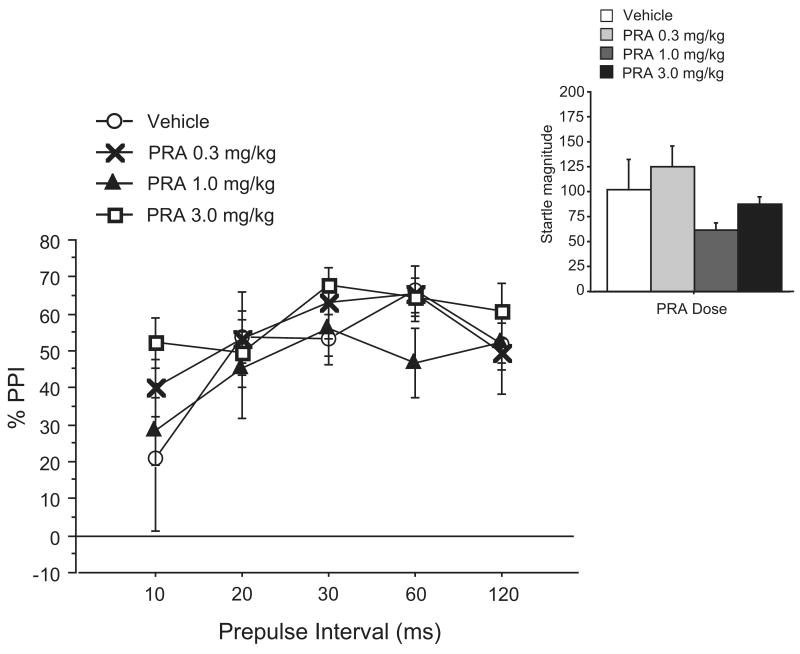
In Experiment 3, the interval between the onset of the (constant intensity) prepulse and the pulse was varied between 10, 20, 30, 60, and 120 msec. PRA doses were also increased to 0.3, 1.0, and 3.0 mg/kg. As expected, there was a significant effect of prepulse interval (F=11.67, df 4, 15; p <0.001) in the pattern that has been reported in rats (e.g. Swerdlow et al., 2009). Again, ANOVA failed to reveal a significant main effect of PRA on PPI (F <1), significant dose × interval interaction (F=1.37, df 12, 15; NS) (Fig. 3) or significant effect of PRA on startle magnitude (F = 1.95, df 3, 15; NS) (Fig. 3, inset).
Figure 3. Effects of 0.3-3.0 mg/kg PRA on PPI and startle magnitude (inset) across varying prepulse time intervals in C57BL/6J mice (Exp. 3).
Due to the absence of evidence of bioactivity in Exps. 1 and 2, PRA doses were shifted higher to 0.3, 1.0, and 3.0 mg/kg for Exp. 3. Prepulses were 8 dB(A) above background, and preceded the pulse by 10, 20, 30, 60, or 120 ms (onset-to-onset). Even at the highest dose, there was no significant effect on startle magnitude. Data are mean ± SEM. (n = 4-5 mice per dose group)
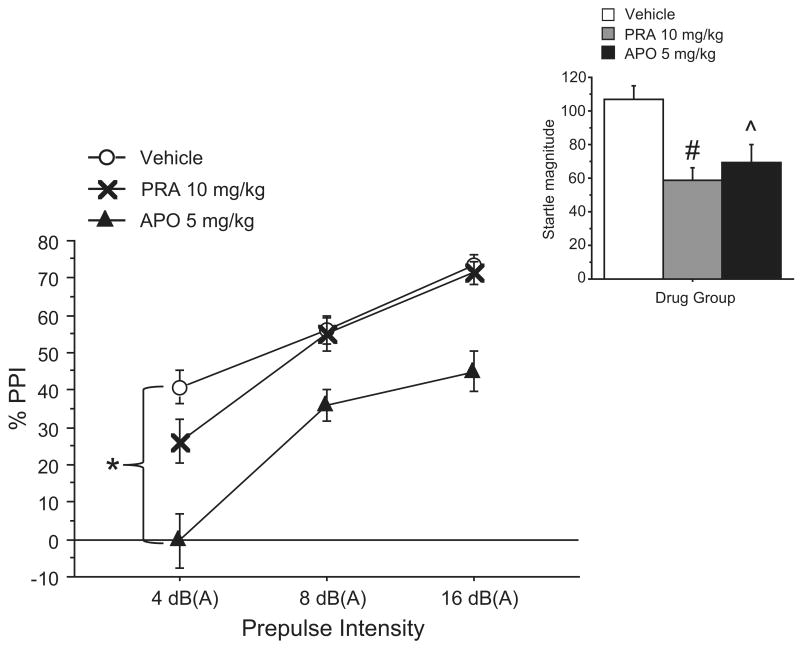
For the final PPI experiment (Exp. 4), the two groups from Exps. 2 and 3 were recombined. Mice were regrouped to balance for baseline PPI and drug history. As no evidence of bioactivity had yet been demonstrated for any doses of PRA used in Exps. 1-3, the PRA dose was increased to 10 mg/kg. Apomorphine (APO, 5 mg/kg) was used as a reference drug to determine whether testing was adequately sensitive to detect a dopaminergic regulation of PPI. ANOVAs revealed a significant main effect of drug on %PPI (p < 0.001). Post hoc tests revealed a significant reduction in %PPI after APO (p < 0.001), but not after 10 mg/kg