Abstract
Purpose of Review
To discuss recent studies which have evaluated determinants of cystatin C and to focus on the relationship of cystatin C with mortality, cardiovascular disease (CVD) and non-cardiovascular outcomes.
Recent Findings
In the Chronic Kidney Disease Epidemiology Study cystatin C was associated with demographic characteristics independent of measured glomerular filtration rate (GFR), although this was to a smaller extent than creatinine. In patients with established CKD, cystatin C was strongly and inversely correlated with measured GFR, suggesting that although cystatin C may have other determinants, it is primarily a measure of kidney function. Several cohort studies, particularly in older adults, have now demonstrated that cystatin C is linearly associated with mortality, CVD and non-CVD outcomes, whereas creatinine is primarily associated with risk in individuals with more advanced kidney disease. A recent study has also shown that changes in kidney function as ascertained by cystatin C, even within the relatively normal range, are associated with subsequent CVD and all-cause mortality among older adults.
Summary
Cystatin C appears to capture an association of mild kidney disease with increased risk of mortality, CVD and non-CVD outcomes. Future studies should evaluate whether cystatin C can improve medical decision-making and lead to favorable patient outcomes.
Keywords: cystatin C, kidney disease, cardiovascular disease, mortality
Introduction
Cystatin C is a measure of kidney function that appears less sensitive than creatinine to factors other than glomerular filtration rate (GFR), particularly muscle mass. Cystatin C is measured in serum, making it a practical alternative to creatinine or estimated GFR (eGFR) based on serum creatinine. In this review we briefly discuss cystatin C as a measure of GFR and then focus our review on studies that have compared cystatin C, eGFR, measured GFR and creatinine as risk factors for mortality, cardiovascular disease (CVD) and non-CVD outcomes in the general population and in those with chronic kidney disease (CKD).
Determinants of cystatin C and its function as a measure of GFR
Determinants of cystatin C
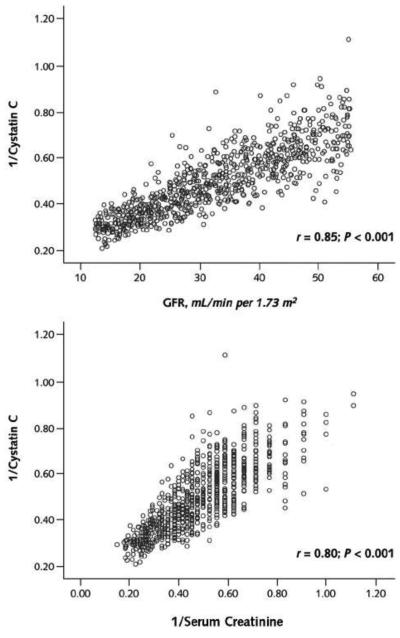
Köttgen and colleagues reported the population distributions of cystatin C in the United States using data from the Third National Health and Nutrition Examination Survey (NHANES III) (1). Abnormal cystatin C levels were defined as the 99th percentile of the cystatin C distribution among 20-40 year olds without hypertension or diabetes (> 1.12 mg/L). Median cystatin C levels were greater in men and non-Hispanic white persons and the prevalence of abnormal levels increased with age from only 1% in the 20-40 year old group to over 50% in persons over 80 years of age. In persons 60 years or older, other than demographics, factors associated with higher cystatin C levels included established risk factors for kidney disease. In 3418 subjects in the Chronic Kidney Disease Epidemiology (CKD Epi) Study (mean age 52 years and mean GFR 48 mL/min/1.73 m2), although both creatinine and cystatin C were associated with demographic factors independent of direct measures of GFR, this was to a larger extent for serum creatinine (2). Increased weight and height, current smoking, higher C reactive protein levels, hyperthyroidism and glucocorticoid use have also been associated higher cystatin C levels (3,4,5) and more recent studies have noted associations with obesity and waist circumference (6,7), as well as secretion of cystatin C by adiposites (8). In the Framingham Study, visceral and subcutaneous adipose tissue were associated with cystatin eGFR but not creatinine eGFR suggesting either that creatinine eGFR is not an accurate measure of kidney function or that cystatin eGFR is associated with adiposity independent of GFR (9). It is important to note that GFR was not directly measured in the latter studies. In the Modification of Diet in Renal Disease (MDRD) Study, a study of patients with mean iothalamate GFR of 32 ml/min/1.73 m2, cystatin C was however highly correlated with GFR (Figure 1), suggesting that kidney function is the primary determinant of cystatin C in CKD (10).
