Abstract
Inflammation is associated with increased sympathetic drive in cardiovascular diseases. Blood-borne pro-inflammatory cytokines, markers of inflammation, induce cyclooxygenase-2 (COX-2) activity in perivascular macrophages of the blood-brain barrier. COX-2 generates prostaglandin E2 (PGE2), which may enter the brain and increase sympathetic nerve activity. We examined the contribution of this mechanism to augmented sympathetic drive in rats following myocardial infarction (MI). Approximately 24h after acute MI, rats received an intracerebroventricular (ICV) injection (1 μl/min over 40 minutes) of clodronate liposomes (MI+CLOD) to eliminate brain perivascular macrophages, liposomes alone (MI+LIPO) or artificial cerebrospinal fluid (MI+aCSF). A week later, COX-2 immunoreactivity in perivascular macrophages and COX-2 mRNA and protein had increased in hypothalamic paraventricular nucleus (PVN) of MI+aCSF and MI+LIPO, compared with sham-operated (SHAM) rats. In MI+CLOD, neither perivascular macrophages nor COX-2 immunoreactivity was seen in PVN, and COX-2 mRNA and protein were similar to SHAM. PGE2 in cerebrospinal fluid, PVN neuronal excitation, and plasma norepinephrine were less in MI+CLOD than MI+aCSF and MI+LIPO but more than in SHAM. ICV CLOD had no effect on interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) mRNA and protein in PVN or plasma IL-1β and TNF-α, which were increased in MI compared with SHAM rats. In normal rats, pretreatment with ICV CLOD reduced (P<0.05) renal sympathetic, blood pressure and heart rate responses to intracarotid artery injection of TNF-α (0.5 μg/kg); ICV LIPO had no effect. The results suggest that pro-inflammatory cytokines stimulate sympathetic excitation after MI by inducing COX-2 activity and PGE2 production in perivascular macrophages of the blood-brain barrier.
Keywords: Cytokines, Cyclooxygenase-2, Perivascular macrophages, Clodronate liposomes, Myocardial infarction, Heart failure, Inflammation
Introduction
There is increasing appreciation for the role of inflammation in cardiovascular and cardiovascular-related diseases, including myocardial infarction (MI), heart failure (HF), hypertension, obesity and diabetes.1-5 Recent studies have established a causal relationship between inflammation and activation of the sympathetic nervous system,6, 7 but exactly how inflammatory mediators trigger an increase in sympathetic activity remains unknown. The present study examined one putative mechanism.
The pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), well-recognized markers of inflammation, are too large to cross the blood-brain barrier. In normal animals, they can affect the brain indirectly by inducing cyclooxygenase-2 (COX-2) activity in endothelial cells and perivascular macrophages of the cerebral microvasculature.8 COX-2 catalyzes the synthesis of prostaglandin E2 (PGE2), which can enter the brain to activate neurohumoral systems. This mechanism has been invoked to explain cytokine activation of the hypothalamic-pituitary-adrenal axis.9, 10
Acute studies in normal rats have demonstrated that the perivascular macrophages are more sensitive to blood-borne pro-inflammatory cytokines than are endothelial cells.8 Moreover, in HF rats that have moderately increased plasma cytokines, COX-2 expression in the paraventricular nucleus (PVN) of the hypothalamus, a key cardiovascular regulatory region of the brain,11, 12 seems largely confined to the perivascular macrophages.3, 4 These observations set the stage for the present study to determine whether eliminating the perivascular macrophages of the blood-brain barrier might lower sympathetic drive in rats with HF. The overall hypothesis guiding this study is that pro-inflammatory cytokines activate the sympathetic nervous system in HF rats by inducing COX-2 activity and the synthesis of PGE2 in perivascular macrophages in the brain.
Perivascular macrophages can be selectively eliminated by administering liposomes containing clodronate.13,14 The method relies upon phagocytosis of the clodronate-containing liposomes by perivascular macrophages, resulting in intracellular release of clodronate and subsequent cell death. In previous work by others,15 intracerebroventricular (ICV) injection of clodronate liposomes has been shown to selectively deplete meningeal and perivascular macrophages in the brain while sparing microglial and other non-phagocytic cells. The effect was nearly complete over the 2-10 day interval following ICV administration of the clodronate liposomes. An associated transient depletion of some particularly sensitive peripheral macrophages (i.e., Kuppfer cells) was also observed, with recovery within a week.
The present study employed this method to examine the functional significance of brain perivascular macrophages in HF rats that have increased plasma cytokines after MI and in normal rats challenged acutely with a systemic injection of TNF-α. The findings suggest that brain perivascular macrophages play a critical role in the relationship between systemic inflammation and sympathetic activation.
