Abstract
Background
Patients with critical limb ischemia (CLI) have multiple comorbidities and limited life spans. The ability of infrainguinal vein bypass to improve quality of life (QoL) in patients with CLI has therefore been questioned. Prospective preoperative and postoperative QoL data for patients undergoing lower extremity vein bypass for CLI are presented.
Methods
A validated, disease-specific QoL questionnaire (VascuQoL) with activity, symptom, pain, emotional, and social domains and responses scored 1 (lowest QoL) to 7 (best QoL) was administered before surgery and at 3 and 12 months after lower extremity vein bypass for CLI. Changes in QoL at 3 and 12 months after lower extremity vein bypass and multiple predetermined variables potentially influencing QoL after lower extremity vein bypass were analyzed to determine the effect of lower extremity vein bypass on QoL in CLI patients.
Results
A total of 1404 patients had lower extremity vein bypass for CLI at 83 centers in the United States and Canada as part of the PREVENT III clinical trial. Surveys were completed in 1296 patients at baseline, 862 patients at 3 months, and 732 patients at 12 months. The global QoL score (mean ± SD) was 2.8 ± 1.1 at baseline and was 4.7 ± 1.4 and 5.1 ± 1.4 at 3 and 12 months, respectively. Mean changes from baseline at 3 and 12 months were statistically significant (P < .0001). Improved QoL scores extended across all domains. Diabetes and the development of graft-related events were associated with decreased improvement in QoL scores, though the mean relative change from baseline remained positive.
Conclusions
Patients with CLI have a low QoL at baseline that is improved at 3 and 12 months after lower extremity vein bypass. QoL improvements are lower in diabetic patients and those who develop graft-related events. Successful revascularization can be expected to improve QoL in patients with CLI, with benefits that are sustained to at least 1 year.
Limb salvage, graft patency, and operative mortality are the traditional outcome measures for assessment of infrainguinal surgical arterial reconstructions. These variables have been the focus of much clinical vascular surgical research for more than 30 years. Anticipated limb salvage and primary, primary assisted, and secondary patency can be stratified for proximal and distal anastomotic sites, conduit, and indication for surgery (claudication vs limb salvage). More recently, however, the focus has shifted from lesion- and graft-oriented results to more patient-oriented results evaluating the effect of arterial reconstructions on functional outcome and quality of life (QoL). The National Heart, Lung, and Blood Institute of the National Institutes of Health states that it “is committed to supporting efforts to measure health-related quality of life as an important end point in trials of cardiovascular interventions.”1
Patients with chronic critical limb ischemia (CLI) are often elderly with many comorbid conditions that, in addition to their limb ischemia, may adversely affect their QoL. Given the advanced age and comorbid conditions of the CLI patient, the overall effect on QoL of a major surgical procedure to correct CLI may be questioned. A report from the Oregon Health & Sciences University indicted that, despite high patency and limb salvage rates, when evaluated in terms of variables that would seem to matter to patients, only a small minority of patients after an infrainguinal arterial vein graft for CLI achieved what was believed to be an ideal result.2
PREVENT III was a large (1404 patients), multicenter (83 North American sites), double-blind, randomized clinical trial evaluating the efficacy of intraoperative treatment of vein grafts with edifoligide to prevent vein graft failure in patients undergoing infrainguinal arterial reconstruction with vein grafts for CLI.3 Edifoligide (E2F Decoy, Congentech, Inc, South San Francisco, Calif) is a short piece of DNA that mimics the binding sequence for E2F.4 The E2F family of transcription factors is important in the expression of genes necessary for smooth muscle cell proliferation.5 Theoretically, inhibition of the E2F binding sequence with edifoligide should decrease smooth muscle cell proliferation in vein grafts and reduce vein graft failure from intimal hyperplasia. Failure of edifoligide to decrease vein graft failure in PREVENT III, and in a companion study of coronary bypass grafts (PREVENT IV), has been recently reported.6,7 To date, to our knowledge, it is the largest randomized clinical trial in patients with CLI.
