Abstract
The development of functional cell populations such hepatocytes and pancreatic beta cells from embryonic stem (ES) cells is dependent on the efficient induction of definitive endoderm early in the differentiation process. To monitor definitive endoderm formation in mouse ES cell differentiation cultures in a quantitative fashion, we generated a reporter cell line that expresses human CD25 from the Foxa3 locus and human CD4 from the Foxa2 locus. Induction of these reporter ES cells with high concentrations of Activin A (activin) led to the development of a CD25-Foxa3+CD4-Foxa2+ population within four to five days of culture. Isolation and characterization of this population revealed that it consists predominantly of definitive endoderm that is able to undergo hepatic specification under the appropriate conditions. To develop reagents that can be used for studies on endoderm development from un-manipulated ES cells, from induced pluripotent stem (iPS) cells, and from the mouse embryo we generated monoclonal antibodies against the CD25-Foxa3+CD4-Foxa2+ population. With this approach, we identified two antibodies that react specifically with endoderm from ES cell cultures as well as from the early embryo. The specificity of these antibodies enables one to quantitatively monitor endoderm development in ES cell differentiation cultures, to study endoderm formation in the embryo and to isolate pure populations of culture- or embryo-derived endodermal cells.
Keywords: Embryonic stem cells, Monoclonal antibodies, Embryoid bodies, Differentiation antigens, Differentiation
Introduction
The ability of ES and iPS cells to generate different cell types in culture provides a powerful model system to study early mammalian development as well as a novel source of cells and tissues for drug discovery and for transplantation for the treatment of disease. Within this context, the endodermal derived tissues including hepatocytes and pancreatic beta cells are of particular interest as the liver is a primary target of drug toxicity and replacement of beta cells is a viable option for the treatment of type I diabetes. The successful generation of these different cell types from ES cells and ultimately from iPS cells is dependent on the efficient induction of definitive endoderm in the differentiation cultures. In the early mouse embryo, endoderm is formed during the process of gastrulation from uncommitted epiblast cells that traverse the anterior region of the primitive streak. While the mechanisms controlling this process are not fully understood, studies using a number of different model systems have demonstrated that nodal signaling plays a pivotal role in the establishment of this lineage [1].
ES cells have been successfully differentiated to the endoderm lineage and these endodermal cells have been further specified to derivative cell types including immature hepatocytes and pancreatic beta cells. The most efficient and reproducible approaches have been those that have recapitulated the key aspects of embryonic development in the tissue culture dish. Using reporter mouse ES cell lines to monitor primitive streak/endoderm formation, several groups demonstrated that signaling through the nodal/activin pathway by addition of activin led to the induction of a population with definitive endoderm properties [2, 3]. To be able to specifically track the formation of the anterior primitive streak population, we generated a dual reporter ES cell line containing the human CD4 cDNA targeted to the Foxa2 locus in the GFP-T line, containing the green fluorescent protein cDNA targeted to the T (brachyury) locus. Using this cell line, we demonstrated that high concentrations of activin preferentially induced a CD4-Foxa2hi-GFP-T+ population and that sustained signaling through this pathway was required for progression of these primitive streak-like cells to definitive endoderm [2, 4]. Studies with human ES cells have also shown that high levels of activin/nodal signaling promotes the development of definitive endoderm, demonstrating that the signaling pathways that regulate primary germ layer induction are conserved in evolution [5].
The reporter ES cell lines established to date have enabled one to monitor the expression of the endodermal genes Foxa2, Sox17, or Hex [4, 6, 7]. While all of the reporter lines have been useful for investigating endoderm development, none of the genes is endoderm specific. Foxa2 and Hex are expressed in the anterior PS prior to endoderm formation [8-10]. In addition, Foxa2 is expressed in subpopulations of neuro-ectodermal tissues [8, 9]. Sox17 is detected some vascular and early hematopoietic progenitors [11, 12], whereas Hex is present in hemangioblasts and yolk sac vascular cells [10, 13]. To be able to quantify and purify endoderm-committed cells from ES cell differentiation cultures, it is necessary to use markers in addition to Foxa2, Sox17 or Hex. Foxa3, another member of the Fox transcription factor family is a good candidate as it is not detected in the primitive streak or neuronal tissues but is expressed as definitive endoderm is formed [9]. Foxa3 is expressed in the endoderm of the liver, pancreas, stomach and intestine as well as in precursors of these tissues [9]. It is not, however, expressed in the most anterior gut tube that forms the lung or tissues anterior to the lung.
In this report, we describe the development of a triple reporter cell line that contains the human CD25 cDNA targeted to the Foxa3 locus in our previously described CD4-Foxa2 GFP-T cell line. We show that the CD25-Foxa3 reporter ES cell line can be used to purify committed endoderm that no longer needs an exogenous activin/nodal signal to maintain endoderm identity. In addition, this reporter line was used to generate 2 monoclonal antibodies with specificity for early endoderm both from differentiating ES cells as well as in the mouse embryo. We show that expression of the cell surface markers recognized by these antibodies can be used to replace the CD25-Foxa3 reporter in the quantification and purification of endoderm.
Materials and Methods
Generation of CD25-Foxa3 Targeted ES Cells
The Foxa3 targeting vector was constructed by isolating PCR amplified arms of homology, 1.7 kb 3′ of the ATG and 6.9 kb 5′ of exon 1. The arms of homology were cloned into a vector containing the human CD25 cDNA, the bovine poly adenylation sequence and a loxp flanked Puromycin resistance cassette (Fig. 1A). The Diphtheria toxin A gene was used for negative selection. The mouse ES cell line GFP-T CD4-Foxa2 [4] was electroporated with the targeting vector and a single clone that had undergone homologous recombination was identified by a PCR screen. The resistance cassette was deleted by transient transfection of a Cre expressing plasmid. The clone was confirmed by southern blot analysis (Fig. 1B). While the Foxa3-CD25 reporter ES cell line was initially found to have a normal number of chromosomes, more detailed analysis revealed the presence of an iso-chromosome 8 which is equivalent to trisomy of chromosome 8. This abnormality did not seem to impair the differentiation capacity of the cells.