Methods
Animals
Adult male Sprague-Dawley rats weighing 250-300 g were obtained from Harlan Sprague-Dawley (Indianapolis, IN). They were housed in temperature (23±2°C) and light controlled animal quarters and were provided with rat chow ad libitum. These studies were performed in accordance with the “Guiding Principles for Research Involving Animals and Human Beings”.16 The experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa and the Research and Development Committee of the Iowa City Department of Veterans Affairs Medical Center.
Drugs Administered
Clodronate liposomes (CLOD) and control liposomes (LIPO) were kind gifts from Dr. Paul E. Sawchenko (The Salk Institute for Biological Studies, La Jolla, CA). The dose and method for injection of the liposomes and the time point for evaluating the effects of CLOD treatment were derived from a previous study demonstrating that perivascular macrophages in all investigated regions of the brain were maximally depleted about 1 week after injection, and then gradually recovered.15
Experimental Protocols
Study I: Effects of Centrally Administered Clodronate Liposomes on MI and Sham-operated Rats
Rats underwent coronary ligation to induce MI or a sham operation (SHAM). Left ventricular function was assessed by echocardiography within 24 hours after recovery from surgery to assign rats to one of 4 treatment groups: SHAM rats that received no treatment (SHAM, n=15) and MI rats that received an ICV infusion (1 μl/min over 40 minutes) of clodronate liposomes (MI+CLOD, n=16), liposomes alone (MI+LIPO, n=16) or artificial cerebrospinal fluid (MI+aCSF, n=17). The ICV infusion was administered immediately after the echocardiographic assessment. One week later, at the conclusion of the study protocol, a second echocardiogram and hemodynamic measurements were obtained in some rats. Rats were then euthanized with an overdose of urethane to collect blood for measurement of plasma cytokines and norepinephrine (NE, a marker of systemic sympathetic nerve activity), cerebrospinal fluid (CSF) for measurement of PGE2 level, brain tissues for molecular analysis, and heart and lungs for anatomical analysis, or were perfused with fixative for immunohistochemical studies.
Study II: Effects of Centrally Administered Clodronate Liposomes on Responses to Intracarotid Artery Injection of TNF-α in Normal Rats
An acute bolus intracarotid artery (ICA) injection of TNF-α elicits an acute sympathetically mediated pressor response in normal rats.17 These studies were performed to determine whether perivascular macrophages in the brain mediate this response. Normal rats received an ICV infusion (1 μl/min over 40 minutes) of CLOD, LIPO, or aCSF, as in Study I. A week later they were anesthetized, prepared for recording of renal sympathetic nerve activity (RSNA), blood pressure (BP) and heart rate (HR), and given a bolus ICA injection of TNF-α (0.5 μg/kg). There were four treatment groups 1) aCSF alone (no TNF-α, n=16); 2) aCSF+TNF-α (n=15); 3) CLOD+TNF-α (n=15); 4) LIPO+TNF-α (n=15). Peak responses of mean BP, HR, RSNA recorded over a 3-minute interval during ICA infusion were compared with a 3-minute baseline value immediately preceding each intervention. RSNA responses are reported as a percent change from baseline.
At the completion of the recording session, 2 hours after the ICA injection, rats were euthanized with an overdose of urethane to collect blood, CSF and brain tissues for biochemical and molecular analyses or perfused with fixative for immunohistochemical studies.
Specific Methods
Please see http://hyper.ahajournals.org.
Statistical Analysis
All data are expressed as mean ± SEM. For most studies, the significance of differences among groups was analyzed by 2-way, repeated-measure ANOVA followed by a post hoc Fisher's least significant difference test. Echocardiographic parameters were analyzed using 1-way ANOVA followed by Fischer's least significant difference test. Differences were considered significant at P<0.05.
Results
Study I: Effects of Centrally Administered Clodronate Liposomes on MI and Sham-operated Rats
Characteristics of the Study Groups
Echocardiography performed within 24 hours of coronary ligation revealed that left ventricular (LV) ejection fraction was reduced and LV end-diastolic volume was increased in the MI rats compared with the SHAM rats. Rats assigned to the MI+CLOD, MI+LIPO and MI+aCSF treatment groups were well-matched with regard to echocardiographically-defined LV function (Table S1 – Please see http://hyper.ahajournals.org).
