Abstract
OBJECTIVE
To examine antidepressant use before and after the diagnosis of diabetes.
RESEARCH DESIGN AND METHODS
This study was a longitudinal analysis of diabetic and nondiabetic groups selected from a prospective cohort study of 151,618 men and women in Finland (the Finnish Public Sector Study, 1995–2005). We analyzed the use of antidepressants in those 493 individuals who developed type 2 diabetes and their 2,450 matched nondiabetic control subjects for each year during a period covering 4 years before and 4 years after the diagnosis. For comparison, we undertook a corresponding analysis on 748 individuals who developed cancer and their 3,730 matched control subjects.
RESULTS
In multilevel longitudinal models, the odds ratio for antidepressant use in those who developed diabetes was 2.00 (95% CI 1.57–2.55) times greater than that in nondiabetic subjects. The relative difference in antidepressant use between these groups was similar before and after the diabetes diagnosis except for a temporary peak in antidepressant use at the year of the diagnosis (OR 2.66 [95% CI 1.94–3.65]). In incident cancer case subjects, antidepressant use substantially increased after the cancer diagnosis, demonstrating that our analysis was sensitive for detecting long-term changes in antidepressant trajectories when they existed.
CONCLUSIONS
Awareness of the diagnosis of type 2 diabetes may temporarily increase the risk of depressive symptoms. Further research is needed to determine whether more prevalent use of antidepressants noted before the diagnosis of diabetes relates to effects of depression, side effects of antidepressant use, or a common causal pathway for depression and diabetes.
Diabetes is a chronic disease with substantial public health importance, but its psychological effects are not well understood (1–4). The diagnosis of diabetes in itself may be a life event that increases risk of depressive symptoms, arising from the awareness of having a pernicious chronic condition (2). However, it is equally possible that psychological impacts are not apparent until patients reach an advanced disease state because at diagnosis type 2 diabetes usually has mild symptoms (3).
The suggestion that depressive symptoms among nondiabetic individuals also increase the risk of diabetes complicates the examination of the effect of diabetes on depression, because this association may be bidirectional (2). To date, the evidence on the status of depressive symptoms as a risk factor for type 2 diabetes is mixed as both supportive and null findings have been reported (2,5). It is therefore important to assess depression both before and after the diagnosis of diabetes in a single methodological setup to ensure adequate estimation of the effect sizes in both directions. To our knowledge, no such study exists.
In this study, we used multiple repeated measurements of antidepressant use, both before and after the diagnosis of type 2 diabetes, to examine whether awareness of diabetes diagnosis is associated with elevations in depression risk and whether individuals who develop type 2 diabetes are more likely to be depressed already before the diagnosis than their nondiabetic counterparts. For comparison, we examine antidepressant use among individuals who developed cancer, a disease that is known to increase risk of depression (6).
RESEARCH DESIGN AND METHODS
Data were drawn from the Finnish Public Sector study (7), which includes the entire public sector personnel of 10 towns (municipalities) and 21 hospitals in the areas where these towns are located (see supplementary Figs. S1 and S2, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2359/DC1). The eligible population comprised 151,347 employees with an employment contract between 1995 and 2005 with a record linkage to national health registers through unique personal identification codes, which are assigned to all citizens in Finland. For all the participants in the eligible population, the linkage to registers was 100% complete, and there was no sample attrition during the follow-up.
We report data from two independent cohorts: 493 participants who developed type 2 diabetes (hereafter referred to as the “diabetes study”) and 748 individuals who developed cancer (the “cancer study”). All participants in both studies had complete data on prescribed antidepressant use and other register measures over a fixed period of 4 years before and 4 years after the diagnosis of type 2 diabetes/cancer, because we limited the study to those participants with incident case subjects who received the diagnosis of diabetes or cancer between 1 January 1999 and 31 December 2001 and were alive a minimum of 4 years after the diagnosis. This ensured an observation period of 4 years before and after the diagnosis without any sample attrition.
