Abstract
Aging is a major risk factor for hypertension and cardiovascular disease. Accumulating evidence suggests that telomere length is a marker for biological aging of the cardiovascular system. Telomere length is determined by genetic and environmental factors. Studies in different racial populations are required to determine the prognostic value of telomere length in hypertension and cardiovascular diseases. The main objective of this study was to investigate the association between leukocyte telomere length and the risk and prognosis of hypertension in a Chinese population. The relative telomere length of leukocytes was determined by a quantitative PCR-based method in 767 subjects: 379 healthy controls and 388 hypertensive patients, ages 30 to 80 years. The median telomere length ratio, 0.57 (interquartile range: 0.48 to 0.72), was shorter in hypertensive than in healthy normotensive subjects (0.67; interquartile range: 0.53 to 0.93; P<0.001). After 5 years of follow-up, subjects with shorter telomeres were at a higher risk of developing coronary artery disease than individuals with longer telomeres (odds ratio: 3.315; 95% CI: 1.662 to 6.609; P<0.001). Multivariate analysis showed that short telomere length and hypertension were independent risk factors for developing coronary artery disease. Our data suggest that mean leukocyte telomere length is a potential predictor of coronary artery disease and support the hypothesis that differences in biological aging can contribute to the risk and variability of developing hypertension and cardiovascular diseases.
Keywords: aging, cardiovascular diseases, risk factor, genetics
Aging is a major risk factor for hypertension. Epidemiological studies have suggested that, relative to normotensive subjects, hypertensive subjects are at higher risk for the development of cardiovascular and cerebrovascular diseases than normotensive subjects. However, there is wide variation in the occurrence and manifestation of cardiovascular and cerebrovascular diseases in hypertensive patients, even in individuals with the same risk factor profiles. The reason for this interindividual variation is largely unknown but may be reflective of the variation in the rate of biological aging.1
Telomere shortening represents one molecular mechanism that appears to contribute to cellular and organismal aging. Telomeres are specialized structures located at the ends of eukaryotic chromosomes. The main function of telomeres is to cap the chromosomal ends and to maintain genome stability and integrity.2 When telomeres reach a critical short length, they lose capping function at the chromosomal ends, resulting in activation of DNA damage checkpoints. These checkpoints limit cell survival by induction of senescence or apoptosis. Telomere shortening limits the life span of human cells in culture3 and occurs in various tissues during human aging.4 Studies in mouse models have demonstrated a crucial role of telomere shortening in the impairment of the stem cell during organismal aging.5,6 Studies in telomerase-deficient mice have also shown a direct link between telomere shortening and hypertension.7 In humans, telomere shortening and cellular senescence have also been correlated with atherosclerosis and cardiovascular aging.8–10 Indeed, epidemiological studies have suggested that age-dependent telomere shortening could be used as a marker for essential hypertension11,12 and related diseases or syndromes, such as insulin resistance,13 diabetes mellitus,14 obesity,15 atherosclerosis,16,17 coronary artery disease,18,19 myocardial infarction,20 and renal dysfunction.21
Telomere length is determined by genetic22–24 and environmental factors.25–28 Differences in leukocyte telomere length have been observed between blacks and whites.29 In addition, polymorphisms in telomerase genes have been reported to be associated with aging and age-dependent pathologies in some but not all studies.30,31 A pilot study of 73 hypertensive patients indicated that telomere length may be associated with hypertension in the Chinese population.32 Other studies have provided evidence that short telomere length is a primary rather than a secondary event in the pathogenesis of coronary artery disease (CAD).33,34 The negative studies could be attributable to inappropriate study design, lack of statistical power, inadequate methodology to measure telomere length, and, especially, to ethnic variability. Therefore, it is important to evaluate the relationship between telomere length and the variables of interest, hypertension and cardiovascular disease, in well-designed cohorts in a homogeneous ethnic group. In the current study, we examined the association between leukocyte telomere length and hypertension and its clinical complications in a cohort of Chinese patients with essential hypertension or normal blood pressure followed prospectively over 5 years. The study shows that the average telomere length of circulating leukocytes is shorter in hypertensive than in normotensive subjects. Moreover, telomere length represents an independent risk factor for the development of CAD both in patients with hypertension and in subjects with normal blood pressure.