Repeat echocardiography a week later, at the conclusion of the study protocol, revealed that all 3 groups of MI rats still had significant increases in LV end-diastolic volume and decreases in LV ejection fraction, compared with SHAM rats. Treatment with CLOD or LIPO, compared with aCSF, had no significant effect on these echocardiographic indicators of LV function (Table S1 – Please see http://hyper.ahajournals.org).
Hemodynamic measurements obtained under anesthesia at the conclusion of the study protocol revealed that systolic blood pressure, LV peak systolic pressure, and the maximum rate of rise of LV pressure were lower, and LV end-diastolic pressure was higher, in MI rats than in SHAM rats (Table S2 – Please see http://hyper.ahajournals.org). There were no significant differences in diastolic blood pressures or heart rates across the experimental groups. MI+CLOD rats had higher maximum rates of rise of LV pressure and lower LV end-diastolic pressure than MI+LIPO and MI+aCSF rats, but these values were still significantly different from SHAM rats.
Anatomical assessment following euthanasia revealed that right ventricle/body weight (BW) and wet lung/BW ratios were substantially higher in MI rats compared with SHAM rats (Table S2 – Please see http://hyper.ahajournals.org). These variables were not affected by treatment with CLOD, LIPO or aCSF.
Effects of Clodronate Liposomes on Perivascular Macrophages
Confocal microscopy revealed ED2 positive cells (perivascular macrophages) in the PVN of MI+LIPO and MI+aCSF rats and in the SHAM rats. There was no difference in the number of ED2 positive cells between MI+aCSF and SHAM rats (data not shown). No ED2 positive cells were found in the PVN in MI+CLOD rats (Figure 1A). In contrast, OX42 positive cells (activated microglia) were present in all four treatment groups, and seemed to be unaffected by treatment with CLOD (Figure 1B).
Figure 1.
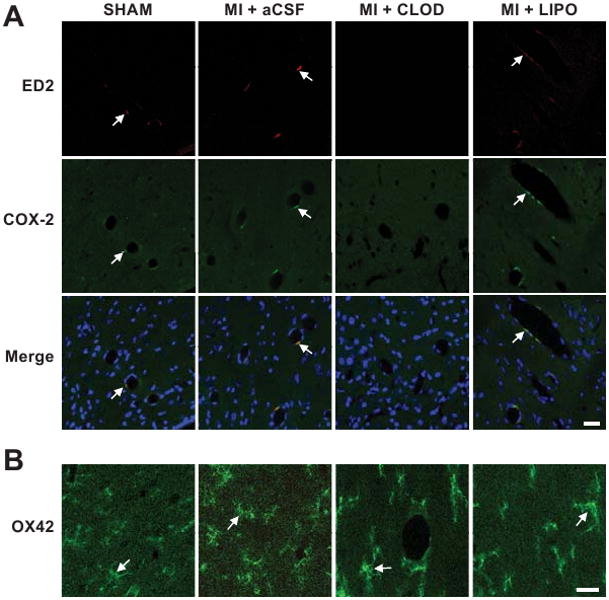
Representative laser confocal images from PVN of SHAM and MI rats treated with artificial cerebrospinal fluid (aCSF), clodronate liposomes (CLOD) and liposomes alone (LIPO). (A) triple immunostaining for the perivascular macrophage marker ED2 (top, bright red), COX-2 (middle, bright green) and combined image (bottom) with nuclear staining (blue). The arrows point to ED2 positive cells expressing COX-2, as indicated by the yellow staining in the merge image. Neither perivascular macrophages nor COX-2 immunoreactivity were found in the MI rats treated with CLOD. (B) OX42 positive microglial cells (bright green). The arrows indicate cells positive for OX42. The microglial cells in the PVN were not affected by CLOD treatment. Scale bar, 20 μm.
As we have shown previously,3, 4 COX-2 immunoreactivity in the PVN of MI rats is found primarily in the cytoplasm of perivascular macrophages. In the present study, MI+CLOD rats, lacking perivascular macrophages, had no COX-2 immunoreactivity in the PVN (Figure 1A). MI+LIPO and MI+aCSF rats had the expected COX-2 immunoreactivity in ED2 positive cells.
Effects of Clodronate Liposomes on the Expression of Central Inflammatory Mediators
COX-2 mRNA (Figure 2A) and protein (Figure 2B) were greater in the PVN of MI+LIPO and MI+aCSF rats than SHAM rats. In MI+CLOD rats, COX-2 mRNA and protein expression were not different from SHAM rats. There were no differences across treatment groups in COX-2 expression in hypothalamic regions immediately adjacent to PVN.
Figure 2.