Figure 1.
Correlations between 1/cystatin C and measured glomerular filtration rate (GFR) (top) and 1/serum creatinine (bottom)
Reproduced with permission from Menon, V, Shlipak, M Wang, X et al. Cystatin C as a risk factor for outcomes in CKD. Ann Intern Med 147 :19-27,2007
Cystatin C as a measure of GFR
Multiple studies have compared cystatin C and creatinine as predictors of GFR. Most studies have found cystatin C to be a better predictor, although others have found no difference. In a meta-analysis by Dharnidharka et al. the reciprocal of cystatin C had a greater approximation of GFR compared with the reciprocal of serum creatinine as measured both by a higher average correlation coefficient (0.82 vs. 0.74; p<0.001) and a higher area under the receiver operator characteristic (ROC) curve (0.93 vs. 0.84; p<0.001) (11).
Investigators from CKD-EPI developed 3 GFR estimating equations for cystatin C: using cystatin C alone, cystatin C with demographic coefficients, and cystatin C with creatinine and demographic coefficients. Cystatin C alone provided GFR estimates that were nearly as accurate as the MDRD equation (82 vs. 83% of the variability in GFR explained, respectively) however an equation including cystatin C, creatinine and demographic coefficients provided the most accurate estimates (87% of variability in GFR explained) (2). Among patients with known CKD, defined as creatinine-based eGFR< 60 ml/min 1.73m2, cystatin C offered only a moderate gain over creatinine for approximating GFR (2); however, in patients with early kidney disease, for example diabetics, changes in GFR over time have a stronger correlation with cystatin C than creatinine (12,13).
Cystatin C may also have advantages over creatinine in conditions of decreased mass including older adults, those with chronic diseases (such as heart failure, cirrhosis, AIDS) and those without established CKD; however since studies in these populations lack direct GFR measurements, validated equations incorporating cystatin C have not been developed (14).
Cystatin C and Mortality
Older adults
Cystatin C and creatinine were compared as risk factors for mortality over 10 years in the Cardiovascular Health Study (CHS), a community-based cohort of ambulatory older adults in the US. Cystatin C had a linear association with mortality risk across its entire distribution. In contrast, associations of creatinine and creatinine eGFR with mortality risk were J-shaped with only the lowest quintile of eGFR associated with increased mortality risk (15). The different associations of cystatin C and creatinine with mortality were confirmed in a second cohort of older adults - the Health, Aging, and Body Composition (Health ABC) Study. Cystatin C, but not creatinine, was independently associated with mortality in adjusted analyses (16).
Chronic Kidney Disease
Menon et al. evaluated cystatin C as a risk factor for long-term outcomes in 825 patients from the MDRD Study. In multivariate-adjusted models, a 1-SD decrease in 1/creatinine, iothalamate GFR, and 1/cystatin C were associated with 1.27 (95% CI, 1.06 to 1.49), 1.27 (95% CI, 1.08 to 1.49) and 1.41 (95% CI, 1.18 to 1.67) increased risk for all cause mortality, and a 1.32 (95% CI, 1.05 to 1.64), 1.28 (CI, 1.04 to 1.59) and 1.64 (CI, 1.28 to 2.08) increased risk for CVD mortality, respectively (10). The authors concluded that the association of cystatin C level with all-cause and CVD mortality is perhaps stronger than that of iothalamate GFR. Two potential explanations for this result were elaborated upon: First, cystatin C has non-kidney influences that are independent of GFR, which are associated with mortality risk. As above cystatin C was however highly correlated with GFR (figure 1) suggesting that other determinants are likely minor. Second, the coefficient of variation of measurement of iothalamate GFR is higher than that of cystatin C; therefore, cystatin C may more accurately represent level of kidney function.
