Abstract
Cognitive neuroscience continues to build meaningful connections between affective behavior and human brain function. Within the biological sciences, a similar renaissance has taken place, focusing on the role of sleep in various neurocognitive processes, and most recently, the interaction between sleep and emotional regulation. In this review, we survey an array of diverse findings across basic and clinical research domains, resulting in a convergent view of sleep-dependent emotional brain processing. Based on the unique neurobiology of sleep, we outline a model describing the overnight modulation of affective neural systems and the (re)processing of recent emotional experiences, both of which appear to redress the appropriate next-day reactivity of limbic and associated autonomic networks. Furthermore, a REM sleep hypothesis of emotional-memory processing is proposed, the implications of which may provide brain-based insights into the association between sleep abnormalities and the initiation and maintenance of mood disturbances.
Keywords: Sleep, REM sleep, Emotion, Affect, Learning, Memory, Depression, PTSD
The ability of the human brain to generate, regulate and be guided by emotions represents a fundamental process governing not only our personal lives, but our mental health as well as our societal structure. The recent emergence of cognitive neuroscience has ushered in a new era of research connecting affective behavior with human brain function, and provided a systems-level view of emotional information processing, translationally bridging animal models of affective regulation and relevant clinical disorders (Labar & Cabeza, 2006; Phelps, 2006).
Independent of this research area, a recent resurgence has also taken place within the basic sciences, focusing on the functional impact of sleep on neurocognitive processes (Walker & Stickgold, 2006; Chee & Chuah, 2008; Walker, 2009). However, surprisingly less research attention has been given to the interaction between sleep and affective brain function. We say surprising considering the remarkable overlap between the known physiology of sleep, especially REM sleep, and the associated neurochemistry and network anatomy that modulate emotions, as well as the prominent co-occurrence of abnormal sleep (including REM sleep) in almost all affective psychiatric and mood disorders.
Despite the relative historical paucity of research, recent work has begun to describe a consistent and clarifying role for sleep in the selective modulation of emotional information and the affective regulation. In the following review, we provide a synthesis of these findings, describing an intimate relationship between sleep, emotional brain function and clinical mood disorders, and offer a tentative first theoretical framework that may account for these observed interactions.
Sleep
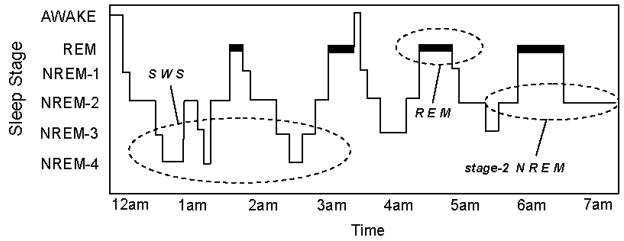
The sleep of mammalian species has been broadly classified into two distinct types; non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, with NREM sleep being further divided in primates and cats into 4 sub-stages (1–4) corresponding, in that order, to increasing depth of sleep (Rechtschaffen & Kales, 1968). In humans, NREM and REM sleep alternate or “cycle” across the night in an ultradian pattern every 90 min (Figure 1). Although this NREM-REM cycle length remains largely stable across the night, the ratio of NREM to REM within each 90 min cycle changes, so that early in the night stages 3 and 4 of NREM dominate, while stage 2 NREM- and REM sleep prevail in the latter half of the night. Interestingly, the functional reasons for this organizing principal (deep NREM early in the night, stage 2 NREM and REM late in the night) remain unknown (Walker, 2009).
Figure 1.
The human sleep cycle. Across the night, NREM and REM sleep cycle every 90 min in an ultradian manner, while the ratio of NREM to REM sleep shifts. During the first half of the night, NREM stages 3 and 4 NREM (SWS) dominate, while stage 2 NREM and REM sleep prevail in the latter half of the night. EEG patterns also differ significantly between sleep stages, with electrical oscillations such as slow delta waves developing in SWS, K-complexes and sleep spindles occurring during stage 2 NREM, and theta waves seen during REM.
As NREM sleep progresses, electroencephalographic (EEG) activity begins to slow in frequency. Throughout stage-2 NREM, there is the presence of phasic electrical events including K-complexes (large electrical sharp waves in the EEG) and sleep spindles (short synchronized bursts of EEG electrical activity in the 11–15 Hz range) (Steriade & Amzica, 1998). The deepest stages of NREM, stages 3 and 4, are often grouped together under the term “slow wave sleep” (SWS), reflecting the occurrence of low frequency waves (0.5–4 Hz), representing an expression of underlying mass cortical synchrony (Amzica & Steriade, 1995). During REM sleep, however, EEG wave forms once again change in their composition, associated with oscillatory activity in the theta band range (4–7 Hz), together with higher frequency synchronous activity in the 30–80 Hz (“gamma”) (Llinas & Ribary, 1993; Steriade, Amzica, & Contreras, 1996). Periodic bursts of rapid eye movement also take place, a defining characteristic of REM sleep, associated with the occurrence of phasic endogenous waveforms. These waveforms are expressed in, among other regions, the pons (P), lateral geniculate nuclei of the thalamus (G), and the occipital cortex (O), and as such, have been termed “PGO waves” (Callaway, et al., 1987).
As the brain passes through these sleep stages, it also undergoes dramatic alterations in neurochemistry (Saper, Chou, & Scammell, 2001). In NREM sleep, subcortical cholinergic systems in the brainstem and forebrain become markedly less active (Hobson, McCarley, & Wyzinski, 1975; Lydic & Baghdoyan, 1988) while firing rates of serotonergic Raphé neurons and noradrenergic locus coeruleus neurons are also reduced relative to waking levels (Aston-Jones & Bloom, 1981; Shima, Nakahama, & Yamamoto, 1986). During REM sleep, both these aminergic populations are strongly inhibited while cholinergic systems become as or more active compared to wake (Kametani & Kawamura, 1990; Marrosu, et al., 1995), resulting in a brain state largely devoid of aminergic modulation and dominated by acetylcholine.
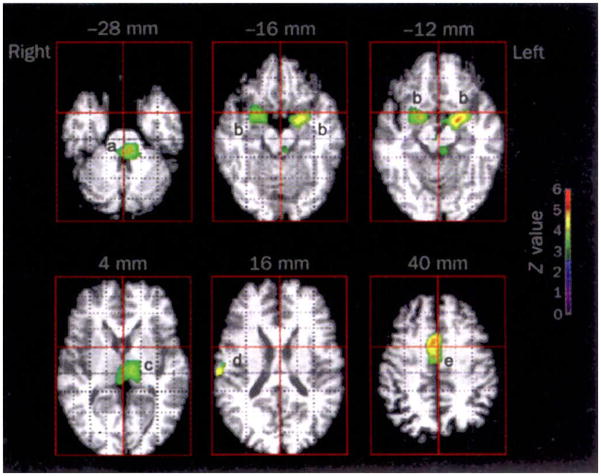
At a whole-brain systems level, neuroimaging techniques have revealed complex and dramatically different patterns of functional anatomy associated with NREM and REM sleep (for review, see Nofzinger, 2005). During NREM SWS, brainstem, thalamic, basal ganglia, prefrontal, and temporal lobe regions all appear to undergo reduced activity. However, during REM sleep, significant elevations in activity have been reported in the pontine tegmentum, thalamic nuclei, occipital cortex, mediobasal prefrontal lobes together with affect-related regions including the amygdala, hippocampus and anterior cingulate cortex (Figure 2). In contrast, the dorso-lateral prefrontal cortex, posterior cingulate, and parietal cortex appear least active in REM sleep.
Figure 2.
Regional brain activation during REM sleep (PET scan). The areas include: (a) the pons; (b) amygdala; (c) thalamus; (d) right parietal operculum and (e) anterior cingulate cortex. The z-value color scale indicates strength of activation. A z-value of 3.09 corresponds to a p-value of less than .001 (From Maquet, et al., 1996).
Although this summary only begins to describe the range of neural processes that are affected by the brain’s daily transit through sleep states, it clearly demonstrates that sleep itself cannot be treated as a homogeneous entity, offering a range of distinct neurobiological mechanisms that can support numerous brain functions. In the following sections, we will examine the role of sleep, and specific stages of sleep, in the modulation of emotional memories, the regulation of affective reactivity, which culminate in a heuristic model of sleep-dependent emotional brain processing.
Sleep and Emotional Memory Processing
The impact of sleep has principally been characterized at two different stages of memory 1) before learning, in the initial formation (encoding) of new information, and 2) after learning, in the long-term solidification (consolidation) of new memories (Marshall & Born, 2007; Walker & Stickgold, 2004, 2006; Walker, 2009). We now consider each of these stages, and focus on reports involving affective learning.
Sleep and Affective Memory Encoding
Emotional memory encoding
The initial stage of memory formation can be strongly modulated by the elicitation of emotion at the time of learning (Phelps, 2004). Emotionally arousing stimuli are consistently remembered better than neutral stimuli both in experimental laboratory studies and in real life accounts (Heuer and Reisberg 1990; Bradley et al. 1992; Buchanan and Lovallo 2001, Christianson, 1992); studies of autobiographical memory have found that individuals are more likely to remember those events that have increased emotional and personal significance (Conway, et al., 1994). However, emotions are not necessarily uni-dimensional, and have commonly been categorized along two dimensions: arousal (ranging from calm to excitement) and valence (ranging from positive to negative with neutral often considered an intermediate value) (Labar & Cabeza, 2006; Lang, Greenwald, Bradley, & Hamm, 1993). Moreover, evidence suggests these two dimensions of emotion influence memory encoding in different ways.
The adrenergic system appears to play a key role in orchestrating the enhancing effect of arousing emotion on memory at the initial moment of learning (and also during consolidation, discussed later). For example, Cahill and colleagues have demonstrated that administration of propanolol, a β-adrenoceptor antagonist, to participants before learning of emotional and neutral narrative texts blocks the memory enhancing effects elicited by arousal (Cahill, Prins, Weber, & McGaugh, 1994). Similarly, propranolol administration before the encoding of affectively arousing word stimuli will subvert the normal facilitation of emotional memory recall when tested shortly after (Strange, Hurlemann, & Dolan, 2003). Interestingly, this autonomic enhancing effect on memory is not observed in patients with amygdala lesions, suggesting a role not only for a specific neurochemical system in affective learning, but also a particular brain region (Adolphs, Cahill, Schul, & Babinsky, 1997; Cahill, Babinsky, Markowitsch, & McGaugh, 1995).