We randomly selected control subjects in a 5:1 ratio for each diabetes case subject and each cancer case subject, matching individually for age-group (25–45, 46–52, and 53–64 years), sex, socioeconomic position (upper nonmanual, lower nonmanual, or manual), type of employment contract (permanent vs. temporary), type of employer (hospital vs. municipality), and geographic area (seven areas based on the location of the workplace), because these characteristics could be related to differences in the likelihood of achieving diagnosis or treatment. The diabetes study included 2,450 matched diabetes-free control subjects and the cancer study included 3,730 matched cancer-free control subjects.
Assessment of antidepressant use
Depression was approximated from antidepressant use for each year of the observation period from 4 years before to 4 years after the diabetes and cancer diagnoses using the nationwide Drug Prescription Register. We used the same period for the incident diabetes/cancer case subjects and their disease-free control subjects to avoid confounding due to secular trends in antidepressant use. In Finland, prescriptions for antidepressant medications are filed by the National Social Insurance Scheme at the Social Insurance Institution, and the available data contain information on the day of purchase, dose, stated as the international standard daily defined dose, and medication classified according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification (8). For each year of observation, we defined antidepressant use as the purchase of antidepressants (ATC code N06A) of at least 30 daily defined doses. In addition, we conducted a sensitivity analysis, limiting the analysis to selective-serotonin reuptake inhibitor (SSRI) (ATC code N06AB) as the outcome because these drugs have a lower risk of cardiotoxicity than tricyclic antidepressants and therefore may be more likely to be selectively prescribed in individuals with diabetes.
Case definition for incident type 2 diabetes and cancer
Since 1965, drug treatment for diabetes has been free of charge in Finland. The Central Drug Register, maintained by the Social Insurance Institution, lists all such individuals with physician-documented evidence of a fasting plasma glucose ≥7.0 mmol/l or a nonfasting plasma glucose ≥11.1 mmol/l and symptoms of diabetes, such as polyuria, polydipsia, and glucosuria. If the symptoms are not present, then evidence of repeatedly measured elevated glucose levels is required. In this study, participants were defined as those with incident case subjects of type 2 diabetes the first time they were listed in the Central Drug Register as eligible for diabetes treatment between 1 January 1999 and 31 December 2001.
To exclude individuals with type 1 diabetes, we additionally linked the data to the Finnish Hospital Discharge Register that lists all discharged hospital patients with information on dates of admission and discharge and to the Drug Prescription Register (Social Insurance Institution) that includes all prescriptions for insulin medications, drugs to lower blood glucose, and other drugs for diabetes in Finland nationwide since 1994, according to the World Health Organization ATC classification. We excluded individuals who were recorded as having type 1 diabetes (ICD-10 code E10) in the Central Drug Register or the Hospital Discharge Register. For these registers, type 1 diabetes is always diagnosed by a diabetes specialist. For sensitivity analyses, we additionally excluded from the case subjects those for whom insulin or its analogs (ATC code A10A, the Drug Prescription Register) were prescribed and who had a diagnosis of diabetes at age ≤35 years. Individuals with type 1 diabetes were also not allowed to be selected as control subjects. From the potential control group, we excluded all individuals with prescriptions for insulin or its analogs, blood glucose–lowering drugs, or other drugs for diabetes during any of the years of observation in the Central Drug Register, Hospital Discharge Register, and Drug Prescription Register.
Individuals with cancer were identified via the nationwide Finnish Cancer Register, which records all patients with any type of cancer. In Finland, all physicians, all hospitals, and other institutions are legally bound to send notifications of all malignant tumors, carcinoid tumors, carcinoma in situ lesions, and tumors with borderline malignancy to the Register. In this study, an individual was defined as a cancer case subject if he or she had the diagnosis of cancer for the first time between 1 January 1999 and 31 December 2001.
