Abstract
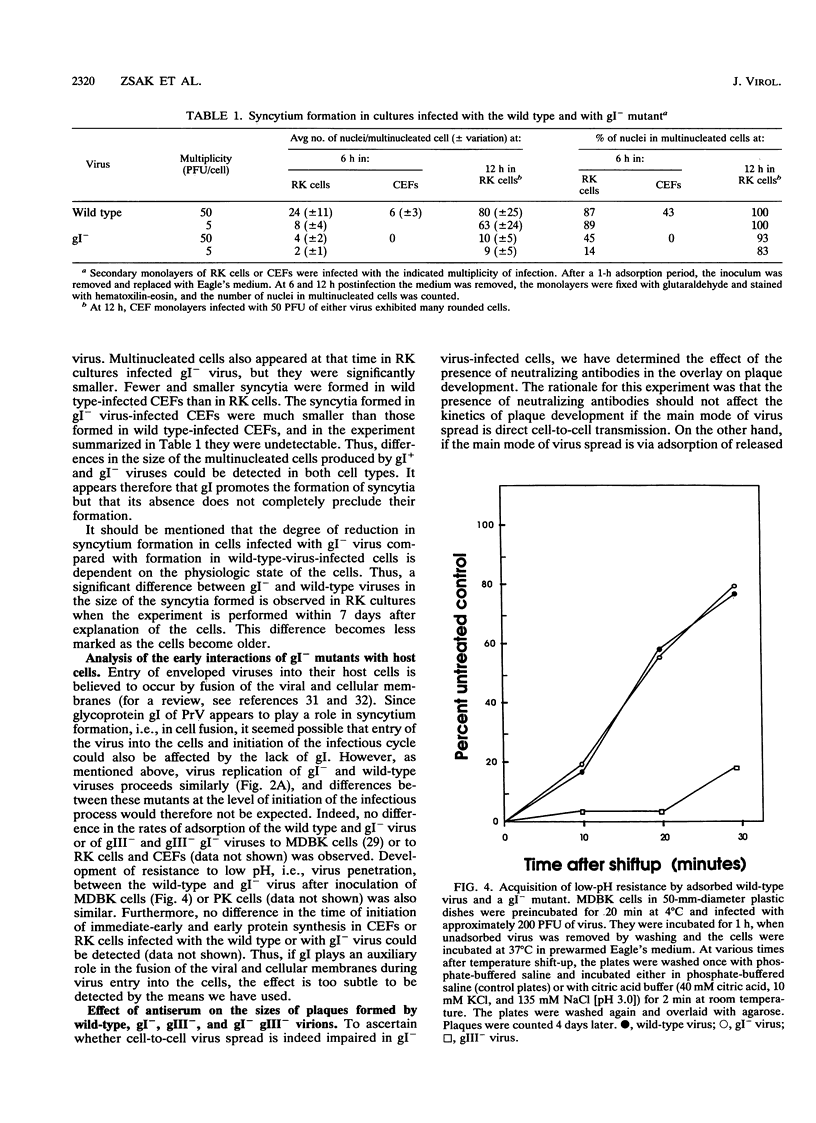
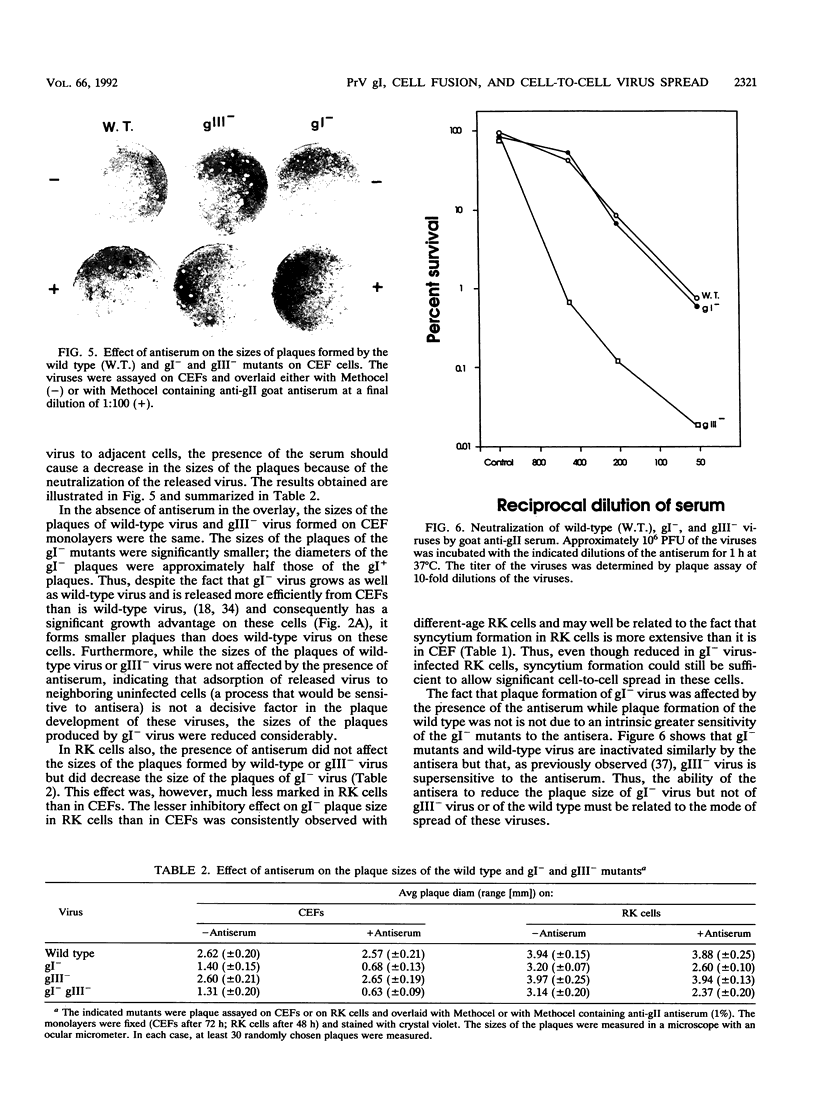
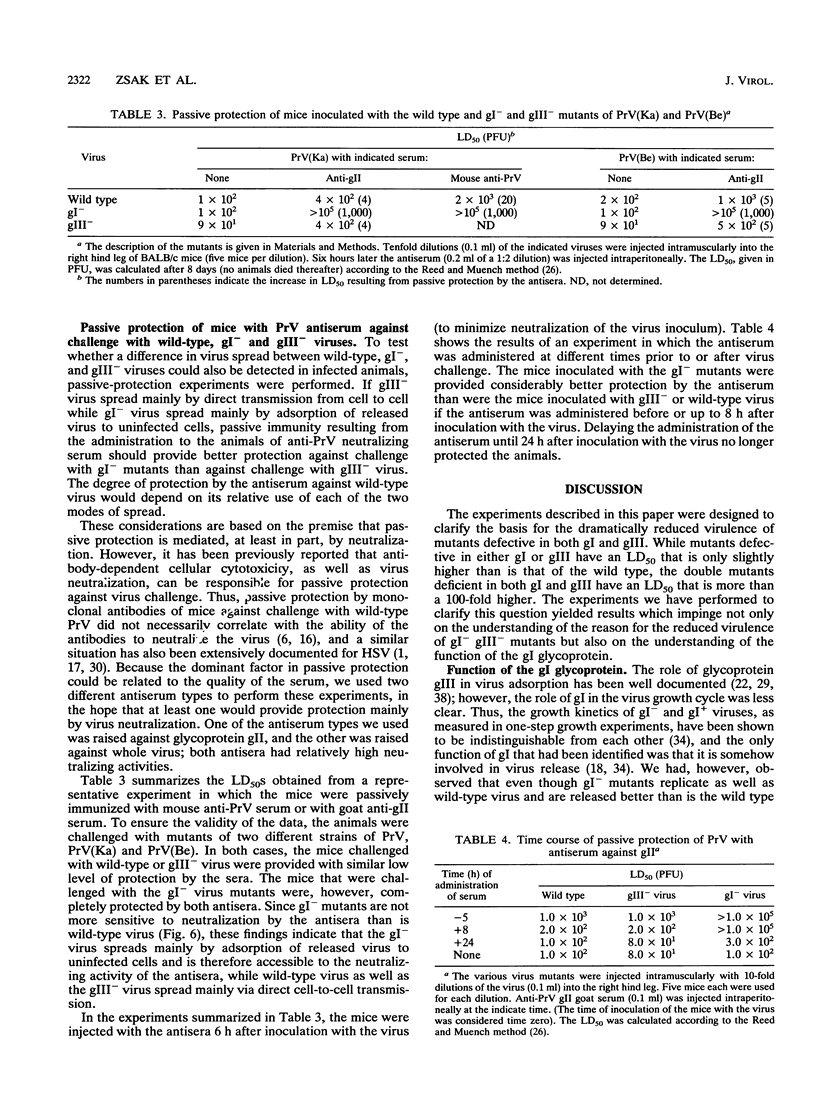
Mutants of pseudorabies virus defective in either glycoprotein gI or gIII are only slightly less virulent for mice and chickens than is wild-type virus, while mutants defective in both gI and gIII are avirulent. To clarify the reason for the lack of virulence of the gI- gIII- mutants, we have analyzed in some detail the interactions of these mutants with their hosts. The results obtained showed that the gI glycoprotein is an accessory protein that promotes cell fusion. This conclusion is based on the findings that in some cell types, syncytium formation is significantly reduced in mutants deficient in gI. Furthermore, despite efficient replication, gI- mutants form significantly smaller plaques on some cell types. Finally, while wild-type and gI- virus are neutralized similarly by antisera, the size of the plaques formed by gI- mutants, but not by wild-type virus, is reduced by the presence of neutralizing antibodies in the overlay. Passive immunization of mice with neutralizing antipseudorabies virus sera is also considerably more effective in protecting them against challenge with gI- mutants than in protecting them against challenge with wild-type virus. These results show that gI- mutants are deficient in their ability to form syncytia and to spread directly by cell-to-cell transmission and that these mutants spread mainly by adsorption of released virus to uninfected cells. Wild-type virus and gIII- mutants, however, spread mainly via direct cell-to-cell transmission both in vivo and in vitro. We postulate that the lack of virulence of the gIII- gI- virus is attributable to its inability to spread by either mode, the defect in gIII affecting virus spread by adsorption of released virus and the defect in gI affecting cell-to-cell spread. Although a gI- gIII- mutant replicates as well as a gIII- mutant, it will be amplified much less well. Our results with in vitro systems show that this is indeed the case.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Avitabile E., Fini S., Stirpe D., Arsenakis M., Roizman B. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology. 1988 Oct;166(2):598–602. doi: 10.1016/0042-6822(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Eloit M., Bouzghaia H., Toma B. Identification of antigenic sites on pseudorabies virus glycoprotein gp50 implicated in virus penetration of the host cell. J Gen Virol. 1990 Sep;71(Pt 9):2179–2183. doi: 10.1099/0022-1317-71-9-2179. [DOI] [PubMed] [Google Scholar]
- Eloit M., Fargeaud D., L'Haridon R., Toma B. Identification of the pseudorabies virus glycoprotein gp50 as a major target of neutralizing antibodies. Arch Virol. 1988;99(1-2):45–56. doi: 10.1007/BF01311022. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp B. G., Mettenleiter T. C. Sequence and expression of the glycoprotein gH gene of pseudorabies virus. Virology. 1991 Jun;182(2):732–741. doi: 10.1016/0042-6822(91)90614-h. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Kaplan A. S., Ben-Porat T. Multiple defects in the genome of pseudorabies virus can affect virulence without detectably affecting replication in cell culture. Virology. 1987 Nov;161(1):181–189. doi: 10.1016/0042-6822(87)90184-x. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Watanabe S., Ben-Porat T., Kaplan A. S. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987 Mar;61(3):796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioli C., Yancey R. J., Jr, Timmins J. G., Post L. E., Young B. R., Povendo D. A. Protection of mice and swine from pseudorabies virus-induced mortality by administration of pseudorabies virus-specific mouse monoclonal antibodies. Am J Vet Res. 1988 Jun;49(6):860–864. [PubMed] [Google Scholar]
- Mester J. C., Glorioso J. C., Rouse B. T. Protection against zosteriform spread of herpes simplex virus by monoclonal antibodies. J Infect Dis. 1991 Feb;163(2):263–269. doi: 10.1093/infdis/163.2.263. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lomniczi B., Sugg N., Schreurs C., Ben-Porat T. Host cell-specific growth advantage of pseudorabies virus with a deletion in the genome sequences encoding a structural glycoprotein. J Virol. 1988 Jan;62(1):12–19. doi: 10.1128/jvi.62.1.12-19.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T., Kaplan A. S. Role of glycoprotein gIII of pseudorabies virus in virulence. J Virol. 