Functional neuroimaging studies further support the critical role of the amygdala in facilitating emotional memory encoding. Cahill et al. have reported greater activity in the right amygdala while viewing emotional versus neutral films; the greater the activity, across subjects, the larger the recollection benefit (Cahill, et al., 1996). Subsequent work, consistent with animal literature, suggests that the amygdala facilitates the initial acquisition of emotional information by influencing key medial temporal lobe structures, including the hippocampal complex (HC; McGaugh, 2004). For example, Dolcos et al. have demonstrated that the amygdala and anterior hippocampus exhibit coupled activation during the successful encoding of emotional scenes, as indexed by later remembering (Dolcos, et al., 2004). Furthermore, emotional arousal can enhance amygdala modulation of not only the ipsilateral parahippocampus, but also the ventrolateral prefrontal cortex; considered part of the extended limbic system (Kilpatrick & Cahill, 2003). Indeed, co-activation of the amygdala and HC during emotional memory encoding has now been reported in a number of studies (Canli, et al., 2000; Dolcos, et al., 2004; Hamann, et al., 1999; Kensinger & Corkin, 2004; Sharot & Phelps, 2004), with the extent of functional connectivity at the time of learning being especially predictive of robust (delayed) memory retention (Ritchey, et al., 2008). Emotionally valenced positive or negative stimuli of low arousal can also result in superior retention relative to neutral events, although this enhancement may occur independently of amygdala activation (Kensinger, 2004). Kensinger and Corkin have described dissociable mechanisms for the encoding of emotional stimuli across these dimensions of valence and arousal (Kensinger & Corkin, 2003). Relative to neutral words, high-arousing negative words generated greater memory encoding activation (difference between successful versus unsuccessful encoding trials) in the hippocampus and amygdala. In contrast, low-arousing negative words generated significantly greater memory encoding activation in the hippocampus and a posterior region of the lateral inferior prefrontal cortex. Therefore, distinct neural and likely cognitive effects underlie the formation of emotional memories, depending on the contribution of arousal and valence strength and direction.
When viewed as a collective, these pharmacological, behavioral and neuroimaging findings indicate that the specific enhancing effects of emotional arousal on memory formation involve interactions between the amygdala and key medial temporal lobe structures, in addition to engagement of central and peripheral neurohormonal systems. Moreover, the facilitation of encoding by autonomically arousing stimuli appears to engage similar brain systems across positive and negative valence domains (Kensinger & Schacter, 2006; Labar & Cabeza, 2006). By contrast, the encoding benefit of emotional valence in the absence of high arousal may be governed, at least in part, by frontally mediated semantic and strategic processes that can modulate memory without key involvement of the amygdala (cf. Labar & Cabeza, 2006).
This account of the beneficial enhancing effects of emotion on the initial process of learning pertains to conditions when the brain is sleep-rested. There is now considerable evidence, in both animals and humans, that sleep deprivation prior to encoding can significantly but also selectively alter and impair the canonical profile of emotional memory enhancement.
Sleep and emotional memory formation
At a behavioral level, pre-training sleep deprivation in rodents has been shown to impair encoding of numerous memory tasks – the evidence for which we only briefly summarize here. Critically, many if not all of these studies involve either appetitive or aversive learning paradigms; meaning these tasks are of an emotional nature.
Sleep deprivation, and specifically REM sleep deprivation, imposes detrimental effects on the encoding of one-way and two-way avoidance learning, taste aversion and passive avoidance learning (McGrath & Cohen, 1978; Smith, 1985). Even short (5 hr) bouts of pre-training REM sleep deprivation appear capable of disrupting the encoding of two-way avoidance learning in rats, reducing the number of avoidances – impairments that cannot be overcome by continued practice during the training session (Gruart-Masso, Nadal-Alemany, Coll-Andreu, Portell-Cortes, & Marti-Nicolovius, 1995).
Building on these behavioral findings, a collection of studies have gone on to explore the potential cellular mechanisms of sleep deprivation-induced encoding deficits, many of which have focused on aspects of the limbic system, and specifically the hippocampus. At the cellular level, REM sleep deprivation (ranging from 24–72 hr) not only reduces the basic excitability of hippocampal neurons, but significantly impairs the formation of long-term potentiation (LTP; a foundational mechanism of memory formation) within these neurons (Davis, Harding, & Wright, 2003; McDermott, et al., 2003). Furthermore, the small amount of LTP that does develop actually decays within 90 min, suggesting that even in the event of successful LTP induction, hippocampal neurons are unable to maintain these plastic changes under conditions of REM sleep deprivation (Davis, et al., 2003). Therefore, sleep prior to learning appears to be necessary in preparing or maintaining the cellular and subcellular ability of key subcortical networks to acquire new memory associations.
While early studies investigating the role of sleep-dependent memory in humans focused primarily on post-learning consolidation (see later sections), more recent data similarly support the need for adequate pre-learning sleep in the formation of new human episodic memories. Some of the first studies of sleep deprivation and human memory encoding focused on neutral forms of learning, indicating that “temporal memory” (memory for when events occur) was significantly disrupted by a night of pre-training sleep deprivation (Harrison & Horne, 2000; Morris, Williams, & Lubin, 1960); even when caffeine was administered to overcome non-specific effects of lower arousal.
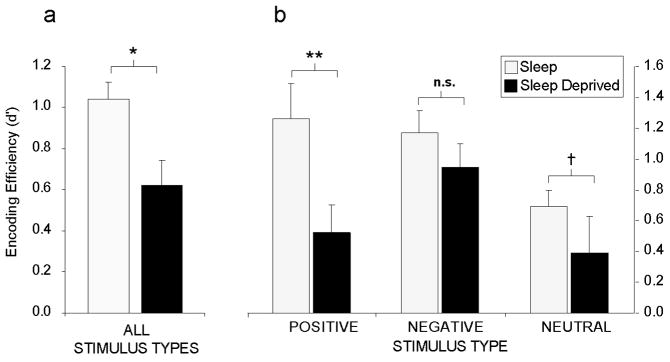
More recent investigations have examined the importance of pre-training sleep for the formation of emotional and neutral memories (Walker et al. unpublished findings). Subjects were either sleep deprived for 36 hr or allowed to sleep normally prior to a learning session composed of emotionally negative, positive and neutral words. Participants were then tested following two recovery nights of sleep so that in both groups, recollection was tested in a sleep rested state. Therefore, any differences observed in performance observed could not be accounted for by the effects of sleep deprivation on retrieval, since neither group was sleep deprived at later testing. Averaged across all memory categories, subjects who were sleep deprived demonstrated a 40% deficit in memory encoding, relative to subjects who had slept normally prior to learning (Figure 3a). However, when these data were separated into the three emotional categories (negative, positive or neutral), selective dissociations became apparent (Figure 3b). In subjects that had slept (control group), both positive and negative stimuli were associated with superior retention levels relative to the neutral condition, consistent with the notion that emotion facilitates memory encoding (Phelps, 2004). In the sleep-deprived group, a severe encoding impairment was evident for neutral and especially positive emotional memories, exhibiting a significant 59% retention deficit, relative to the control condition. Most interesting was the relative resistance of negative emotional memory to sleep deprivation, showing a markedly smaller and non-significant impairment.
Figure 3.
Sleep deprivation and encoding of emotional and non-emotional declarative memory. Effects of 38 hr of total sleep deprivation on encoding of human declarative memory a) When combined across all emotional and non-emotional categories, b) When separated by emotional (positive and negative valence) and non-emotional (neutral valence) categories, demonstrating a significant group [sleep, sleep-deprivation] × emotion category [positive, negative, neutral] interaction (F(1,18) 3.58, p < .05). Post-hoc t-test comparisons:
†p < .08, *p < .05, **p < .01, n.s. not significant, error bars represent s.e.m.
These data indicate that sleep loss impairs the ability to commit new experiences to memory, and has recently been associated with dysfunction throughout the hippocampal complex (Yoo, Hu, Gujar, Jolesz, & Walker, 2007). They also suggest that, while the effects of sleep deprivation are directionally consistent across emotional subcategories, the most profound impact is on the encoding of positive emotional stimuli, and to a lesser degree, emotionally neutral stimuli. In contrast, the encoding of negative memory appears to be more resistant to the effects of prior sleep loss. Moreover, such results may offer novel learning and memory insights into affective mood disorders that express co-occurring sleep abnormalities (Buysse, 2004). Indeed, if one compares the two profiles of memory encoding in Figure 3b, it is clear that the sleep control group completes the encoding session with a balanced mix of both positive and negative memories. However, those in the deprivation group have a skewed distribution, finishing the encoding session with an overriding dominance of negative memories, and far fewer positive or neutral memories; an issue with clinical relevance discussed below.
The mechanistic cause of these differential encoding impairments requires greater elucidation. For example, at a brain level, the arousal strength of such word stimuli can place different demands upon the amygdala or the prefrontal cortices (Kensinger & Corkin, 2004) – an issue of particular relevance considering the marked hypo-frontality commonly reported in sleep deprivation (Chee & Chuah, 2008; Thomas, et al., 2000, 2003), yet a hyper-active state of amygdala function following sleep loss (Yoo, Gujar, Hu, Jolesz, & Walker, 2007). Moreover, positive materials may be more associated with greater relational processing (Kensinger, 2004), which could also account for the susceptibility to interference following sleep deprivation. Additionally, there may be contributions due to the negative affective state of sleep deprived individuals, leading to a mood-congruent memory bias of encoding (Lewis, Critchley, Smith, & Dolan, 2005). Subsequent studies combining the assessment of brain activity, mood state and task stimulus properties will be necessary to distinguish between these possibilities.
In summary, studies indicate that prior sleep loss significantly impairs the ability for effective next-day learning of new experiences across numerous species. Furthermore, sleep loss appears to disrupt the learning of different affective categories to different extents, potentially creating an imbalance in negative emotional memory dominance. Most intriguing, animal models indicate that affective learning demonstrates a particular sensitivity to REM sleep deprivation, suggesting a dependency on a specific physiological stage of prior sleep for next-day emotional learning.
Sleep and Affective Memory Consolidation
Mechanisms of emotional memory consolidation
In addition to substantial evidence suggesting that emotion facilitates initial memory encoding, emotion is also known to modulate the subsequent stage of latent memory consolidation. A number of behavioral studies in humans have demonstrated diminished forgetting of emotional compared to neutral stimuli. This benefit of emotion on later memory retention increases as the time delay between learning and testing increases. This effect of offline retention has been shown over varying post-learning intervals, including comparisons between recollection at: 20 min to 1 week (Kleinsmith & Kaplan, 1963), immediate to 1h (LaBar & Phelps, 1998), immediate to 24 h (Sharot & Phelps, 2004) and 15 min to 2 weeks (Anderson, Yamaguchi, Grabski, & Lacka, 2006). Furthermore, this latent emotional memory enhancement may be more prevalent for items receiving more detailed remembering than simply a sense of familiarity (Dolcos, LaBar, & Cabeza, 2005; Kensinger & Corkin, 2003; Ochsner, 2000; Ritchey, et al., 2008).
