Abstract
OBJECTIVE
Many of the metabolic benefits of Roux-en-Y gastric bypass (RYGB) occur before weight loss. In this study we investigated the influence of caloric restriction on the improvements in the metabolic responses that occur within the 1st week after RYGB.
RESEARCH METHODS AND DESIGN
A mixed meal was administered to nine subjects before and after RYGB (average 4 ± 0.5 days) and to nine matched, obese subjects before and after 4 days of the post-RYGB diet.
RESULTS
Weight loss in both groups was minimal; the RYGB subjects lost 1.4 ± 5.3 kg (P = 0.46) vs. 2.2 ± 1.0 kg (P = 0.004) in the calorically restricted group. Insulin resistance (homeostasis model assessment of insulin resistance) improved with both RYGB (5.0 ± 3.1 to 3.3 ± 2.1; P = 0.03) and caloric restriction (4.8 ± 4.1 to 3.6 ± 4.1; P = 0.004). The insulin response to a mixed meal was blunted in both the RYGB and caloric restriction groups (113 ± 67 to 65 ± 33 and 85 ± 59 to 65 ± 56 nmol · l−1 · min−1, respectively; P < 0.05) without a change in the glucose response. Glucagon-like peptide 1 levels increased (9.2 ± 8.6 to 12.2 ± 5.5 pg · l−1 · min−1; P = 0.04) and peaked higher (45.2 ± 37.3 to 84.8 ± 33.0 pg/ml; P = 0.01) in response to a mixed meal after RYGB, but incretin responses were not altered after caloric restriction.
CONCLUSIONS
These data suggest that an improvement in insulin resistance in the 1st week after RYGB is primarily due to caloric restriction, and the enhanced incretin response after RYGB does not improve postprandial glucose homeostasis during this time.
Bariatric surgical procedures achieve a large and sustained improvement in insulin sensitivity and a high resolution rate in type 2 diabetes. The metabolic benefits of Roux-en-Y gastric bypass surgery (RYGB) are observed very early and precede substantial weight loss (1). It has been proposed that the long-term improvements are related to a reduction in fat mass (2); however, the mechanisms for the early improvements remain uncertain. The surgical bypass of the foregut and/or rapid nutrient exposure of the distal gut alters enterokine release, which has been proposed to result in metabolic improvements (3) and in particular glucose homeostasis. However, caloric restriction in the absence of weight loss has metabolic benefits (4) and could also contribute to the early improvements in glucose homeostasis.
The incretins, namely glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide (GIP), are gut hormones that contribute to postprandial insulin secretion (5). RYGB augments GLP-1 secretion, whereas its impact on GIP is less consistent (3). In contrast, bariatric procedures that induce weight loss by caloric restriction in the absence of intestinal bypass, such as adjustable gastric banding, do not alter postprandial incretin levels (3). Ghrelin is another enterokine that has a primary role in appetite stimulation but also has glucose and insulin modulatory effects (6). The presence of an acyl group is considered necessary for biological activity of ghrelin, although the desacyl form probably has biological functions as well (7). Ghrelin levels are abnormally low in obese individuals and remain suppressed after RYGB, whereas weight loss by diet enhances ghrelin levels (8). Leptin and adiponectin, adipocyte-derived hormones, are thought to be mediators of weight-related improvements in insulin resistance. The concentrations of these hormones are aberrant in obesity and normalize after RYGB. A recent study indicated that >5% weight loss by consumption of a hypocaloric diet (∼40% energy restriction) is required to favorably change circulating adipokines and metabolic parameters (9).
A limited number of reports have directly compared the contribution of duodenal bypass versus caloric restriction on enterokine responses and hormone levels after RYGB (10–12). These studies all incorporated moderate weight loss (∼10 kg) and were conducted 2–4 weeks postoperatively. In the present study, we compared the immediate, weight loss–independent effects of RYGB and caloric restriction on fasting hormone levels and meal-stimulated enterokine release. RYGB were evaluated within the 1st week after surgery, and a matched group of subjects were evaluated after 4 days of the equivalent post–bariatric surgery diet.
RESEARCH DESIGN AND METHODS
RYBG subjects were recruited from the Center for Surgical Weight Loss at Vanderbilt University Medical Center after approval for surgery. Diet control were matched to the surgery group (Table 1) for age (P = 0.37), weight (P = 0.09), diabetes status and duration (P = 0.80), and A1C (P = 0.56). All subjects provided written, informed consent to participate in the study. The study protocol was approved by the Vanderbilt University Institutional Review Board.