As part of PREVENT III, the effect of infrainguinal vein grafting for limb salvage in CLI patients on health-related QoL was prospectively assessed by using the Vascular Quality of Life Questionnaire (VascuQol), a validated, standardized questionnaire. This article details the QoL data from PREVENT III and suggests an overall favorable effect of infrainguinal vein grafting on QoL in patients with CLI. In addition, QoL improvements were positively associated with maintained graft patency at 1 year.
METHODS
Trial design
PREVENT III was a multicenter, double-blinded, randomized, placebo-controlled trial of edifoligide for the prevention of vein graft failure in patients undergoing lower extremity bypass for the treatment of CLI (gangrene, ischemic ulcer, or rest pain). Details of the trial design have been previously reported.3 The trial was sponsored by Corgentech Incorporated (South San Francisco, Calif) and Bristol-Myers Squibb (Princeton, NJ).
Eighty-three sites in the United States and Canada randomized 1404 patients aged more than 18 years with CLI to receive either edifoligide or placebo during intraoperative preparation of the vein graft. Patients presenting with only rest pain were required to have vascular laboratory evidence of CLI (ankle systolic pressure <50 mm Hg, an ankle-brachial index of ≤0.4, or a toe pressure of <30 mm Hg or a pulse volume recording at the transtarsal level suggesting severe ischemia).
In addition to grafts arising from a native artery, permissible reconstructions also included vein grafts that arose from a prosthetic patch on the inflow artery and vein grafts that arose from a prosthetic graft providing inflow to the groin. Redo vein grafts, spliced vein grafts, and infrainguinal bypass in the setting of simultaneous inflow (catheter-based or surgical) reconstruction were all permitted. Exclusion criteria included claudication alone as an indication for intervention, a nonautogenous component of the infrainguinal graft, and an in situ vein graft (treatment with edifoligide required ex vivo delivery to the vein graft). Secondary end points included all-cause failure, primary graft patency, freedom from clinically significant stenosis, and assessment of QoL improvement over preoperative status.
Baseline data
Detailed history and physical examinations were performed at study entry. Comorbid conditions were characterized from the patient's interview and prior medical records, including a history of advanced coronary artery disease (history of myocardial infarction, coronary artery bypass grafting, or a percutaneous coronary intervention), symptomatic cerebrovascular disease (prior stroke or transient ischemic attack), diabetes, hypertension, hyperlipidemia, renal failure, prior lower extremity arterial reconstruction (including prior infrainguinal bypass grafts), chronic obstructive pulmonary disease, and smoking status.
QoL assessment
VascuQol was used to assess the effect largest of CLI and revascularization on health-related QoL. VascuQol is a disease-specific questionnaire developed to assess the entire spectrum of chronic limb ischemia, including CLI. It was developed by Morgan et al in a rigorous two-stage procedure of initial item selection followed by testing for validity, reliability, and responsiveness to within-patient change. VascuQol consists of 25 questions in 5 domains: pain (4 questions), symptoms (4 questions), activities (8 questions), social (two questions), and emotional (7 questions).8 There is a seven-point response scale for each question. Responses are averaged for composite overall and domain-specific scores from 1 to 7. A score of 1 is the worst possible QoL, and a score of 7 is the best possible QoL.
Changes in global and domain-specific QoL scores from baseline were assessed at 3 and 12 months. Patients with no composite scores were considered nonresponders. Effect of patient- and procedure-related variables on QoL were also assessed at 3 and 12 months. The effect of a graft-related event (GRE; development of a >70% graft stenosis or having undergone a percutaneous or surgical revision or a major amputation) on QoL was also assessed at 3 and 12 months. Nonresponders and responders at 3 and 12 months were compared at each time interval.
Statistical methods
Changes from baseline of each of the VascuQol domains and the VascuQol global score were treated as continuous variables and assessed by using paired t tests for each time interval. Univariate analysis of the effect of patient variables (all listed in Table I) and GRE (Table II) on composite scores was performed with linear regression for continuous variables (age and weight) and an analysis of variance via Generalized Linear Models for categorical variables. Factors that reached a P value of .20 in univariate analysis (age, baseline weight, indication, prior infrainguinal reconstruction, diabetes, dyslipidemia, hypercholesterolemia, critical stenosis, and loss of patency) were used in a multivariable mixed effects regression model for repeated measures as a function of time. Because GREs were covariates, GREs were analyzed individually with patient variables in the multivariable models. This model also incorporated survey nonresponders, although a separate and detailed analysis of survey nonresponders is described below. Other mixed effects models were created to test time-variable interactions.