With the knowledge that emotion triggers improvements in memory performance over time, elegant work in animal models has substantively characterized the underlying anatomical and neurochemical mechanisms contributing to these effects; findings with implications relvant to the role of sleep neurobiology in emotional memory processing. A striking finding from a series of early consolidation experiments was that post-training drug manipulations, such as sub-cutaneous amphetamine injections, could facilitate memory retention when animals were tested 24hr after learning. Under these conditions, animals were drug free during the initial learning and subsequent testing, so that pharmacological treatment did not affect performance at encoding or recall, but specifically the intervening consolidation phase. Moreover, it was discovered that administration of certain endogenous compounds could facilitate memory consolidation – namely, peripheral post-training injections of the stress hormones epinephrine and glucocorticoids (Cahill, 2000; McGaugh, 2000).
It is now proposed that stress hormones constitute a plausible protracted means of modifying latent consolidation by way of emotional arousal, promoting the adaptive reorganization of long-term memory representations. Recent human studies have confirmed these predictions. Using post-training pharmacological and pain manipulations to elicit responses involving the amygdala (Cahill & Alkire, 2003; Cahill, Gorski, & Le, 2003), Cahill and co-workers have demonstrated that induction of stress hormones can selectively enhance long-term memory of emotional stimuli when retested several days later (and see later sections). Thus, the hormones adrenaline and corticosterone appear to offer two important adaptive functions in response to arousing experiences – 1) they aid immediate responses to a potentially stressful experience, and 2) they aid future responses by enhancing consolidation of declarative memory for those arousing events.
In addition to neurohormonal effects, there is also substantial evidence that several neurotransmitters co-regulate the effects of emotion on consolidation; including adrenergic transmitters and acetylcholine (ACh). The cholinergic effects are of particular note in relationship to sleep and specifically REM sleep discussed later. Acetylcholine appears to enhance amygdala-dependent memory consolidation. For example, in rats, post-training infusions of muscarinic cholinergic agonists and antagonists into the amygdala enhance and impair, respectively, memory across numerous tasks, including inhibitory avoidance, fear conditioning and change in reward magnitude (Introini-Collison, Dalmaz, & McGaugh, 1996; Passani, et al., 2001; Power & McGaugh, 2002; Schroeder & Packard, 2002). Furthermore, post-training cholinergic stimulation of the amygdala using either muscarinic cholinergic agonists or the acetylcholinesterase inhibitor physostigmine has been shown to attenuate this emotional memory impairment (Power & McGaugh, 2002).
Collectively, these studies suggest that a cascade system of neurohormonal and neurochemical mechanisms can either jointly act in the endeavor of facilitating consolidation, or may independently contribute to this process at different times across the later consolidation period, or during different brain states, such as wake or sleep.
Sleep and emotional memory consolidation
The role of sleep after learning in subsequent memory consolidation has now been demonstrated across a range of phylogeny (Walker & Stickgold, 2004, 2006). Here we focus on affective learning paradigms, especially in humans.
Animal models support a role for sleep in the consolidation of both contextual fear and shock avoidance tasks (for more detailed reviews beyond the scope of the current article, see (Smith, 1985; Walker & Stickgold, 2004), all known to depend on intact hippocampal function. Daytime training on these tasks triggers alterations in sleep-stage characteristics, especially REM sleep (Ambrosini, et al., 1993; Ambrosini, Sadile, Gironi Carnevale, Mattiaccio, & Giuditta, 1988; Hennevin & Hars, 1987; Mandai, Guerrien, Sockeel, Dujardin, & Leconte, 1989; Sanford, Silvestri, Ross, & Morrison, 2001; Sanford, Tang, Ross, & Morrison, 2003; Smith, Young, & Young, 1980), possibly reflecting homeostatic demands on REM sleep-dependent mechanisms of consolidation.
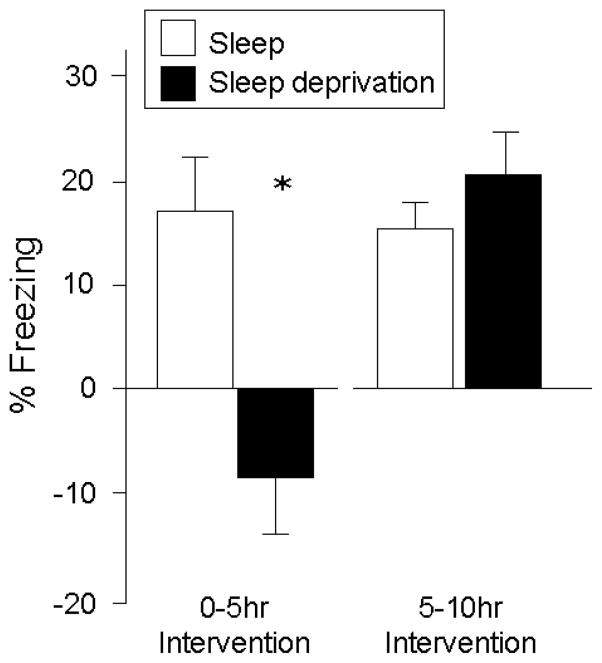
Conversely, sleep deprivation after learning of such tasks has also been shown to disrupt consolidation and impair next day memory retention (Smith, 1985; Walker & Stickgold, 2004). Moreover, these effects are apparent following selective REM sleep deprivation, rather than total sleep deprivation (Beaulieu & Godbout, 2000; Fishbein, Kastaniotis, & Chattman, 1974; Hennevin & Hars, 1987; Marti-Nicolovius, Portell-Cortes, & Morgado-Bernal, 1988; Oniani, Lortkipanidze, & Maisuradze, 1987; Pearlman, 1969; Shiromani, Gutwein, & Fishbein, 1979; Smith & Kelly, 1988). Interestingly, the timing of when sleep deprivation occurs appears to be important. For example, Graves et al. (Graves, Heller, Pack, & Abel, 2003) have demonstrated that sleep deprivation 0–5hr post-training selectively impairs consolidation of contextual fear conditioning (as measured at a later 24hr retest; Figure 4). However, sleep deprivation 5–10hr post-training did not block consolidation, resulting in similar memory performance at retest (Figure 4). These temporal dynamics appear to overlap with studies investigating the effects of adrenergic antagonists on the consolidation of contextual fear conditioning, describing a window of sensitivity several hours post-learning, but become less effective approximately 6hr and beyond (depending on the learning paradigm used) (Bevilaqua, et al., 1997; Izquierdo, et al., 1998; Ji, Wang, & Li, 2003).
Figure 4.
Difference in percent freezing during contextual fear conditioning with or without sleep deprivation, across either 0–5 hr post-training, or 5–10 hr post-training (Graves, et al., 2003).
*p < .05, error bars represent s.e.m.
These findings suggest that the consolidation of fear-associated memory occurs soon after learning, potentially during discreet brain-state time windows. The implications of this temporal sensitivity are informative from a mechanistic standpoint, but also from a clinical treatment perspective if the targeted disruption of sleep to negate overnight consolidation becomes a goal; a situation perhaps most pertinent to conditions such as PTSD (discussed below).
In humans, the role of sleep in declarative memory consolidation, rather than being absolute, may depend on more intricate aspects of the information being learned, such as novelty, meaning to extract, and also the affective salience of the material. The wealth of evidence demonstrating that human emotional experiences tend to be remembered better than neutral ones (Cahill, 2000; McGaugh, 2004; Phelps, 2004) may help clarify the potential contribution of sleep to episodic memory processing.
Many of these findings describe an offline consolidation benefit (reduction in forgetting) for emotional compared to neutral information that appears to persist and even improve over time across periods containing a night of sleep (Kleinsmith & Kaplan, 1963; LaBar & Phelps, 1998; Levonian, 1972; Sharot & Phelps, 2004; Walker & Tarte, 1963), and several have directly examined whether it is time, with sleep, that preferentially modulates these effects. More specifically, and based on the coincident neurophysiology that REM sleep provides and the neurobiological requirements of emotional memory processing (Cahill, 2000; McGaugh, 2004), work has now begun to test a selective REM sleep-dependent hypothesis of affective human memory consolidation. For example, Hu et al. have compared the consolidation of emotionally arousing and non-arousing picture-stimuli following a 12 hr period across a day or following a night of sleep (Hu, Stylos-Allen, & Walker, 2006). A specific emotional memory benefit was observed only following sleep and not across an equivalent time awake. Atienza and Cantero have also demonstrated that total sleep deprivation the first night after learning significantly impairs later one-week retention of emotional as well as neutral visual stimuli (Atienza & Cantero, 2008). Interestingly, this difference was greatest for neutral relative to emotional items. Such a difference may indicate that emotional items are more resistant to the impact of first night sleep deprivation (a finding with clinical treatment consequences), or that subsequent post-deprivation recovery sleep is more capable of salvaging consolidation of emotional relative to neutral memories.
Wagner and colleagues (Wagner, Gais, & Born, 2001) have also shown that sleep selectively favours the retention of previously learned emotional texts relative to neutral texts, and that this affective memory benefit is only present following late-night sleep (a time period rich in REM sleep). This emotional memory benefit was found to persist in a follow-up study performed four years later (Wagner, et al., 2006). It has also been demonstrated that the speed of recognizing emotional face expressions presented prior to sleep is significantly improved the next day, a benefit that is positively correlated with the amount of intervening REM sleep (Wagner, Kashyap, Diekelmann, & Born, 2007).
Sleep has been shown to target the consolidation of specific aspects of emotional experiences, as well as mediate the extinction of human fear memories. By experimentally varying the foreground and background elements of emotional picture stimuli, Payne et al. have demonstrated that sleep can target the strengthening of negative emotional objects in a scene, but not the peripheral background (Payne, Stickgold, Swanberg, & Kensinger, 2008). In contrast, equivalent time awake did not afford any benefit to emotional object memory (or the background scene). This may suggest that sleep-dependent processing can selectively separate episodic experience into component parts, preferentially consolidating those of greatest affective salience. Using a conditioning paradigm in humans, Pace-Schott and colleagues recently investigated the effects of sleep and wake on fear extinction and generalization of fear extinction (Pace-Schott, et al., 2009). Concurrent fear conditioning to two different stimuli was followed by targeted extinction of conditioned responding to only one of the stimuli. Participants were then tested following a 12hr offline delay period across the day or following a night of sleep. Upon returning 12hr later, generalization of extinction from the target stimuli to the non-targeted stimuli occurred only following a night of sleep, and not across an equivalent waking period. Therefore, sleep can not only modulate the learned emotional associations between stimuli, but also may actively generalize these associational values across related contexts.