Cystatin C and CVD
CVD risk factors
Cystatin C has been associated with incident hypertension and pre-diabetes (17,18). In the Multi-Ethnic Study of Atherosclerosis, a community-based study of subclinical cardiovascular disease in adults age 45 to 84 years, cystatin C was a risk factor for incident hypertension amongst patients without CKD or hypertension at baseline (17) (Figure 2), while in a nested case-control investigation from the Western New York Study cystatin C (but not creatinine or albuminuria) was associated with an increased risk for development of pre-diabetes (defined as a fasting glucose < 100 mg/dl at baseline and 100-125 mg/dl in follow-up) (18). The authors suggested that level of kidney function may be involved in the pathogenesis of hypertension and that mild kidney dysfunction may occur early and in parallel with the natural history of diabetes.
Figure 2.
Incident hypertension rates within quartiles of serum cystatin C and sex-specific urinary albumin–creatinine ratio
Rates are adjusted for sex, age and race
Reproduced with permission from Kestenbaum, B, Rudser, K, de Boer, I et al. Differences in Kidney Function and Incident Hypertension: The Multi-Ethnic Study of Atherosclerosis. Ann Intern Med 148 (7): 501-508, 2008
CVD outcomes
Heart Failure
Cystatin C and creatinine were compared as risk factors for heart failure in CHS. As for mortality, cystatin C had linear associations with heart failure, whereas creatinine and eGFR were associated with increased risk only for those in the quintile with the worst kidney function (19). Cystatin C was also more strongly associated with mortality risk in those with established heart failure than creatinine or creatinine eGFR (20).
More recently cystatin C was evaluated as a risk factor for incident diastolic heart failure (ejection fraction >50%) or systolic heart failure (ejection fraction < 50%) in CHS. After 8 years of follow up, 167 and 206 patients developed diastolic and systolic heart failure, respectively. In adjusted analysis higher quartiles of cystatin C were associated with systolic heart failure but the risk of diastolic heart failure was only apparent in the highest cystatin C quartile (21). This study was however limited by a moderate group of subjects who developed heart failure but who did not have echocardiographic data available.
In a case control study (n=440) from the Physicians Health Study cystatin C was associated with incident heart failure amongst hypertensive but not non-hypertensive individuals (22). The authors raised the question whether cystatin C may be a biomarker of earlier blood pressure effects on heart failure.
Coronary Artery Disease
Several studies have compared creatinine and cystatin C as predictors of outcomes in subjects with established coronary heart disease (23-25). In 726 persons hospitalized with acute coronary syndromes, after a median follow-up of 40 months, the fourth quartile of cystatin C was independently associated with increased mortality. Cystatin C had an area under the ROC curve of 0.79 (95% CI 0.74 to 0.83), whereas creatinine had an area under the ROC curve of 0.66 (95% CI 0.61 to 0.72) for predicting death (23). Koenig and colleagues also compared cystatin C and creatinine as predictors of CVD events among 1,033 patients with established coronary heart disease. Cystatin C again had a stronger and more linear association with CVD risk than creatinine or creatinine eGFR (24). A recent study using data from the Uppsala Longitudinal Study of Adult Men (ULSAM), a community-based cohort of older men, investigated whether a combination of biomarkers that reflect myocardial cell damage, left ventricular dysfunction, kidney disease, and inflammation (troponin I, N-terminal pro–brain natriuretic peptide, cystatin C, and C-reactive protein, respectively) improved risk stratification beyond established risk factors for CVD. After adjustment for established risk factors for CVD, all of the biomarkers were significantly associated with CVD mortality. Each 1-SD increase in cystatin C was associated with a 1.43 (95% CI 1.22-1.66) higher hazard ratio for CVD mortality. In addition, when the four biomarkers were incorporated into a model with established risk factors, the C statistic increased (C statistic with biomarkers vs. without biomarkers, 0.766 vs. 0.664; P<0.001) (25).
