Abstract
OBJECTIVE
To evaluate the efficacy of a removable cast walker compared with that of a nonremovable fiberglass off-bearing cast in the treatment of diabetic plantar foot ulcer.
RESEARCH DESIGN AND METHODS
Forty-five adult diabetic patients with nonischemic, noninfected neuropathic plantar ulcer were randomly assigned for treatment with a nonremovable fiberglass off-bearing cast (total contact cast [TCC] group) or walker cast (Stabil-D group). Treatment duration was 90 days. Percent reduction in ulcer surface area and total healing rates were evaluated after treatment.
RESULTS
A total of 48 patients were screened; however, 2 patients in the TCC group and 1 patient in the Stabil-D group did not complete the study and were considered dropouts. There were no significant differences in demographic and clinic characteristics of the 45 patients completing the study. Ulcer surface decreased from 1.41 to 0.21 cm2 (P < 0.001) in the TCC group and from 2.18 to 0.45 cm2 (P < 0.001) in the Stabil-D group, with no significant differences between groups (P = 0.722). Seventeen patients (73.9%) in the TCC group and 16 patients (72.7%) in the Stabil-D group achieved healing (P = 0.794). Average healing time was 35.3 ± 3.1 and 39.7 ± 4.2 days in the TCC and Stabil-D group, respectively (P = 0.708).
CONCLUSIONS
The Stabil-D cast walker, although removable, was equivalent in efficacy to the TCC in terms of ulcer size reduction and total healing rate. The easier use of Stabil-D may help increase the use of off-loading devices in the management of plantar neuropathic diabetic foot ulcers.
The importance of excessive pressure on the sole of the foot in the pathogenesis of neuropathic plantar ulcers is well established (1). It is also known that complete relief of pressure from the ulcerated area is key to effective healing (2). Use of a total contact cast (TCC) is considered the gold standard for management of neuropathic plantar ulcers (3). Nevertheless, management of patients with a TCC poses several problems (4). Proper TCC application with avoidance of iatrogenic lesions requires skilled cast technicians and is an expensive and time-consuming process (5). The use of a TCC is absolutely contraindicated in patients with infection or critical ischemia. A TCC is also contraindicated in patients who are very elderly, have visual or equilibrium problems, have a contralateral foot ulcer, or have varicose veins. For these reasons, TCCs are rarely used (6).
In an attempt to overcome these problems, recent studies have evaluated the efficacy of commercially available nonremovable cast walkers (7–10). The aim of this study was to evaluate healing outcomes in diabetic patients managed with a nonremovable TCC and a new removable off-loading device specifically designed for the management of neuropathic plantar ulcers.
RESEARCH DESIGN AND METHODS
Two centers specializing in diabetic foot management (located in Sesto San Giovanni and Milan, Italy) participated in this open, randomized clinical trial. The ethics committee approved the study on 10 January 2008. Consecutive patients were enrolled from February 2008 through March 2009. Study inclusion criteria were the presence of a neuropathic plantar forefoot ulcer with an area graded IA according to the University of Texas Classification of Diabetic Wounds (11). Peripheral neuropathy was diagnosed based on insensitivity to a 10-g Semmes-Weinstein monofilament in more than six of nine areas of the foot and by a vibration perception threshold measured by biothesiometer (Neurothesiometer SLS, Nottingham, U.K.) at the malleolus of >25 V. Exclusion criteria were the presence of an ankle-brachial pressure index <0.9 and/or transcutaneous oxygen tension <50 mmHg tested on the dorsum of the foot and clinical signs of infection. Both the probe-to-bone maneuver and standard X-ray examination of the foot were required to be negative for osteomyelitis (12). Additional exclusion criteria included use of steroids or antimitotic drugs, the presence of visual problems that could impair balance, an active ulcer on the contralateral foot, previous major amputation of the contralateral limb, previous or current deep venous thrombosis of the leg, or mental disorder interfering with patient compliance.