Table I.
Univariate analysis of patient variables regarding change in quality of life from baseline after infrainguinal vein bypass
|
3 mo |
12 mo |
|||
|---|---|---|---|---|
| Variable | Change* | P value† | Change* | P value† |
| Sex | .6258 | .6910 | ||
| Race | .5263 | .3081 | ||
| Weight (per 10-kg increase) | <–0.01 | .1712 | –0.02 | .0185 |
| Age (per 10-yr increase) | –0.01 | .040 | <–0.01 | .8302 |
| Indication | .1677 | .1375 | ||
| Rest pain | 2.03 | 2.16 | ||
| Ulceration | 1.84 | 2.13 | ||
| Gangrene | 1.90 | 2.39 | ||
| Prior inflow reconstruction | .3058 | .2985 | ||
| Prior infrainguinal reconstruction | .0401 | .0469 | ||
| Yes | 1.79 | 2.10 | ||
| No | 1.96 | 2.29 | ||
| Prior myocardial infarction | .0977 | .8043 | ||
| Prior coronary bypass procedure | .2586 | .6952 | ||
| Prior stroke | .2477 | .8338 | ||
| Diabetes | <.0001 | .0968 | ||
| Yes | 1.75 | 2.18 | ||
| No | 2.18 | 2.32 | ||
| Dialysis | .6219 | .1859 | ||
| Dyslipidemia | <.0001 | .1181 | ||
| Yes | 1.77 | 2.19 | ||
| No | 2.09 | 2.32 | ||
| Hypertension | .2898 | .7424 | ||
Mean change in global score from baseline.
P value for intravariable comparison.
Table II.
Univariate analysis of graft-related events regarding change in quality of life at 12 months from baseline after infrainguinal vein bypass
| Graft-related event | Change* | P value† |
|---|---|---|
| Critical stenosis | <.0001 | |
| Yes | 1.98 | |
| No | 2.40 | |
| Primary patency | .0006 | |
| Yes | 2.33 | |
| No | 2.03 | |
| Primary assisted patency | <.0001 | |
| Yes | 2.34 | |
| No | 1.70 | |
| Secondary patency | <.0001 | |
| Yes | 2.31 | |
| No | 1.70 | |
| Freedom from graft-related events | <.0001 | |
| Yes | 2.42 | |
| No | 2.00 |
Mean change in global score from baseline.
P value for intravariable comparison.
Comparisons between survey responders and nonresponders at 12 months were also performed. Patients who were deceased at the time of the survey were excluded from this aspect of the analysis. The Wilcoxon rank-sum test was used for continuous variables, and the Fisher exact test was used for categorical variables in univariate analysis. Logistic regression with backward stepwise elimination was used to model survey completion in multivariable analysis.
An α value of .05, corresponding to P = .05 and 95% confidence intervals, was used as a criterion for statistical significance. Analysis was performed with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient demographics
The details of the demographics and comorbidities of patients enrolled in the PREVENT III trial have been previously reported.7,8 Pertinent demographic variables are summarized here. There were 1404 patients who had lower extremity vein bypass as part of the PREVENT III trial (897 men and 507 women; mean age, 69 ± 12 years). The primary indication for surgery was ischemic rest pain in 25%, nonhealing ulceration in 39%, and ischemic gangrene in 36%. The mean preoperative ankle-brachial index was 0.5 ± 0.4. Sixty-four percent had diabetes, 73% were smokers, 12% were on dialysis, and 28% had undergone a previous infrainguinal bypass. Overall, 222 patients (15.8%) died during the study, 18 were lost to follow-up, and 26 withdrew from the study.