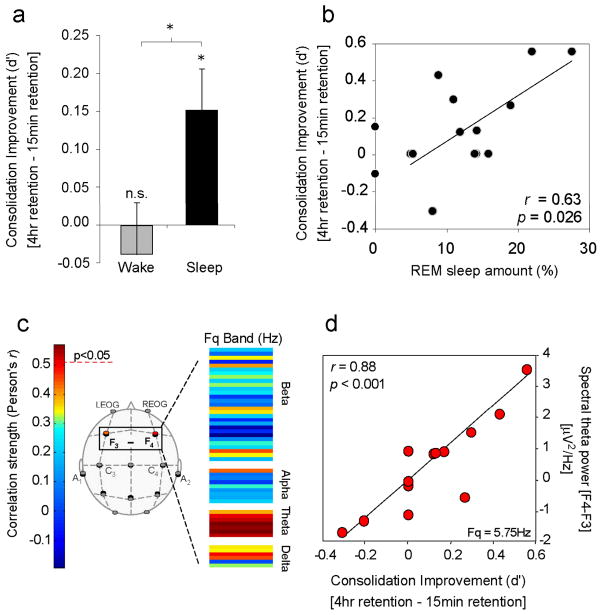
Using a nap paradigm, Nishida et al. recently demonstrated that sleep, and specifically REM sleep neurophysiology, may underlie such consolidation benefits (Nishida, Pearsall, Buckner, & Walker, 2009). Subjects performed two study sessions in which they learned emotionally arousing negative and neutral picture stimuli; one 4 hr prior, and one 15 min prior to a recognition memory test. In one group, participants slept (90 min nap) after the first study session, while in the other group, participants remained awake. Thus, items from the first (4 hr) study sessions transitioned through different brain-states in each group prior to testing, containing sleep in the Nap group and no sleep in the No-Nap group, yet experienced identical brain-state conditions following the second (15 min) study session prior to testing. No change in memory for emotional (or neutral stimuli), occurred across the offline delay in the no-nap group. However, a significant and selective offline enhancement of emotional memory was observed in the nap group (Figure 5a), the extent of which was correlated with the amount of REM sleep (Figure 5b), and the speed of entry into REM sleep (latency; not shown in figure). Most striking, spectral analysis of the EEG demonstrated that the magnitude of right-dominant prefrontal theta power during REM sleep (activity in the frequency range of 4.0–7.0 Hz) exhibited a significant and positive relationship with the amount of emotional memory improvement (Figure 5c&d).
Figure 5.
REM sleep enhancement of negative emotional memories. a) Offline benefit (change in memory recall for 4 hr versus 15 min old memories) across the day (wake, grey bar) or following a 90 min nap (sleep, filled bar); b) Correlation between the amount of offline emotional memory improvement in the nap group (i.e. the offline benefit expressed in filled bar of figure a), and the amount of REM sleep obtained within the nap; c) Correlation strength (Pearson’s r-value) between offline benefit for emotional memory in the sleep group (the benefit expressed in filled bar of figure a) and the relative right versus left prefrontal spectral-band power ([F4 – F3]) within the delta, alpha, theta and beta spectral bands, expressed in average 0.5 Hz bin increments. Correlation strength is represented by the color range, demonstrating significant correlations within the theta frequency band (hot colors), and d) exhibiting a maximum significance at the 5.75 Hz bin.
*p < .05; error bars indicate s.e.m. (Modified from Nishida et al. In Press).
These findings move beyond simply demonstrating that affective memories are preferentially enhanced across periods of sleep, and indicate that the extent of emotional memory improvement is associated with specific REM sleep characteristics – both quantity and quality (and independent of nocturnal hormonal changes). Corroborating these correlations, it has previously been hypothesized that REM sleep represents a brain-state particularly amenable to emotional memory consolidation, based on its unique biology (Hu, et al., 2006; Pare, Collins, & Pelletier, 2002; Walker, 2009). Neurochemically, levels of limbic and forebrain ACh are markedly elevated during REM sleep (Vazquez & Baghdoyan, 2001), reportedly quadruple those seen during NREM and double those measured in quiet waking (Marrosu, et al., 1995). Considering the known importance of ACh in the long-term consolidation of emotional learning (McGaugh, 2004), this pro-cholinergic REM sleep state may promote the selective memory facilitation of affective memories, similar to that reported using experimental manipulations of ACh (Power, 2004). Neurophysiologically, theta oscillations have been proposed as a carrier frequency, allowing disparate brain regions that initially encode information to selectively interact offline, in a coupled relationship. By doing so, REM sleep theta may afford the ability to strengthen distributed aspects of specific memory representations across related but different anatomical networks (Buzsaki, 2002; Jones & Wilson, 2005).
Together, these results demonstrate that offline time containing sleep, especially REM sleep, may offer a neurobiological state that is especially well suited for the preferential processing of emotional experiences. It should be noted, however, that the majority of studies have principally used negative and arousing emotional stimuli. An important next step will be the inclusion of positive stimuli of different arousal magnitudes in such paradigms, offering an increasingly refined understanding of the interaction between valence asymmetry and sleep-dependent emotional memory consolidation.
Sleep and Emotional Regulation
Despite substantial research focusing on the interaction between sleep and affective memory, the impact of sleep loss on basic emotional regulation and perception has received limited research attention. This absence of research is also striking considering that nearly all psychiatric and neurological mood disorders express co-occurring abnormalities of sleep, suggesting an intimate relationship between sleep and emotion. Nevertheless, a number of studies evaluating subjective as well as objective measures of mood and affect, combined with insights from clinical domains, offer an emerging understanding for the critical role sleep plays in regulating emotional brain function.
Sleep loss, mood stability and emotional brain (re)activity
Together with impairments of attention and alertness, sleep deprivation is commonly associated with increased subjective reports of irritability and affective volatility (Horne, 1985). Using a sleep restriction paradigm (5hr/night), Dinges et al. have reported a progressive increase in emotional disturbance across a one-week period on the basis of questionnaire mood scales (Dinges, et al., 1997). In addition, subjective descriptions in participants’ daily journals also indicated increasing complaints of emotional difficulties. Zohar et al. have investigated the effects of sleep disruption on emotional reactivity to daytime work events in medical residents (Zohar, Tzischinsky, Epstein, & Lavie, 2005). Sleep loss was shown to amplify negative emotional consequences of disruptive daytime experiences while blunting the positive benefit associated with rewarding or goal-enhancing activities.
While these findings help to characterize the behavioral irregularities imposed by sleep loss, evidence for the role of sleep in regulating psychophysiological reactivity and emotional brain networks is only now starting to emerge.
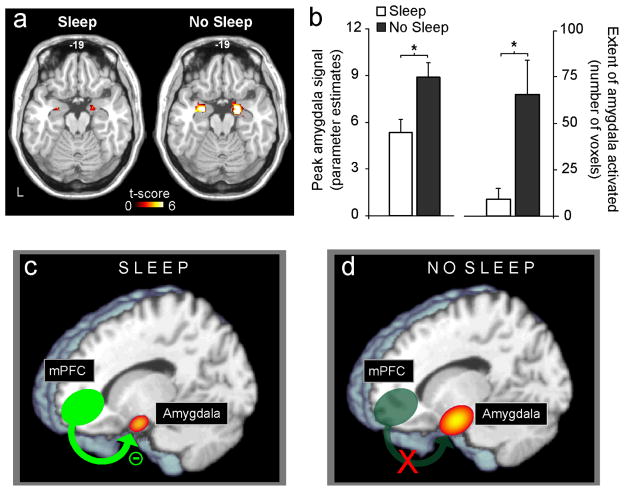
To date, only two studies have addressed this interaction. Using functional MRI (fMRI), Yoo and colleagues examined the impact of one night of sleep deprivation on emotional brain reactivity in healthy young adults (Yoo, Gujar, et al., 2007). During scanning, participants performed an affective stimulus-viewing task involving the presentation of picture slides ranging in a gradient from emotionally neutral to increasingly negative and aversive. While both groups expressed significant amygdala activation in response to increasingly negative picture stimuli, those in the sleep-deprivation condition exhibited a remarkable +60% greater magnitude of amygdala reactivity, relative to the control group (Figure 6a&6b). In addition to this increased intensity of activation, there was also a three-fold increase in the extent of amygdala volume recruited in response to the aversive stimuli in the sleep-deprivation group (Figure 6b). Perhaps most interestingly, relative to the sleep-control group, there was a significant loss of functional connectivity identified between the amygdala and the medial prefrontal cortex (mPFC) in those who were sleep deprived – a region known to have strong inhibitory projections to the amygdala (Sotres-Bayon, Bush, & LeDoux, 2004) (Figure 6c&6d). In contrast, significantly greater connectivity was observed between the amygdala and the autonomic-activating centers of the locus coeruleus in the deprivation group.
Figure 6.
The impact of sleep deprivation on emotional brain reactivity and functional connectivity. a) Amygdala response to increasingly negative emotional stimuli in the Sleep deprivation and Sleep control groups, and b) Corresponding differences in intensity and volumetric extent of amygdala activation between the two groups (average ± s.e.m. of left and right amygdala), c) Depiction of associated changes in functional connectivity between the medial prefrontal cortex (mPFC) and the amygdala. With sleep, the prefrontal lobe was strongly connected to the amygdala, regulating and exerting and inhibitory top-down control, d) Without sleep, however, amygdala-mPFC connectivity was decreased, potentially negating top-down control and resulting in an overactive amygdala.
*p < .01; error bars indicate s.e.m. (Modified from Yoo, Gujar, et al., 2007).
Thus, without sleep, an amplified hyper-limbic reaction by the human amygdala was observed in response to negative emotional stimuli. Furthermore, this altered magnitude of amygdala activity was associated with a loss of functional connectivity with the mPFC in the sleep-deprivation condition; implying a failure of top-down inhibition by the prefrontal lobe. It would therefore appear that a night of sleep may “reset” the correct affective brain reactivity to next-day emotional challenges by maintaining functional integrity of this mPFC-amygdala circuit and thus govern appropriate behavioral repertoires (e.g. optimal social judgments and rational decisions). Interestingly, a similar pattern of anatomical dysfunction has been implicated in a number of psychiatric mood disorders, which express co-occurring sleep abnormalities (Davidson, 2002; Davidson, Pizzagalli, Nitschke, & Putnam, 2002; New, et al., 2007), and directly raises the issue of whether such factors (sleep loss and clinical mood disorders) are causally related. It will be important to identify if a similar dysregulation is observed for positive emotional stimuli, examining the full bi-directional impact of sleep loss on affective reactivity.
More recently, Franzen et al. measured the impact of total sleep deprivation on pupil diameter responses (a measure of autonomic reactivity) during a passive affective picture-viewing task containing positive, negative and neutral stimuli (Franzen & Buysse, 2008). Relative to a sleep control group, there was a significantly larger pupillary response to negative pictures compared to positive or neutral stimuli in the deprivation group. Furthermore, those in the sleep-deprived condition expressed earlier reactivity to negative images prior to the stimulus onset cue. These data suggest that sleep deprivation not only alters emotional reactivity, but may also change the anticipation of these of these events. It is interesting to note the similarity between these findings and those observed in depressed individuals (Siegle, Granholm, Ingram, & Matt, 2001; Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003), as well as those from the study by Yoo et al. reporting increased connectivity between the amygdala and autonomic activating brainstem centers under condition of sleep loss (Yoo, Gujar, et al., 2007).