Eligible patients were fully informed of the study aim and procedures, and written consent was obtained before study participation. Patients were randomly assigned to one of the two treatment groups by opening randomization codebreak envelopes containing one of the two options. Separate randomization was performed for each center, and a copy of all randomization envelopes was kept at the statistical department of the Multimedica center. The two arms were composed of patients managed with a nonremovable fiberglass off-bearing cast (TCC group) and patients managed with the Stabil-D (Podartis, Montebelluna, Treviso, Italy) walker cast (Stabil-D group).
TCC
Patients in the TCC group were casted according to the technique described previously by Caravaggi et al. (13). All casts were made by personnel with particular expertise in the use of this device (W.V. in Sesto San Giovanni and D.S. in Milan). Two types of fiberglass bandages were used for construction of the pressure-relief apparatus. The first type of bandage (Softcast3M; 3M Health Care, St. Paul, MN) was composed of fiberglass imbued with a polyurethane resin with characteristics of flexibility and resistance. The other bandage (Scotchcast3M; 3M Health Care) was composed of fiberglass imbued with a polyurethane resin of two different concentrations that confers high resistance to loading. A bandage with German cotton and tubular stockinet was placed on the limb. To further protect bony protrusions, such as the malleolus and tibial crista, pieces of protective rubber foam (Microfoam 3M; 3M Health Care) were also applied. The structure was then reinforced with a stick made of a Scotchcast bandage placed in the middle of the two malleoli, extending beyond them for at least 20 cm to give rigidity to the cast. The same material was used to build a rigid plantar sole. The number of layers applied to construct the sole depended on the weight of the patient (range 3–8 layers). An aluminum stirrup was anchored to the structure as a support to allow walking. The side supports were secured with an outer layer of Softcast3M. After very brief training, all patients were able to walk properly without crutches.
Stabil-D
The Stabil-D device is composed of a specifically designed rigid, boat-shaped, and fully rocker bottom sole: its rounded extremities (at the heel and tiptoe) facilitate gait, and its middle section improves the mid-stance phase. The insole height (24 mm) avoids excessive lifting of the contralateral limb during walk, thus lowering the barycenter and favoring more stable walking. The cover is made of Elastam (Lycra), a yarn composed of polyurethane segments and block copolymers that confer high transparency and stability to the system, mixed with polyethylene glycol segments with the characteristic of elasticity. At the ankle, the cast is provided with removable, lateral stabilizer inserts made of ABS, which ensure stability to the tibiotarsal joint and/or adequate support during gait. Moreover, a rigid brace made of a thermoformable polymer material properly supports the Achilles tendon and contributes to stability during rolling steps; such a brace can be adapted to the foot deformity using a hot air gun and malleolar forceps. The cast is closed dorsally with Velcro wrap placed over the forefoot to relieve skin pressure and Velcro straps with self-fitting rings placed against the instep to secure perfect fastening, provide foot stability, and ensure a perfect fit of the heel in the rigid brace. Finally, more Velcro straps are placed or secured with rings against the tibia to provide a secure fit.
The cast has a special foot arch support (Modus) with small adaptable inserts. This modular insole is made of multiple layers of different stiffness and is specifically designed to allow proper off-loading by removing the small inserts from the ulcerated area, without the need for traditional milling procedures. The bottom layer is composed of chemically knitted closed cell polyphenylic foam (Evaform 167). The middle layer is composed of knitted, expandable, and moldable closed cell polystyrene foam (plastazole). The Diapod cover, specifically designed for feet at risk of ulcer formation, is composed of chemically knitted dermocompatible Eva Diflex Vibram (closed cell polyphenylic foam; tested by ABICH Laboratories, Verbania, Italy), which also has bactericidal and fungicidal properties (tested by Fresenius Institute, Taunusstein, Germany). Figure 1 shows the Stabil-D device and the Modus insole. Patients randomly assigned to the Stabil-D group were carefully trained for proper cast wearing, in particular for accurate closure of Velcro straps, and were prescribed continuous cast wear of the Stabil-D; patients were allowed to remove the cast only during nocturnal rest.
Figure 1.
“Stabil-D” device and the “Modus” insole. (A high-quality digital representation of this figure is available in the online issue.)