Other variables
Age, sex, socioeconomic position (upper nonmanual, lower nonmanual, or manual), type of employment contract (permanent vs. temporary), type of employer (hospital vs. municipality) and geographic area (seven areas based on the location of the workplace) were obtained from employers' registers. Age at diagnosis was calculated from the dates of diagnosis and birth, using register data. We assessed the status of coronary heart disease at each year of observation, because this condition is known to be associated with both depression and diabetes. Information on coronary heart disease was obtained from the Finnish Hospital Discharge Register and Central Drug Register (ICD-10 codes I20–I25).
Statistical analysis
We analyzed the diabetes study and the cancer study separately. The observation period started at the date of diagnosis (year 0) for those who developed type 2 diabetes or had cancer (i.e., case subjects) and at a matched year for the control subjects. Participants were then traced backward and forward from year 0 to assess antidepressant use for a period covering 4 years before and 4 years after the diagnosis (i.e., years −4 to +4). We applied a repeated-measures logistic regression analysis using the generalized estimating equations method to estimate trajectories of antidepressant use before and after the diagnosis (for details, see supplementary data, available in an online appendix). We conducted all analyses using STATA statistical software (version 10.1 for Windows). Statistical significance was inferred at a two-tailed P < 0.05.
RESULTS
Table 1 shows the characteristics of participants at baseline, i.e., 4 years before the diagnosis in case subjects. In the studies of type 2 diabetes and cancer, there were no differences in baseline characteristics between the incident case subjects and disease-free participants (P > 0.11), including job type and geographical area that are not shown in the tables (P > 0.95 in the diabetes study and P > 0.93 in the cancer study). Comparison of the two studies shows that participants in the diabetes study were 2.4 (95% CI 2.2–2.7) years older, more likely to be male (odds ratio [OR] 2.80 [95% CI 2.64–2.97]) and with a manual occupation (2.25 [2.15–2.38]) compared with participants from the cancer study. These differences were expected, given that male sex and low socioeconomic position tend to be stronger risk factors for type 2 diabetes than cancer in working populations.
Table 1.
Baseline characteristics of study participants
| Diabetes study* |
Cancer study* |
|||||
|---|---|---|---|---|---|---|
| Incident case subjects | Control subjects | P value | Incident case subjects | Control subjects | P value | |
| Total N | 2,943 | 4,478 | ||||
| n | 493 | 2,450 | 748 | 3,730 | ||
| Male sex | 42.0 | 41.8 | 0.95 | 18.7 | 18.5 | 0.83 |
| Age-group | 0.11 | 0.39 | ||||
| 26–44 years | 18.9 | 23.8 | 31.2 | 34.3 | ||
| 45–54 years | 48.1 | 45.7 | 42.4 | 41.8 | ||
| 45–64 years | 30.6 | 28.7 | 24.1 | 21.9 | ||
| 65–70 years | 2.4 | 1.8 | 2.1 | 2.0 | ||
| Age (years) | 50.9 ± 7.4 | 50.0 ± 8.1 | 47.9 ± 9.6 | 47.7 ± 8.9 | ||
| Socioeconomic position | 0.95 | 1.00 | ||||
| Upper nonmanual | 19.1 | 19.2 | 33.2 | 33.2 | ||
| Lower nonmanual | 39.2 | 39.2 | 43.6 | 43.7 | ||
| Manual | 41.8 | 41.6 | 23.3 | 23.2 | ||
| Prevalent coronary heart disease | 3.3 | 2.4 | 0.28 | 2.1 | 1.5 | 0.17 |
Data are means ± SD or %.
*There were no missing data in any of the variables.
Antidepressant use before and after diagnosis of diabetes
Crude ORs for antidepressant use among incident diabetes case subjects versus nondiabetic control subjects at each year of observation were 2.19 (year −4), 1.95, 1.90, 2.33, 2.66 (year 0, diagnosis), 1.93, 1.73, 1.87, and 1.97 (year 4). The 95% CIs for the lowest OR, 1.73, were 1.2–2.38 and for the highest OR (year of diagnosis) were 1.94–3.65 (complete results available upon request from the first author).