Methods
Participants
A total of 900 participants (30 to 80 years old) were recruited from Henan province, China. Exclusion criteria were a history of alcohol abuse, diabetes mellitus, secondary hypertension, and renal disease. After excluding subjects with incomplete data or poor DNA quality, 767 individuals (388 patients with essential hypertension and 379 healthy controls) were analyzed. The study was approved by an institutional review committee, and informed consent was obtained from all of the subjects at entry or at specified intervals during the course of the study. The study was designed in accordance with the principles of the Declaration of Helsinki, and patient data were evaluated anonymously.
A detailed medical history of each subject was obtained, including the use of antihypertensive drugs. Blood pressure was measured using a mercury sphygmomanometer after resting for >5 minutes in the sitting position, according to the recommendations of the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Three consecutive blood pressure measurements were taken at each of 2 separate visits 4 to 6 weeks apart. The mean of the 6 readings was used for analysis. Subjects were classified as hypertensive cases if they did the following: (1) had systolic blood pressure of ≥140 mm Hg; (2) had diastolic blood pressure of ≥90 mm Hg; or (3) were taking antihypertensive medication. Subjects were classified as controls if they: (1) had a systolic blood pressure ≤120 mm Hg; (2) had a diastolic blood pressure ≤80 mm Hg; and (3) were not receiving treatment for hypertension, heart disease, or hormone replacement therapy. A venous blood sample was obtained from the antecubital vein for measurement of fasting plasma glucose and lipid levels and DNA extraction. Medical charts collected from local hospitals were examined for the evidence of stroke, CAD, hypertensive renal disease, hypertensive heart disease, and hypertensive retinopathy. Hypertension complications were defined as newly diagnosed secondary symptoms or diseases (see below) that developed within the follow-up period of 5 years. The diagnosis of hypertension complications was first established by clinical signs and symptoms by the field physicians and re-examined by physician specialists. Specifically, stroke was confirmed by neurologic examination, computed tomography, or MRI. CAD was confirmed by stress test with ECG or coronary angiography. Hypertensive heart disease was confirmed by transthoracic echocardiography and radiograph examination. Renal disease was confirmed by blood, urine, and kidney function tests. Hypertensive retinopathy was confirmed by funduscopy.
Measurement of Telomere Length
The integrity of the sample DNA was assessed by the electrophoresis of 0.5 μg of undigested DNA on 1.0% agarose gel (200 V for 2 hours) and ethidium bromide staining. Telomere length ratio (T/S ratio) was measured using a quantitative PCR method that compared the ratio of the telomere repeat copy number (T) and single-copy gene copy number (S) in a given sample.35 Specifically, DNA samples were amplified in 20-μL PCRs with ABI Step One Real-Time Thermal Cycler. The primer sequences used for the telomeres and the single-copy gene human β-globin were as follows: telomere forward 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′; telomere reverse 5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′; β-globin forward 5′-GCTTCTGACACAACTGTGTTCACTAGC-3′; and β-globin reverse 5′-CACCAACTTCATCCACGTTCACC-3′. The thermal cycling profiles started with 95°C incubation for 10 minutes, followed by 30 cycles of 15 s at 95°C and 1 minute at 56°C for the telomere PCR or followed with 35 cycles of 15 s at 95°C and 1 minute at 54°C for the β-globin PCR. The specificity of all of the amplification was determined by melting curve analysis. A reference DNA sample (transformed kidney epithelial cells: 293T cells) was included with each measurement to control interassay variability. Individual DNA samples were measured in triplicate. The average SD for the triplicate measurement was 6.8%. A dilution series (1.56 to 100.00 ng; 2-fold dilution; 7 points) run for both the telomere and β-globin PCRs was linear (R2>0.98). Test samples with threshold cycle numbers that fell outside the range defined by the standard curves were rerun at different concentrations to ensure that they were amplified within the linear range. All of the analyses were done blinded as to disease status of the individuals. The reliability of the telomere length obtained with the PCR assay was verified by comparing the data with those determined by conventional Southern blotting (please see Figure S1 in the online data supplement available at http://hyper.ahajournals.org).