Initial visit
At the initial visit, ulcers were debrided to remove all nonviable tissue and to expose the entire surface lesion. The ulcers were then photographed and measured using a Visitrak system (Smith & Nephew, Hull, U.K.). Ulcers were dressed with paraffin gauze and covered with sterile gauze before the application of the off-loading device.
Follow-up
Patients were followed weekly for 90 days after application of the TCC or Stabil-D. At each follow-up visit, off-loading devices were removed, and dressings were changed. The ulcer was photographed and measured with the Visitrak system. Afterward, patients in the TCC group were provided with a newly manufactured cast; patients in the Stabil-D group had their cast walker and arch support carefully controlled before reapplication of the off-loading device.
End point
The primary end point was decrease in ulcer size. The secondary end point was rate of complete healing at study completion. Ulcers were considered healed if they showed complete reepithelization of the ulcerated area.
Statistical analysis
Homogeneity of the initial distribution of baseline primary variables between groups was tested using a Fisher exact test for dichotomous variables and Student t test for continuous variables. The differences in ulcer size reduction between the two groups were compared using the Mann-Whitney test. The Wilcoxon test was used for analysis of ulcer size reduction over time within groups. Healing rate over time was analyzed by the Kaplan-Meier test, and the log-rank test was used to detect differences between the two groups.
RESULTS
A total of 48 patients were enrolled. Two patients in the TCC group and one patient in the Stabil-D group did not complete the study and were considered dropouts. Of these three dropout patients, one patient in the TCC group withdrew consent, one patient in the TCC group stopped treatment because of the development of an ulcer on the contralateral foot, and one patient in the Stabil-D group was unable to complete the off-loading treatment because of ulcer infection requiring antibiotic therapy and more frequent clinic visits. Of the remaining 45 patients who completed the study, there were 23 patients in the TCC group and 22 patients in the Stabil-D group. Table 1 reports demographic and clinical characteristics of participants.
Table 1.
Patient characteristics
| TCC group | Stabil-D group | P value | |
|---|---|---|---|
| n | 23 | 22 | |
| Age (years) | 59.0 ± 8.5 | 61.7 ± 10.4 | 0.35 |
| Sex: female/male | 8 (34.8)/15 (65.2) | 7 (31.8)/15 (68.2) | 0.83 |
| Diet/insulin/oral therapy | 4 (17.4)/16 (69.6)/3 (13.0) | 5 (22.7)/10 (45.5)/7 (31.8) | 0.21 |
| Duration of diabetes (years) | 17.7 ± 11.2 | 17.2 ± 10.7 | 0.88 |
| BMI (kg/m2) | 32.3 ± 4.5 | 30.3 ± 1.1 | 0.16 |
| A1C (% Hb) | 9.1 ± 2.1 | 7.5 ± 1.1 | 0.18 |
| Previous foot ulcer | 15 (65.2) | 15 (68.2) | 0.82 |
| Previous minor amputation | 11 (47.8) | 12 (54.5) | 0.65 |
| Mean area of lesion (cm2) | 1.4 ± 1.2 | 2.2 ± 2.2 | 0.47 |
Data are means ± 1 SD or n (%).
During the study some minor treatment complications occurred, none of which required cessation or change in treatment. In the TCC group one patient developed partial rupture of the stirrup, which was replaced without removing the cast. One patient showed hitching, which resolved after removal of the German cotton. One patient in the Stabil-D group complained of odor and perilesional skin maceration; however, these were resolved at subsequent follow-up visits.
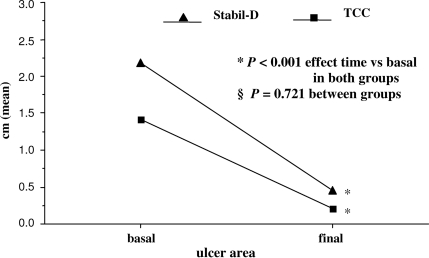
Ulcer surface area decreased from 1.41 to 0.21 cm2 (P < 0.001) in the TCC group and from 2.18 to 0.45 cm2 (P < 0.001) in the Stabil-D group; there was no significant difference between groups (P = 0.72, Mann-Whitney test). The percent reductions were 73.6 and 90.0% in the TCC and Stabil-D groups, respectively, with no statistically significant difference between groups (P = 0.321). The time course of reduction in ulcer area did not significantly differ between the two groups, as shown in Fig. 2 (P < 0.721, Wilcoxon test).