Procedures
The details of the procedures performed and the results of the PREVENT III trial with respect to the primary end point of the trial have been previously reported6,7 and are summarized here. A single segment of great saphenous vein (reversed or nonreversed) was used in 81% of cases. Fifteen percent of the grafts were composite vein grafts, and 5% were single-segment non–great saphenous vein grafts. The proximal anastomosis was to the common femoral, superficial femoral, or profunda femoris artery in 78%, the popliteal artery (above or below the knee) in 18%, and other sites in 4%. The distal anastomosis was to the popliteal artery in 30%, a tibial artery in 55%, and the dorsal pedal or a plantar artery in 13%.
Surgical outcomes
The details of the postoperative outcomes of patients enrolled in the PREVENT III trial have been previously reported6,7 and are summarized here to provide context for the QoL results. The perioperative (30-day) death rate was 2.7%. Major morbidity occurred in 17.6%. The 30-day graft occlusion rate was 5.2%, and the 30-day major amputation rate was 1.8%. Major wound complications (dehiscence, infection, and necrosis) occurred in 5% of patients. During the 1-year follow-up, reinterventions were performed on 435 grafts. Assisted primary patency at 1 year was 77%. Limb salvage at 1 year was 88%, and survival at 1 year was 84%. There was no beneficial effect observed in the edifoligide treatment group for protocol-defined study end points, primary or primary assisted patency, or limb salvage. Secondary graft patency was improved at 1 year in the treatment group (82.6% vs 77.5%; P = .02).7
QoL analysis
QoL evaluations were available from 1296 (92.3%) of patients at baseline, from 862 (61.4%) patients at 3 months, and from 732 (52.1%) patients at 12 months. There were no differences in changes of QoL scores from baseline with regard to the global scores or scores of the individual domains at 3 or 12 months in the patients whose grafts were treated with edifoligide or placebo (all P values >.221). All comparisons of QoL scores with baseline values were therefore performed independently of the patient's vein graft treatment group.
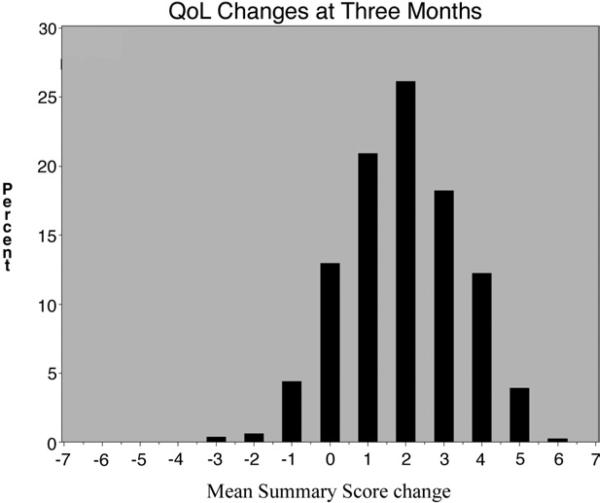
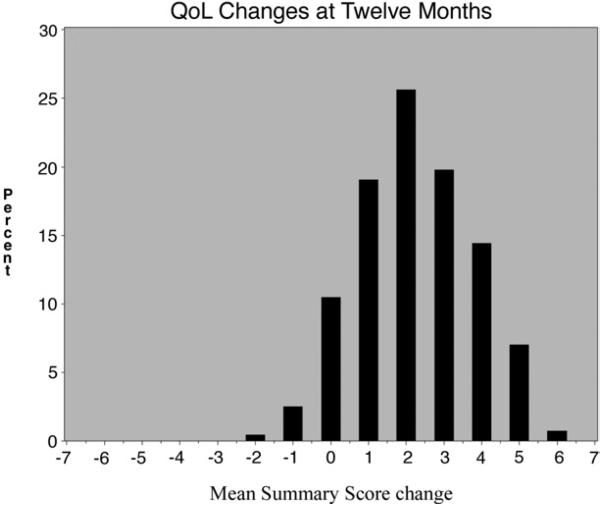
Mean global QoL scores increased significantly during the study period (2.8 ± 1.1 at baseline, 4.7 ± 1.4 at 3 months, and 5.1 ± 1.4 at 12 months), resulting in changes of +1.93 from baseline to 3 months, +0.34 from 3 months to 12 months, and +2.27 from baseline to 12 months (all P < .0001). Histograms showing the distribution of the changes from baseline at 3 and 12 months are shown in Figs 1 and 2. Benefit extended across all domains at 3 and 12 months (all P values <.05; Table III).