Table 1.
Baseline subject characteristics
| RYGB | Diet | |
|---|---|---|
| n | 9 | 9 |
| Age (years) | 41.1 ± 11.5 | 46.6 ± 6.7 |
| Sex (male/female) | 3/6 | 2/7 |
| Weight (kg) | 153.2 ± 32.2 | 127.0 ± 36.5 |
| Type 2 diabetes (yes/no) | 5/4 | 4/5 |
| Diabetes duration (years) | 3.7 ± 4.7 | 2.6 ± 1.5 |
| A1C (%) | 6.5 ± 1.3 | 6.2 ± 1.0 |
Data are means ± SD or count. All comparisons between groups were nonsignificant (P > 0.05).
Subjects were studied at baseline and then after RYGB (surgery group) or after caloric restriction (diet group). After the baseline study, subjects in the surgery group underwent either open or laparoscopic RYGB (13). The average time for the postoperative study was 4 ± 0.5 days (range 2–7 days). The diet group was studied after 4 days of caloric restriction that replicated the post-RYGB diet. The diet consisted of 2.5 liters of fluid/day for 3 days; the 1st day included water only, followed by water and sugar-free clear liquids (e.g., gelatin, juices, and/or broths equivalent to 200–300 kcal/day) on days 2 and 3. For each study visit, subjects were admitted after a 12-h overnight fast for measurement of fasting and meal-induced metabolic and hormonal responses. Blood samples were collected from a heated forearm vein at 0700 h (time 0), immediately after completion of a meal (time 20), and every subsequent hour for 4 h. Subjects were asked to take 15–20 min to complete the meal to account for the reduced stomach capacity after RYGB. The meal was a standardized 250-kcal liquid mixed-meal containing 40 g carbohydrates, 6 g fat, and 9 g protein (8 oz of Ensure).
Sample collection and analysis
Blood was collected in chilled EDTA tubes and immediately centrifuged, and plasma was stored at −80°C until analysis. Glucose was measured via the glucose oxidase method (Beckman glucose analyzer). The plasma designated for GLP-1 measurement was supplemented with aprotinin (1,000 kIU/ml) and dipeptidyl peptidase 4 inhibitor (20 μl/ml plasma). Plasma designated for acylated ghrelin measurement was treated with 1 N hydrochloric acid (50 μl/ml plasma) and phenylmethylsulfonyl fluoride (0.1 mg/ml plasma). Plasma insulin, leptin, adiponectin, and active GLP-1 were measured using multiplex immunoassays (Luminex xMAP). Total (acylated and desacyl) and acylated ghrelin were determined by radioimmunoassay. Plasma concentrations of total GIP were measured by enzyme-linked radioimmunoassay. A1C was assayed using high-pressure liquid chromatography.
Calculations
The homeostasis model assessment of insulin resistance index (HOMA-IR) is derived from the inverse of insulin sensitivity based on Levy's nonlinear computer model (14). Total area under the curve (AUC) was calculated according to the trapezoidal rule in GraphPad Prism (version 5.02).
Statistical analyses
The Wilcoxon signed rank test was performed to compare data from the same subjects. The nonparametric Mann-Whitney test was used for comparisons between RYGB and diet groups. All analyses were performed in R 2.6.2 (www.r-project.org). Data are means ± SD, except for graphs which are presented as means ± SEM.
RESULTS
Weight loss
RYGB subjects lost 1.4 ± 5.3 kg or 1.0 ± 3.4% of initial body weight (P = 0.46) in the 1st week postoperatively. Caloric-restricted control subjects lost 2.2 ± 1.0 kg or 2.2 ± 1.0% of initial body weight (P = 0.004). Comparison of the weight changes with RYGB and diet was not significant (P = 0.09) (Table 2).
Table 2.