Figure 2.
Ulcer size reduction at study completion. *Wilcoxon test. §Mann-Whitney test.
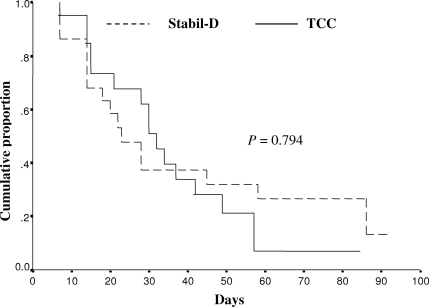
Seventeen patients (73.9%) in the TCC group and 16 patients (72.7%) in the Stabil-D group achieved complete healing. Figure 3 shows the Kaplan-Meier estimate of complete healing rates at the end of the study (P = 0.794). The mean duration of healing time was 35.3 ± 3.1 day in the TCC group and 39.7 ± 4.2 days in the Stabil-D group (P = 0.708).
Figure 3.
Kaplan-Meier estimate of complete healing rate at study completion.
The average manufacturing time for the TCC was 25 min, and the time needed for cast removal using an oscillating cast saw was 10 min. A very short period of time was required to remove and reposition the Stabil-D device.
The cost of the Stabil-D device was €130 each plus €20 for the Modus plantar sole. The cost of the TCC was €73.50 per cast (€22 for the stockinet, €4 for the Microfoam, and € 47.5 for the bandages). For a very obese patient an extra bandage was required, increasing the cost to €89.5. Twenty-two off-loading devices were applied to patients in the Stabil-D group, and total costs were €3,300.00. A total of 91 casts were applied to patients in the TCC group for a total cost of €6,688.50.
CONCLUSIONS
Among available methods to relieve plantar ulcers resulting from overpressure, the use of off-loading casts, which can be fabricated in different manners and with different materials, is considered the gold standard (14,15). However, it is well known that TCCs are not widely used and that wheelchairs, crutches, or therapeutic shoes with unloaded insoles are more commonly prescribed in the management of patients with plantar ulcers (16). What reasons underlie these therapeutic choices? Cutaneous ulcers caused by friction of the cast on bony protrusions are the most frequent side effects from the use of rigid casts (17). This problem could be solved using materials whose rigidity can be modulated; however, the whole procedure for preparing such a type of TCC requires significant expertise and still remains a costly and time-consuming process. Another important issue is the high percentage of patients, such as those with vascular disease, bilateral ulcers, or lower limb amputation, who cannot tolerate TCCs (18).
The results of our study indicate that the use of Stabil-D is as effective as use of a TCC in the treatment of neuropathic plantar forefoot ulcers. Similar results were obtained in previous studies reported by Piaggesi et al. (19) and Caravaggi et al. (20); however, these two reports differ from our study in two important ways.
First, Piaggesi et al. used a novel, off-the-shelf nonremovable device, and we used a removable cast. The efficacy of nonremovable off-loading devices has been emphasized in previous studies. Armstrong et al. (21) reported significant differences in the healing rates obtained using removable or nonremovable cast walkers. Katz et al. (22) found comparable efficacy of different types of equally nonremovable cast walkers (22). Why we obtained different results is uncertain. In the literature, the superiority of nonremovable casts over removable ones is due to poor patient compliance in the proper use of an off-loading device (23,24). In the two above-mentioned studies, the percentage of patients with previous ulcers was not reported, suggesting that all patients enrolled presented with their first episode of ulcer. In our study population, a high percentage of patients reported a previous foot ulcer, and a high percentage of patients had previously undergone minor amputation. It can be reasonably supposed that patients with a history of ulcer may be more aware of the serious consequences of plantar ulcers, and therefore their compliance might be higher than that of patients with a first ulcer episode. One could argue that those patients with recurring ulcers were inherently less compliant because they did, indeed, have a recurrent ulcer. However, only data on reulceration outcomes during long-term follow-up would allow us to draw a reasonable conclusion.