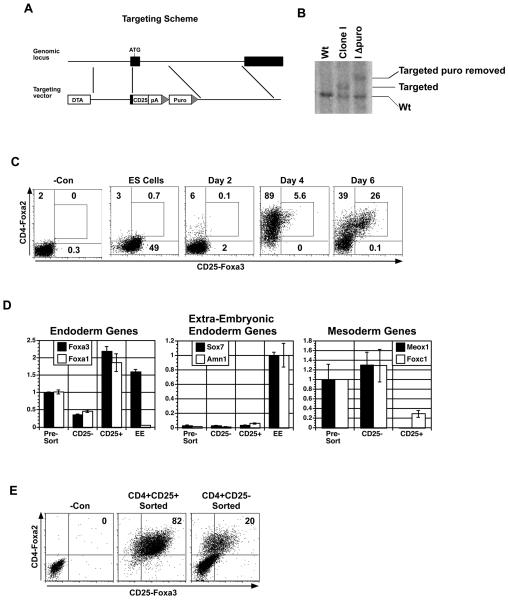
Figure 1. Generation and characterization of the CD25-Foxa3 reporter ES cell line.
(A) Schematic of Foxa3 targeting vector. (B) Southern blot analyses identifying a targeted clone and a targeted clone following removal of the selection cassette (I delta puro). (C) Kinetic analyses of CD4-Foxa2 and CD25-Foxa3 expression during endoderm formation in EBs generated from the CD25-Foxa3 reporter cell line. (D) QPCR-based gene expression analysis of CD4+CD25+ and CD4+CD25− populations isolated from day six EBs. Extra-embryonic tissue from day 8.25 mouse embryos was used as a control. Relative expression is shown with the presort population set to 1 except for the extra-embryonic endoderm genes where EE was set to 1. (E) Expression of CD4-Foxa2 and CD25-Foxa3 on CD4+CD25− and CD4+CD25+-derived populations following 12 days of culture in hepatic inducing conditions. One of three independent experiments is shown (C-E). Abbreviations: DTA, diphtheria toxin-A gene; pA, bovine poly-adenylation sequence; Puro, puromycin resistance cassette; −Con, negative control; CD25−, CD4+CD25− population; CD25+, CD4+CD25+ population; EE, extra-embryonic tissue.
ES Cell Maintenance and Differentiation
ES cells were maintained in a serum-free/feeder-free culture system described by Ying et al [14] with modifications as described previously [4]. In addition to the CD25-Foxa3 reporter, wild type E14 and F1 (C57Bl/6 × 129Sv) (v6.5) (Open Biosystems) ES cells were used as indicated. ES cells were differentiated in serum-free media as described previously [4]. Briefly, ES cells were trypsinized and allowed to form EBs (3 × 104 cells/ml) in non-tissue cultured treated dishes in serum free media without any exogenous growth factors added. After 48 hours in culture, the EBs were dissociated by trypsin and allowed to re-aggregate (used different cell densities depending on day of harvest) with different factors to induce the different germ layers. For endoderm the cells were cultured in human Activin A (25-50 ng/ml) (R&D systems) and mouse Wnt3a (3-5 ng/ml) (R&D systems), for mesoderm, they were cultured in Activin A (2 ng/ml), Wnt3a (3-5 ng/ml), and human BMP4 (0.3-1 ng/ml) (R&D systems) and for ectoderm they were cultured in human bFGF (10 ng/ml) (R&D systems). The cultures were harvested at times indicated in the text. Hepatocyte induction was performed as described previously [15], utilizing sorted populations as indicated. Briefly, endodermal cells were plated onto gelatin coated dishes (2 × 105 cells/ml) in serum free differentiation media containing BMP4 (50 ng/ml), human TGFα (20 ng/ml) (R&D systems), bFGF (10 ng/ml), human EGF (10 ng/ml) (R&D systems), human HGF (10 ng/ml) (R&D systems), dexamethasone (10−7 M) (Sigma), and MTG (4.5 × 10−4 M) (Sigma). Cells were isolated for analyses at times indicated in the text.
Monoclonal Antibody Generation
Animal care and immunization procedures were performed in accordance with standards established by the Oregon Health & Science University institutional animal care and use committee. For the hybridomas described in this report, two immunization strategies were used. A standard immunization protocol was used to prime the animal used for generation of the DMBC0-8-E10 (ENDM2) hybridoma, and a modified subtractive immunization protocol [16, 17] was used for generation of the DMBC2-8-G10 (ENDM1) hybridoma.
Standard Immunization
A Fisher 344 female rat was immunized 3 times at 21 day intervals with 2 × 106 cells recovered from 4 day ES cultures of sorted CD4-Foxa2+ cells. Cells were injected ip in combination with the adjuvant Imject Alum (Thermo Scientific; as per manufacturer's instructions).
Subtractive Immunization
A Fisher 344 female rat was immunized with 2.65 × 106 undifferentiated ES cells, a source of undesirable antigen. At 24 and 48 hours after the initial immunization, cyclophosphamide (Sigma-Aldrich; 51 mg/kg body weight) was injected ip to eliminate B lymphocytes reacting against antigens on these cells. On day 18 following the initial immunization, animals received 1.6 × 106 sorted Foxa2+Foxa3+ cells (a source of desired markers), and were dosed 21 days later with 1.2 × 106 sorted Foxa2+Foxa3+ cells. All cells were delivered ip in combination with Imject Alum.
For hybridoma generation, both animals were sacrificed and their spleens were harvested four days after the final immunization. Splenocytes were fused with SP2/0 Ag14 myeloma cells [18] and fused cells were selected by growth in methylcellulose-containing HAT (hypoxanthine-aminopterin-thymidine) medium [19] (Stem Cell Technologies Inc., Vancouver, Canada). Clones were transferred to liquid media in 96-well plates and supernatants were subsequently collected for antibody screening by flow cytometry, with the initial screening performed on ES cells cultured in endoderm inducing conditions for either 4 or 6 days (4,704 clones total). Clones of interest were expanded and cryopreserved.