PRA (NS) (Fig. 4). However, there was also a significant main effect of drug on startle magnitude (p < 0.001), and post hoc tests indicated that both PRA (p < 0.01) and APO (p < 0.05) significantly reduced startle magnitude (Fig. 4, inset). Additionally, it was observed grossly that mice receiving 10 mg/kg of PRA appeared lethargic and had reduced activity as soon as 5 minutes after drug administration.
Figure 4. Effects of 10 mg/kg PRA and 5 mg/kg APO on PPI and startle magnitude (inset) across varying prepulse intensities in C57BL/6J mice (Exp. 4).
PRA was administered at a maximum dose of 10 mg/kg for comparison against 5 mg/kg of APO with the same test parameters used in Exp.1. 10 mg/kg PRA demonstrated evidence of bioactivity via startle reduction with no change in PPI, and APO served as a positive control of dopamine agonist-induced PPI disruption. Data are mean ± SEM. *p < 0.0001, #p < 0.009, ˆp < 0.04. (n = 9 mice per dose group)
Locomotor and exploratory activity
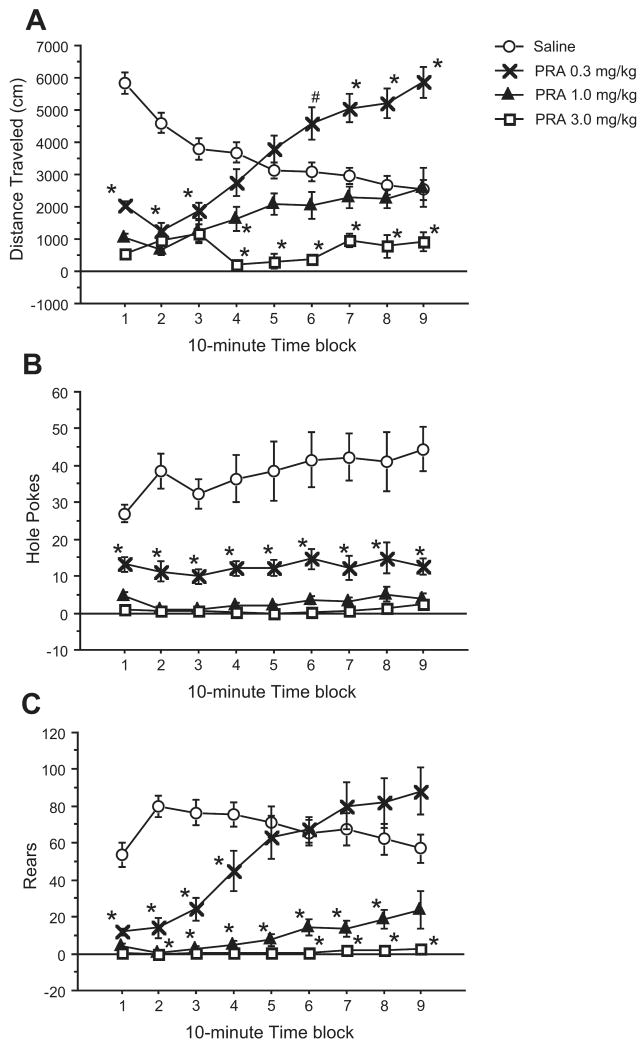
In Exp. 5, a different set of mice were tested for locomotor activity at doses of PRA (0.3, 1, 3 mg/kg) that did not demonstrate any effects on either PPI or startle magnitude. ANOVAs revealed a significant main effect of drug dose (p < 0.001) and dose × time interaction (p < 0.001) on locomotor activity and both measures of exploratory activity. All doses caused significant reductions in locomotor activity during the first 30 minutes after administration compared to the vehicle condition. At 0.3 mg/kg PRA, mice showed decreased locomotor activity during the first 30 minutes and hyperactivity during the last 40 minutes compared to the vehicle condition. Mice receiving 1.0 mg/kg PRA had decreased locomotor activity during the first 40 minutes that gradually increased to similar levels as vehicle-treated