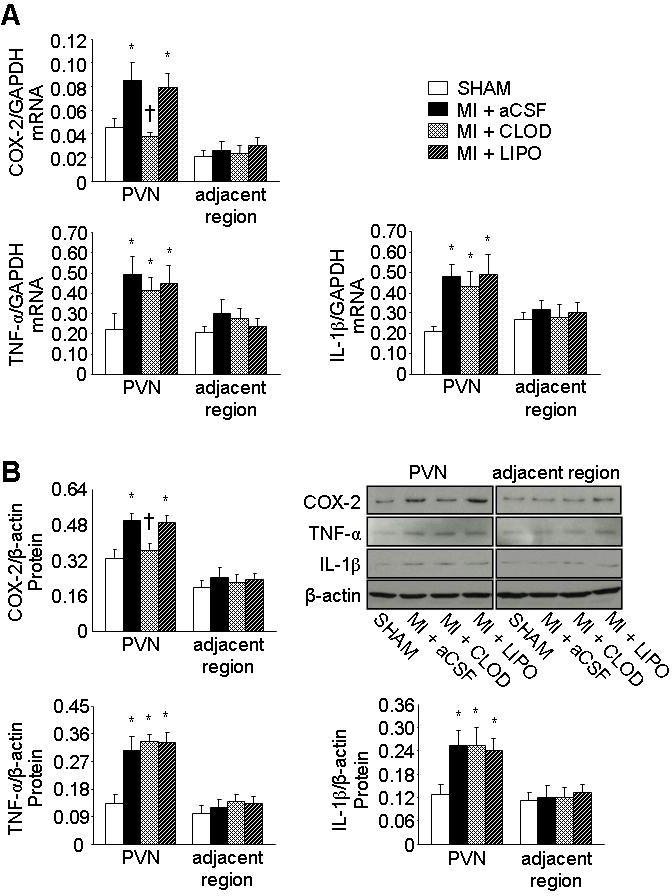
Quantitative comparison of mRNA expression (A) and protein levels (B) for COX-2, TNF-α and IL-1β from the PVN and adjacent regions of hypothalamus of each treatment group. Representative Western blots of COX-2, TNF-α, IL-1β and β-actin are shown in figure B. Values were expressed as mean ± SEM (n=5 to 8 for each group). *P<0.05 vs SHAM in same region, †P<0.05, MI+treatment vs MI+aCSF in same region.
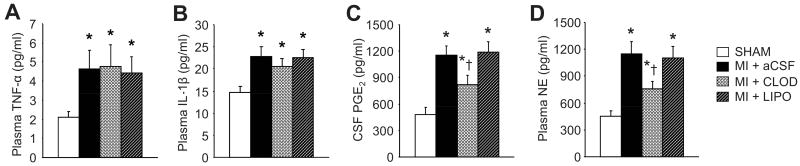
The PGE2 level in the CSF was increased in all 3 MI groups, compared with SHAM (Figure 3). MI+CLOD rats had less CSF PGE2 than MI+LIPO and MI+aCSF rats, but more than SHAM rats. There was no difference in CSF PGE2 between the MI+LIPO and the MI+aCSF groups.
Figure 3.
Plasma TNF-α (A) and IL-1β (B), CSF PGE2 (C) and plasma NE (D) levels in each group. Values were expressed as mean ± SEM (n=6 to 8 for each group). *P<0.05 vs SHAM, †P<0.05, MI+treatment vs MI+aCSF.
IL-1β and TNF-α mRNA (Figure 2A) and protein (Figure 2B) was greater in the PVN of all 3 MI groups than in SHAM rats. Neither CLOD nor LIPO affected IL-1β or TNF-α expression in PVN or adjacent hypothalamic regions.
Effects of Clodronate Liposomes on Neuronal Excitation in PVN
Fra-Like activity, an indicator of chronic neuronal excitation, was significantly increased in medial parvocellular, ventrolateral parvocellular and dorsal parvocellular regions in MI+LIPO and MI+aCSF rats, compared with SHAM rats (Figure 4). MI+CLOD rats had significantly less Fra-Like activity in the medial parvocellular and ventrolateral parvocellular regions of PVN. Fra-Like activity in dorsal parvocellular region was not affected. MI+LIPO and MI+aCSF rats had similar Fra-Like activity in all 3 parvocellular regions. There was no difference across treatment groups in Fra-Like activity in the posterior magnocellular region of PVN.
Figure 4.