Fig 1.
Quality-of-life changes at 3 months.
Fig 2.
Quality-of-life changes at 12 months.
Table III.
VascuQol domain scores
| Survey domain | Baseline | 3 mo | 12 mo |
|---|---|---|---|
| Total, n (%) | 1296 (92.3%) | 832 (65.7%) | 732 (62.5%) |
| Activity | 2.4 (2.1) | 4.2 (4.1) | 4.5 (4.5) |
| Emotional | 3.0 (2.8) | 5.0 (5.3) | 5.4 (5.7) |
| Pain | 2.6 (2.3) | 4.8 (5.0) | 5.3 (5.7) |
| Social | 3.4 (3.0) | 5.1 (5.5) | 5.5 (6.0) |
| Symptoms | 3.3 (3.3) | 5.3 (5.5) | 5.5 (5.8) |
| Overall | 2.8 (2.6) | 4.7 (4.9) | 5.1 (5.3) |
Scores are expressed as mean (median) unless otherwise noted. The percentage at each time point was calculated as completed surveys/living enrolled patients. All paired t test comparisons between 3 months and baseline had P values <.0001. All paired t test comparisons between 12 months and baseline had P values <.0001.
Results of univariate analysis of the influence of baseline patient variables on QoL scores are presented in Table I. At 3 months, older age, prior infrainguinal reconstruction, diabetes, and dyslipidemia were associated with lesser degrees of improvement in QoL scores. At 12 months, higher patient weight and prior infrainguinal reconstruction were associated with a reduced gain in QoL. Of note, sex, race, the indication for surgery (rest pain vs tissue loss), coronary artery disease, stroke, hypertension, and dialysis did not have a significant effect on QoL changes.
The effect of GRE on QoL was significant (Table II). At 12 months, there was a reduction in the magnitude of QoL improvements in patients who developed critical graft stenosis (>70% on ultrasonography or angiography; +1.98 vs +2.40; P < .0001), loss of primary patency (+2.03 vs +2.33; P = .0006), loss of primary assisted patency (+1.70 vs +2.34; P < .0001), and loss of secondary patency (+1.70 vs +2.31; P < .0001). Overall, patients free from any GRE had a greater increase in 12-month QoL than patients with a GRE (+2.40 vs +2.0; P < .0001). Patients who underwent successful graft revision had lower QoL at 12 months than patients free from any GRE (+2.17 vs + 2.40; P = .0164). Multivariable analysis showed that diabetes (P < .001 for all models) and GRE (P < .0001 for all models) were related to a reduction in the QoL benefit experienced at 12 months.
To assess the potential for bias from incomplete surveys, an analysis of survey nonresponders was performed. Univariate analysis results comparing responders and nonresponders are presented in Table IV. Nonwhite patients (P = .0034), diabetics (P = .0338), and patients without hypertension (P = .0264) were more likely to be survey nonresponders at 12 months. Patients with GRE were also more likely to be survey nonresponders: stenosis (P = .0014), loss of primary assisted patency (P < .0001), and loss of secondary patency (P < .0001) were associated with survey nonresponse at 12 months. Amputation had the greatest effect on 12-month survey nonresponse (88% nonresponse among eligible amputees vs 31.5% of patients free of amputations; P < .0001), thus effectively precluding any meaningful measurement of the effect of amputation on QoL. Multivariable analysis showed that nonwhite race (P < .01), diabetes (P < .01), loss of primary assisted patency (P < .0001), loss of secondary patency (P < .0001), and amputation (P < .0001) were associated with survey nonresponse, whereas critical stenosis or loss of primary patency did not have a significant effect.
Table IV.