Early effects of RYGB and short-term diet restriction on body weight and fasting metabolic parameters
| Before RYGB | After RYGB | Before diet | After diet | |
|---|---|---|---|---|
| Weight (kg) | 153.2 ± 32.2 | 151.8 ± 33.1 | 127.0 ± 36.5 | 124.2 ± 36.5* |
| BMI (kg/m2) | 51.9 ± 6.0 | 51.4 ± 6.6 | 44.2 ± 9.9 | 43.2 ± 10.0* |
| Glucose (mmol/l) | 6.4 ± 1.5 | 6.0 ± 1.8 | 6.8 ± 1.8 | 5.4 ± 1.1* |
| Insulin (pmol/l) | 236 ± 159 | 155 ± 102* | 220 ± 196 | 178 ± 217 |
| HOMA-IR | 5.0 ± 3.1 | 3.3 ± 2.1* | 4.8 ± 4.1 | 3.6 ± 4.1* |
| GLP-1 (pg/ml) | 34.9 ± 32.4 | 36.5 ± 32.6 | 39.4 ± 14.9 | 39.3 ± 32.8 |
| GIP (pg/ml) | 58.0 ± 31.6 | 42.4 ± 21.1 | 53.3 ± 29.0 | 33.7 ± 25.5 |
| Leptin (ng/ml) | 72.4 ± 15.3 | 49.5 ± 15.1* | 61.1 ± 30.6 | 38.0 ± 21.9 |
| Adiponectin (μg/ml) | 7.3 ± 3.0 | 6.4 ± 2.0 | 4.5 ± 2.6 | 4.7 ± 2.6 |
| Acylated ghrelin (pg/ml) | 68.2 ± 33.6 | 48.5 ± 26.9* | 34.7 ± 23.5 | 26.1 ± 20.2 |
| Total ghrelin (pg/ml) | 585 ± 272 | 414 ± 107 | 623 ± 205 | 559 ± 268 |
Data are means ± SD.
*P < 0.05 compared with baseline within each group (RYGB or Diet).
Fasting measures of insulin resistance
Within 1 week after RYGB, fasting glucose levels were similar to preoperative levels (−6%; P = 0.14); however, a decrease in insulin levels was observed (−25%; P = 0.04). HOMA-IR also decreased by 25% (P = 0.03). Diet control subjects exhibited a disparate decrease in glucose levels (−20%; P = 0.004) but a similar decrease in fasting insulin levels (−27%; P = 0.07) and improvement in the HOMA-IR index (−30%; P = 0.004). Changes in HOMA-IR with RYGB and diet were not different (P = 0.45) (Table 2).
Fasting levels of enterokines and adipokines
Fasting plasma levels of GLP-1 and GIP were not altered either by RYGB (P = 0.65 and 0.16, respectively) or after caloric restriction (P = 0.73 and 0.13, respectively). On the other hand, there were decreases in the fasting levels of acylated (−21%; P = 0.03) and total ghrelin (−20%; P = 0.05) after RYGB, with no changes in the caloric-restricted group (P > 0.2). The fasting levels of leptin declined 1 week after RYGB (−31%; P = 0.004) and after caloric restriction (−26%; P = 0.05), whereas adiponectin levels were not altered in either group (P > 0.1) (Table 2).
Metabolic response to a mixed meal
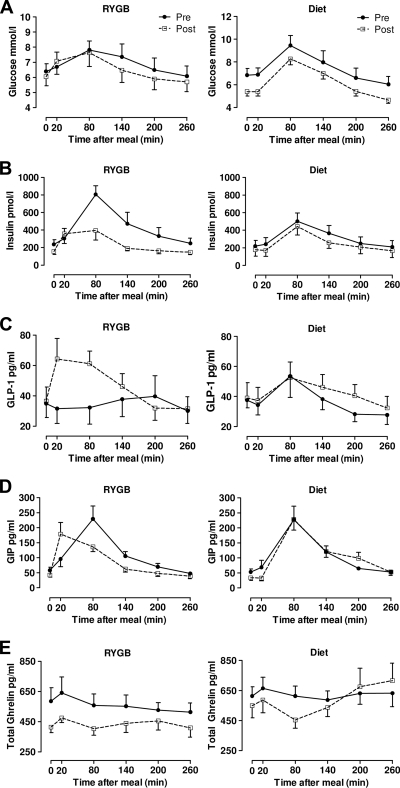
RYGB did not result in differences in the glucose AUC (P = 0.13) or peak glucose levels (P = 0.21) achieved after a mixed meal. The insulin response was reduced after RYGB, evidenced by decreases in AUC (P = 0.004) and peak levels of insulin (P = 0.02). Conversely, there were increases in the AUC (P = 0.04) and peak (P = 0.01) GLP-1 levels postoperatively. Whereas the AUC and peak levels of GIP were not altered in response to a mixed meal after RYGB, the peak in GIP occurred earlier. Despite a decrease in the AUC and nadir for total ghrelin in the early postoperative period (P = 0.004 for both), ghrelin remained at fasting levels throughout the study (Fig. 1, Table 3).