Dreams and Emotion
The strong emotional tone of mental activity that occurs during sleep (often referred to as dream mentation; Hobson, Pace-Schott, & Stickgold, 2000), has long encouraged speculation of sleep-dependent affective processing (for recent review, see Levin & Nielsen, 2009). Indeed, the notion that dreams are intimately linked to our emotional state is of course not new, and was perhaps most famously conveyed in the theories of Sigmund Freud, the utility of which are reviewed elsewhere (Hobson, et al., 2000; Reiser, 2001). Between 75 % and 95 % of dreams contain emotional contexts and largely emerge from REM sleep (Hobson, et al., 2000; Hobson, Stickgold, & Pace-Schott, 1998). Furthermore, and discounting the often-stated hypothesis that dreams depict a veridical replay of previous episodic experience, the most apparent link between prior daytime events and subsequent dream content are current emotional concerns and themes (Fosse, Fosse, Hobson, & Stickgold, 2003). Such findings have taken on added clinical importance in the context of reactive depression. For example, Cartwright et al. have shown that recently divorced women who initially suffered depression dreamt of their ex-spouses more frequently and with stronger emotion than those not depressed (Cartwright, Kravitz, Eastman, & Wood, 1991). Most remarkable, those who were in remission one year later were the same patients that had significantly more such dreams, indicating a potential functional connection between dreaming (and/or the neurophysiological state from which they emerge – REM sleep) and recovery from emotional conflict or trauma.
Sleep and mood disorders
The implication of such dream studies raises the more general issue of sleep abnormalities in affective disorders, and whether the high degree of co-occurrence is more than epiphenomenal. Indeed, it is difficult to identify any psychiatric mood disorder where sleep disturbance is not a listed formal symptom or a common feature of the condition ([DSM-IV], 1994) – a relationship that may be bi-directional (for reviews, see Benca, Obermeyer, Thisted, & Gillin, 1992; Bliwise, 2004; Harvey, 2001; Harvey, Jones, & Schmidt, 2003). Here we focus on two psychiatric disorders of most relevance to the topic of sleep-dependent emotional information processing; major depression and post-traumatic stress disorder (PTSD).
Major Depression
As the most prevalent mood disorder, major depression has consistently been linked to sleep abnormalities, found in up to 90% of patients, aspects of which are among the diagnostic criteria for this condition ([DSM-IV], 1994). The inability to initiate and maintain sleep (insomnia) is a robust risk factor for the development of both the first episode of depression, as well as recurrent episodes (Harvey, 2001; Perlis, et al., 2006). Polysomnographic (PSG) recordings of sleep in major depression are often marked by increased sleep latency, wake time after sleep onset and nocturnal awakenings (Berger, Doerr, Lund, Bronisch, & von Zerssen, 1982; Gillin, Duncan, Pettigrew, Frankel, & Snyder, 1979; Kupfer, et al., 1985; Waller, et al., 1989). Intriguingly, an additional hallmark feature appears to be reduced REM sleep latency (faster entry into REM), a prolonged first REM period and an increase in REM density (Armitage, 2007; Gottesmann & Gottesman, 2007; Tsuno, Besset, & Ritchie, 2005). Moreover, the normalization of sleep architecture abnormalities have been associated with a reduced risk of relapse into depression, yet the persistence of abnormally short REM sleep latency has been related to an increased risk of relapse (Ohayon, 2007). Short (< 65 min) REM sleep latency has similarly been shown to predict both response to antidepressant treatment and risk of relapse in major depression (Giles, Jarrett, Roffwarg, & Rush, 1987; Grunhaus, et al., 1994; Kupfer, Frank, McEachran, & Grochocinski, 1990; Rush, et al., 1989). Indeed, a recent review by Krystal et al. indicated that the likelihood of relapse and/or a more indolent course of both major depression and bipolar disorder (together with alcoholism) were strongly predicted by sleep disruption, including the traits of shorter REM sleep latency, greater REM density and greater percentage of REM sleep (Krystal, Thakur, & Roth, 2008). Furthermore, successful psychological treatments such as interpersonal therapy and cognitive behavioral therapy have been found to decrease REM density (Buysse, Frank, Lowe, Cherry, & Kupfer, 1997; Nofzinger, et al., 1994).
Interestingly, depressed patients show increased activity in the midbrain reticular formation and in the anterior paralimbic cortex from waking to REM sleep (Nofzinger, et al., 2000). Given the negative affect of depressed patients during waking, Nofzinger et al. have suggested that the over-activation of limbic structures during REM sleep may reflect a susceptibility of depressed patients to experience (and possibly encode) stimuli in a more affectively intense, negative context – findings discussed in greater detail in the next section.
Post-traumatic stress disorder
Another patient population commonly linked to sleep disturbance is that of post-traumatic stress disorder (PTSD), characterized by intrusive re-experiencing, avoidance, and hyperarousal reactions that persist after exposure to a traumatic event ([DSM-IV], 1994). Increasing attention has been paid to the repeated incorporation of emotionally charged waking episodes into sleep mentation – a defining characteristic in the DSM-IV criteria for diagnosis. Perhaps not surprisingly, PTSD has also been associated with a dysregulation of REM sleep, together with reports of significantly increased sympathetic autonomic tone (Harvey, et al., 2003; Mellman & Hipolito, 2006). In fact, the sleep disruptions that occur following trauma exposure may constitute a specific mechanism involved in the pathophysiology of chronic PTSD and poor clinical outcome. Subjective and objective sleep disturbances occurring early after trauma exposure, as well as heightened sympathovagal tone during REM sleep, are all associated with an increased risk of meeting criteria for PTSD at subsequent assessments conducted up to 1 year later (Koren, Arnon, Lavie, & Klein, 2002; Mellman, Bustamante, Fins, Pigeon, & Nolan, 2002). It is still unclear whether changes in sleep composition and dream characteristics in PTSD reflect attempted functional or dysfunctional processes. However, it appears that emotional episodic memory events pervade the mental experiences of dreaming in these patients, potentially related to aberrant consolidation mechanisms and the etiology of the disorder itself; issues discussed in more detail below.
A heuristic model of sleep-dependent emotional processing – explanatory clinical insights and predictive associations
Based on the emerging interaction between sleep and emotion at the basic experimental as well as clinical level, we next provide a synthesis of these findings, which converge on a functional role for sleep in affective brain modulation. We describe a model of sleep-dependent emotional information processing, offering provisional brain-based explanatory insights regarding the impact of sleep abnormalities in the initiation and maintenance of certain mood disorders, and leading to testable predictions for future experimental investigations.
A nexus of experimental and clinical observation
The findings discussed above suggest a predisposition for the encoding of negative emotional memories and a hyper-limbic reactivity to negative emotional events under conditions of sleep loss, together with a strengthening of negative memories during subsequent REM sleep, all of which have potential relevance for the understanding of major depression.
The reduction of sleep caused by insomnia (Buysse, 2004; Shaffery, Hoffmann, & Armitage, 2003) may predispose patients with depression to an imbalance in memory encoding. Although based on findings from acute sleep deprivation, chronic accumulated sleep debt associated with depression may impair the ability to form and retain memories of positive (and neutral) affective valence, yet leave preserved the formation and hence long-term dominance of negative experiences. This encoding bias would result in a perceived autobiographical history dominated by negative life events, despite being potentially filled with both positive and negative daily life experiences. Indeed, this imbalance may provide a converse mechanistic explanation for the higher incidence of depression in populations expressing impairments in sleep.
Beyond an imbalance in emotional memory formation, there may be another sleep-dependent mnemonic dysfunction potentiating disease severity. Mounting data suggests that patients suffering from depression exhibit both a faster progression into REM sleep (reduced REM sleep latency) and an increase in the amount of REM sleep, particularly early in the night (Armitage, 2007; Tsuno, et al., 2005). When considered on the foundation of evidence described above, indicating a strong positive correlation between the amplification of negative emotional memories and the amount and speed of entry into REM sleep, this signature alteration of REM sleep in depression may instigate maladaptive and disproportionate consolidation of prior negative affective experiences. This would be especially pronounced when combined with the preexisting dominance of negative memories due to the biased encoding noted above.
Consistent with this hypothesis, many anti-depressant medications are known to be REM sleep suppressants (Winokur, et al., 2001), which by their action, would curtail such offline emotional memory processing, and in doing so, reduce the strength (consolidation) of associated affective experiences. Indeed, total sleep deprivation is known to be a rapid yet short-lived treatment for a subset of depressed patients. Improvement in depressive symptoms has also been shown to occur after a single night of sleep deprivation, although this is only apparent in 40–60% of patients (Giedke & Schwarzler, 2002; Wirz-Justice & Van den Hoofdakker, 1999), and maybe related to the extent of resting baseline over-activity within the amygdala (Clark, et al., 2006). Most remarkable, selective deprivation of late night sleep, rich in REM sleep, appears to be particularly efficacious in these sub-group populations (Clark, et al., 2006). It is also interesting to note the speed with which symptoms relapse following recovery sleep, likely containing a strong REM sleep rebound, which may contribute to the rapid reversal of this therapeutic effect (Wu & Bunney, 1990). It still remains unclear why the efficacy of REM sleep suppressing anti-depressants takes a number of weeks to produce clinical improvement, yet the effects of experimental sleep deprivation affords rapid symptom alterations. It may be that different mechanistic routes underlie each effect, or that the magnitude of physiological REM sleep suppression induced by anti-depressant medications is significantly less than classical sleep-stage scoring imply (bound by strict criteria and perhaps missing still present physiological signatures of REM sleep), and hence these medications require a more protracted time frame to achieve the same mechanistic impact.
Thus, at both stages of early memory processing – encoding and consolidation – the architectural sleep abnormalities expressed in major depression may facilitate an adverse prevalence and strengthening of prior negative episodic memories. Yet, there may be an additional consequence of sleep-dependent memory processing, beyond the strengthening of the experience itself, and one that has additional implications for mood disorders – that is, sleeping to forget
Emotional-memory processing: A sleep to forget and sleep to remember (SFSR) hypothesis
Founded on the emerging interaction between sleep and emotion, below we outline a model of affective information processing that may offer brain-based explanatory insights regarding the impact of sleep abnormalities, particularly REM sleep, for the initiation or maintenance of mood disturbance.
Although there is abundant evidence to suggest that emotional experiences persist in our autobiographies over time, an equally remarkable but far less noted change is a reduction in the affective tone associated with their recall. The reason that affective experiences appear to be encoded and consolidated more robustly than neutral memories is due to autonomic neurochemical reactions elicited at the time of the experience (McGaugh, 2004), creating what we commonly term an “emotional-memory”. However, the later recall of these memories tends not to be associated with anywhere near the same magnitude of autonomic (re)activation as that elicited at the moment of experience – suggesting that, overtime, the affective “blanket” previously enveloped around the memory during learning has been removed, whereas the information contained within that experience (the memory) remains.