Univariate analysis of factors associated with survey response versus nonresponse at 12 months
| Variable | NR/total (%)* | P value† |
|---|---|---|
| Patient-related variables | ||
| Sex | .9497 | |
| Race | .0034 (nonwhite) | |
| White | 291/848 (34.3) | |
| Nonwhite | 148/323 (45.8) | |
| Weight | .1933 | |
| Age | .8355 | |
| Indication (tissue loss vs rest pain) | .2506 | |
| Prior inflow reconstruction | .9999 | |
| Prior infrainguinal reconstruction | .6882 | |
| Prior myocardial infarction | .6865 | |
| Prior coronary bypass procedure | .9438 | |
| Prior stroke | .9390 | |
| Diabetes | .0338 (yes) | |
| Yes | 293/736 (39.8) | |
| No | 146/435 (33.6) | |
| Dialysis | .3912 | |
| Dyslipidemia | .5828 | |
| Hypertension | .0264 (no) | |
| Yes | 340/946 (35.9) | |
| No | 99/225 (44.0) | |
| Surgical outcomes | ||
| Critical stenosis | .0014 (yes) | |
| Yes | 209/487 (42.9) | |
| No | 230/684 (33.6) | |
| Primary patency | .1741 | |
| Primary assisted patency | <.0001 (no) | |
| Yes | 260/883 (29.5) | |
| No | 179/288 (62.15) | |
| Secondary patency | <.0001 (no) | |
| Yes | 275/925 (29.7) | |
| No | 164/246 (66.7) | |
| Amputation | <.0001 (yes) | |
| Yes | 110/125 (88.0) | |
| No | 329/1046 (31.5) |
NR indicates nonresponders; total represents patient is alive at the time of the survey.
The factor related to survey nonresponse is given in parentheses for significant P values.
DISCUSSION
Over the last 40 years, vascular surgeons have focused on what can be done to salvage ischemic limbs. The focus of limb salvage surgery is, however, broadening not only to focus on what can be done, but to also consider what should be done to salvage an ischemic limb. The effectiveness of limb salvage surgery can be measured not only in terms of graft patency, survival, and limb salvage, but also in terms of functional outcome and overall QoL.9 Prior studies have looked at QoL outcomes after surgical bypass for lower extremity ischemia. Studies combining claudication with CLI patients have shown positive QoL benefits with successful lower extremity vein bypass.10,11 Early attempts to focus on expanded parameters of assessing limb salvage surgery have shown that ideal results, as defined as no further need for any additional intervention, complete healing of wounds, and rapid return to independent status, are seldom achieved long-term in limb-salvage patients. However, such results do not exclude the fact that interventions for limb salvage may still improve QoL for the limb-salvage patient. Indeed, earlier single-center studies involving only CLI patients showed QoL improvements with successful lower extremity vein bypass.12 However, to date, to our knowledge, no large, multicenter, prospective trial has examined QoL outcomes after lower extremity vein bypass for CLI.
Although PREVENT III failed to demonstrate a benefit of edifoligide in preventing vein graft failure,6 it has provided information regarding outcomes of infrainguinal limb salvage surgery. It is the first large-scale trial to prospectively assess QoL in patients with CLI. The results suggest that infrainguinal vein graft surgery substantially improves QoL in patients with CLI by 3 months after surgery and that this benefit is maintained at 1 year. Limb-salvage surgery is therefore appropriate in achieving results that matter to patients and their families. In addition, these results can be achieved across a broad spectrum of patients with CLI and are not limited by most comorbidities present in patients with CLI or by the complexity of the required operation. The study also indicates that GRE adversely affects QoL, thus suggesting that maintained QoL benefits are linked to successful surgery and maintenance of graft patency, at least in the mid-term. However, some improvement from baseline is still seen with most patients who experience GRE, a finding seen by previous authors.13 Patients free from GRE have more QoL benefits than patients who undergo graft revision, even when graft revision is successful. Additional studies to assess the ability of pharmacologic interventions and other adjuncts to reduce vein graft stenosis and failure are therefore clearly indicated.