Figure 1.
Metabolic responses during a mixed-meal before and after RYGB and diet. Blood was drawn before (time 0), immediately after the ingestion of a mixed-meal (time 20), and every subsequent hour for 4 h. Plasma levels of glucose (A), insulin (B), GLP-1 (C), GIP (D), and total ghrelin (E) were measured at each time point at baseline (●) and 4 days after RYGB (□) or 3 days after a post–bariatric surgery diet (□). Data are means ± SEM.
Table 3.
Metabolic responses to a mixed-meal before and after RYGB and diet
| Before RYGB | After RYGB | Before diet | After diet | |
|---|---|---|---|---|
| Glucose AUC (mmol · l−1 · min−1) | 1,829 ± 510 | 1,714 ± 568 | 1,966 ± 641 | 1,637 ± 297 |
| Peak glucose (mmol/l) | 8.4 ± 2.1 | 7.9 ± 2.5 | 9.4 ± 2.6 | 8.2 ± 1.5 |
| Insulin AUC (mmol · l−1 · min−1) | 113 ± 67 | 65 ± 33* | 85 ± 59 | 65 ± 56* |
| Peak insulin (pmol/l) | 742 ± 347 | 485 ± 307* | 532 ± 287 | 406 ± 307* |
| GLP-1 AUC (pg · l−1 · min−1) | 9.2 ± 8.4 | 12.2 ± 5.5* | 10.2 ± 5.9 | 11.6 ± 6.4 |
| Peak GLP-1 (pg/ml) | 45.2 ± 37.3 | 84.8 ± 33.0* | 58.8 ± 43.2 | 57.2 ± 28.4 |
| GIP AUC (pg · l−1 · min−1) | 28.7 ± 12.3 | 23.5 ± 8.6 | 29.5 ± 13.5 | 30.1 ± 13.8 |
| Peak GIP (pg/ml) | 227 ± 115 | 193 ± 111 | 235 ± 128 | 228 ± 105 |
| Ghrelin AUC (pg · l−1 · min−1) | 145 ± 53 | 112 ± 36* | 162 ± 58 | 142 ± 52 |
| Nadir ghrelin (pg/ml) | 456 ± 165 | 341 ± 84* | 543 ± 217 | 393 ± 201 |
Data are means ± SD.
*P < 0.05 compared with baseline within each group (RYGB and diet).
The subjects who underwent caloric restriction similar to that for the subjects undergoing RYGB displayed similar changes in meal-stimulated glucose and insulin release after the diet; the AUC (P = 0.13) and peak (P = 0.34) glucose concentrations did not change and the AUC (P = 0.02) and peak (P = 0.04) insulin concentrations decreased. Although the AUC for insulin was decreased in both the RYGB and diet groups, the change in the RYGB group was greater (P = 0.04). Caloric restriction alone did not induce any changes in enterokine responses to a mixed meal (all P > 0.05), with the exception of a decreased nadir in ghrelin release after diet (P = 0.05). Interestingly, caloric restriction altered the pattern of ghrelin release. At baseline, ghrelin levels did not vary after the mixed meal similar to that in the RYGB subjects; however, after the diet, ghrelin release was suppressed after the mixed meal followed by a steady increase in ghrelin release above fasting levels.