For example, neuroimaging studies have shown that initial exposure and learning of emotional stimuli is associated with substantially greater activation in the amygdala and hippocampus, relative to neutral stimuli (Dolcos, et al., 2004; Dolcos, et al., 2005; Kilpatrick & Cahill, 2003). In one of these studies (Dolcos, et al., 2004), however, when participants were re-exposed to these same stimuli during recognition testing many months later, a change in the profile of activation occurred (Dolcos, et al., 2005). Although the same magnitude of differential activity between emotional and neutral items was observed in the hippocampus, this was not true in the amygdala. Instead, the difference in amygdala (re)activity to emotional items compared with neutral items had dissipated over time. This would support the idea that the strength of the memory (hippocampal-associated activity) remains at later recollection, yet the associated emotional reactivity to these items (amygdala activity) is reduced over time.
Our hypothesis predicts that this decoupling preferentially takes place overnight, such that we sleep to forget the emotional tone, yet sleep to remember the tagged memory of that episode (SFSR model; Figure 7). The model further argues that if this process is not achieved, the magnitude of affective “charge” remaining within autobiographical memory networks would persist, resulting in the potential condition of chronic anxiety.
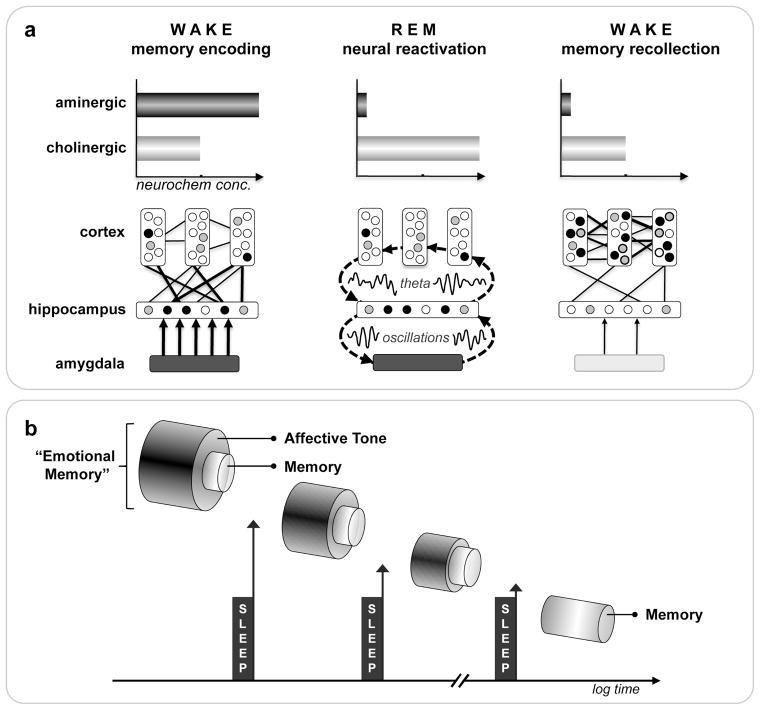
Figure 7.
The sleep to forget and sleep to remember (SFSR) model of emotional memory processing: a) Neural dynamics. Waking formation of an episodic emotional memory involves the coordinated encoding of hippocampal-bound information within cortical modules, facilitated by the amygdala, and modulated by high concentrations of aminergic neurochemistry. During subsequent REM sleep, these same neural structures are reactivated, the coordination of which is made possible by synchronous theta oscillations throughout these networks, supporting the ability to reprocess previously learned emotional experiences. However, this reactivation occurs in a neurochemical milieu devoid of aminergic modulation, and dominated by cholinergic neurochemistry. As a consequence, emotional memory reprocessing can achieve, on the one hand, a depotentiation of the affective tone initially associated with the event(s) at encoding, while on the other, a simultaneous and progressive neocortical consolidation of the information. The latter process of developing stronger cortico-cortical connections additionally supports integration into previous acquired autobiographical experiences, further aiding the assimilation of the affective event(s) in the context of past knowledge, the conscious expression of which may contribute to the experience of dreaming. Cross-connectivity between structures is represented by number and thickness of lines. Circles within cortical and hippocampal structures represent information nodes; shade reflects extent of connectivity: strong (filled), moderate (grey) and weak (clear). Color fill of amygdala and arrow thickness represents magnitude of co-activation with and influence on the hippocampus. b) Conceptual outcome. Through multiple iterations of this REM mechanism across the night, and/or across multiple nights, the long-term consequence of such sleep-dependent reprocessing would allow for the strengthening and retention of salient information previously tagged as emotional at the time of learning. However, recall no longer maintains an affective, aminergic charge, allowing for post-sleep recollection with minimal autonomic reactivity (unlike encoding), thereby preventing a state of chronic anxiety.
Based on the unique neurobiology of REM, here we propose a REM sleep hypothesis of emotional brain processing (Figure 7a). We suggest that the state of REM provides an optimal biological theater, within which, can be achieved a form of affective “therapy”. Specifically, increased activity within limbic and paralimbic structures (including the hippocampus and amygdala) during REM sleep may first offer the ability for reactivation of previously acquired affective experiences. Second, the neurophysiological signature of REM sleep involving dominant theta oscillations within subcortical as well as cortical nodes may offer large-scale network cooperation at night, allowing the integration and, as a consequence, greater understanding of recently experienced emotional events in the context of pre-existing neocortically stored semantic memory. Third, these interactions during REM sleep (and perhaps through the conscious process of dreaming) critically and perhaps most importantly take place within a brain that is devoid of aminergic neurochemical concentration (Pace-Schott & Hobson, 2002), particularly noradrenergic input from the locus coeruleus; the influence of which has been linked to states of high stress and anxiety disorders (Sullivan, Coplan, Kent, & Gorman, 1999).
In summary, the described neuroanatomical, neurophysiological and neurochemical conditions of REM sleep offer a unique biological milieu in which to achieve, on one hand, a balanced neural facilitation of the informational core of emotional experiences (the memory), yet may also depotentiate and ultimately ameliorate the autonomic arousing charge originally acquired at the time of learning (the emotion), negating a long-term state of anxiety (Figure 7).
The model complements previous psychological theories of dreaming by Greenberg (Greenberg, Pearlman, & Gampel, 1972; Greenberg, Pillard, & Pearlman, 1972) and also Cartwright (Cartwright, Agargun, Kirkby, & Friedman, 2006; R. Cartwright, Luten, Young, Mercer, & Bears, 1998; Cartwright, et al., 1991), which suggest that the process of REM-sleep mental activity aids in the resolution of previous emotional conflict, resulting in reduced next-day negative mood. Moreover, pioneering work by Cartwright et al. have demonstrated that not only the occurrence of dreaming, but the actual content of dreams, plays an important role in the recovery from emotional trauma, and can be predictive of clinical remission months later (Cartwright, et al., 2006; Cartwright, et al., 1998; Cartwright, et al., 1991). The current model offers a neurobiological framework for the overnight modulation and alteration of emotional memories and next-day affective brain reactivity, and does not discount the potential contribution that the mental operation of dreaming itself, beyond the physiological underpinnings of REM sleep, may afford to this process.
Emotional memory processing: Time (wake) vs. sleep
Although many studies have described an enhancement of emotional memory across time periods containing sleep (and even when comparing sleep and wake time periods directly), several reports have demonstrated the facilitation of emotional recollection across shorter intervals (up to several hours) that are unlikely to contain sleep (Dolcos, et al., 2004; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002; LaBar & Phelps, 1998). This may suggest that sleep represents a preferential, although not exclusive, time when emotional memories are consolidated, and that both time and sleep modulate affective experiences by way of similar underlying mechanisms. For example, theta electrical oscillations throughout subcortical and across cortical areas appear to play an important role in promoting the strengthening and consolidation of emotional memory. Although dominant during REM sleep, such oscillatory activity could occur during the wake state, driven by prior affective learning experience.
Alternatively, emotional memories may be modulated by two different mechanisms; one during wake and one during sleep, which at a behavioral level (memory recollection), may appear quantitatively similar, but at a mechanistic brain and autonomic-body level, are qualitatively different. The contrasting neurobiology of wake and sleep states, especially REM sleep, would support this latter hypothesis. For example, across time awake, emotional memories may be processed and modulated predominately by adrenergic mechanisms which are prolific during wakefulness (Saper, et al., 2001), enabling more shorter term memory benefits without the necessity of sleep. Therefore, suppression of aminergic systems following affective learning blocks these emotional memory improvements (Cahill & McGaugh, 1998; McGaugh, 2004). The second, REM sleep-dependent process, while also facilitating the consolidation of emotional experience, may take place by way of cholinergic modulation, in the absence of adrenergic influence. It is in this neurochemical distinction that the qualitative difference between wake and sleep mechanisms may emerge. Specifically, by being processed in a network that is now devoid of adrenergic neurochemistry, the visceral autonomic charge associated with the memory at the time of the emotional learning may be de-potentiated during the reactivation and reprocessing of information during REM sleep (Figure 7a). As a consequence, the memory representation is not only more robust, leading to enhanced recall, the strength of the bound autonomic charge will be reduced (Figure 7b).
Predictions of the model
If this process of divorcing emotion from memory is not achieved across the first night following such an experience, the model would predict that a repeat attempt of affective demodulation would occur on the second night, since the strength of the emotional “tag” associated with the memory would remain high. If this process failed a second time, the same events would continue to repeat across ensuing nights. It is just such a cycle of REM-sleep dreaming (nightmares) that represents a diagnostic key feature of post-traumatic stress disorder (Lavie, 2001). It may not be coincidental, therefore, that these patients continue to display hyperarousal reactions to associated trauma cues (Harvey, et al., 2003; Pole, 2007), indicating that the process of separating the affective tone from the emotional experience has not been accomplished. The reason why such a REM mechanism may fail in PTSD remains unknown, although the exceptional magnitude of trauma-induced emotion at the time of learning may be so great that the system is incapable of initiating/completing one or both of these processes, leaving some patients unable to integrate and depotentiate the stored experience. Alternatively, it may be the hyperarousal status of the brain during REM sleep in these patients (Harvey, et al., 2003; Pole, 2007; Strawn & Geracioti, 2008), potentially lacking sufficient aminergic demodulation, that prevents the processing and separation of emotion from memory.
This model also makes specific experimental predictions as to the fate of these two components – the memory and the emotion. As partially demonstrated, the first prediction would be that, overtime, the veracity of the memory itself would be maintained or improved, and the extent to which these [negative] emotional experiences are strengthened would be proportional to the amount of post-experience REM sleep obtained, as well as how quickly it is achieved (REM latency).