Embryo Dissections
Embryos were isolated from pregnant swiss webster mice (Taconic) at ages indicated in the text. Dissections were performed under a dissection microscope (Leica) using forceps and tungsten needles. Whole embryos were isolated at day 7.25, 8.25, or 9.25. Day 8.25 embryos were also dissected into extra-embryonic and embryonic regions as indicated in the text.
Whole Mount Staining
5-6 somite stage (E.8.25) mouse embryos were isolated in cold PBS and subsequently fixed in methanol-acetone (1:1) at −20°C for 2 h. Fixed embryos were washed twice for 10 min in TBS (50mM Tris-HCl, 150 mM NaCl, pH 7.5) and blocked for 2 h in TBS containing 15% heat inactivated FBS. Next, embryos were incubated with non-diluted primary antibody supplemented with 5% FBS and incubated O/N at 4-8°C. Embryos were then washed at least 6 times for 5 min in TBS-T (TBS containing 0.1 % Triton X-100) by gently moving them from one well to another in a 6-well plate, with each well containing at least 3 ml of TBS-T. This step was followed by 1 h incubation with a secondary antibody (Alexa Fluor 488 donkey anti-rat IgG diluted 1:400, from Invitrogen) in TBS containing 15% FBS. The final washing steps were performed as before. DAPI (diluted 1:1000) was added to one of the washes. All incubation and washing steps were performed on ice or at 4°C. Embryos were then photographed whole under fluorescence imaging.
Gene Expression Analysis
Total RNA was prepared with the RNeasy mini or micro kits (Qiagen) and treated with RNase-free DNase (Qiagen). 100ng to 1ug RNA was reverse transcribed into cDNA using random hexamers with Superscript II Reverse Transcriptase (Invitrogen). Real-time quantitative PCR was performed in triplicate for all samples using the ABI 7900HT (Applied Biosystems) with SYBR GreenER qPCR SuperMix (Invitrogen). A 10 fold dilution series of mouse genomic DNA ranging from 30ng to 3pg per reaction was used to evaluate the efficiency of the PCR and calculate the copy number of each gene relative to the house keeping gene Actb. The oligonucleotide sequences for Actb have been described previously [20] and the other oligonucleotide sequences are listed in the supporting text (Supplemental Table 1).
Flow Cytometry and Cell Sorting
Various ES cell derived cultures or mouse embryos were dissociated by incubation with trypsin for 1-3 minutes and stained for the following cell surface antigens: anti-human CD4-phycoerythrin, −allophycocyanin, or −phycoerythrin/Cy5.5 (Invitrogen), anti-human CD25-phycoerythrin or −allophycocyanin (Invitrogen), anti-mouse EpCAM-phycoerythrin (Santa Cruz Biotechnology), or anti-mouse CXCR4-biotin (Becton Dickenson) followed by streptavidin–phycoerythrin/Cy5.5 (Invitrogen). ENDM1, ENDM2, and a rat IgG1 control (Invitrogen) (were stained using un-conjugated antibodies and visualized with Donkey anti-rat-allophycocyanin (Jackson ImmunoResearch). Samples stained with both EpCAM or CXCR4 and ENDM1 or ENDM2 were first stained with ENDM antibodies, blocked with normal Rat IgG (Jackson ImmunoResearch) then stained with the other antibodies. Intracellular staining for Afp and Albumin was performed as described previously [15]. The cells were acquired using a LSR II or FacsCalibur flow cytometer (Becton Dickenson) or sorted on a MoFlo (Cytomation Systems), FacsVantage (Becton Dickenson), or Influx (Becton Dickenson) cell sorter. Analysis was performed using FlowJo software (Tree Star Inc.).
Results
To be able to monitor endoderm development and isolate cells of this primary germ layer from ES cell differentiation cultures, we generated a reporter cell line that contained the human CD25 cDNA targeted to the Foxa3 locus in our CD4-Foxa2/GFP-T ES cell line [4]. This cell line enables one to monitor distinct developmental stages including primitive streak formation based on CD4-Foxa2 and GFP-T expression and endoderm induction by CD4-Foxa2 and CD25-Foxa3 expression. The targeting strategy used for the generation of the CD25-Foxa3 cells as well as the Southern blot analysis confirming the targeting event are shown in Figs. 1A and 1B. The CD25-Foxa3/CD4-Foxa2/GFP-T ES cells (hereafter referred to as CD25-Foxa3 cells) were differentiated in serum free media in the presence of high concentrations of activin, conditions that we have previously shown support the efficient formation of anterior primitive streak and definitive endoderm [2, 4, 15]. A kinetic analysis of CD4-Foxa2 and CD25-Foxa3 expression on the activin induced EB cells is shown in Fig. 1C. CD25-Foxa3 is expressed at moderate levels in undifferentiated ES cells and then down regulated (by day 2) with the onset of differentiation. As we have previously shown, CD4-Foxa2 is induced by day 4 at which time almost all of the population expressed this marker. Expression of CD25-Foxa3 is first detected within the CD4-Foxa2 population at day 4 of differentiation and by day 6, greater than 25% of the entire EB population co-expresses these markers. To determine if the Foxa2+Foxa3+ population represents definitive endoderm, these cells together with the Foxa2+Foxa3− population were isolated by cell sorting and subjected to QPCR based gene expression analyses (Fig. 1D). Foxa3 was expressed at much higher levels in the CD25+ fraction compared to the CD25− cells, indicating that the reporter accurately reflects endogenous Foxa3 gene expression. Foxa1, another member of the Fox family that is expressed in definitive but at much lower levels in visceral endoderm in the early embryo [8, 9] was also expressed at higher levels in the CD25+ fraction compared to the negative and pre-sort populations. Sox7 and Amn, two genes expressed in extra-embryonic (visceral) endoderm were detected at very low levels in all ES derived populations compared to extra-embryonic tissue isolated from a day 8.25 mouse embryo, indicating that the induction conditions did not support the development of this lineage. The mesodermal genes Meox1 and Foxc1 were expressed at lower levels in the Foxa2+Foxa3+ fraction compared to the Foxa2+Foxa3− or presort fractions suggesting that the population is not contaminated with mesodermal cells. Taken together, the findings from these analyses strongly suggest that the Foxa2+Foxa3+ population is highly enriched for definitive endoderm.