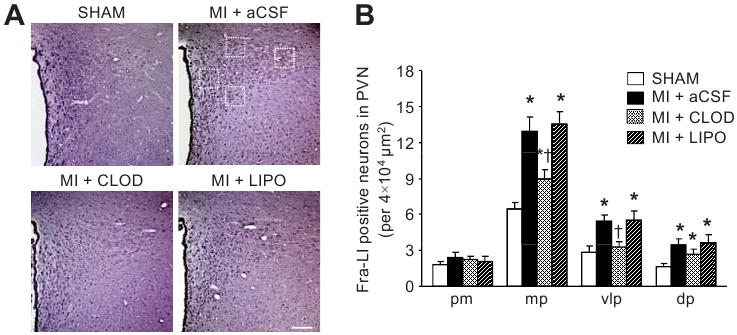
Expression of Fra-LI activity in the PVN. (A) Representative sections from each group showing Fra-LI immunoreactivity in PVN neurons. Dark dots indicate Fra-LI positive neurons. Squares (dotted lines) indicate the regions in panel A from which the data in panel B are derived. Scale bar=200 μm. (B) Quantification of Fra-LI positive neurons in 4 different regions of the PVN. Values are expressed as mean ± SEM (n=4 for each group). *P<0.05 vs SHAM. †P<0.05, MI+treatment vs MI+aCSF. pm indicates posterior magnocellular; mp, medial parvocellular; vlp, ventrolateral parvocellular; dp, dorsal parvocellular.
Effects of Clodronate Liposomes on Plasma Levels of IL-1ß, TNF-α and NE
Plasma levels of IL-1ß, TNF-α and NE were higher (P<0.05) in all three MI treatment groups, as compared with SHAM rats (Figure 3). MI+CLOD rats had significantly lower plasma NE levels than MI+LIPO and MI+aCSF, but higher levels than SHAM. Plasma levels of IL-1ß and TNF-α were similar across all three MI treatment groups.
Study II: Effects of Centrally Administered Clodronate Liposomes on Responses to Intracarotid Artery Injection of TNF-α in Normal Rats
Effects of Clodronate Liposomes on Perivascular Macrophages
As in Study I, confocal microscopy revealed that central injection of CLOD depleted perivascular macrophages in the PVN, but had no apparent effect on activated microglia (data not shown).
As expected, TNF-α induced increased COX-2 immunoreactivity in perivascular macrophages of the PVN in aCSF- and LIPO-treated rats, compared with the aCSF (no TNF-α) group, but no COX-2 activity was induced in the CLOD-treated rats in which the perivascular macrophages were depleted (data not shown).
Real time PCR and western blot showed significantly increased COX-2 mRNA and protein expression in the PVN of aCSF+TNF-α rats, compared with the aCSF (no TNF-α) rats. CLOD+TNF-α rats had less COX-2 mRNA and protein expression than LIPO+TNF-α or aCSF+TNF-α (Figure 5). There were no differences in expression of COX-2 mRNA and protein between LIPO+TNF-α and aCSF+TNF-α rats.
Figure 5.
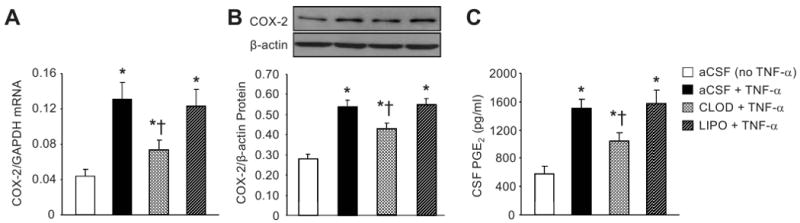
Effects of bolus ICA injection of TNF–α on COX-2 mRNA (A) and protein (B) expression in PVN, and PGE2 level in CSF (C) in normal rats pre-treated a week earlier with ICV CLOD, LIPO or aCSF. aCSF-treated rats not receiving TNF–α injection were used as control. Representative Western blots are aligned with the matching grouped data (B). Values were expressed as mean ± SEM (n=5 to 7 for each group). *P<0.05 vs aCSF (no TNF-α); †P<0.05, Treatment+TNF–α vs aCSF+TNF–α.
Similarly, as predicted by the COX-2 results, aCSF+TNF-α and LIPO+TNF-α rats had an increased PGE2 level in CSF, as compared with aCSF (no TNF-α) rats. CLOD+TNF-α rats had lower PGE2 levels in CSF than LIPO+TNF-α or aCSF+TNF-α rats. In LIPO+TNF-α rats, PGE2 level in CSF was no different from that in aCSF+TNF-α rats.