Functional outcome can be measured by using a series of standardized performance evaluations testing a patient's physical abilities.14,15 Changes in QoL after an intervention are generally measured by using questionnaires administered before and after an intervention There are both overall (eg, Short Form-36, EuroQol, and Nottingham Health Profile) and disease-specific (eg, VascuQol and Walking Impairment Questionnaire) instruments. When an intervention is studied for a specific disease process, disease-specific questionnaires may have an advantage over more global assessments of QoL, because disease-specific questionnaires are designed to assess variables affecting QoL that are most likely to be influenced by the disease process being studied.16 The PREVENT III trial was a large, prospective, multicenter trial that used a disease-specific questionnaire (VascuQol) designed specifically to assess both intermittent claudication and CLI.17 PREVENT III differs significantly in magnitude and design from previous, much smaller, retrospective or single-institution studies in this field that also suggested improved QoL after limb-salvage surgery.8,18 Whereas other questionnaires exist to assess limb ischemia,19 at the time of the design of PREVENT III, VascuQol was the only disease-specific questionnaire designed to assess CLI. Other existing validated questionnaires at the time of PREVENT III design assessed only intermittent claudication.
There are several potentially important limitations to this study. First and foremost among these is the potential bias induced by survey nonresponders. Most survey nonresponse was due to patients not completing the VascuQol questionnaire at each study visit, although the database does not provide information on the reasons why. We do not know whether patients who did not fill out the form were incapable of filling out the form, were not offered the opportunity to fill out the form, or refused to fill out the form. An additional cause of survey nonresponse was patient exit from the trial, because many patients died (16%) during the study, and a very few (1.3%) were lost to follow-up or withdrew (1.9%) from participation in the study. The analysis of the characteristics of the patients who did and did not fill out QoL forms at months 3 and 12 found that nonwhite patients, diabetics, patients with loss of patency after revision, and patients with amputations were more likely to be survey nonresponders. Thus, although conclusions about QoL improvements in patients with successful limb salvage surgery can be made, conclusions about the magnitude of effect on patients who experienced a subsequent GRE must be made with great caution. In particular, amputations were so highly associated with survey nonresponse (88%) that no meaningful conclusions can be made about the QoL of amputees. Survey nonresponse is an issue that concerns all survey-based studies, and our subanalysis of nonresponders identifies potential omission biases so as to better frame the main study results.
Although VascuQol was developed by using rigorous methods common to questionnaires designed to assess QoL, it is nonetheless a fairly new questionnaire, and experience with its use is relatively limited. Questionnaires also do not directly measure functional impairments secondary to CLI and do not measure the ability of surgery to improve patient function. Functional outcome (eg, pain-free walking distance) was not studied in PREVENT III. We do not know whether the magnitude of changes in QoL measured in this study will correlate with actual patient functional improvement. In addition, because QoL was not a primary end point of the study, the number of survey time points was limited to three, and no time points beyond the 1-year study follow-up were available. Additional studies to specifically assess the ability of limb-salvage surgery to improve patient function are required. Those limitations notwithstanding, PREVENT III provides the most convincing evidence to date that vein bypass surgery is effective in salvaging limbs in patients with CLI and can improve patient QoL, particularly in those patients who experience maintained patency of their grafts.
CONCLUSIONS
Patients with CLI have a low QoL at baseline that is improved at 3 and 12 months after lower extremity vein bypass. QoL improvements are lower in diabetic patients and those who develop GRE. Successful revascularization can be expected to improve QoL in patients with CLI, with benefits that are sustained to at least 1 year.
Footnotes
Competition of interest: Drs Moneta, Conte, Bandyk, and Clowes served on the PREVENT III Steering Committee and were paid consulting fees. Dr Seely was an employee of Corgentech Inc, sponsor of the study.
Presented at the Twentieth Annual Meeting of the Western Vascular Society, Park City, Utah, September 24-27, 2005.