To demonstrate that the expression of CD25-Foxa3 marks the commitment of endoderm in the ES cell differentiation system, we analyzed the ability of the CD25-Foxa3+CD4-Foxa2+ sorted cells to maintain endoderm cell fate when cultured in hepatocyte promoting conditions [15]. Our previous studies demonstrated that sustained activin stimulation is required to induce definitive endoderm from CD4-Foxa2+ primitive streak like cells [4]. As activin is not a component of the hepatocyte maturation media, we would predict that only committed endoderm would be maintained in these culture conditions. Following 12 days of culture in the hepatocyte maturation media, greater than 80% of the CD25-Foxa3+-derived cells maintained an endoderm phenotype as demonstrated by the expression of CD4-Foxa2 and CD25-Foxa3 (Fig. 1E). In contrast, the majority of the Foxa2+Foxa3− derived cells down-regulated expression of Foxa2 indicating a lack of progression to definitive endoderm in the absence of activin. The up-regulation of Foxa3 expression in a portion of the Foxa2+Foxa3− derived population may be due to the presence of limiting levels of endogenously produced Activin/nodal or to induction by an Activin/nodal-independent pathway. These findings indicate that expression of Foxa3 in combination with Foxa2 marks definitive endoderm in ES cell differentiation cultures.
The above analyses highlight the power of transgene reporters in defining distinct developmental stages during ES cell differentiation. When analyzing endoderm induction and differentiation from different pluripotent stem cells including both ES and iPS cells, the generation of a reporter line for each cell type is not feasible. To be able to quantify endoderm induction and isolate endodermal cells from un-manipulated pluripotent stem cell differentiation cultures, we generated monoclonal antibodies specific for this germ layer. Two populations were used for the immunizations: CD4-Foxa2+ cells isolated from ES cell cultures differentiated for 4 days or CD4-Foxa2+ CD25-Foxa3+ cells isolated from 6-day-old cultures (Fig. 1C). From this approach, 2 antibodies, ENDM1 and ENDM2, were identified that displayed a staining pattern similar to that of CD25-Foxa3 on day 6 EB cells (Fig. 2A). These antibodies did not react with undifferentiated ES cells or with populations induced to a mesoderm or ectoderm fate (Fig. 2A). Time course experiments showed that the antigens recognized by both antibodies displayed an expression pattern similar, but not identical, to that of CD25-Foxa3 (Supplemental Fig. 1A&B). The ENDM1 antigen was expressed earlier than CD25-Foxa3 as it was already detected on greater than 10% (~13%) of the cells at day 4 of differentiation. Comparable time course experiments performed under mesoderm or ectoderm inducing conditions revealed very little staining with either antibody. The findings from these analyses indicate that these two antibodies are highly specific for ES cell-derived endoderm.
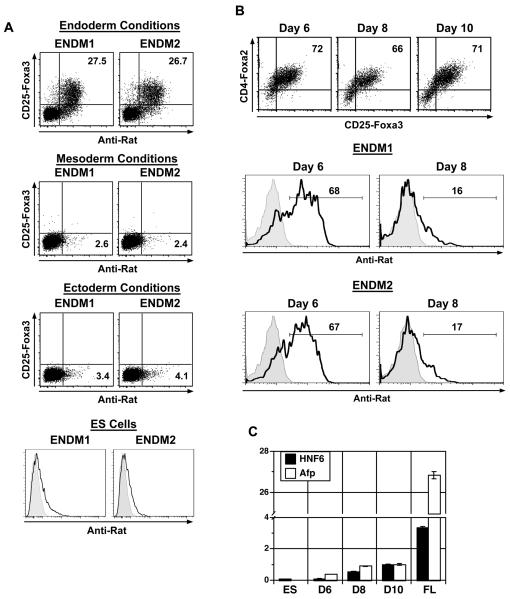
Figure 2. Analysis of endoderm specific monoclonal antibodies on ES cell cultures.
(A) Staining patterns of CD25-Foxa3, ENDM1 and ENDM2 on ES cell-derived endoderm, mesoderm, ectoderm and on undifferentiated ES cells. EBs differentiated in various conditions for 6 days were analyzed. (B) Staining patterns of CD4-Foxa2 versus CD25-Foxa3, ENDM1 and ENDM2 on sorted endoderm cells cultured in hepatocyte conditions for 1 day (day 6 total), 3 days (day 8 total) or 5 days (day 10 total). Histograms shown are gated on CD4-Foxa2+ CD25-Foxa3+ cells. (C) Afp and HNF6 expression (QPCR) in cells cultured as described in B. Relative expression is shown with the D10 population set to 1. Abbreviations: ES, ES cells; FL, day 15 fetal liver; D6, Day 6; D8, Day 8; D10, Day 10.
To determine the stage-specificity of these antibodies, endodermal cells were sorted from day 5 ES cell differentiation cultures by expression of a combination of ENDM1 and ENDM2, and induced to a hepatic fate as previously described [15]. Cells were analyzed for Foxa2 versus Foxa3 and ENDM1 or ENDM2 expression following one, three or five days of culture in hepatocyte inducing conditions (day 6, day 8, or day 10 total). We observed that Foxa2 and Foxa3 expression was maintained over this time period (Fig. 2B). Slightly less than 70% of the CD4-Foxa2+ CD25-Foxa3+ endoderm population stained with either ENDM1 or ENDM2 after one day of hepatic culture (day 6 total)(Fig. 2B). After three days in the hepatocyte inducing conditions (day 8 total), the proportion of cells that stained with either antibody dropped significantly to represent less than 20% of the CD4-Foxa2+ CD25-Foxa3+ population. During these cultures ENDM1 and ENDM2 only stained CD4-Foxa2+ CD25-Foxa3+ cells (data not shown). Expression analyses showed an up-regulation of Afp and HNF6 expression during this time period, indicating that the cells were beginning to undergo specification to a hepatic fate (Fig. 2C). The levels of expression of both genes were considerably less than observed in fetal liver indicating the immaturity of these cells. These observations show that the ENDM1 and ENDM2 antibodies react with endodermal cells during a narrow window of development and that the antigens recognized by them are down-regulated as the cells differentiate to immature hepatocytes.