mice for the remainder of the 90-minute testing period. At the highest dose of PRA tested, 3.0 mg/kg, mice showed a steady and decreased level of locomotor activity throughout the testing period. Habituation -- reduced locomotor activity in vehicle-treated mice as they acclimated to the BPM chamber -- was not observed in mice treated with PRA (Fig. 5a).
Figure 5. Effects of PRA on locomotor activity (A) and exploratory activity (B,C).
Nonacclimated, drug-naïve mice were placed in Behavior Pattern Monitor (BPM) chambers immediately after administration of saline vehicle or 0.3, 1.0, or 3.0 mg/kg of PRA. Locomotor and exploratory behavior was measured for 90 minutes, divided into 10 minute time blocks. (a) Dose response of PRA effects on distance traveled (in cm). Mice used were male C57BL/6J. (b) Dose response of PRA effects on number of hole pokes. (c) Dose response of PRA effects on number of rears. Data are mean ± SEM. *p < 0.01, #p < 0.05. (n = 9 mice per dose group)
ANOVA of hole pokes revealed a significant main effect of drug dose, with a steady, dose-dependent decrease in the number of hole pokes throughout the 90 minute test session (Fig. 5b). There was no significant dose × time interaction (F = 1.164, df 24, 32; NS). The pattern of rearing looked similar to that of locomotion, with a significant dose × time interaction (p < 0.001). The group treated with 0.3 mg/kg PRA demonstrated fewer rears during the first 40 minutes, which increased by the end of the test session. Mice treated with 1.0 or 3.0 mg/kg PRA reared less than saline treated mice for the entire session, though this difference failed to reach significance for the last time block of the middle dose, due to a slight increase in rears by this treatment group as well as a gradual decrease in rears by vehicle-treated mice. (Fig. 5c).