Statistical Analysis
Characteristics of cases and controls were compared by χ2 tests for categorical variables and ANOVA for quantitative variables. The telomere length ratios were log transformed, because the T/S ratios were not normally distributed. The relationships between telomere length and quantitative variables were analyzed with Spearman correlation. Because older individuals tend to have shorter telomeres than younger individuals, we stratified the samples into 5 categories of age at recruitment (30 to 39 years, 40 to 49 years, 50 to 59 years, 60 to 69 years, and ≥70 years). The telomere length distribution was determined independently within each category, individuals in the lower half for telomere length in each age group were pooled together, and their characteristics were compared with those of the pooled upper-half individuals. The effects of telomere length, age, case-control status, and other individual risk factors of hypertension and its complications were assessed by logistic regression models. All of the analyses were performed using SPSS 13.
Results
The characteristics of 388 subjects with essential hypertension and 379 healthy normotensive controls at recruitment are shown in Table 1. In the original study design, 450 hypertensive patients were recruited with age- and gender-matched controls. After the exclusion of 133 participants with incomplete clinical data or poor quality of DNA samples, the compositions of age and gender in cases and controls were still comparable. The characteristics of the 133 excluded participants and 767 included participants were also comparable (please see Table S1).
Table 1.
Characteristics of Participants at Recruitment
| Characteristics | Controls | Cases | P |
|---|---|---|---|
| No., men/women | 186/193 | 207/181 | 0.282 |
| Age, y | 52.4 (9.7) | 53.7 (9.4) | 0.061 |
| BMI, kg/m2 | 24.4 (3.0) | 26.4 (3.3) | <0.001 |
| SBP, mm Hg | 105 (9.8) | 143 (15.6) | <0.001 |
| DBP, mm Hg | 66 (6.7) | 92 (9.9) | <0.001 |
| Fasting glucose, mmol/L | 5.37 (1.34) | 5.79 (1.45) | <0.001 |
| Triglycerides, mmol/L | 1.56 (1.26) | 2.01 (1.59) | <0.001 |
| TC, mmol/L | 4.80 (0.91) | 5.06 (0.98) | <0.001 |
| HDL, mmol/L | 1.71 (0.51) | 1.62 (0.54) | 0.012 |
| Family history, % | 18.3 | 61.8 | <0.001 |
| BUN, mmol/L | 4.63 (1.70) | 4.86 (1.89) | 0.074 |
| SC, μmol/L | 76.0 (10.2) | 81.8 (13.6) | <0.001 |
BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, serum total cholesterol; HDL, high-density lipoprotein; BUN, blood urine nitrogen; SC, serum creatinine.