Stroke
Cystatin C has also been associated with cerebrovascular events. In a case control cohort of 293 stroke patients and 894 controls, higher cystatin C levels were associated with increased risk of stroke (26). In CHS cystatin C was associated with subclinical brain infarcts (SBI) (defined as an infarct-like lesion ≥3 mm in a participant without a previous stroke or TIA) in a cross-sectional study that included 2784 subjects with MRI imaging. There was a strong and linear association with SBI for 1/cystatin C (P < 0.001) but not for 1/Creatinine ((P = 0.14), for which a quadratic U-shaped association was suggested (P = 0.004) (27).
Peripheral Artery Disease
Some (28, 29) but not all (30) studies have noted a relationship between cystatin C and peripheral artery disease (PAD). In 4025 individuals from CHS cystatin C was associated with incident PAD (hazard ratio 2.5 (95% CI, 1.2-5.1) for the highest vs lowest quintile of cystatin C). The highest quintiles of serum creatinine level and creatinine eGFR were not associated with PAD in either unadjusted or adjusted analyses (28). In contrast, in a nested case– control study of healthy men who subsequently developed symptomatic PAD, cystatin-C levels were virtually identical among case and control subjects (median 0.83 mg/l in both groups, p = 0.84) (30).
Non-CVD outcomes
Aging Success
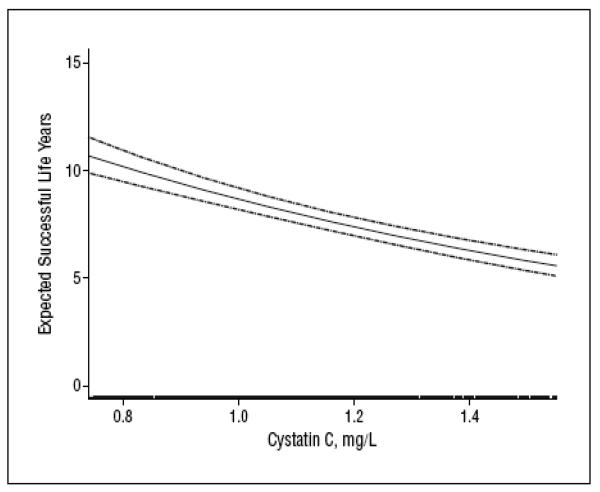
Cystatin C was evaluated as a risk factor for aging success during a 6-year follow up of CHS. Successful aging was defined as remaining free of CVD, cancer, and chronic obstructive pulmonary disease and having intact physical and cognitive functioning. A total of 2140 patients free of the above-mentioned conditions were included. 873 patients reached a first event in follow-up. The adjusted percentage reduction in successful life years in the highest vs the lowest quartile of cystatin C was 27% (95% confidence interval, 11%-39%) (Figure 3). The risk in the highest quartile of cystatin C was as strongly associated with unsuccessful aging as a decreased arm-ankle index, left ventricular hypertrophy, or prevalent diabetes mellitus, and more strongly associated than a history of hypertension or an increase in age of 5 years (31).
Figure 3.
Smoothed spline of expected successful life years and baseline cystatin C level. Pointwise 95% confidence intervals (dashed lines) and a rug of the middle 95% of cystatin C measures are shown
Reproduced with permission from Sarnak M, Katz, R, Fried L et al. Cystatin C and Aging Success. Arch Intern Med. 168(2):147-153,2008
Non-CVD mortality
In 4637 individuals from CHS who were followed for 8 years, rates of non-CVD mortality attributed primarily to pulmonary disease, cancer and infection were increased with increasing quartiles of cystatin C (16.8, 17.1, 21.6, and 50.0 per 1000 person-years, respectively) and the association persisted after adjusting for potential confounders (32).
Musculoskeletal Disease and Physical Function
Patients with kidney failure are known to be at increased risk for hip fractures. However, recent studies have also noted an association between mild to moderate kidney disease with hip fractures (33). In CHS after a mean follow up time of 7.1 years, 195 and 79 hip fractures occurred in women and men, respectively. In multivariate models, higher cystatin C levels were significantly associated with hip fracture in women but not in men (34). Cystatin C was also associated with more rapid loss of bone mineral density at the hip using DEXA, especially in men (35).