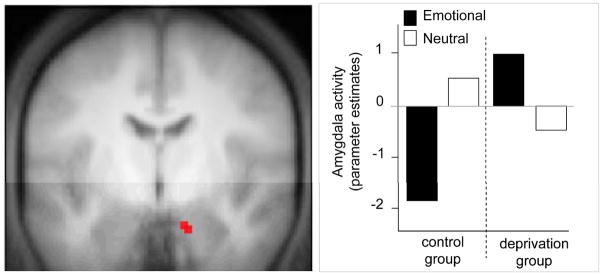
Second, using physiology measures, these same predictions would hold in the inverse direction for the magnitude of emotional reactivity induced at the time of recall. Together with the neuroimaging studies of emotional memory recall over time, and psychological studies investigating the role of REM sleep dreaming in mood regulation, a recent fMRI study offers perhaps the strongest preliminary support of this sleep-dependent model of emotional-memory processing (Sterpenich, et al., 2007). Relative to a control group that slept, participants who were deprived of sleep the first night after learning arousing emotion picture slides not only showed reduced recall of the information 72hr later (the sleep to remember component of the hypothesis), but also showed a lack of reduction in amygdala reactivity when re-exposed to these same negative emotional picture slides at recognition testing (Figure 8; the sleep to forget component of the hypothesis). Thus, sleep after learning facilitated improved recollection of these prior emotional experiences, yet this later recollection was conversely associated with a reduction in amygdala reactivity. In contrast, those who did not sleep the first night after the emotional learning session, despite obtaining two full recovery nights of sleep, exhibited no such depotentiation of subsequent amygdala reactivity.
Figure 8.
Impact of sleep deprivation on limbic brain activity during subsequent emotional memory retrieval. a) Higher degree of amygdala reactivity during delayed (72hr) recollection of previously learned negative emotion picture slides in participants that were sleep deprived the first night after learning, compared to a control group that slept the first night after learning, and b) The associated MR signal from the amygdala in both groups of subjects, demonstrating a significant reduction in limbic reactivity in those that slept the first night after learning, together with the magnitude of response from the same region to neutral stimulus recollection. Modified from (Sterpenich, et al., 2007).
There is, however, a related study that does not conform to these trends. Wagner and colleagues had participants subjectively rate and re-rate emotional picture slides after 3hr of early-night sleep, or 3hr of late-night sleep (Wagner, Fischer, & Born, 2002). Valence ratings of unpleasantness actually increased following sleep, specifically late night sleep (rich in REM), compared to new picture slides not seen before. This was also true in a group that was allowed to sleep the entire night. The difference between this finding and those describing a decrease in emotional reactivity remains unclear. It is of note that when compared to the same items before sleep (baseline ratings, instead of new items), no increase in valence rating was evident. This discrepancy may also be due to the dimension of valence not assessing autonomic emotional reactivity. In fact, subjects also rated these picture stimuli on the basis of arousal strength. There was no such amplification of arousal reactivity following sleep, demonstrating a numerical but non-significant decrease overtime, relative to baseline measures. Alternatively, it may be that the assessment of valence is associated with the veracity of the memory, which is strengthened overnight, thereby promoting the recall of perceived pleasantness (or unpleasantness). Arousal (the visceral, autonomic dimension of emotion), in contrast, appears to be reduced over time, despite the beneficial strengthening of memory recall.
The third tenet of the model predicts that a pathological increase in REM, as commonly occurs in depression (Armitage, 2007; Gottesmann & Gottesman, 2007; Tsuno, et al., 2005), may disproportionately amplify the strength of negative memories, so much so that, despite concomitant attempts at ameliorating the associated affective tone, would still create a perceived autobiographical history dominated by negative memory excess (which may also facilitate disadvantageous waking rumination). In contrast, the selective decrease of REM, as occurs with many anti-depressants, would predict a reduction of such negative memory consolidation and bias, although it may curtail the degree of affective decoupling that can occur. Long-term, the balanced extent of accumulated REM should therefore correlate not only with the persistence, in memory, of the emotional experience, it should also be associated with a decreased magnitude of autonomic response associated with recall – all of which are testable experimental questions.
Conclusion
When viewed as a whole, findings at the cellular, systems, cognitive and clinical level all point to a crucial role for sleep in the affective modulation of human brain function. Based on the remarkable neurobiology of sleep, and REM sleep in particular, a unique capacity for the overnight modulation of affective networks and previously encountered emotional experiences may be possible, redressing and maintaining the appropriate connectivity and hence next-day reactivity throughout limbic and associated autonomic systems. However, if the canonical architecture and amount of sleep is disrupted, as commonly occurs in mood disorders, particularly major depression and PTSD, this symbiotic alliance of sleep-dependent emotional brain processing may fail. The predicted consequences of this failure appear to support the development and/or maintenance of a number of clinical symptoms expressed in mood disorders, while the changes in sleep associated with common pharmacological treatments of these cohorts support a relief of these aberrant overnight processes, all of which lead to experimentally testable hypotheses which can serve to guide future research. Ultimately, the timeless wisdom of mothers alike may never have been more relevant: that is, when troubled “get to bed, you’ll feel better in the morning”
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AG31164 [M.P.W.]); and the University of California, Berkeley [M.P.W.]).
References
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4(3):291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Ambrosini MV, Mariucci G, Colarieti L, Bruschelli G, Carobi C, Giuditta A. The structure of sleep is related to the learning ability of rats. Eur J Neurosci. 1993;5(3):269–275. doi: 10.1111/j.1460-9568.1993.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Ambrosini MV, Sadile AG, Gironi Carnevale UA, Mattiaccio M, Giuditta A. The sequential hypothesis on sleep function. I. Evidence that the structure of sleep depends on the nature of the previous waking experience. Braz J Med Biol Res. 1988;21(1):141–145. doi: 10.1016/0031-9384(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol. 1995;73(1):20–38. doi: 10.1152/jn.1995.73.1.20. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Yamaguchi Y, Grabski W, Lacka D. Emotional memories are not all created equal: evidence for selective memory enhancement. Learn Mem. 2006;13(6):711–718. doi: 10.1101/lm.388906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007;(433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-IV) 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res. 2008;17(3):285–294. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu I, Godbout R. Spatial learning on the Morris Water Maze Test after a short-term paradoxical sleep deprivation in the rat. Brain Cogn. 2000;43(1–3):27–31. [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- Berger M, Doerr P, Lund R, Bronisch T, von Zerssen D. Neuroendocrinological and neurophysiological studies in major depressive disorders: are there biological markers for the endogenous subtype? Biol Psychiatry. 1982;17(11):1217–1242. [PubMed] [Google Scholar]
- Bevilaqua L, Ardenghi P, Schroder N, Bromberg E, Schmitz PK, Schaeffer E, et al. Drugs acting upon the cyclic adenosine monophosphate/protein kinase A signalling pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav Pharmacol. 1997;8(4):331–338. doi: 10.1097/00008877-199708000-00006. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6(Suppl 1A):S16–28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59(2):47–51. quiz 52. [PubMed] [Google Scholar]
- Buysse DJ, Frank E, Lowe KK, Cherry CR, Kupfer DJ. Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression. Biol Psychiatry. 1997;41(4):406–418. doi: 10.1016/S0006-3223(96)00041-8. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cahill L. Neurobiological mechanisms of emotionally influenced, long-term memory. Prog Brain Res. 2000;126:29–37. doi: 10.1016/S0079-6123(00)26004-4. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79(2):194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003;10(4):270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci U S A. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21(7):294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371(6499):702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Lydic R, Baghdoyan HA, Hobson JA. Pontogeniculooccipital waves: Spontaneous visual system activity during rapid eye movement sleep. Cellular & Molecular Neurobiology. 1987;7(2):105–149. doi: 10.1007/BF00711551. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20(19):RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright R, Agargun MY, Kirkby J, Friedman JK. Relation of dreams to waking concerns. Psychiatry Res. 2006;141(3):261–270. doi: 10.1016/j.psychres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Cartwright R, Luten A, Young M, Mercer P, Bears M. Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Res. 1998;81(1):1–8. doi: 10.1016/s0165-1781(98)00089-4. [DOI] [PubMed] [Google Scholar]
- Cartwright RD, Kravitz HM, Eastman CI, Wood E. REM latency and the recovery from depression: getting over divorce. Am J Psychiatry. 1991;148(11):1530–1535. doi: 10.1176/ajp.148.11.1530. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21(4):417–423. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, et al. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146(1):43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA, Anderson SJ, Larsen SF, Donnelly CM, McDaniel MA, McClelland AG, et al. The formation of flashbulb memories. Mem Cognit. 1994;22(3):326–343. doi: 10.3758/bf03200860. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973(2):293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the Amygdala and the Medial Temporal Lobe Memory System Predicts Better Memory for Emotional Events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102(7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W, Kastaniotis C, Chattman D. Paradoxical sleep: prolonged augmentation following learning. Brain Res. 1974;79(1):61–75. doi: 10.1016/0006-8993(74)90566-6. [DOI] [PubMed] [Google Scholar]
- Fosse MJ, Fosse R, Hobson JA, Stickgold RJ. Dreaming and episodic memory: a functional dissociation? J Cogn Neurosci. 2003;15(1):1–9. doi: 10.1162/089892903321107774. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361–377. [PubMed] [Google Scholar]
- Giles DE, Jarrett RB, Roffwarg HP, Rush AJ. Reduced rapid eye movement latency. A predictor of recurrence in depression. Neuropsychopharmacology. 1987;1(1):33–39. doi: 10.1016/0893-133x(87)90007-8. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Duncan W, Pettigrew KD, Frankel BL, Snyder F. Successful separation of depressed, normal, and insomniac subjects by EEG sleep data. Arch Gen Psychiatry. 1979;36(1):85–90. doi: 10.1001/archpsyc.1979.01780010091010. [DOI] [PubMed] [Google Scholar]
- Gottesmann C, Gottesman I. The neurobiological characteristics of rapid eye movement (REM) sleep are candidate endophenotypes of depression, schizophrenia, mental retardation and dementia. Prog Neurobiol. 2007;81(4):237–250. doi: 10.1016/j.pneurobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10(3):168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R, Pearlman CA, Gampel D. War neuroses and the adaptive function of REM sleep. Br J Med Psychol. 1972;45(1):27–33. doi: 10.1111/j.2044-8341.1972.tb01416.x. [DOI] [PubMed] [Google Scholar]
- Greenberg R, Pillard R, Pearlman C. The effect of dream (stage REM) deprivation on adaptation to stress. Psychosom Med. 1972;34(3):257–262. doi: 10.1097/00006842-197205000-00007. [DOI] [PubMed] [Google Scholar]
- Gruart-Masso A, Nadal-Alemany R, Coll-Andreu M, Portell-Cortes I, Marti-Nicolovius M. Effects of pretraining paradoxical sleep deprivation upon two-way active avoidance. Behav Brain Res. 1995;72(1–2):181–183. doi: 10.1016/0166-4328(96)00082-4. [DOI] [PubMed] [Google Scholar]