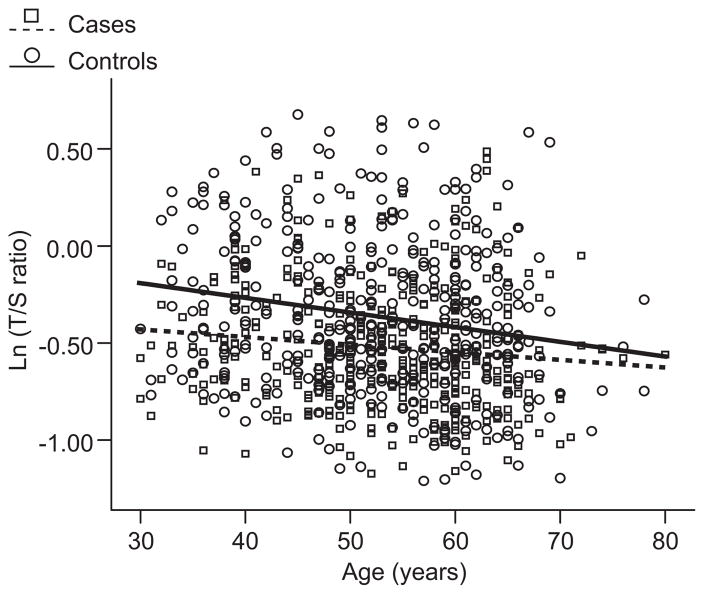
Figure 1 shows the distribution of log T/S ratio as a function of chronological aging in cases and controls. Overall, an inverse correlation between telomere length ratio with age was found in all of the individuals (regression coefficient: −0.006; SE: 0.001; r=−0.163; P<0.001). In subjects with essential hypertension, the telomere length ratio decreased steadily with age at a mean yearly rate of 0.004±0.002 (r=−0.106; P=0.038). The decrease in telomere length ratio in controls was 0.008±0.002 (r=−0.192; P<0.001).
Figure 1.
Telomere length as a function of age in controls and cases. Controls are shown as circles and solid line (n=379) and cases as squares and dotted line (n=387). Telomere length is plotted as log T/S ratio. r values were −0.192 (P<0.001) for controls and −0.106 (P=0.038) for cases.
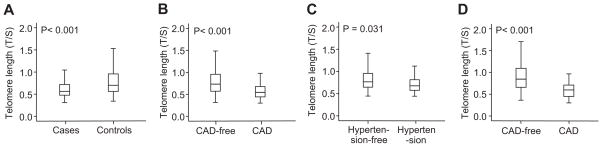
A highly significant difference was found in the average telomere length ratio between hypertensive and normotensive subjects (median telomere length ratio: 0.57 [interquartile range {IQR}: 0.48 to 0.72] and 0.67 [IQR: 0.53 to 0.93], respectively; P<0.001; Figure 2A). Of the hypertension risk factors measured, inverse associations were found between telomere length and body mass index, fasting glucose, and diastolic blood pressure after adjusting for age and gender (please see Table S2). However, after inclusion of the hypertension status into the regression model, the association between telomere length and body mass index, fasting glucose, or diastolic blood pressure was no longer significant (please see Table S3). Therefore, we reclassified the participants according to their disease status. The presence of short telomeres did not correlate with any of these risk factors in either cases or controls (Table 2). Thus, short telomeres correlated with hypertension independent of other risk factors and can be taken as an independent risk factor for essential hypertension (odds ratio: 1.831; 95% CI: 1.375 to 2.425).
Figure 2.
Telomere length in hypertension and CAD. Median and IQR (box) of telomere length were measured using the DNA collected at recruitment. A, Telomere lengths were shorter in hypertensive cases than in healthy, normotensive controls. B, Individuals who developed CAD within 5 years had shorter telomeres at the time of recruitment than the CAD-free individuals. C, Among the normotensive controls (according to the baseline blood pressure), those who developed hypertension within 5 years had shorter telomeres at the time of recruitment than those who did not develop hypertension. D, Normotensive controls (according to the baseline blood pressure) who developed CAD within 5 years also had shorter telomeres at the time of recruitment than the CAD-free individuals.
Table 2.