Several studies have noted an association between cystatin C and physical function (31, 36). In a cross sectional analysis of patients with established coronary heart disease, high cystatin C concentrations were associated with worse exercise capacity and delayed heart rate recovery (37). In Health ABC high cystatin C levels were associated with worse physical function in cross-sectional analyses (36). In longitudinal analyses the association between cystatin C and incident functional limitation was attenuated after adjustment for inflammatory markers, suggesting that inflammation may be a mediating factor (38).
Cognitive impairment
Cognitive impairment is common in all stages of CKD. In the general population, the two most common causes of dementia in adults are Alzheimer's disease and vascular dementia (39). Although higher cystatin C is a risk factor for cognitive impairment (31,40), it may also be protective for the development of Alzheimer's disease (41). In 3030 individuals enrolled in Health ABC, those with high cystatin C had worse baseline cognitive scores on the modified Mini-Mental State Exam (p=0.01) or Digit Symbol Substitution Test (p=0.02) compared to those with intermediate or low levels of cystatin C. After 7 years of follow up, those with higher cystatin C levels also had a more pronounced decline and higher incidence of cognitive impairment (40). In contrast, in ULSAM, lower cystatin C was associated with higher risk of Alzheimers Disease (41). Experimental models in mice have shown that over-expression of human cystatin C in brains of amyloid precursor protein-transgenic mice reduces cerebral amyloid-beta deposition and binds amyloid beta inhibiting fibril formation (42). In combination, these results suggest that high levels of cystatin C may be associated with cognitive impairment mediated through cystatin C effect on vascular disease; however, low cystatin C levels may be associated with higher risk of Alzheimers Disease through mechanisms independent of kidney function.
Change in Cystatin C
The studies described above evaluated baseline cystatin C and its association with outcomes. In a recent analysis from CHS longitudinal declines in kidney function and their association with subsequent outcomes was evaluated. In 4,380 participants followed over 9.9 years, the slope of annual decline in eGFR was estimated using both serum creatinine and cystatin C. Rapid decline in eGFR was defined by a loss of GFR greater than 3ml/min/1.73 m2/year as this change is 3 times the rate previously described in studies of normal aging (43), and beyond the range of noise in measurement. Both rapid decline by creatinine and cystatin C eGFR (Figure 3) were significantly associated with all-cause and CVD mortality. Rapid decline by cystatin C eGFR was however more sensitive in detecting rapid decline than creatinine eGFR (25% vs 16% of the cohort, respectively) (44).
Cystatin C and a “preclinical state of CKD”
One of the important uses of cystatin C may be to capture the gradient of kidney function among persons who do not meet conventional definitions of CKD (creatinine eGFR < 60 ml/min/1.73 m2). The term “preclinical kidney disease” has been defined as creatinine eGFR >60 ml/min/1.73 m2 with cystatin C levels ≥1.0mg/L. This group is at higher risk for development of creatinine eGFR < 60 ml/min/1.73 m2 as well as CVD and mortality outcomes (45).
Conclusions and Future directions
Cystatin C is a promising measure of GFR that may be an alternative or a complement to serum creatinine. It may provide a more accurate estimate of kidney function than serum creatinine, particularly in individuals susceptible to muscle loss. Cystatin C is also a more powerful risk factor for outcomes in these same individuals. The use of cystatin C has led to appreciation of small declines in kidney function as an important prognostic indicator. The question remains whether identification of subjects at high risk through use of cystatin C should lead to more aggressive targeting of risk factors and whether this will translate into improved patient outcomes.