As a next step in the characterization of the ENDM1 and ENDM2 antibodies, we used them to isolate cells from day 5 EBs induced to an endoderm fate. ENDM1+, ENDM1−, ENDM2+, and ENDM2− fractions were isolated and cultured in hepatocyte inducing conditions. As a control, CD25-Foxa3+ and CD25-Foxa3− cells were isolated and cultured under the same conditions. Following 10 days in culture, the populations were harvested and analyzed for CD4-Foxa2 and CD25-Foxa3 as a measure of definitive endoderm maintenance and for Alb and Afp as an indication of hepatic specification (Fig. 3A-C). The ENDM1+, ENDM2+ and CD25-Foxa3+-derived populations all maintained expression of CD4-Foxa2 and CD25-Foxa3. In addition, greater than 50% of the cells in each population expressed Afp and between 30% and 60% expressed Alb (Fig. 3B and C). In contrast, less than 20% of the populations derived from the ENDM1−, ENDM2− or CD25-Foxa3− cells expressed CD4-Foxa2 and CD25-Foxa3 following the 10-day culture period and only a small fraction were Afp+ or Alb+ (Supplemental Fig. 2). QPCR-based expression analysis confirmed these flow cytometric data and showed that the ENDM1+, ENDM2+, and CD25-Foxa3+-derived populations expressed approximately 10 fold more Afp and Hnf6 than the populations generated from the corresponding negative fractions (Fig. 3D). Collectively, these findings demonstrate that populations isolated from activin-induced EBs with either ENDM1 or ENDM2 represent definitive endoderm that can undergo specification to a hepatocyte fate.
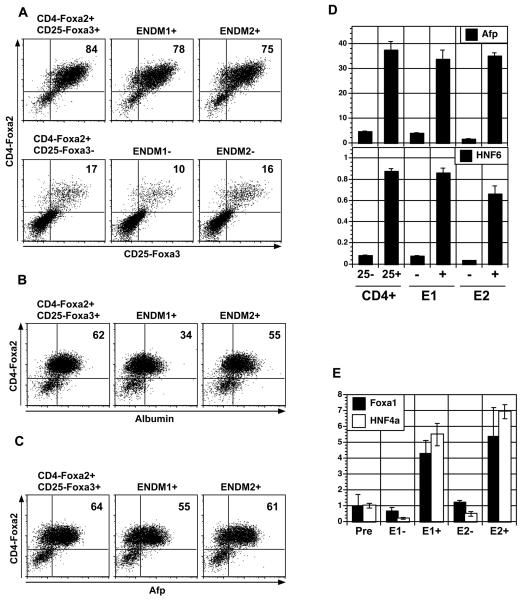
Figure 3. Isolation and characterization of ES cell-derived ENDM1+ and ENDM2+ cells.
(A) CD4-Foxa2 versus CD25-Foxa3 expression on CD4+CD25+, CD4+CD25−, ENDM1+, ENDM1−, ENDM2+, and ENDM2− derived cells following 10 days of culture in hepatocyte inducing conditions. Populations were isolated from day 5 EBs induced under endoderm conditions. (B) Intracellular flow cytometry measuring the proportion of CD4-Foxa2+ and albumin expressing cells in the CD4+CD25+, ENDM1+ and ENDM2+-derived populations shown in A. (C) Intracellular flow cytometry measuring the proportion of CD4-Foxa2+ and Afp expressing cells in the CD4+CD25+, ENDM1+ and ENDM2+-derived populations shown in A. (D) Afp and HNF6 expression in the different populations described in A by QPCR. (E) Expression of Foxa1 and HNF4a in ENDM1+, ENDM1−, ENDM2+, and ENDM2− populations sorted from 5-day-old EBs generated from E14 wild type ES cells differentiated in endoderm inducing conditions (QPCR). Relative expression is shown with the presort population set to 1. Abbreviations: CD4, CD4-Foxa2; 25, CD25-Foxa3; E1, ENDM1; E2, ENDM2; Pre, presort.
ENDM1+ and ENDM2+ populations were also detected in day 5 EBs generated from the parental ES cell line from which the CD25-Foxa3 cells were created, un-manipulated wild type E14 ES cells. These populations were isolated by cell sorting and found to express higher levels of Foxa1 and HNF4a than the corresponding negative fractions, indicating that they represent populations of definitive endoderm (Fig. 3E). The antibodies also stained endoderm generated from the F1(v6.5) ES cell line (Supplemental Fig. 3). As observed with the E14 derived ES cells (CD25-Foxa3 cells), ENDM1 and ENDM2 did not react with F1 ES cell-derived mesoderm or ectoderm indicating that expression of the corresponding epitopes was also endoderm specific in derivatives of this cell line. While the antibodies recognize endoderm generated from different mouse ES cells, they do not stain human ES cell derived endoderm (data not shown).
To verify that the antibodies recognize definitive endoderm in vivo, we performed whole mount staining on mouse embryos at day 8.25 of gestation (5-6 somite pairs). At this stage, the endoderm is multipotent, and has not yet committed to hepatic and pancreatic fates [21]. Both antibodies labeled the ventral foregut endoderm at the anterior intestinal portal (Fig. 4A, white arrows), corresponding precisely to the endodermal domain competent to activate the hepatic and pancreatic fates. In addition, the ENDM2 antibody also labeled the dorsal endoderm, tracking near the embryo midline (Fig. 4A, yellow arrow). ENDM1 and ENDM2 also stained visceral endoderm in vivo (data not shown). These studies establish that the monoclonal antibodies derived from ES cell differentiation studies appropriately label definitive organogenic progenitors in the mouse embryo. In addition, the antibodies usefully label intact endodermal cells in their native epithelium.