Distribution of Demographic Characteristics and Hypertension Risk Factors at Recruitment by Telomere Length in Controls and Cases
| Characteristics | Controls |
Cases |
||||
|---|---|---|---|---|---|---|
| Upper Half | Lower Half | P | Upper Half | Lower Half | P | |
| Age, y | 52.6 (9.7) | 52.2 (9.8) | 0.663 | 53.8 (9.5) | 53.7 (9.2) | 0.236 |
| BMI, kg/m2 | 24.4 (3.1) | 24.5 (3.0) | 0.609 | 26.4 (3.4) | 26.2 (3.2) | 0.236 |
| SBP, mm Hg | 104 (9) | 106 (10) | 0.089 | 142 (15) | 143 (16) | 0.808 |
| DBP, mm Hg | 65 (6.9) | 68 (6.4) | 0.082 | 91 (9.6) | 92 (10.2) | 0.688 |
| Fasting glucose, mmol/L | 5.35 (1.3) | 5.40 (1.5) | 0.723 | 5.78 (1.4) | 5.80 (1.5) | 0.868 |
| Triglycerides, mmol/L | 1.52 (0.91) | 1.61 (1.64) | 0.480 | 1.83 (0.96) | 2.14 (1.91) | 0.051 |
| TC, mmol/L | 4.80 (0.86) | 4.80 (0.98) | 0.974 | 4.98 (0.96) | 5.12 (0.99) | 0.184 |
| HDL, mmol/L | 1.67 (0.48) | 1.77 (0.55) | 0.046 | 1.66 (0.50) | 1.59 (0.57) | 0.238 |
| Family history, % | 15.4 | 22.6 | 0.069 | 62.7 | 61.1 | 0.739 |
| BUN, mmol/L | 4.68 (1.74) | 4.56 (1.65) | 0.495 | 5.01 (2.08) | 4.75 (1.74) | 0.187 |
| SC, μmol/L | 75.6 (9.4) | 76.4 (11.3) | 0.435 | 81.6 (11.6) | 81.9 (14.8) | 0.817 |
BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, serum total cholesterol; HDL, high-density lipoprotein; BUN, blood urine nitrogen; SC, serum creatinine.
The prognostic value of telomere length in hypertension and its complications was studied next. Among the total of 767 individuals recruited at baseline, 411 individuals (221 hypertensive patients and 190 controls according to baseline measurement) with complete 5-year follow-up data were analyzed for the risk of developing hypertension complications, including stroke, CAD, hypertensive heart disease, hypertensive renal disease, and hypertensive retinopathy. The dropout subjects were comparable to the subjects included in the follow-up analysis in terms of age, gender, and conventional vascular risk factors (please see Table S4). The incidence of developing complications of hypertension within 5 years was significantly higher in hypertensive than in healthy, normotensive subjects (stroke: 14.5% versus 1.6%, P<0.001; CAD: 15.8% versus 6.3%, P<0.001; and for hypertensive retinopathy: 9.1% versus 2.6%, P<0.001). Overall mean telomere length ratio was shorter in individuals who developed ≥1 complication within 5 years (median: 0.54 [IQR: 0.44 to 0.86]) than those who did not show any complication (median: 0.73 [IQR: 0.57 to 0.96]; P<0.001; Figure 2B). A stepwise linear regression analysis with forced entry of age and gender showed that only CAD, stroke, and hypertensive retinopathy remained independently associated with telomere length. Univariate logistic analysis showed that the relative risk of developing CAD for individuals with shorter telomeres compared with those with longer telomeres at baseline was 3.315 (95% CI: 1.662 to 6.609; P<0.001), whereas the risk of developing hypertensive retinopathy was 5.741 (95% CI: 1.922 to 17.075; P<0.001), and the risk of developing stroke was 2.031 (95% CI: 0.991 to 4.221; P=0.05). Multivariate analysis revealed that the risk of developing stroke within 5 years was attributed only to the presence of hypertension at entry. The risk of developing hypertensive retinopathy was attributed only to telomere length, whereas the risk of developing CAD was attributed to both the presence of hypertension at entry and telomere length (Table 3). Although hypertension was found to be an independent risk factor for CAD, not all of the hypertensive patients developed CAD. Therefore, we examined the risk of developing CAD in hypertensive patients. After adjustment for chronological age and gender, short telomeres remained as an independent risk factor for hypertensive patients in the development of CAD within 5 years (Table 4).
Table 3.