Acknowledgements
RO1 AG 027002 and K24 DK078204
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Köttgen A, Selvin E, Stevens LA, et al. Serum cystatin C in the United States: The Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. This analysis from the Third National Health and Nutrition Examination Survey (NHANES III) evaluates the determinants of cystatin C across a wide age range and different ethnicities. Serum cystatin C level was related to sex and ethnicity, even in young healthy individuals. The prevalence of increased cystatin C levels increased dramatically with age, reaching greater than 50% after the age of 80 years in both sexes and all ethnic groups. [DOI] [PubMed] [Google Scholar]
- 2*.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. In a pooled analyses of 3,418 individuals with CKD (mean GFR 48 ml/min/1.73 m2), serum cystatin C level alone provided GFR estimates that were nearly as accurate as serum creatinine level adjusted for age, sex, and race. An equation including serum cystatin C level in combination with serum creatinine level, age, sex, and race provided the most accurate estimate of GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 4.Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28(4):346–9. doi: 10.1007/BF03347201. 2005. [DOI] [PubMed] [Google Scholar]
- 5.Risch L, Herklotz R, Blumberg A, et al. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47(11):2055–9. [PubMed] [Google Scholar]
- 6.Muntner P, Winston J, Uribarri J, et al. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med. 2008;121:341–348. doi: 10.1016/j.amjmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Retnakaran R, Connelly PW, Harris SB, et al. Cystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youth. Pediatr Nephrol. 2007;22:1007–1013. doi: 10.1007/s00467-007-0471-9. [DOI] [PubMed] [Google Scholar]
- 8.Taleb S, Cancello R, Clement K, et al. Cathepsin s promotes human preadipocyte differentiation: Possible involvement of fibronectin degradation. Endocrinology. 2006;147:4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 9.Young JA, Hwang S, Sarnak M, et al. Association of Visceral and Subcutaneous Adiposity with Kidney Function. Clin J Am Soc Nephrol. 2008;3:1786–1791. doi: 10.2215/CJN.02490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Menon V, Shlipak M, Wang X, et al. Cystatin C as a risk factor for outcomes in CKD. Ann Intern Med. 2007;147:19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. This study using data from the Modification in Diet in Renal Disease (MDRD) Study compared cystatin C, serum creatinine, estimated glomerular filtration rate (GFR) and measured GFR as risk factors for death and kidney failure in chronic kidney disease (CKD). The association of cystatin C level with all cause and CVD mortality was perhaps stronger than that of iothalamate GFR. [DOI] [PubMed] [Google Scholar]
- 11.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 12.Premaratne E, MacIsaac RJ, Finch S, et al. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;5:971–3. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]
- 13.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16(5):1404–12. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlipak MG. Cystatin C: research priorities targeted to clinical decision making. Am J Kidney Dis. 2008;51(3):358–61. doi: 10.1053/j.ajkd.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and Mortality Risk in the Elderly: The Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2006;17(1):254–61. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 17**.Kestenbaum B, Rudser K, de Boer I, et al. Differences in Kidney Function and Incident Hypertension: The Multi-Ethnic Study of Atherosclerosis. Ann Intern Med. 2008;148(7):501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. Using data from the Multi-Ethnic Study of Atherosclerosis (MESA), the authors examined the association between cystatin C and incident hypertension amongst 2767 participants without prevalent hypertension. Cystatin C was associated with incident hypertension among individuals without clinical kidney or cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donahue RP, Stranges S, Rejman K, et al. Elevated cystatin C concentration and progression to pre-diabetes: the Western New York study. Diabetes Care. 2007;(7):1724–9. doi: 10.2337/dc07-0040. [DOI] [PubMed] [Google Scholar]
- 19.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 20.Shlipak MG, Katz R, Fried LF, et al. Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005;45(2):268–71. doi: 10.1016/j.jacc.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 21.Moran A, Katz R, Smith NL, Fried LF, et al. Cystatin C concentration as a predictor of systolic and diastolic heart failure. J Card Fail. 2008;14(1):19–26. doi: 10.1016/j.cardfail.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djoussé L, Kurth T, Gaziano JM. Cystatin C and risk of heart failure in the Physicians' Health Study (PHS) Am Heart J. 2008;155(1):82–6. doi: 10.1016/j.ahj.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jernberg T, Lindahl B, James S, et al. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation. 2004;110(16):2342–8. doi: 10.1161/01.CIR.0000145166.44942.E0. [DOI] [PubMed] [Google Scholar]