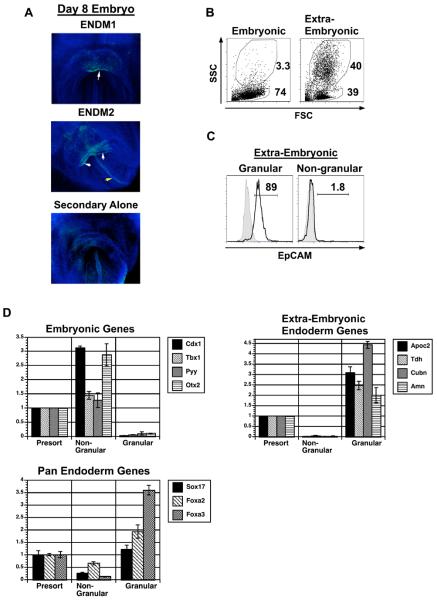
Figure 4. Whole mount staining of ENDM1 and ENDM2 and isolation of extra-embryonic endoderm by the side scatter flow cytometry parameter.
(A) Whole mount staining analyses of day 8.25 mouse embryos showing ENDM1 and ENDM2 positive populations. White arrow indicates positive ventral endoderm and yellow arrow indicates dorsal endoderm. Embryos were co-stained with DAPI. (B) Analyses of forward and side scatter properties of the embryonic- and extra-embryonic-dissected tissues isolated form E 8.25 embryos. (C) EpCAM expression on SSChigh (granular) and SSClow (non-granular) populations from the extra-embryonic region of the embryo. Filled histogram is staining control, open histogram is EpCAM staining. (D) Gene expression analysis of the SSChigh and SSClow populations isolated from pooled whole day 8.25 mouse embryos. Populations were isolated by cell sorting based on forward and side scatter using the gates shown in B. CDNA was generated and expression of the indicated genes analyzed by QPCR. Relative expression is shown with the presort population set to 1. Data shown is the average from 3 independent experiments (60-80 embryos per experiment). Abbreviations: SSC, side scatter; FSC, forward scatter.
To further investigate the native embryonic cells that are recognized by the monoclonal antibodies, we next analyzed the staining patterns of ENDM1 and ENDM2 during embryonic development using flow cytometry. The examination of endoderm by flow cytometry in vivo can be complicated by the fact that both definitive (embryonic) and visceral (extra-embryonic) endoderm express many of the same genes, including the surface molecules recognized by ENDM1 and ENDM2 (Fig. 5A and Supplemental Fig. 4). As an initial approach to distinguish these two types of endoderm, we analyzed the forward-scatter (FSC) and side-scatter (SSC) properties of the embryonic and extra-embryonic populations of day 8.25 embryos by flow cytometry. Given that extra-embryonic endodermal cells contain large numbers of granules which are involved in nutrient exchange to the embryo [22], we reasoned that it may be possible to distinguish extra-embryonic from early embryonic endoderm based on SSC, a parameter that is reflective of cellular granularity. Analysis of the two populations revealed that the embryonic region consists predominantly of SSC low cells whereas the extra-embryonic region contains both SSC low and SSC high populations (Fig. 4B). The SSC high (granular) extra-embryonic subpopulation expressed EpCAM, a marker found on both extra-embryonic and embryonic endoderm [23]. By contrast, the SSC low (non-granular) extra-embryonic cells did not express EpCAM (Fig. 4C). These observations suggest that SSC may be a defining characteristic of extra-embryonic endoderm in the early embryo.
Figure 5. Expression of ENDM1 and ENDM2 during mouse embryogenesis and endoderm isolation ex vivo by cell sorting.
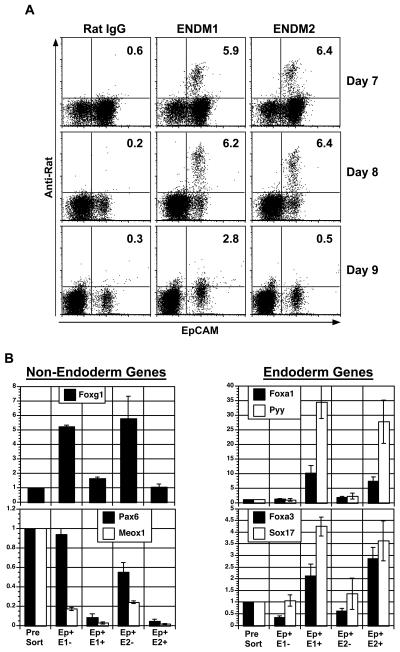
(A) Expression of EpCAM, ENDM1 and ENDM2 on cells from embryos of indicated ages. Plots shown are gated on SSClow, non-granular cells to exclude extra-embryonic endoderm. (B) QPCR-based expression analyses of EpCAM+ ENDM1−, EpCAM+ ENDM1+, EpCAM+ ENDM2−, and EpCAM+ ENDM2+ populations isolated from day 8.25 mouse embryos. Cells were isolated by cell sorting, with gating on SSClow, non-granular cells to exclude extra-embryonic endoderm. Relative expression is shown with the presort population set to 1. Abbreviations: Ep, EpCAM; E1, ENDM1; E2, ENDM2.
To further investigate this possibility, SSC high and SSC low cells were isolated from whole day 8.25 mouse embryos by cell sorting and analyzed for the expression of genes found either in visceral endoderm (Apoc2, Tdh, Cubn, and Amn [24, 25]) or definitive endoderm as well as in other embryonic tissue (Cdx1, Tbx1, Pyy, and Otx2 [24]) and pan-endodermal genes (Foxa2, Foxa3, Sox17). As shown in Fig. 4D, the SSC high granular cells expressed Apoc2, Tdh, Cubn, and Amn but not Cdx1, Tbx1, Pyy, and Otx2. The SSC low population displayed an opposite pattern and expressed the definitive but not the visceral endoderm set of genes (Fig. 4D). Both populations express the pan-endodermal genes Foxa2, Foxa3, and Sox17 (Fig. 4D). The expression is higher in the SSC high population, which is expected if SSC high cells are pure visceral endoderm and SSC low cells are a mixture of definitive endoderm as well as other embryonic and extra-embryonic tissues. These findings demonstrate that SSC can be used as one parameter to distinguish visceral from definitive endoderm in the early mouse embryo.