Discussion
The present studies expand upon previous reports of DA agonist effects on PPI and locomotion in mice by testing the preferential D3 agonist pramipexole for behavioral effects in C57BL/6J mice. The ability of APO to reduce PPI confirms several previous findings in this mouse strain (Ralph-Williams et al., 2002, 2003; Yee et al., 2004; Russig et al., 2005, van den Buuse et al., 2005; Semenova et al., 2008; Martin et al., 2008; Caldwell et al., 2009) and other strains (Curzon and Decker, 1998; Ukai and Okuda, 2003; Malone et al., 2004; Park et al., 2005; Brea et al., 2006; Depoortère et al., 2007; Yano et al., 2009). While no effect of PRA on PPI was shown in this report, bioactivity was demonstrated by a reduction in startle magnitude at the highest dose tested (10 mg/kg) and by changes in locomotor and exploratory behavior across lower doses. Startle magnitude reduction after administration of PRA at doses inducing PPI deficits is often observed in rats (Weber et al., 2008, 2009). A reduction in startle magnitude (indicating bioactivity) in the absence of any PPI effects in these C57BL/6J mice suggests that the pharmacological actions of PRA do not regulate PPI in this strain/species. Indeed, the mixed D2/D3 agonists quinpirole and quinelorane fail to disrupt PPI in several strains of mice, including C57BL/6J (Ralph-Williams et al., 2003; Ralph and Caine, 2005). It should be noted, though, that quinelorane can disrupt PPI in some mouse strains (Ralph and Caine, 2007), and quinpirole has been shown to increase PPI in mice when infused directly into the nucleus accumbens. In contrast, rats reliably exhibit reduced PPI after systemic pramipexole, quinpirole, or quinelorane administration, at least at the most commonly used prepulse intervals (Culm et al., 2004; Ralph and Caine 2005, Qu et al., 2008; Weber et al., 2008). It has been reported through knockout studies in C57BL/6J mice that the DA D2 receptor (D2R) subtype is necessary for amphetamine-induced PPI disruptions, while the DA D1 (D1R), D3 (D3R), and D4 (D4R) receptor subtypes are not (Ralph et al, 1999). D3R knockout mice have been shown to have exaggerated effects of cocaine on PPI (Doherty et al., 2008) perhaps due to unopposed effects of D1R activation. However, pharmacological manipulation of D3R activity does not always produce effects that are consistent with specific genetic manipulations of receptor subtypes (Corbin et al., 1998; Le Foll et al., 2002; Xi et al, 2005). These results suggest that, while PRA may primarily be stimulating D3Rs in mice, this pharmacological activation does not alter PPI, and furthermore, D3Rs may not play a prominent role in PPI regulation in this mouse strain.
Locomotion and exploratory behavior findings in the present studies are in keeping with previously published reports on pramipexole effects in other strains of mice (Lehr et al., 2002; Siuciak and Fujiwara 2004; Maj et al., 1997). 7-OH-DPAT and PD 128907, two other D3-preferential DA agonists, also reduce locomotor activity in wild-type C57BL/6J mice; this effect is not present in D3 receptor knockout mice (Pritchard et al., 2003). Additionally, D3 receptor antagonists enhance locomotor hyperactivity due to cocaine (Piercey et al., 1992) and amphetamine (Pritchard et al., 2007), and mice deficient in D3 receptors exhibit an augmented cocaine-stimulated locomotion. Thus, compared to their role in regulating PPI in mice, D3 receptors appear to play a more prominent role in the regulation of locomotor activity.
The main goal of this study was to establish an assay in mice that could be used via molecular manipulations to assess the D3 specificity of PRA in its impact on PPI. Our findings, however, suggest that such an assay may not be informative, based on the surprising insensitivity of PPI to apparent D3 stimulation in mice. While “negative results” must always be interpreted with caution, this study used a number of different approaches to demonstrate that the lack of PRA effects on PPI did not reflect the insensitivity of the PPI paradigm, lack of PRA bioactivity, or a restricted range of stimulus parameters or time course. In so doing, it is conceivable that these results identify a fundamental difference in the dopaminergic regulation of PPI across rat vs. mouse species, analogous to other species differences in PPI previously reported in rats vs. mice (Ralph and Caine, 2005; Ralph-Williams et al., 2002, 2003). Although differential sex effects of pramipexole were not explored in this study or other reports, pramipexole has not demonstrated sexually dimorphic effects or sensitivity to estrous phase in Sprague Dawley rats (Chang et al., 2009). Given the disparate effects of pramipexole on PPI between male rats and mice, however, this lack of sex effect in rats is not necessarily translatable to mice. If molecular manipulations are to be applied towards clarifying the basis for the PPI-disruptive effects of D3 stimulation, it would appear that such studies may need to be pursued in rats, or at least in a different mouse strain.