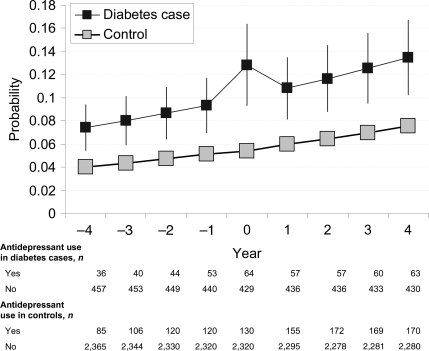
Figure 1 shows the final model to describe the trajectory of antidepressant use among incident diabetes case subjects and nondiabetic control subjects (for model parameters, see supplementary Table S1, available in an online appendix). There was an overall upward trend in the use of antidepressants across the 9-year observation period in diabetes case subjects and control subjects (time P < 0.0001), reflecting the nationwide increase in prescription of these drugs (15). Across the entire observation period, the OR of antidepressant use was 2.00 (95% CI 1.57–2.55) times higher for the incident diabetes case subjects than for the control subjects (P < 0.0001). There were no differences in the antidepressant slopes between the groups before or after the diagnosis (P = 0.32), except for the temporary increase in antidepressant use during the year of diagnosis among the incident diabetes case subjects (P = 0.01).
Figure 1.
Probability (95% CI) of antidepressant use before and after diagnosis of type 2 diabetes.
In four sensitivity analyses we repeated the main analysis first, after exclusion of all incident case subjects who were receiving insulin treatment and those aged ≤35 years at the time of diagnosis; second, after exclusion of subjects with prevalent coronary heart disease; third, with adjustment for socioeconomic position, job contract, and geographical area in the model; and fourth, with SSRIs as the outcome. These sensitivity analyses largely replicated the findings in the main analysis (supplementary Tables S2 and S3, available in an online appendix).
Antidepressant use before and after diagnosis of cancer
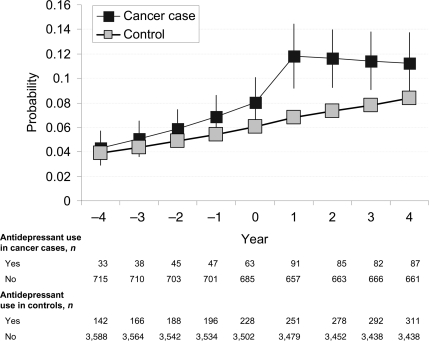
Figure 2 presents the final model to describe trajectories in antidepressant use before and after the diagnosis of cancer (for model parameters, see supplementary Table S1). The slope in antidepressant use did not differ between case subjects and control subjects before year 0 (P = 0.21); antidepressant use during the year of diagnosis was slightly higher among incident cancer case subjects than among control subjects (P = 0.03). There was a substantial increase in antidepressant use in case subjects after the diagnosis (12.2% 1 year after the diagnosis compared with 6.3% in the year before the diagnosis; caseness × period P = 0.004). The OR of antidepressant use was 1.92 (95% CI 1.49–2.48) times higher in incident cancer case subjects 1 year after the diagnosis compared with that in control subjects. Antidepressant use declined somewhat in the 2nd year after the diagnosis of cancer (caseness × time × period P = 0.01) but remained higher in incident cancer case subjects compared with cancer-free control subjects for the whole 4-year period after diagnosis.
Figure 2.
Probability (95% CI) of antidepressant use before and after diagnosis of cancer.
CONCLUSIONS
Serial measurements show that antidepressant use among men and women who develop type 2 diabetes was ∼2 times greater than that in nondiabetic individuals. Except for a temporary change in risk at the year of diagnosis, the relative difference in antidepressant use between these groups was similar during the 4 years before and 4 years after the diagnosis of diabetes. In contrast, there was no difference in antidepressant use before diagnosis of cancer between the incident case subjects and cancer-free participants, but the use of antidepressants increased sharply after diagnosis and remained higher in case subjects throughout the 4 years after diagnosis.