To verify that ENDM1 and ENDM2 recognize definitive endoderm in vivo, their staining patterns were analyzed on the non-granular (SSClo) cells from E7.25, E8.25, and E9.25 embryos (Fig. 5A). These patterns were compared to that of an anti-EpCAM antibody, as EpCAM has been reported to be expressed on endoderm in the early embryo [23]. Both ENDM1 and ENDM2 stained approximately 6% of non-granular cells from E7.25 and E8.25 embryos. These populations were also EpCAM+. By E9.25, the proportion of cells stained with both antibodies dropped to almost undetectable levels, despite the persistence of EpCAM+ cells. These antibodies were also examined in adult pancreas and were not found to stain this tissue (data not shown). The staining patterns of the antibodies were also analyzed in conjunction with that of an anti-CXCR4 antibody as Cxcr4 is expressed on embryonic but not on extra-embryonic endoderm [26]. Both the ENDM1+ and ENDM2+ populations from E8.25 embryos (gated on non-granular cells) were also CXCR4+ (Supplemental Fig. 4B). Taken together, these findings are consistent with the interpretation that both antibodies recognize antigens expressed on definitive endoderm in the early embryo. As observed in the ES cell differentiation cultures, the epitopes are rapidly down-regulated as the endodermal cells undergo lineage specification at day 9.25. In addition to definitive endoderm, the ENDM1 and ENDM2 antibodies also stained the granular (SSChi) EpCAM+ CXCR4− extra-embryonic population from E8.25 embryos, indicating that they also recognize visceral endoderm (Supplemental Fig. 4).
To verify that the antibodies recognize definitive endoderm in vivo, non-granular EpCAM+ ENDM1+/− or ENDM2+/− cells were isolated from day 8.25 mouse embryos by cell sorting and analyzed for gene expression patterns by QPCR. The ENDM1+ and ENDM2+ EpCAM+ cells expressed higher levels of Sox17, Foxa3, Foxa1, and Pyy compared to ENDM− and ENDM2− EpCAM+ or presort populations (Fig. 5B). Pyy, a marker specific for posterior foregut endoderm showed the most dramatic differential in expression (30-fold) between the antibody+ and presort populations. This suggests that these antibodies both preferentially stain the Pyy+ foregut endoderm. Expression of the ectoderm gene Pax6 and the somitic mesoderm gene Meox1 were substantially lower in the ENDM1+ and ENDM2+ cells compared to the corresponding negative populations. As the ENDM1− and ENDM2− populations were also sorted on the basis of EpCAM expression, these findings demonstrate that EpCAM is not an endoderm specific marker at this stage. It has been reported that EpCAM is expressed on both surface ectoderm in addition to endoderm at E8.25 of gestation [23]. Higher levels of expression of the surface ectoderm marker Foxg1 [27] in the EpCAM+ ENDM1− or ENDM2− cells compared to the ENDM1+ or ENDM2+ or the unsorted cells, suggests that this population does contain these ectodermal cells. Taken together, the findings from the cell sorting studies demonstrate that the antibodies ENDM1 and ENDM2 specifically recognize a subpopulation of definitive endoderm in the early mouse embryo.
Discussion
Studies on lineage diversification in the early embryo are dependent on the availability of markers that enable one to identify and monitor distinct developmental steps. The traditional approaches used in developmental biology involve the analyses of RNA and protein expression patterns in situ to define commitment steps and establish lineage relationships. While studies based on these approaches have established many of the current principles of developmental biology, they typically do not allow one to isolate the cell population of interest. With recent advances in our ability to direct the differentiation of ES cells in culture [28], it is now possible to access relatively large number of cells at early developmental stages, including those representing the establishment of the three primary germ layers; mesoderm, endoderm and ectoderm. The ability to isolate these cells to purity will provide unprecedented opportunities to investigate the molecular pathways that regulate their induction as well as their specification to derivative cell types. To be able to study definitive endoderm development, we developed a reporter ES cell line that enabled us to specifically monitor induction of this lineage as well as to isolate highly enriched populations of these cells. Using this reporter ES cell line, we generated two monoclonal antibodies that recognize antigens expressed on endoderm generated from ES cells as well as endoderm in the early embryo.
Previous studies have relied on the creation of reporter ES cell lines to track early development in differentiation cultures and to isolate populations representing early stages of lineage commitment [4, 6, 7]. Although this strategy has been effectively used to define the key signaling pathways regulating different stage of ES cell differentiation, applying it to a rapidly increasing number of pluripotent stem cells lines is a formidable task. Stage- and lineage- specific antibodies represent an attractive alternative, as they should recognize comparable populations generated from different stem cell lines. Previous studies have identified several other antibodies that recognize antigens present on endoderm. For instance, Cxcr4 has been shown to be expressed on anterior primitive streak cells as well as on cells that represent the earliest stages of endoderm commitment in ES cell cultures [6, 15]. While Cxcr4 does provide a useful endoderm marker under certain conditions, it is not specific to this lineage as it is also expressed on mesodermal cells in the embryo as well as in ES cell differentiation cultures (PG, unpublished observations and [26]). EpCAM is another candidate endoderm marker that has been shown to be expressed on this lineage in the mouse embryo [23]. Specificity for endoderm lineage appears to be stage specific, however, as it is broadly expressed on most embryo cells at E7.0 and is found on surface ectoderm ([23] and Fig. 5A, 5B) in addition to endoderm at E8.0. By E9.0 EpCAM does appear to be more specific for the endoderm lineage [23]. While useful for monitoring specific stages of endoderm development in vivo, it is less useful for monitoring this lineage in the ES cell differentiation cultures, as it is widely expressed during in vitro differentiation of ES cells to endoderm ([23] and PG, unpublished results).