Acknowledgments
The authors gratefully acknowledge the assistance of Maria Bongiovanni in manuscript preparation.
Research was supported by awards from the Tourette Syndrome Association (MW) and NARSAD (MW), and by MH068366 (NRS) and MH042228 (NRS and MAG). MAG holds an equity interest in San Diego Instruments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bitsios P, Giakoumaki SG, Frangou S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. Psychopharmacology (Berl) 2005;182:144–52. doi: 10.1007/s00213-005-0056-x. [DOI] [PubMed] [Google Scholar]
- Brea J, Castro M, Loza MI, Masaguer CF, Ravina E, Dezi C, Pastor M, et al. QF2004B, a potential antipsychotic butyrophenone derivative with similar pharmacological properties to clozapine. Neuropharmacology. 2006;51:251–62. doi: 10.1016/j.neuropharm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14:190–6. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- Carta AR, Gerfen CR, Steiner H. Cocaine effects on gene regulation in the striatum and behavior: increased sensitivity in D3 dopamine receptor-deficient mice. Neuroreport. 2000;11:2395–9. doi: 10.1097/00001756-200008030-00012. [DOI] [PubMed] [Google Scholar]
- Chang WL, Swerdlow NR, Breier MR, Thangaraj N, Weber M. Parametric approaches towards understanding the effects of the preferential D3 receptor agonist pramipexole on prepulse inhibition in rats. Abstr, Soc Neurosci. 2009 doi: 10.1016/j.pbb.2010.04.001. No 843.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin AE, Pugsley TA, Akunne HC, Whetzel SZ, Zoski KT, Georgic LM, et al. Pharmacological characterization of PD 152255, a novel dimeric benzimidazole dopamine D3 antagonist. Pharmacol Biochem Behav. 1998;59:487–493. doi: 10.1016/s0091-3057(97)00442-5. [DOI] [PubMed] [Google Scholar]
- Culm KE, Lugo-Escobar N, Hope BT, Hammer RP., Jr Repeated quinpirole treatment increases cAMP-dependent protein kinase activity and CREB phosphorylation in nucleus accumbens and reverses quinpirole-induced sensorimotor gating deficits in rats. Neuropsychopharmacology. 2004;29:1823–30. doi: 10.1038/sj.npp.1300483. [DOI] [PubMed] [Google Scholar]
- Curzon P, Decker MW. Effects of phencyclidine (PCP) and (+)MK-801 on sensorimotor gating in CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:129–46. doi: 10.1016/s0278-5846(97)00184-x. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Bardin L, Auclair AL, Kleven MS, Prinssen E, Colpaert F, Vacher B, et al. F15063, a compound with D2/D3 antagonist, 5-HT 1A agonist and D4 partial agonist properties. II. Activity in models of positive symptoms of schizophrenia. Br J Pharmacol. 2007;151:253–65. doi: 10.1038/sj.bjp.0707159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, Geyer MA. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–56. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2003;Chapter 8(Unit 8 17) doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Schmauss C. Effect of methamphetamine on cognition and repetitive motor behavior of mice deficient for dopamine D2 and D3 receptors. Ann N Y Acad Sci. 2004;1025:110–8. doi: 10.1196/annals.1316.014. [DOI] [PubMed] [Google Scholar]
- Harrison SJ, Nobrega JN. Differential susceptibility to ethanol and amphetamine sensitization in dopamine D3 receptor-deficient mice. Psychopharmacology (Berl) 2009;204:49–59. doi: 10.1007/s00213-008-1435-x. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–50. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Kitamura Y, Miyazaki T, Miyaoka J, Kawasaki H, Asanuma M, Sendo T, et al. Effects of pramipexole on the duration of immobility during the forced swim test in normal and ACTH-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:59–66. doi: 10.1007/s00210-009-0405-0. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–26. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Micale V, Drago F. Increased sensitivity to antidepressants of D3 dopamine receptor-deficient mice in the forced swim test (FST) Eur Neuropsychopharmacol. 2008;18:271–7. doi: 10.1016/j.euroneuro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Lehr E. Potential antidepressant properties of pramipexole detected in locomotor and operant behavioral investigations in mice. Psychopharmacology (Berl) 2002;163:495–500. doi: 10.1007/s00213-002-1199-7. [DOI] [PubMed] [Google Scholar]
- Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. The behavioural effects of pramipexole, a novel dopamine receptor agonist. Eur J Pharmacol. 1997;324:31–7. doi: 10.1016/s0014-2999(97)00066-6. [DOI] [PubMed] [Google Scholar]
- Malone DT, Long LE, Taylor DA. The effect of SR 141716 and apomorphine on sensorimotor gating in Swiss mice. Pharmacol Biochem Behav. 2004;77:839–45. doi: 10.1016/j.pbb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Martin S, Markus MA, Morris BJ, Davisson RL, Lawrence AJ, van den Buuse M. Does angiotensin interact with dopaminergic mechanisms in the brain to modulate prepulse inhibition in mice? Neuropharmacology. 2008;54:399–404. doi: 10.1016/j.neuropharm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Levant B, Taylor B, Ahlbrand R, Liu Y, Sullivan JR, et al. C57BL/6J mice exhibit reduced dopamine D3 receptor-mediated locomotor-inhibitory function relative to DBA/2J mice. Neuroscience. 2006;143:141–53. doi: 10.1016/j.neuroscience.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Park WK, Jeong D, Cho H, Lee SJ, Cha MY, Pae AN, Choi KI, et al. KKHA-761, a potent D3 receptor antagonist with high 5-HT1A receptor affinity, exhibits antipsychotic properties in animal models of schizophrenia. Pharmacol Biochem Behav. 2005;82:361–72. doi: 10.1016/j.pbb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Piercey MF, Lum JT, Hoffmann WE, Carlsson A, Ljung E, Svensson K. Antagonism of cocaine's pharmacological effects by the stimulant dopaminergic antagonists, (+)-AJ76 and (+)-UH232. Brain Res. 1992;588:217–22. doi: 10.1016/0006-8993(92)91578-3. [DOI] [PubMed] [Google Scholar]
- Piercey MF, Hoffmann WE, Smith MW, Hyslop DK. Inhibition of dopamine neuron firing by pramipexole, a dopamine D3 receptor-preferring agonist: comparison to other dopamine receptor agonists. Eur J Pharmacol. 1996;312:35–44. doi: 10.1016/0014-2999(96)00454-2. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, et al. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology. 2003;28:100–7. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA, et al. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol Biochem Behav. 2007;86:718–26. doi: 10.1016/j.pbb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Qu Y, Swerdlow NR, Weber M, Stouffer D, Parsons LH. Quinelorane, a dopamine D3/D2 receptor agonist, reduces prepulse inhibition of startle and ventral pallidal GABA efflux: time course studies. Pharmacol Biochem Behav. 2008;90:686–90. doi: 10.1016/j.pbb.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–41. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Effects of selective dopamine D1-like and D2-like agonists on prepulse inhibition of startle in inbred C3H/HeJ, SPRET/EiJ, and CAST/EiJ mice. Psychopharmacology (Berl) 2007;191:731–9. doi: 10.1007/s00213-006-0511-3. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, Grandy DK, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–33. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–11. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–18. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Bitsios P. The dopamine D(3) receptor Ser9Gly polymorphism modulates prepulse inhibition of the acoustic startle reflex. Biol Psychiatry. 2008;64:235–40. doi: 10.1016/j.biopsych.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Russig H, Spooren W, Durkin S, Feldon J, Yee BK. Apomorphine-induced disruption of prepulse inhibition that can be normalised by systemic haloperidol is insensitive to clozapine pretreatment. Psychopharmacology (Berl) 2004;175:143–7. doi: 10.1007/s00213-004-1810-1. [DOI] [PubMed] [Google Scholar]