In the study of Ha Van et al. (25), the presence of a persistent ulcer prompted clinicians to provide patients with a TCC rather than an orthopedic cast walker boot, and patients treated with a TCC achieved better outcomes. It could be hypothesized that the “persistent ulcer” in the study of Ha Van et al. might have played a role similar to that of the “previous ulcer” in our study. However, this study, although commonly quoted to support the importance of patient compliance, was not a randomized clinical trial. In fact, compliance was taken into account in choosing the most adequate off-loading device for each patient. Therefore, compliance played a critical role in treatment allocation.
The effectiveness of the Stabil-D action in promoting ulcer healing is related to the ability of the rigid, boat-shaped, and fully rocker bottom sole to redistribute most of the pressure from the metatarsal heads to the back foot. Therefore, the effectiveness of the Stabil-D relates to pressure redistribution by diverting most of the pressure from the sole of the foot to the leg muscles. This is a mechanism different from that of the TCC, which produces a reduction of mechanical loading.
As in our study, the study reported by Caravaggi et al. (21) indicated that cast removability per se does not influence ulcer healing. In contrast to the results of that study, we did not find a difference in healing time between groups. We speculate that this may be due to differences in device structure.
Distribution of variables did not significantly vary between the two groups, as one would hope to see in a randomized study. However, better A1C levels, although not statistically significant, were observed in the Stabil D group. Could improved glucose control have positively influenced patient compliance in general and, perhaps, the rate of ulcer healing in patients wearing the removable device? Only further studies in larger populations might resolve this issue.
The results of our study indicate that pressure off-loading using the Stabil-D and pressure off-loading using total contact casting are equally effective in the treatment of neuropathic forefoot plantar ulcers, thus proving that optimal results may be obtained with a removable cast walker. Moreover, considering that the Stabil-D device is less bulky than a TCC and therefore may cause fewer sleep problems, we believe that these results are also important in terms of patient quality of life. Above all, our results suggest that more effective options may be available in the management of neuropathic forefoot plantar ulcers, particularly in centers that do not have the technology and/or investments available to provide TCCs.
Acknowledgments
We acknowledge the contribution of Podartis, Montebelluna, Treviso, Italy, manufacturers of the Stabil-D walkers used in this study.
No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT01005264, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Boulton AJ: The diabetic foot: from art to science: the 18th Camillo Golgi Lecture. Diabetologia 2004; 47: 1343–1353 [DOI] [PubMed] [Google Scholar]
- 2. Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A: Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care 1998; 21: 1714–1719 [DOI] [PubMed] [Google Scholar]
- 3. Rathur HM, Boulton AJ: Pathogenesis of foot ulcers and the need for offloading. Horm Metab Res 2005; 37(Suppl 1): 61–68 [DOI] [PubMed] [Google Scholar]
- 4. Boulton AJ, Bowker JH, Gadia M, Lemerman R, Caswell K, Skyler JS, Sosenko JM: Use of plaster casts in the management of diabetic neuropathic foot ulcers. Diabetes Care 1986; 9: 149–152 [DOI] [PubMed] [Google Scholar]
- 5. Brem H, Sheehan P, Boulton AJ: Protocol for treatment of diabetic foot ulcers. Am J Surg 2004; 187: S1–S10 [DOI] [PubMed] [Google Scholar]
- 6. Nabuurs-Franssen MH, Sleegers R, Huijberts MS, Wijnen W, Sanders AP, Walenkamp G, Schaper NC: Total contact casting of the diabetic foot in daily practice: a prospective follow-up study. Diabetes Care 2005; 28: 243–247 [DOI] [PubMed] [Google Scholar]