CONCLUSIONS
It is well established that RYGB is effective in improving insulin resistance and ameliorating type 2 diabetes. The beneficial metabolic effects of RYGB were initially attributed to the substantial weight reduction achieved with surgery; however, subsequent investigations revealed an improvement in insulin sensitivity at 6 days after RYGB without appreciable weight loss (15). We have confirmed these findings by demonstrating a 25% improvement in insulin sensitivity (HOMA-IR) within 1 week after RYGB before any apparent weight loss. Interestingly, obese subjects, albeit with a nonsignificantly lower body weight, who consumed a post–bariatric surgery liquid diet for 4 days replicated the improved insulin sensitivity observed in the RYGB subjects. The parallel improvements occurred with minimal noticeable differences in weight loss between the two groups. HOMA-IR is a fasting measure of whole-body insulin resistance that has been shown to correlate with other dynamic measures of insulin sensitivity in obese subjects, such as the hyperinsulinemic-euglycemic clamp (16). Such studies suggest that a short duration, very-low-calorie diet can reduce hepatic glucose production (17) and improve skeletal muscle insulin sensitivity (4). Our data demonstrate that the improvement in insulin sensitivity, as measured by HOMA-IR, precedes appreciable weight loss and is largely achieved with caloric restriction. However, we must consider the possibility that the immediate improvements in insulin sensitivity after RYGB could have been blunted consequent to the associated stress/inflammatory responses of surgery, thus masking a greater improvement in insulin sensitivity with RYGB than with caloric restriction.
Fasting levels of GLP-1 have been reported to remain stable 2–10 weeks after RYGB (1,11,18,19), consistent with our current observations within the 1st postoperative week. Our data show increases in peak GLP-1 levels and total GLP-1 release after ingestion of a mixed meal 1 week after RYGB, in agreement with previous short-term follow-up investigations (11,19–21). This increase in GLP-1 could not be attributed to the restrictive nature of the surgical procedure, because the caloric-restricted diet group did not show enhanced an GLP-1 release in response to the mixed meal. The findings with GIP are novel; GIP is released sooner but not to a greater extent with a meal within the 1st week after RYGB. Laferrère et al. (11) and Campos et al. (10) compared incretin levels in two groups of subjects after a 10-kg weight loss via RYGB or diet and reported an increase in GLP-1 only after RYGB; their findings for GIP were disparate, with one reporting an increase (11) and the other reporting no change (10). Our results, however, show altered incretin release after RYGB before substantial weight loss. The proposed mechanism of enhanced incretin release after RYGB is most likely related to the increased and more rapid nutrient stimulus to the intestinal neuroendocrine cells.
The effects of the changes in incretin levels on the improved metabolic responses after RYGB remain controversial. In our study, the observed increase in GLP-1 and shift to an earlier GIP peak within 1 week after RYGB was accompanied by improved insulin sensitivity, whereas the improvement in insulin sensitivity after caloric restriction occurred without alterations in either GLP-1 or GIP. Thus, the improvements in insulin sensitivity in our study are unlikely to be due to altered incretin-induced insulin release but rather to the caloric restriction. In fact, in both surgical and caloric-restricted diet groups we observed similar decreases in insulin release (Fig. 1) accompanied by improved insulin sensitivity. These findings contrast with previous reports of either no early changes in insulin release (10) or an increase in insulin release (11) after RYGB. The discrepancy with our findings may be related to the associated losses (10 kg) in body weight in these studies (10,11). In addition, GLP-1 could exert extrapancreatic effects on improving insulin sensitivity (22), which in our RYGB group could have been blunted as a result of the associated inflammatory responses immediately after surgery. Lastly, perhaps the improved GLP-1 response in the 1st week after RYGB is not robust enough to elicit increased insulin secretion and glucose-lowering effects and may take longer to exert such effects.
Levels of the orexiogenic hormone ghrelin are diminished in obesity (23), perhaps indicating a positive energy balance. The short-term effect of RYGB on fasting total ghrelin is controversial. We observed 20% decreases in fasting levels of acylated and total ghrelin within 1 week after RYGB; these are consistent with previously reported decreases in fasting total ghrelin at 6 weeks (24) but different from another report showing no change 1 month after RYGB (12). Whether the decreased levels of acylated and total ghrelin levels play a role in the improvement in insulin sensitivity after RYGB remains to be determined. Vestergaard et al. (6) demonstrated that exogenous infusion of a pharmacological dose of acyl-ghrelin acutely induced insulin resistance independent of growth hormone and cortisol.
Alterations in adipokines, such as leptin and adiponectin, after RYGB have been attributed to fat mass loss and are responsible for the long-term improvements in insulin resistance (2). The similarities in the decrease in plasma leptin in the RYGB and diet groups suggest that this immediate change in leptin after surgery can be accounted for by caloric restriction. A similar finding was reported 1 month after RYGB and diet, coinciding with a 10-kg weight loss (12). The stable adiponectin concentrations during caloric restriction via RYGB and diet could indicate dissociation in the regulation of these two adipokines between nutrient exposure and fat mass.