The two antibodies identified in this study, ENDM1 and ENDM2 show similar staining patterns that display remarkable specificity for an early stage of endoderm development in ES cell differentiation cultures. Our kinetic analyses demonstrate that the antigens recognized by the antibodies are down-regulated as the cells are specified to a hepatic fate. Importantly, the cell sorting studies show that the endoderm population recognized by ENDM1 and ENDM2 does have the potential to generate cells of the hepatic lineage. Whether or not the ENDM1+ and ENDM2+ ES cell-derived populations are able to generate a broad range of endoderm-derived cell types remains to be determined. The staining patterns in vivo confirm those observed in vitro as both antibodies specifically recognize ventral foregut endoderm that will contribute to the formation of the liver and pancreas. Expression of the antigens in the early embryo is also transient, and is no longer detectable at significant levels by E9.0. The loss of expression of the markers recognized by ENDM1 and ENDM2 coincides with the emergence of EpCAM as a more specific definitive endoderm marker, suggesting that these antibodies may be a complementary set of reagents for monitoring endoderm development in the early mouse embryo.
While ENDM1+ and ENDM2+ did not appear to recognize any mesoderm or ectoderm-derived lineages within the embryo, they both stained extra-embryonic visceral endoderm. This is not surprising, given that visceral and definitive endoderm do share expression of many markers, a fact that has made it difficult to distinguish these two populations [29]. Although these endoderm populations show overlapping expression of many markers, the physiology of the cells is quite different as extra-embryonic endodermal cells contain large numbers of granules required for nutrient exchange to the embryo [22]. Taking advantage of this characteristic, we were able to demonstrate that the two populations could be easily distinguished based on side scatter properties visualized by flow cytometry. Combined with either ENDM1 or ENDM2 staining, the distinction based on granularity enables one to easily isolate pure populations of definitive endoderm from the early mouse embryo. Contamination of the ES cell-derived ENDM1+ and ENDM2+ populations with visceral endoderm does not appear to be of great concern, as the conditions used for induction did not induce this population (Fig. 1D). When different induction conditions are used, antibodies to CXCR4 can be used in combination with ENDM1+ and ENDM2+ as CXCR4 is expressed on ES cell-derived definitive but not visceral endoderm [6].
Conclusion
In summary, we have identified two new monoclonal antibodies that display remarkable specificity for mouse endoderm. The availability of these antibodies enables one to easily monitor endoderm induction in ES cell differentiation cultures as well as to isolate cells that represent this early developmental stage. The ability to isolate this population in high purity from the ES cell differentiation cultures and the early mouse embryo will allow for detailed molecular comparisons to ensure that the populations are comparable. Finally, the success in generating anti-mouse endoderm antibodies paves the way for generating antibodies to other germ layers in the mouse system, as well as to those derived from human embryonic stem cells.
Supplementary Material
Acknowledgements
We would like to thank Lill Mårtensson-Bopp at the Babraham Institute, Cambridge, United Kingdom, for the human CD25 cDNA. We would like to thank members of the Keller laboratory for critical discussions of the manuscript.
Grant support: NIH U01DK072513 (G.M.K.), NIDDK U01DK072477 (M.G.), and NIDDK U01DK072473 (P.R.S.).
Footnotes
The authors declare that they have no competing financial interests.
References
- 1.Conlon FL, Lyons KM, Takaesu N, et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120(7):1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 2.Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131(7):1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 3.Tada S, Era T, Furusawa C, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132(19):4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- 4.Gadue P, Huber TL, Paddison PJ, et al. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103(45):16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 6.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23(12):1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 7.Morrison GM, Oikonomopoulou I, Migueles RP, et al. Anterior Definitive Endoderm from ESCs Reveals a Role for FGF Signaling. Cell Stem Cell. 2008;3(4):402–415. doi: 10.1016/j.stem.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118(1):47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Monaghan AP, Kaestner KH, Grau E, et al. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119(3):567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- 10.Thomas PQ, Brown A, Beddington RS. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125(1):85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T, Kanai-Azuma M, Hara K, et al. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119(Pt 17):3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- 12.Kim I, Saunders TL, Morrison SJ. Sox17 Dependence Distinguishes the Transcriptional Regulation of Fetal from Adult Hematopoietic Stem Cells. Cell. 2007;130(3):470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubo A, Chen V, Kennedy M, et al. The homeobox gene HEX regulates proliferation and differentiation of hemangioblasts and endothelial cells during ES cell differentiation. Blood. 2005;105(12):4590–4597. doi: 10.1182/blood-2004-10-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115(3):281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 15.Gouon-Evans V, Boussemart L, Gadue P, et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24(11):1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- 16.Williams CV, Stechmann CL, McLoon SC. Subtractive immunization techniques for the production of monoclonal antibodies to rare antigens. BioTechniques. 1992;12(6):842–847. [PubMed] [Google Scholar]
- 17.Sleister HM, Rao AG. Strategies to generate antibodies capable of distinguishing between proteins with >90% amino acid identity. J Immunol Methods. 2001;252(1-2):121–129. doi: 10.1016/s0022-1759(01)00346-5. [DOI] [PubMed] [Google Scholar]
- 18.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 19.Davis JM, Pennington JE, Kubler AM, et al. A simple, single-step technique for selecting and cloning hybridomas for the production of monoclonal antibodies. J Immunol Methods. 1982;50(2):161–171. doi: 10.1016/0022-1759(82)90222-8. [DOI] [PubMed] [Google Scholar]
- 20.Nostro MC, Cheng X, Keller GM, et al. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2(1):60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutsch G, Jung J, Zheng M, et al. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128(6):871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 22.Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43(3):183–205. [PubMed] [Google Scholar]
- 23.Sherwood RI, Jitianu C, Cleaver O, et al. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304(2):541–555. doi: 10.1016/j.ydbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Charters AM, Lee SC, et al. A systematic screen for genes expressed in definitive endoderm by Serial Analysis of Gene Expression (SAGE) BMC Dev Biol. 2007;7(1):92. doi: 10.1186/1471-213X-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalantry S, Manning S, Haub O, et al. The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nat Genet. 2001;27(4):412–416. doi: 10.1038/86912. [DOI] [PubMed] [Google Scholar]
- 26.McGrath KE, Koniski AD, Maltby KM, et al. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213(2):442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 27.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124(14):2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 28.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 29.Pfister S, Steiner KA, Tam PP. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr Patterns. 2007;7(5):558–573. doi: 10.1016/j.modgep.2007.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.