REFERENCES
- 1.Czajkowski SM. Health-related quality of life outcomes in clinical research: NHLBI policy and perspectives. Ann Thorac Surg. 1998;66:1486–7. doi: 10.1016/s0003-4975(98)00837-6. [DOI] [PubMed] [Google Scholar]
- 2.Nicoloff AD, Taylor LM, Jr, McLafferty RB, et al. Patient recovery after infrainguinal bypass grafting for limb salvage. J Vasc Surg. 1998;27:256–63. doi: 10.1016/s0741-5214(98)70356-8. discussion 264-6. [DOI] [PubMed] [Google Scholar]
- 3.Conte MS, Lorenz TJ, Bandyk DF, et al. Design and rationale of the PREVENT III clinical trial: edifoligide for the prevention of infrainguinal vein graft failure. Vasc Endovasc Surg. 2005;39:15–23. doi: 10.1177/153857440503900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehsan A, Mann MJ, Dell'Acqua G, Dzau VJ. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121:714–22. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- 5.Mann MJ, Dzau VJ. Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest. 2000;106:1071–5. doi: 10.1172/JCI11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte MS, Bandyk DF, Clowes AW, et al. Risk factors, medical therapies and perioperative events in limb salvage surgery: observations from the PREVENT III multicenter trial. J Vasc Surg. 2005;42:456–64. doi: 10.1016/j.jvs.2005.05.001. discussion 464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multi-center, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. doi: 10.1016/j.jvs.2005.12.058. submitted. [DOI] [PubMed] [Google Scholar]
- 8.Morgan MBF, Crayford T, et al. Developing the vascular quality of life questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33:679–87. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Zamzam AM, Jr, Lee RW, Moneta GL, et al. Functional outcome after infrainguinal bypass for limb salvage. J Vasc Surg. 1997;25:287–95. doi: 10.1016/s0741-5214(97)70350-1. discussion 295-7. [DOI] [PubMed] [Google Scholar]
- 10.Klevsgard R, Risberg BO, Thomsen MB, Hallberg IR. A 1-year follow-up quality of life study after hemodynamically successful or unsuccessful surgical revascularization of lower limb ischemia. J Vasc Surg. 2001;33:114–22. doi: 10.1067/mva.2001.109769. [DOI] [PubMed] [Google Scholar]
- 11.Klevsgard R, Froberg BL, Risberg B, Hallberg IR. Nottingham Health Profile and Short-Form 36 Health Survey questionnaires in patients with chronic lower limb ischemia: before and after revascularization. J Vasc Surg. 2002;36:310–7. doi: 10.1067/mva.2002.125747. [DOI] [PubMed] [Google Scholar]
- 12.Thorsen H, McKenna S, Tennant A, Holstein P. Nottingham health profile scores predict the outcome and support aggressive revascularisation for critical ischaemia. Eur J Vasc Endovasc Surg. 2002;23:495–9. doi: 10.1053/ejvs.2002.1648. [DOI] [PubMed] [Google Scholar]
- 13.Chetter IC, Spark JI, Scott DJ, et al. Prospective analysis of quality of life in patients following infrainguinal reconstruction for chronic critical ischaemia. Br J Surg. 1998;85:951–5. doi: 10.1046/j.1365-2168.1998.00752.x. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Liu K, O'Brien E, et al. Measuring physical activity in peripheral arterial disease: a comparison of two physical activity questionnaires with an accelerometer. Angiology. 2000;51:91–100. doi: 10.1177/000331970005100201. [DOI] [PubMed] [Google Scholar]
- 15.Simonsick EM, Gardner AW, Poehlman ET. Assessment of physical function and exercise tolerance in older adults: reproducibility and comparability of five measures. Aging (Milano) 2000;12:274–80. doi: 10.1007/BF03339847. [DOI] [PubMed] [Google Scholar]
- 16.de Vries M, Ouwendijk R, Kessels AG, et al. Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J Vasc Surg. 2005;41:261–8. doi: 10.1016/j.jvs.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33:679–87. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BF, Singh S, Evans L, et al. A prospective study of the effect of limb-threatening ischaemia and its surgical treatment on the quality of life. Eur J Vasc Endovasc Surg. 1997;13:306–14. doi: 10.1016/s1078-5884(97)80103-7. [DOI] [PubMed] [Google Scholar]
- 19.Mehta T, Venkata Subramaniam A, Chetter I, McCollum P. Disease-specific quality of life assessment in intermittent claudication: review. Eur J Vasc Endovasc Surg. 2003;25:202–8. doi: 10.1053/ejvs.2002.1837. [DOI] [PubMed] [Google Scholar]