In summary, our present data suggest that caloric restriction without substantial weight loss is of primary importance in the rapid improvement of insulin sensitivity within the 1st week after RYGB. Early alterations in the incretin response can be attributed to the surgery; however, the enhanced incretin response does not seem to have any additional benefit beyond caloric restriction on glucose homeostasis and insulin sensitivity. It is important to note that our cohorts of obese subjects were balanced for type 2 diabetes, and the measured parameters were similar at baseline (except for HOMA-IR, which was higher in the subjects with type 2 diabetes) and changed similarly after intervention. Further investigations in the immediate postoperative period with more dynamic measures of insulin resistance are also warranted to determine the mechanisms/site of improved insulin sensitivity, along with a direct assessment of the incretin effect on insulin production.
Acknowledgments
This work was supported by the following National Institutes of Health grants: National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-070860 (to N.N.A.), Vanderbilt Clinical and Translational Science Award grant 1-UL1-RR-024975 from the National Center for Research Resources, grant DK-20593 to the Vanderbilt Diabetes Research and Training Center, grant DK-058404 to the Vanderbilt Digestive Disease Research Center, and grant T32-DK-007061-31A1 (to J.M.I.).
No potential conflicts of interest relevant to this article were reported.
We thank Marcy Buckley for nursing support and Kareem Jabbour and Nadine Saliba for laboratory assistance.
Footnotes
Clinical Trial reg. no. NCT00765596, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E: The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004; 240: 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gumbs AA, Modlin IM, Ballantyne GH: Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obes Surg 2005; 15: 462–473 [DOI] [PubMed] [Google Scholar]
- 3.Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B: Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg 2009; 19: 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lara-Castro C, Newcomer BR, Rowell J, Wallace P, Shaughnessy SM, Munoz AJ, Shiflett AM, Rigsby DY, Lawrence JC, Bohning DE, Buchthal S, Garvey WT: Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism 2008; 57: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B: Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 2004; 113: 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO: Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 2008; 57: 3205–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares JB, Leite-Moreira AF: Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides 2008; 29: 1255–1270 [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ: Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346: 1623–1630 [DOI] [PubMed] [Google Scholar]
- 9.Varady KA, Tussing L, Bhutani S, Braunschweig CL: Degree of weight loss required to improve adipokine concentrations and decrease fat cell size in severely obese women. Metabolism 2009; 58: 1096–1101 [DOI] [PubMed] [Google Scholar]
- 10.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K: Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 2010; 14: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B: Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliván B, Teixeira J, Bose M, Bawa B, Chang T, Summe H, Lee H, Laferrère B: Effect of weight loss by diet or gastric bypass surgery on peptide YY3–36 levels. Ann Surg 2009; 249: 948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saliba J, Wattacheril J, Abumrad NN: Endocrine and metabolic response to gastric bypass. Curr Opin Clin Nutr Metab Care 2009; 12: 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy JC, Matthews DR, Hermans MP: Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21: 2191–2192 [DOI] [PubMed] [Google Scholar]
- 15.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS: Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005; 15: 474–481 [DOI] [PubMed] [Google Scholar]
- 16.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495 [DOI] [PubMed] [Google Scholar]
- 17.Jazet IM, Pijl H, Frölich M, Romijn JA, Meinders AE: Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism 2005; 54: 705–712 [DOI] [PubMed] [Google Scholar]
- 18.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL: Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg 2004; 70: 1–4; discussion 4–5 [PubMed] [Google Scholar]
- 19.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B: Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T: Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246: 780–785 [DOI] [PubMed] [Google Scholar]
- 21.Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J: GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 2006; 16: 1594–1601 [DOI] [PubMed] [Google Scholar]
- 22.Vella A, Rizza RA: Extrapancreatic effects of GIP and GLP-1. Horm Metab Res 2004; 36: 830–836 [DOI] [PubMed] [Google Scholar]
- 23.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML: Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707–709 [DOI] [PubMed] [Google Scholar]
- 24.Morínigo R, Casamitjana R, Moizé V, Lacy AM, Delgado S, Gomis R, Vidal J: Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res 2004; 12: 1108–1116 [DOI] [PubMed] [Google Scholar]