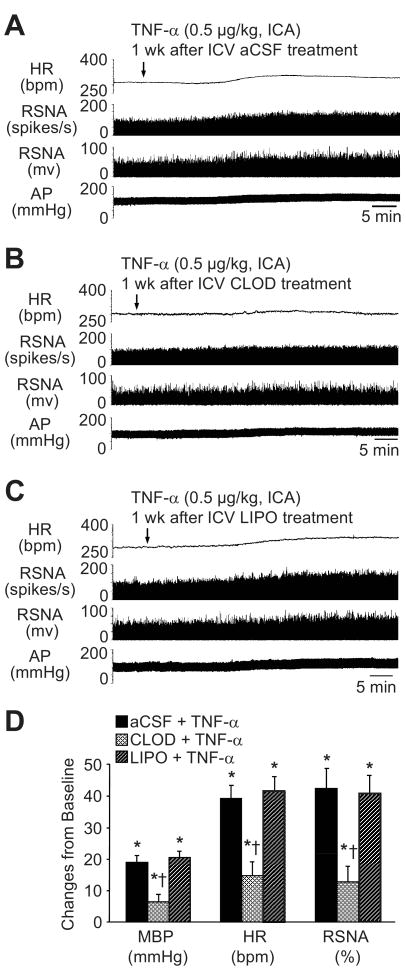
Effects of Clodronate Liposomes on Sympathetic Responses to TNF-α
ICA injection of TNF-α (0.5 μg/kg) in the aCSF-treated rats dramatically (p<0.05) increased integrated RSNA (42.4 ± 6.3 %), HR (39.3 ± 4.1 bpm, from baseline of 328.5 ± 16.6 bpm), and MBP (19.0 ± 2.2 mmHg, from baseline of 95.1 ± 5.8 mmHg). The onset latency for this response was ∼10-15 min with duration at least 120 min recorded. The peak responses occurred ∼30-40 min after TNF-α administration. Compared with aCSF-treated rats, CLOD-treated rats had significantly (p<0.05 vs aCSF+TNF-α) diminished sympatho-excitatory responses to ICA TNF-α: integrated RSNA (12.8 ± 5.4%), MBP (6.4 ± 2.4 mmHg), and HR (14.7 ± 4.4 bpm). There were no differences in the responses to TNF-α between LIPO- and aCSF-treated rats (Figure 6).
Figure 6.
Effects of CLOD on cardiovascular and sympathetic responses to intracarotid artery (ICA) injection of TNF-α. Heart rate (HR), renal sympathetic nerve activity (RSNA, shown both as integrated nerve activity and as windowed spike activity), and arterial pressure (AP) responses to ICA bolus injection of TNF–α in normal rats pre-treated a week earlier with (A) intracerebroventricular (ICV) aCSF, (B) ICV CLOD, or (C) ICV LIPO. (D) Grouped data showing peak changes from baseline in mean blood pressure (MBP), HR and RSNA in each group. Values were expressed as mean ± SEM (n=6 to 7 for each group). *P<0.05 vs baseline; †P<0.05 vs aCSF+TNF–α.
Discussion
The novel findings of this study are: 1) in rats with increased circulating cytokines after MI, a treatment that selectively eliminates a single cell type - the perivascular macrophage of the blood brain barrier - normalizes COX-2 expression in the microvessels of the PVN and reduces both PGE2 levels in CSF and sympathetic nerve activity; and 2) in normal rats, selective depletion of these perivascular macrophages prevents the increases in heart rate, blood pressure, and sympathetic nerve activity induced by ICA administration of TNF-α. Together, these results demonstrate that perivascular macrophages of the blood brain barrier contribute to the augmented sympathetic nerve activity in rats with HF following MI and suggest that the pro-inflammatory cytokines, which increase in the blood and the brain of rats with HF following MI,3, 4, 18, 19 are a likely driving mechanism. The data also implicate perivascular macrophages as a primary source of cytokine-induced COX-2 activity and PGE2 production in the PVN, an important presympathetic nucleus, in rats early after MI. A broader interpretation might be that the perivascular macrophages provide a crucial link between systemic inflammation and sympathetic drive.
These results complement earlier studies from our laboratory demonstrating that rats with ischemia-induced HF have an increase in COX-2 expression in perivascular macrophages in the microvasculature of the PVN 3, 18, 19 that can be reduced by lowering circulating levels of TNF-α and IL-1ß.3, 18 Interestingly, COX-2 activity in the perivascular macrophages of the PVN also responds to changes in the proinflammatory cytokines content inside the blood-brain barrier.4 In those earlier studies, a reduction in COX-2 activity in the perivascular macrophages was uniformly associated with reductions in CSF PGE2 and plasma NE in HF rats, suggesting a tight association between COX-2 activity in perivascular macrophages and sympathetic drive that was confirmed in the present study.