Our findings provide support for the hypothesis that awareness of the diagnosis of type 2 diabetes may temporarily increase the risk of depressive symptoms. However, because antidepressant use was similarly elevated both before and after the diagnosis among diabetes case subjects, it seems likely that awareness of the diagnosis has no lasting effect on depression risk. These findings do not support the concern that overlap of symptoms between type 2 diabetes and depression (e.g., fatigue) would make it less likely for depression to be appropriately recognized in diabetic patients (9).
It is possible that regular contact with a physician after diagnosis of type 2 diabetes makes detection of unrecognized depression more likely, explaining the observed increase in the use of antidepressants. However, if this were to be the case, prescription rates would have remained high after diagnosis over the 4-year observation period. This was not the case. Therefore, we believe that the temporary elevation in antidepressant use represents a true temporary increase in depression risk as a result of the diagnosis. The results obtained for cancer, showing elevated depression rates over the entire postdiagnosis period, demonstrate that our methodology was sensitive for detecting long-term changes in depression trajectories when these were present.
There are several possible explanations for more prevalent use of antidepressants already before the diagnosis of diabetes: First, it is possible that depression, as indicated by antidepressant use, increases the risk of type 2 diabetes. Most of the existing evidence provides support for such an association (2,5). Indeed, depression is associated with several behavioral and metabolic factors that can increase the risk of diabetes and insulin resistance, including obesity-promoting health behaviors, such as physical inactivity and hypercaloric diets (10,11) and activation of the neuroendocrine (12) and inflammatory responses (13).
Second, it is also conceivable that the bidirectional association between depression and diabetes in adulthood is a consequence of early factors, such as low birth weight and childhood adversity, which predispose individuals to both depression (14) and obesity/type 2 diabetes (15,16). However, further research is needed to test this hypothesis empirically.
Third, there is some, although not completely consistent, evidence that specific antidepressant drugs may increase the risk of diabetes (17–19). In the Diabetes Prevention Program (DPP) for individuals at high risk for developing type 2 diabetes, a strong association between antidepressant use and subsequent diabetes onset was not accounted for by measured confounders or mediators (17). It is also known that tricyclic antidepressants and noradrenergic and specific serotonin antidepressants may induce weight gain and promote hyperglycemia (20,21). In contrast, SSRIs and related agents may, at least in the short-term, be related to reduced weight gain and improved insulin sensitivity (22). However, in our investigation those treated with SSRIs had an increased risk of incident diabetes and in the DPP study the association between antidepressant use and diabetes risk was not accounted for by weight changes (17).
Finally, the notion that undiagnosed diabetes is more likely to be picked up in individuals who see their physician for depression could explain the more prevalent use of antidepressants before the diagnosis of diabetes. However, this interpretation is not consistent with recent studies showing both treated and untreated depression to be related to an elevated risk of diabetes (19).
Strengths and limitations
Nine repeated measurements of antidepressant drug use, encompassing the period both before and after diagnosis, enabled a better characterization of the association between diabetes diagnosis and antidepressant use than was possible in previous studies with fewer measurement points. Comprehensive records from national registers made it possible to avoid biases related to sample attrition. Because we selected matched control subjects for socioeconomic position and other key confounding factors, major confounding due to social stratification of type 2 diabetes or treatment of this disease is unlikely. Our study design is more effective in reducing confounding than a simple multivariate adjustment in the total cohort, the most widely used method in this field of research. However, no observational study can exclude the possibility of residual confounding.