1988 Aug;62(8):2712–2717. doi: 10.1128/jvi.62.8.2712-2717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987 Sep;61(9):2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Kaplan A. S., Ben-Porat T., Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987 Dec;61(12):4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Dorney D. J., Wathen M. W., Whealy M. E., Gold C., Watson R. J., Holland L. E., Weed S. D., Levine M., Glorioso J. C. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987 Sep;61(9):2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Nash A. A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985 Mar;53(3):944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus glycoprotein gIII is required for efficient virus growth in tissue culture. J Virol. 1988 Jul;62(7):2512–2515. doi: 10.1128/jvi.62.7.2512-2515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Mettenleiter T. C., Sugg N., Ben-Porat T. Effect of polylysine on the early stages of infection of wild type pseudorabies virus and of mutants defective in gIII. Virology. 1990 Nov;179(1):330–338. doi: 10.1016/0042-6822(90)90301-7. [DOI] [PubMed] [Google Scholar]
- Zsak L., Mettenleiter T. C., Sugg N., Ben-Porat T. Release of pseudorabies virus from infected cells is controlled by several viral functions and is modulated by cellular components. J Virol. 1989 Dec;63(12):5475–5477. doi: 10.1128/jvi.63.12.5475-5477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Sugg N., Ben-Porat T., Robbins A. K., Whealy M. E., Enquist L. W. The gIII glycoprotein of pseudorabies virus is involved in two distinct steps of virus attachment. J Virol. 1991 Aug;65(8):4317–4324. doi: 10.1128/jvi.65.8.4317-4324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F. A., Mettenleiter T. C., Schreurs C., Sugg N., Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988 Dec;62(12):4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F., Zsak L., Reilly L., Sugg N., Ben-Porat T. Early interactions of pseudorabies virus with host cells: functions of glycoprotein gIII. J Virol. 1989 Aug;63(8):3323–3329. doi: 10.1128/jvi.63.8.3323-3329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]