- Grunhaus L, Shipley JE, Eiser A, Pande AC, Tandon R, Remen A, et al. Shortened REM latency PostECT is associated with rapid recurrence of depressive symptomatology. Biol Psychiatry. 1994;36(4):214–222. doi: 10.1016/0006-3223(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Sleep loss and temporal memory. Q J Exp Psychol. 2000;53(1):271–279. doi: 10.1080/713755870. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Insomnia: symptom or diagnosis? Clin Psychol Rev. 2001;21(7):1037–1059. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23(3):377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Hars B. Is increase in post-learning paradoxical sleep modified by cueing? Behav Brain Res. 1987;24(3):243–249. doi: 10.1016/0166-4328(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: Reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23(6):793–842. doi: 10.1017/s0140525x00003976. discussion 904–1121. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM sleep dreaming. Neuroreport. 1998;9(3):R1–14. doi: 10.1097/00001756-199802160-00033. [DOI] [PubMed] [Google Scholar]
- Horne JA. Sleep function, with particular reference to sleep deprivation. Ann Clin Res. 1985;17(5):199–208. [PubMed] [Google Scholar]
- Hu P, Stylos-Allen M, Walker MP. Sleep facilitates consolidation of emotionally arousing declarative memory. Psychol Sci. 2006;17(10):891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Dalmaz C, McGaugh JL. Amygdala beta-noradrenergic influences on memory storage involve cholinergic activation. Neurobiol Learn Mem. 1996;65(1):57–64. doi: 10.1006/nlme.1996.0006. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem. 1998;69(3):219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Wang XM, Li BM. Deficit in long-term contextual fear memory induced by blockade of beta-adrenoceptors in hippocampal CA1 region. Eur J Neurosci. 2003;17(9):1947–1952. doi: 10.1046/j.1460-9568.2003.02620.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: the contribution of valence and arousal. Rev Neurosci. 2004;15(4):241–251. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2(2):118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: are emotional words more vividly remembered than neutral words? Mem Cognit. 2003;31(8):1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci U S A. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20(4):2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. J Exp Psychol. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Thakur M, Roth T. Sleep disturbance in psychiatric disorders: effects on function and quality of life in mood disorders, alcoholism, and schizophrenia. Ann Clin Psychiatry. 2008;20(1):39–46. doi: 10.1080/10401230701844661. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47(12):1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Ulrich RF, Coble PA, Jarrett DB, Grochocinski VJ, Doman J, et al. Electroencephalographic sleep of younger depressives. Comparison with normals. Arch Gen Psychiatry. 1985;42(8):806–810. doi: 10.1001/archpsyc.1985.01790310068009. [DOI] [PubMed] [Google Scholar]
- Labar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- Levin R, Nielsen T. Nightmares, bad dreams, and emotion dysregulation: A review and new neurocognitive model of dreaming. Curr Dir Psychol Sci. 2009;18(2):84–88. [Google Scholar]
- Levonian E. Retention over time in relation to arousal during learning: an explanation of discrepant results. Acta Psychol (Amst) 1972;36(4):290–321. doi: 10.1016/0001-6918(72)90013-3. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 2005;25(4):1214–1223. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90(5):2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Handbook Of Behavioral State Control: Cellular And Molecular Mechanisms. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- Mandai O, Guerrien A, Sockeel P, Dujardin K, Leconte P. REM sleep modifications following a Morse code learning session in humans. Physiol Behav. 1989;46(4):759–762. doi: 10.1016/0031-9384(89)90344-2. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J-M, Aerts J, Delfiore G, Degueldre C, Luxen A, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11(10):442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Marti-Nicolovius M, Portell-Cortes I, Morgado-Bernal I. Improvement of shuttle-box avoidance following post-training treatment in paradoxical sleep deprivation platforms in rats. Physiol Behav. 1988;43(1):93–98. doi: 10.1016/0031-9384(88)90103-5. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23(29):9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGrath MJ, Cohen DB. REM sleep facilitation of adaptive waking behavior: a review of the literature. Psychol Bull. 1978;85(1):24–57. [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Hipolito MM. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 2006;11(8):611–615. doi: 10.1017/s1092852900013663. [DOI] [PubMed] [Google Scholar]
- Morris GO, Williams HL, Lubin A. Misperception and disorientation during sleep. Arch Gen Psychiatry. 1960;2:247–254. [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32(7):1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. Prefrontal theta during REM sleep enhances emotional memory. Cereb Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA. Functional neuroimaging of sleep. Semin Neurol. 2005;25(1):9–18. doi: 10.1055/s-2005-867070. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Price JC, Meltzer CC, Buysse DJ, Villemagne VL, Miewald JM, et al. Towards a neurobiology of dysfunctional arousal in depression: the relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry. 2000;98(2):71–91. doi: 10.1016/s0925-4927(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Schwartz RM, Reynolds CF, 3rd, Thase ME, Jennings JR, Frank E, et al. Affect intensity and phasic REM sleep in depressed men before and after treatment with cognitive-behavioral therapy. J Consult Clin Psychol. 1994;62(1):83–91. doi: 10.1037//0022-006x.62.1.83. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. J Exp Psychol Gen. 2000;129(2):242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Insomnia: a ticking clock for depression? J Psychiatr Res. 2007;41(11):893–894. doi: 10.1016/j.jpsychires.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Oniani TN, Lortkipanidze ND, Maisuradze LM. Interaction between learning and paradoxical sleep in cats. Neurosci Behav Physiol. 1987;17(4):304–310. doi: 10.1007/BF01183059. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3(8):591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6(7):306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Passani MB, Cangioli I, Baldi E, Bucherelli C, Mannaioni PF, Blandina P. Histamine H3 receptor-mediated impairment of contextual fear conditioning and in-vivo inhibition of cholinergic transmission in the rat basolateral amygdala. Eur J Neurosci. 2001;14(9):1522–1532. doi: 10.1046/j.0953-816x.2001.01780.x. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19(8):781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman CA. Effect of rapid eye movement (dreaming) sleep deprivation on retention of avoidance learning in rats. Rep No 563. Rep US Nav Submar Med Cent. 1969:1–4. [PubMed] [Google Scholar]
- Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133(5):725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Power AE. Muscarinic cholinergic contribution to memory consolidation: with attention to involvement of the basolateral amygdala. Curr Med Chem. 2004;11(8):987–996. doi: 10.2174/0929867043455558. [DOI] [PubMed] [Google Scholar]
- Power AE, McGaugh JL. Cholinergic activation of the basolateral amygdala regulates unlearned freezing behavior in rats. Behav Brain Res. 2002;134(1–2):307–315. doi: 10.1016/s0166-4328(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, Maryland, USA: U.S. Department of Health; 1968. [DOI] [PubMed] [Google Scholar]
- Reiser MF. The dream in contemporary psychiatry. Am J Psychiatry. 2001;158(3):351–359. doi: 10.1176/appi.ajp.158.3.351. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: an event-related FMRI investigation. Cereb Cortex. 2008;18(11):2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Jarrett RB, Feldman-Koffler F, Debus JR, Weissenburger J, et al. Reduced REM latency predicts response to tricyclic medication in depressed outpatients. Biol Psychiatry. 1989;26(1):61–72. doi: 10.1016/0006-3223(89)90008-5. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Silvestri AJ, Ross RJ, Morrison AR. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139(3):169–183. [PubMed] [Google Scholar]
- Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33(1):43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Posttraining intra-basolateral amygdala scopolamine impairs food- and amphetamine-induced conditioned place preferences. Behav Neurosci. 2002;116(5):922–927. doi: 10.1037//0735-7044.116.5.922. [DOI] [PubMed] [Google Scholar]
- Shaffery J, Hoffmann R, Armitage R. The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist. 2003;9(1):82–98. doi: 10.1177/1073858402239594. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: disentangling the effects of attention and retention. Cogn Affect Behav Neurosci. 2004;4(3):294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Shima K, Nakahama H, Yamamoto M. Firing properties of two types of nucleus raphe dorsalis neurons during the sleep-waking cycle and their responses to sensory stimuli. Brain Res. 1986;399(2):317–326. doi: 10.1016/0006-8993(86)91522-2. [DOI] [PubMed] [Google Scholar]
- Shiromani P, Gutwein BM, Fishbein W. Development of learning and memory in mice after brief paradoxical sleep deprivation. Physiol Behav. 1979;22(5):971–978. doi: 10.1016/0031-9384(79)90343-3. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biol Psychiatry. 2001;49(7):624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Smith C. Sleep states and learning: a review of the animal literature. Neurosci Biobehav Rev. 1985;9(2):157–168. doi: 10.1016/0149-7634(85)90042-9. [DOI] [PubMed] [Google Scholar]
- Smith C, Kelly G. Paradoxical sleep deprivation applied two days after end of training retards learning. Physiol Behav. 1988;43(2):213–216. doi: 10.1016/0031-9384(88)90240-5. [DOI] [PubMed] [Google Scholar]
- Smith C, Young J, Young W. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep. 1980;3(1):67–81. [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11(5):525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res Online. 1998;1(1):1–10. [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. Journal of Neuroscience. 1996;16(1):392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5(11):e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proc Natl Acad Sci U S A. 2003;100(23):13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46(9):1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus & Related Systems. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Vazquez J, Baghdoyan HA. Basal forebrain acetylcholine release during REM sleep is significantly greater than during waking. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R598–601. doi: 10.1152/ajpregu.2001.280.2.R598. [DOI] [PubMed] [Google Scholar]
- Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002;64(4):627–634. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional Memory Formation Is Enhanced across Sleep Intervals with High Amounts of Rapid Eye Movement Sleep. Learn Mem. 2001;8(2):112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60(7):788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Wagner U, Kashyap N, Diekelmann S, Born J. The impact of post-learning sleep vs. wakefulness on recognition memory for faces with different facial expressions. Neurobiol Learn Mem. 2007;87(4):679–687. doi: 10.1016/j.nlm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Walker EL, Tarte RD. Memory storage as a function of arousal and time with homogeneous and heterogeneous lists. Journal of Verbal Learning and Verbal Behavior. 1963;2:113–119. [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, Memory and Plasticity. Annu Rev Psychol. 2006;10(57):139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Waller DA, Hardy BW, Pole R, Giles D, Gullion CM, Rush AJ, et al. Sleep EEG in bulimic, depressed, and normal subjects. Biol Psychiatry. 1989;25(5):661–664. doi: 10.1016/0006-3223(89)90233-3. [DOI] [PubMed] [Google Scholar]
- Winokur A, Gary KA, Rodner S, Rae-Red C, Fernando AT, Szuba MP. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14(1):19–28. doi: 10.1002/da.1043. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46(4):445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep - a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10(3):385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28(1):47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]