Logistic Regression of Risk Factors for Hypertensive Complications
| Complications | Variables | B | SE | Wald | P | OR |
|---|---|---|---|---|---|---|
| Stroke | Age | −0.033 | 0.020 | 2.706 | 0.100 | 0.967 |
| Gender | 0.776 | 0.383 | 4.102 | 0.043 | 2.173 | |
| Hypertension | 2.381 | 0.628 | 14.396 | 0.000 | 10.820 | |
| Short telomere | 0.139 | 0.392 | 0.126 | 0.722 | 1.150 | |
| Constant | 0.020 | 1.328 | 0.000 | 0.988 | ||
| CAD | Age | −0.095 | 0.022 | 19.228 | 0.000 | 0.910 |
| Gender | 0.254 | 0.331 | 0.588 | 0.443 | 1.289 | |
| Hypertension | 0.813 | 0.378 | 4.614 | 0.032 | 2.254 | |
| Short telomere | 1.001 | 0.376 | 7.083 | 0.008 | 2.721 | |
| Constant | 5.478 | 1.400 | 15.314 | 0.000 | ||
| Retinopathy | Age | −0.045 | 0.025 | 3.209 | 0.073 | 0.956 |
| Gender | 0.027 | 0.427 | 0.004 | 0.950 | 1.027 | |
| Hypertension | 0.911 | 0.532 | 2.935 | 0.087 | 2.487 | |
| Short telomere | 1.485 | 0.574 | 6.697 | 0.010 | 4.413 | |
| Constant | 3.464 | 1.630 | 4.514 | 0.034 |
Table 4.
Risk of Developing CAD in Hypertensive Patients Within 5 Years After Blood Test
| Variables | B | SE | Wald | P | OR |
|---|---|---|---|---|---|
| Age | −0.089 | 0.026 | 11.920 | 0.001 | 0.915 |
| Gender | 0.617 | 0.402 | 2.358 | 0.125 | 1.853 |
| Short telomere | 1.858 | 0.700 | 7.048 | 0.008 | 6.413 |
| Constant | 6.724 | 1.538 | 19.117 | 0.000 |
The regression coefficients of short telomere remained statistically significant after adjustment for chronological age and gender. OR indicates odds ratio.
Among the 190 healthy, normotensive controls, 65 individuals developed hypertension within 5 years. The telomere length of these hypertensive subjects was significantly shorter compared with participants who did not develop hypertension within the 5-year follow-up period (Figure 2C). This result recapitulated the finding in the case-control study that individuals with short telomeres had an increased risk of developing essential hypertension. During the 5-year follow-up period, normotensive subjects who developed CAD had shorter telomeres at entry than the normotensive subjects who remained CAD free (Figure 2D).
Discussion
Essential hypertension and cardiovascular diseases are closely linked to the aging of human beings. Although classic risk factors, such as smoking, overweight, hyperlipidemia, and diabetes mellitus, have been found to predict the occurrence of hypertension or cardiovascular disease, there is wide variation in disease manifestation in individuals who have the same risk profile. Telomere length fulfills several of the criteria for robust biomarkers of human aging because it does the following: (1) decreases progressively with chronological age; (2) varies considerably among individuals; (3) registers the life cycle of proliferative cells; and (4) is strongly linked to inflammation and oxidative stress.36
The present study showed that leukocyte telomere length is significantly shorter in patients with essential hypertension than in normotensive subjects, in agreement with a previous study in hypertensive men.37 More importantly, hypertensive subjects who developed CAD within 5 years had even shorter telomeres than hypertensive subjects who did not develop CAD. This finding provides evidence that short telomeres portend a worse prognosis in essential hypertension. The mechanisms by which leukocyte telomere length predicts the evolution of hypertension and its prognosis remain to be defined. Decreased telomere length in circulating leukocytes may reflect age-related turnover of the bone marrow stem cells.38 Telomere shortening–induced endothelial cell senescence has been reported to be associated with increased intercellular adhesion molecule 1 and decreased endothelial NO synthase activity, conditions that are implicated in the pathogenesis of atherosclerosis.9 Bone marrow-derived endothelial progenitor cells have been considered as important agents of vascular repair. A recent study showed that telomere shortening in endothelial progenitor cells played an important role in the pathogenesis of CAD via increased oxidative-related DNA damage.39 In addition to the direct effects on vascular repair and endothelial progenitor cell function, telomere shortening can increase the level of circulating proinflammatory cytokines, possibly affecting atherosclerosis and vascular disease.6,40 Thus, age-dependent telomere attrition may not only trigger cellular senescence but may also be related to chronic inflammation and increased oxidative burden, which are central to the pathogenesis of age-related cardiovascular disorders, including atherosclerosis and arterial stiffness.