It is surprising that the destruction of the perivascular macrophages normalized COX-2 mRNA and protein in the PVN of the HF rats and eliminated COX-2 immunofluorescence. Microglial cells, which are activated in the HF rats as they are under conditions of chronic brain injury or inflammation,20 were apparently unaffected by treatment with CLOD. Microglia can produce a wealth of inflammatory mediators,21 including COX-2, PGE2 and the pro-inflammatory cytokines TNF-α and IL-1β.22, 23 Why microglia do not appear to contribute to overall COX-2 activity in the HF rats in these studies remains unexplained. A predominant influence of the perivascular macrophages is suggested, but the incomplete reduction in the PGE2 level in the CSF suggests that COX-2 activity in perivascular macrophages is not the only source of PGE2 production in the HF brain.
In normal rats challenged acutely with systemically administered TNF-α, there was a direct relationship between the induction of COX-2 and CSF PGE2 levels – pre-treatment with CLOD prevented the TNF-α-induced increase in COX-2, PGE2 and sympathetic drive. These new findings are consistent with previous work demonstrating that acutely administered pro-inflammatory cytokines induce COX-2 activity in perivascular macrophages8 and activate the sympathetic nervous system via a cyclooxygenase-dependent mechanism.17 In addition, they implicate the perivascular macrophage as the single cellular element responsible for acute cytokine-induced sympathetic activation. However, they leave open the question of how a sympathetic response dependent on the induction of COX-2 protein can occur so quickly – i.e., the induction of a pressor response within 10-15 min after injection of TNF-α. COX-2 is constitutively expressed in brain tissue, including in PVN,24 and our data demonstrate some COX-2 present in the perivascular macrophages of the sham-operated animals. It is conceivable that constitutively present COX-2 might somehow contribute to these very early responses. COX-1 seems an unlikely contributor since it does not increase in HF rats that have chronic elevation of pro-inflammatory cytokines.4
Finally, it is notable that CLOD had no effect on the ambient lower levels of COX-2 expression in immediately adjacent hypothalamic tissues. This may simply be a function of the dense vascularity of the PVN,25 compared with surrounding regions, providing a substrate for higher numbers of perivascular macrophages. Nevertheless, it suggests a heightened responsiveness of this particular region of the brain to inflammatory signals from the periphery. This consistent with the known influence of cytokines on the hypothalamic-pituitary-adrenal axis9, 26 and with our previous observation that COX-2 expression in the cortex, where it is more diffusely distributed3 does not seem to be affected by heart failure or by treatments for heart failure that reduce COX-2 expression in PVN.3
An intriguing finding of this study is the absence of any effect of CLOD on the expression of pro-inflammatory cytokines within the PVN. The mechanism responsible for the increase in the pro-inflammatory cytokines in cardiovascular regions of the brain in HF rats19 has not been elucidated. The present study argues against cytokine signaling across the blood-brain barrier, via PGE2 production, as a potential mechanism. Signaling via the renin-angiotensin-aldosterone system,27 or via cardiovascular afferent fibers,28 remain viable possibilities.
This study focused on the role of COX-2 activity and PGE2 production by the perivascular macrophages of the blood brain barrier, a mechanism by which circulating pro-inflammatory cytokines are thought to activate the hypothalamic-pituitary-adrenal axis.8-10 A caveat to be considered is that stimulated macrophages can also produce reactive oxygen species, including superoxide29 which may also activate the sympathetic nervous system. Thus, the present study does not exclude the possibility that other products of perivascular macrophages might contribute to cytokine-induced sympathetic discharge in heart failure.
Central interventions that chronically reduce sympathetic nerve activity in rats with HF after myocardial infarction can reduce LV remodeling30, 31 and improve LV function.4, 30-32 These beneficial effects likely result from reductions in sympathetically mediated renin release with activation of the renin-angiotensin-aldosterone system,33 sympathetically mediated renal reabsorption of sodium and water,33-35 and sympathetically mediated direct detrimental effects on cardiac myocytes.36 Cardiac remodeling is a chronic progressive process evolving over weeks following myocardial infarction.37, 38 Significant effects of central intervention on LV remodeling and function in rat models of heart failure have typically been demonstrated after at least 4 weeks of continuous ICV drug administration.4, 30-32 In the present study, in which LV function was re-assessed only 1 week after myocardial infarction and only 1 week after a single ICV injection of CLOD, small but significant improvements in LV systolic function (LV dP/dtmax) and volume regulation (LV end-diastolic pressure) were observed. The short duration of treatment and the re-assessment of LV function so early in the course of cardiac remodeling may have minimized our ability to demonstrate greater improvements in LV function. Another factor to consider is that depleting the brain perivascular macrophages reduced but did not normalize plasma NE, the surrogate marker of sympathetic drive used in this study. Thus, the continued influence of other neurohumoral systems on sympathetic drive may also have impaired our ability to demonstrate larger changes in LV function.