- Schmauss C. A single dose of methamphetamine leads to a long term reversal of the blunted dopamine D1 receptor-mediated neocortical c-fos responses in mice deficient for D2 and D3 receptors. J Biol Chem. 2000;275:38944–8. doi: 10.1074/jbc.M005064200. [DOI] [PubMed] [Google Scholar]
- Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. Inactivation of the 5-HT(7) receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol Psychiatry. 2008;63:98–105. doi: 10.1016/j.biopsych.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Fujiwara RA. The activity of pramipexole in the mouse forced swim test is mediated by D2 rather than D3 receptors. Psychopharmacology (Berl) 2004;175:163–9. doi: 10.1007/s00213-004-1809-7. [DOI] [PubMed] [Google Scholar]
- Svensson K, Carlsson A, Huff RM, Kling-Petersen T, Waters N. Behavioral and neurochemical data suggest functional differences between dopamine D2 and D3 receptors. Eur J Pharmaco. 1994;263:235–43. doi: 10.1016/0014-2999(94)90718-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Lelham SA, Sutherland Owens AN, Chang WL, Sassen SD, Talledo JA. Pramipexole effects on startle gating in rats and normal men. Psychopharmacology (Berl) 2009;205:689–98. doi: 10.1007/s00213-009-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talledo JA, Owens ANS, Schortinghuis T, Swerdlow NR. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology. 2009;204:165–175. doi: 10.1007/s00213-008-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai M, Okuda A. Endomorphin-1, an endogenous mu-opioid receptor agonist, improves apomorphine-induced impairment of prepulse inhibition in mice. Peptides. 2003;24:741–4. doi: 10.1016/s0196-9781(03)00123-2. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Martin S, Brosda J, Leck KJ, Matthaei KI, Hendry I. Enhanced effect of dopaminergic stimulation on prepulse inhibition in mice deficient in the alpha subunit of G(z) Psychopharmacology (Berl) 2005;183:358–67. doi: 10.1007/s00213-005-0181-6. [DOI] [PubMed] [Google Scholar]
- Weber M, Chang WL, Breier M, Ko D, Swerdlow NR. Heritable strain differences in sensitivity to the startle gating-disruptive effects of D2 but not D3 receptor stimulation. Behav Pharmacol. 2008;19:786–795. doi: 10.1097/FBP.0b013e32831c3b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang WL, Durbin JP, Park PE, Luedtke RR, Mach RH, Swerdlow NR. Using prepulse inhibition to detect functional D3 receptor antagonism: effects of WC10 and WC44. Pharmacol Biochem Behav. 2009;93:141–7. doi: 10.1016/j.pbb.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Koda K, Ago Y, Kobayashi H, Kawasaki T, Takuma K, Matsuda T. Galantamine improves apomorphine-induced deficits in prepulse inhibition via muscarinic ACh receptors in mice. Br J Pharmacol. 2009;156:173–80. doi: 10.1111/j.1476-5381.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Russig H, Feldon J. Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology. 2004;29:240–8. doi: 10.1038/sj.npp.1300323. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Adomako-Mensah J, Yuferov V, Ho A, Zhang J, Xu M, Kreek M. Effects of acute “binge” cocaine on mRNA levels of mu opioid receptor and neuropeptides in dopamine D1 or D3 receptor knockout mice. Synapse. 2007;61:50–9. doi: 10.1002/syn.20340. [DOI] [PubMed] [Google Scholar]