One cross-sectional study has suggested that short telomere length was an independent predictor of the presence of atherosclerotic lesions in 163 hypertensive men.17 In the current study, we analyzed the 5-year outcomes of 411 individuals (221 hypertensive patients and 190 controls). We found that, in addition to hypertension, telomere length was independently associated with the occurrence of CAD within 5 years. Our data suggested that telomere length could be used as a potential predictor of CAD in both hypertensive subjects and healthy controls. However, we did not observe an independent role of telomere length in predicting the occurrence of stroke but rather that high blood pressure was the predominant predictor of stroke. The disease spectrum of stroke in Chinese subjects is different from non-Chinese subjects: there is a greater occurrence of hemorrhagic stroke than ischemic stroke in Chinese subjects than in other ethnic groups. In the current study, the association of a particular type of stroke and telomere length was not examined because of a paucity of events. So far, there has been no investigation on the relationship between telomere length and different types of stroke. A large-scale study of the relationship between telomere length and stroke needs to be conducted.
In the current study, we used a quantitative PCR–based method to measure the relative telomere length. The quantitative PCR method is less time consuming than other methods. In contrast to a previous study,15 we did not observe a relationship between gender or smoking and telomere length. This could indicate that the quantitative PCR method is relatively less sensitive than classical Southern blotting. T/S ratios difference between studies usually relates to different DNA references, underscoring the lack of uniformity of results derived from this measurement across studies.19 Using DNA from a common cell line with stable telomere length might facilitate a uniform expression of the T/S ratio. We observed a linear correlation between telomere shortening and chronological age. The decrease in telomere length tended to be faster in controls than that in hypertensive cases. A possible explanation could be that hypertensive subjects already had excessive telomere attrition at an early age, making the additional decrease in telomere length with aging less obvious than those decreases observed in age-matched normotensive controls. However, this does not mean that the decrease in telomere length in hypertensive subjects is less important than in healthy controls, because the incidence of 5-year CAD increased to a greater extent in hypertensive subjects with shorter telomeres than in those with longer telomeres. Thus, our data support the hypothesis that leukocyte telomere length could be taken as an independent risk factor for hypertension and cardiovascular aging. Indeed, another recent study suggested that mean leukocyte telomere length could identify patients with coronary heart disease who would benefit most from statin treatment.1
Perspectives
Age-dependent telomere shortening in leukocytes mirrors the replicative history of the hematopoietic stem cells. Our prospective study revealed that subjects with hypertension have shortened telomeres, and the occurrence of CAD is associated with shorter telomeres in hypertensive subjects. These observations support the hypothesis that telomere shortening can be used as a prognostic marker for age-related cardiovascular disease.
Supplementary Material
Acknowledgments
We thank Dr Lianfeng Zhang for advice and discussion.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (grant 30771189) and the Max Planck Society–Chinese Academy of Medical Sciences Partner Group Program on stem cell aging. Z.J. is supported by a fund of the Max Planck Society, and K.L.R. is supported by funding of the Deutsche Forschungsgemeinschaft (RU745/10-1 and RU745/7-1), the Deutsche Krebshilfe e.V. (project program grant on tumor stem cells), and the European Union (GENINCA, TELOMARKER).
Footnotes
Disclosures
None.