- 7. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB: Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care 2001; 24: 1019–1022 [DOI] [PubMed] [Google Scholar]
- 8. Cavanagh PR, Lipsky BA, Bradbury AW, Botek G: Treatment for diabetic foot ulcers: review. Lancet 2005; 336: 1725–1735 [DOI] [PubMed] [Google Scholar]
- 9. Lavery LA, Vela SA, Lavery DC, Quebedeaux TL: Reducing dynamic foot pressures in high-risk diabetic subjects with foot ulcerations. A comparison of treatments. Diabetes Care 1996; 19: 818–821 [DOI] [PubMed] [Google Scholar]
- 10. Armstrong DG, Short B, Espensen EH, Abu-Rumman PL, Nixon BP, Boulton AJM: Technique for fabrication of an “instant total contact cast” for treatment of neuropathic diabetic foot ulcers. J Am Podistr Assoc 2002; 92: 405–408 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong DG, Lavery LA, Harkless LB: Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1999; 21: 855–859 [DOI] [PubMed] [Google Scholar]
- 12. Dinh MT, Abad CL, Safdar N: Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis 2008; 47: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caravaggi C, Faglia E, De Giglio R, Mantero M, Quarantiello A, Sommariva E, Gino M, Pritelli C, Morabito A: Effectiveness and safety of a nonremovable fiberglass off-bearing cast versus a therapeutic shoe in the treatment of neuropathic foot ulcers: a randomized study. Diabetes Care 2000; 23: 1746–1751 [DOI] [PubMed] [Google Scholar]
- 14. Birke JA, Pavich MA, Patout CA, Jr, Horswell R: Comparison of forefoot ulcer healing using alternative off-loading methods in patients with diabetes mellitus. Adv Skin Wound Care 2002; 15: 210–215 [DOI] [PubMed] [Google Scholar]
- 15. Mueller MJ, Lott DJ, Hastings MK, Commean PK, Smith KE, Pilgram TK: Efficacy and mechanism of orthotic devices to unload metatarsal heads in people with diabetes and a history of plantar ulcers. Phys Ther 2006; 86: 833–842 [PubMed] [Google Scholar]
- 16. Sinacore DR: Total contact casting for diabetic neuropathic ulcers. Phys Ther 1996; 76: 296–301 [DOI] [PubMed] [Google Scholar]
- 17. Guyton GP: An analysis of iatrogenic complications from the total contact cast. Foot Ankle Int 2005; 26: 903–907 [DOI] [PubMed] [Google Scholar]
- 18. Lavery LA, Vela SA, Lavery DC, Quebedeaux TL: Total contact casts: pressure reduction at ulcer sites and the effect on the contralateral foot. Arch Phys Med Rehabil 1997; 78: 1268–1271 [DOI] [PubMed] [Google Scholar]
- 19. Piaggesi A, Macchiarini S, Rizzo L, Palumbo F, Tedeschi A, Nobili LA, Leporati E, Scire V, Teobaldi I, Del Prato S: An off-the-shelf instant contact casting device for the management of diabetic foot ulcers: a randomized prospective trial versus traditional fiberglass cast. Diabetes Care 2007; 30: 586–590 [DOI] [PubMed] [Google Scholar]
- 20. Caravaggi C, Sganzaroli A, Fabbi M, Cavaiani P, Pogliaghi I, Ferraresi R, Capello F, Morabito A: Nonwindowed nonremovable fiberglass off-loading cast versus removable pneumatic cast (AircastXP Diabetic Walker) in the treatment of neuropathic noninfected plantar ulcers: a randomized prospective trial. Diabetes Care 2007; 30: 2577–2578 [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Lavery LA, Wu S, Boulton AJ: Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care 2005; 28: 551–554 [DOI] [PubMed] [Google Scholar]
- 22. Katz IA, Harlan A, Miranda-Palma B, Prieto-Sanchez L, Armstrong DG, Bowker JH, Mizel MS, Boulton AJ: A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care 2005; 28: 555–559 [DOI] [PubMed] [Google Scholar]
- 23. Chantelau E, Haage P: An audit of cushioned diabetic footwear: relation to patient compliance. Diabet Med 1994; 11: 114–116 [DOI] [PubMed] [Google Scholar]
- 24. Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJ: Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care 2003; 26: 2595–2597 [DOI] [PubMed] [Google Scholar]
- 25. Ha Van G, Siney H, Hartmann-Heurtier A, Jacqueminet S, Greau F, Grimaldi A: Nonremovable, windowed, fiberglass cast boot in the treatment of diabetic plantar ulcers: efficacy, safety, and compliance. Diabetes Care 2003; 26: 2848–2852 [DOI] [PubMed] [Google Scholar]