Perspectives
The present study utilized a unique pharmacological tool to target one particular cell type and demonstrate its role as mediator of increased sympathetic drive in heart failure. The study unequivocally demonstrates that COX-2 expression and PGE2 production by perivascular macrophages of the blood-brain barrier increase in rats with HF following MI, and that selective elimination of these perivascular macrophages reduces cytokine-induced sympathetic drive. Based on what is already known about the effect of pro-inflammatory cytokines on COX-2 expression by perivascular macrophages in the brain,3, 8, 10, 18, 32 the effects of cytokine-induced PGE2 production in the brain on sympathetic drive17 and the present results from both normal and MI rats, it seems reasonable to speculate that the pro-inflammatory cytokines contribute to the augmented sympathetic drive in rats with HF following MI by inducing COX-2 activity and PGE2 production by the perivascular macrophages. However, a role for other products of perivascular macrophages29 that might stimulate the sympathetic nervous system cannot be excluded.
A more global implication of these findings might be that the perivascular macrophages signal the brain to increase sympathetic activity in response to systemic inflammation, not only in heart failure but also in other cardiovascular conditions such as hypertension, obesity and diabetes in which both pro-inflammatory cytokines and sympathetic drive are increased.1, 2, 5, 39 Further studies are needed to determine whether perivascular macrophages of the blood-brain barrier increase COX-2 expression, or the expression of other substances29 that might activate the sympathetic nervous system, in these disease processes. Ultimately, the identification of discrete cellular mechanisms that augment sympathetic drive raises the possibility of novel targeted interventions to disrupt the progression of heart failure, hypertension and other devastating cardiovascular diseases.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Kathy Zimmerman, RDMS/RDCS/FASE, for diligent and expert assistance in the performance of the echocardiograms.
Funding Sources: This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, by NIH HL-073986, and by institutional funds provided by the University of Iowa.
Footnotes
Disclosures: None
References
- 1.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 2.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23:45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension. 2007;49:511–518. doi: 10.1161/01.HYP.0000257356.20527.c5. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res. 2007;101:304–312. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- 5.Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–447. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- 6.Felder RB. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol. 2010;95:19–25. doi: 10.1113/expphysiol.2008.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther. 2009;86:216–220. doi: 10.1038/clpt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26:761–788. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- 10.Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci. 2003;8:s1321–s1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- 11.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 12.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2003;284:R259–R276. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- 13.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–1243. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- 15.Polfliet MM, Goede PH, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. J Neuroimmunol. 2001;116:188–195. doi: 10.1016/s0165-5728(01)00282-x. [DOI] [PubMed] [Google Scholar]
- 16.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R916–R927. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 18.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 19.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–H2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 20.Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 21.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- 23.Eder C. Regulation of microglial behavior by ion channel activity. J Neurosci Res. 2005;81:314–321. doi: 10.1002/jnr.20476. [DOI] [PubMed] [Google Scholar]
- 24.Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Pol AN. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol. 1982;206:317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]
- 26.Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 27.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H791–H797. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- 29.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang BS, Leenen FH. Blockade of brain mineralocorticoid receptors or Na+ channels prevents sympathetic hyperactivity and improves cardiac function in rats post-MI. Am J Physiol Heart Circ Physiol. 2005;288:H2491–H2497. doi: 10.1152/ajpheart.00840.2004. [DOI] [PubMed] [Google Scholar]
- 31.Huang BS, Ahmad M, Tan J, Leenen FH. Sympathetic hyperactivity and cardiac dysfunction post-MI: Different impact of specific CNS versus general AT(1) receptor blockade. J Mol Cell Cardiol. 2007;43:479–486. doi: 10.1016/j.yjmcc.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 32.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension. 2000;36:1083–1088. doi: 10.1161/01.hyp.36.6.1083. [DOI] [PubMed] [Google Scholar]
- 34.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2138–H2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 35.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2241–H2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- 36.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors--alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 37.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1734–R1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi P, Raizada MK, Sumners C. Brain Cytokines as Neuromodulators in Cardiovascular Control. Clin Exp Pharmacol Physiol. 2010;37:e52–e57. doi: 10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.