References
- 1.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 3.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Ju Z, Rudolph KL. Telomere shortening and ageing. Z Gerontol Geriatr. 2007;40:314–324. doi: 10.1007/s00391-007-0480-0. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 6.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Rivero G, Ruiz-Torres MP, Rivas-Elena JV, Jerkic M, Diez-Marques ML, Lopez-Novoa JM, Blasco MA, Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114:309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. [DOI] [PubMed] [Google Scholar]
- 8.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 10.Edo MD, Andres V. Aging, telomeres, and atherosclerosis. Cardiovasc Res. 2005;66:213–221. doi: 10.1016/j.cardiores.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Aviv A. Chronology versus biology: telomeres, essential hypertension, and vascular aging. Hypertension. 2002;40:229–232. doi: 10.1161/01.hyp.0000027280.91984.1b. [DOI] [PubMed] [Google Scholar]
- 12.Vasan RS, Demissie S, Kimura M, Cupples LA, Rifai N, White C, Wang TJ, Gardner JP, Cao X, Benjamin EJ, Levy D, Aviv A. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation. 2008;117:1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 14.Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram JH, Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- 15.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 16.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 17.Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 18.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 19.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 21.van der Harst P, Wong LS, de Boer RA, Brouilette SW, van der Steege G, Voors AA, Hall AS, Samani NJ, Wikstrand J, van Gilst WH, van Veldhuisen DJ. Possible association between telomere length and renal dysfunction in patients with chronic heart failure. Am J Cardiol. 2008;102:207–210. doi: 10.1016/j.amjcard.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 23.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 24.Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson JR, Mason A, Bodycote CL, Raleigh SM, Louis E, Samani NJ. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 26.Huda N, Tanaka H, Herbert BS, Reed T, Gilley D. Shared environmental factors associated with telomere length maintenance in elderly male twins. Aging Cell. 2007;6:709–713. doi: 10.1111/j.1474-9726.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 27.Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 28.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsubara Y, Murata M, Watanabe K, Saito I, Miyaki K, Omae K, Ishikawa M, Matsushita K, Iwanaga S, Ogawa S, Ikeda Y. Coronary artery disease and a functional polymorphism of hTERT. Biochem Biophys Res Commun. 2006;348:669–672. doi: 10.1016/j.bbrc.2006.07.103. [DOI] [PubMed] [Google Scholar]
- 31.Nordfjall K, Osterman P, Melander O, Nilsson P, Roos G. hTERT (-1327)T/C polymorphism is not associated with age-related telomere attrition in peripheral blood. Biochem Biophys Res Commun. 2007;358:215–218. doi: 10.1016/j.bbrc.2007.04.099. [DOI] [PubMed] [Google Scholar]
- 32.Lung FW, Ku CS, Kao WT. Telomere length may be associated with hypertension. J Hum Hypertens. 2008;22:230–232. doi: 10.1038/sj.jhh.1002314. [DOI] [PubMed] [Google Scholar]
- 33.Brouilette SW, Whittaker A, Stevens SE, van der Harst P, Goodall AH, Samani NJ. Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart. 2008;94:422–425. doi: 10.1136/hrt.2007.139675. [DOI] [PubMed] [Google Scholar]
- 34.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 37.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 38.Brummendorf TH, Rufer N, Holyoake TL, Maciejewski J, Barnett MJ, Eaves CJ, Eaves AC, Young N, Lansdorp PM. Telomere length dynamics in normal individuals and in patients with hematopoietic stem cell-associated disorders. Ann N Y Acad Sci. 2001;938:293–303. doi: 10.1111/j.1749-6632.2001.tb03598.x. discussion 303–294. [DOI] [PubMed] [Google Scholar]
- 39.Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H, Schiffer E, Song Z, Wang J, Zurbig P, Thedieck K, Moes S, Bantel H, Saal N, Jantos J, Brecht M, Jeno P, Hall MN, Hager K, Manns MP, Hecker H, Ganser A, Dohner K, Bartke A, Meissner C, Mischak H, Ju Z, Rudolph KL. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.