- 24.Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51(2):321–7. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- 25**.Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107–16. doi: 10.1056/NEJMoa0707064. In this study that included 1135 patients from the Uppsala Longitudinal Study of Adult Men (ULSAM), the simultaneous addition of several biomarkers of cardiovascular and kidney abnormalities including cystatin C substantially improved prediction of CVD death beyond that of a model based only on established risk factors. [DOI] [PubMed] [Google Scholar]
- 26.Ni L, Lü J, Hou LB, et al. Cystatin C, associated with hemorrhagic and ischemic stroke, is a strong predictor of the risk of cardiovascular events and death in Chinese. Stroke. 2007;38(12):3287–8. doi: 10.1161/STROKEAHA.107.489625. [DOI] [PubMed] [Google Scholar]
- 27.Seliger SL, Longstreth WT, Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16(12):3721–7. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare AM, Newman AB, Katz R, et al. Cystatin C and incident peripheral arterial disease events in the elderly: results from the Cardiovascular Health Study. Arch Intern Med. 2005;165(22):2666–70. doi: 10.1001/archinte.165.22.2666. [DOI] [PubMed] [Google Scholar]
- 29.Arpegård J, Ostergren J, de Faire U, et al. Cystatin C--a marker of peripheral atherosclerotic disease? Atherosclerosis. 2008;199(2):397–401. doi: 10.1016/j.atherosclerosis.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Albert MA, Rifai N, Ridker PM. Plasma levels of cystatin-C and mannose binding protein are not associated with risk of developing systemic atherosclerosis. Vasc Med. 2001;6(3):145–9. doi: 10.1177/1358836x0100600304. [DOI] [PubMed] [Google Scholar]
- 31**.Sarnak M, Katz R, Fried L, et al. Cystatin C and Aging Success. Arch Intern Med. 2008;168(2):147–153. doi: 10.1001/archinternmed.2007.40. This study evaluated the association of cystatin C with successful aging in a cohort of 2140 patients from the Cardiovascular Health Study. Cystatin C, even within the range of relatively normal kidney function, was associated with a reduction in successful life years. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16(12):3728–35. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 33.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–9. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 34.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18(1):282–6. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 35.Fried LF, Shlipak MG, Stehman-Breen C, et al. Kidney function predicts the rate of bone loss in older individuals: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2006;61(7):743–8. doi: 10.1093/gerona/61.7.743. [DOI] [PubMed] [Google Scholar]
- 36.Odden MC, Chertow GM, Fried LF, et al. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006;164:1180–1189. doi: 10.1093/aje/kwj333. [DOI] [PubMed] [Google Scholar]
- 37.McManus D, Shlipak M, Ix JH, et al. Association of cystatin C with poor exercise capacity and heart rate recovery: data from the heart and soul study. Am J Kidney Dis. 2007;49(3):365–72. doi: 10.1053/j.ajkd.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: Health, Aging and Body Composition study. J Am Geriat Soc. 2006;54(5):750–6. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 39.Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 40.Yaffe K, Lindquist K, Shlipak MG, et al. Cystatin C as a marker of cognitive function in elders: findings from the health ABC study. Ann Neurol. 2008;63(6):798–802. doi: 10.1002/ana.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundelöf J, Arnlöv J, Ingelsson E, et al. Serum cystatin C and the risk of Alzheimer disease in elderly men. Neurology. 2008;71(14):1072–9. doi: 10.1212/01.wnl.0000326894.40353.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaeser SA, Herzig MC, Coomaraswamy J, et al. Cystatin C modulates cerebral beta-amyloidosis. Nat Genet. 2007;39(12):1437–9. doi: 10.1038/ng.2007.23. [DOI] [PubMed] [Google Scholar]
- 43.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 44**.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Int Med. 2008;168(20):2212–8. doi: 10.1001/archinte.168.20.2212. Longitudinal changes in kidney function were evaluated as risk factors for mortality in 4380 participants from the Cardiovascular Health Study. Kidney function was ascertained by creatinine and cystatin C estimates of GFR. Those patients with rapid kidney function decline over time (loss greater than 3 mL/min/1.73 m2 per year) had increased mortality risk, independent of baseline eGFR and other demographic variables. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]