We included in the study participants alive 4 years after diagnosis. This approach implies that patients with the most aggressive cancers leading to death within the first 4 years were excluded. Because these patients are particularly likely to experience depression, our findings may provide an underestimate of postcancer antidepressant use. In contrast, for diabetic patients 4 years of follow-up probably did not capture all of the potentially detrimental psychological impact that may become most apparent when patients have reached an advanced state of diabetes and experience the burden of dealing with its complications. Our findings on postdiabetes antidepressant use may therefore provide a conservative estimate.
Misclassification between type 1 and type 2 diabetes is a source of potential error in epidemiological studies on type 2 diabetes. Our sensitivity analyses, excluding all individuals with diabetes who are receiving insulin treatment and who are aged ≤35 years, suggest that our findings are not due to falsely coded type 1 diabetes. In this study, 25% of the incident diabetes case subjects were receiving insulin therapy 4 years after the diagnosis. It is likely that most of these case subjects were true type 2 diabetic patients, as the projected need for insulin therapy for patients with type 2 diabetes is >20% in this time window.
The Finnish nationwide registries validly identified individuals pharmacologically treated for type 2 diabetes and depression but did not capture undiagnosed disease or conditions treated without medication. Furthermore, in addition to depression, antidepressants are used in the treatment of other disorders, such as chronic pain and sleeping disorders, and they are sometimes prescribed for smoking cessation. When the present data are interpreted, it is important to recognize that antidepressant treatment is not exactly the same as a diagnosis of depression, although patients with such disorders represent a vast majority of those taking antidepressants.
We could not address some potentially important issues, such as the precise timing of diabetes onset (rather than when it was recognized), the severity of depression, and the status of other clinical conditions, because all of these would require a clinical examination. With repeated clinical examinations, however, the present study design with nine serial assessments around the diagnosis would be very expensive to undertake (the current study is based on 151,618 individuals followed up for 11 years). Therefore, future researchers should seek solutions to overcome these limitations.
Implications
Depression is known to be associated with poor glycemic control and negative clinical outcomes (23,24). We found that individuals with a recent diagnosis of type 2 diabetes had an excess use of antidepressants similar in size to that among 4-year cancer survivors immediately after diagnosis. Our findings support the recommendation of the American Diabetes Association to screen diabetic patients for depression (25).
Our finding that elevated antidepressant use exists already before the diagnosis of diabetes warrants further research. Because these agents are routinely offered to patients with moderate and severe depression, it would be important to determine whether antidepressants have side effects that increase diabetes risk and, if this were the case, make appropriate modifications to the treatment of patients. It is also possible that depression rather than antidepressant use is a risk factor for diabetes or that the two share common risk factors, indicating that research is needed to assess the potential benefits of diabetes prevention interventions targeted especially to patients treated for depression.
Supplementary Material
Acknowledgments
M.K. and J.V. are supported by the Academy of Finland. M.K. is additionally supported by a BUPA Foundation Specialist Research Grant, U.K., the National Heart, Lung, and Blood Institute (grant R01-HL-036310), and the National Institute on Aging, National Institutes of Health (grant R01AG034454). D.A.L. works in a center that receives some core funding from the U.K. Medical Research Council (MRC). G.D.B. is a Wellcome Trust Research Fellow, U.K. A.S.-M. is supported by a EURYI award from the European Science Foundation. The MRC Social and Public Health Sciences Unit receives funding from the U.K. MRC and the Chief Scientist Office at the Scottish Government Health Directorates. The Centre for Cognitive Ageing and Cognitive Epidemiology is supported by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, the Economic and Social Research Council, the MRC, and the University of Edinburgh as part of the cross-council Lifelong Health & Wellbeing initiative.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ: The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24: 1069–1078 [DOI] [PubMed] [Google Scholar]
- 2. Mezuk B, Eaton WW, Albrecht S, Golden SH: Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31: 2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lee HB, Lyketsos C: Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008; 299: 2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kivimäki M, Tabak AG, Batty GD, Singh-Manoux A, Jokela M, Akbaraly TN, Witte DR, Brunner EJ, Marmot MG, Lawlor DA: Hyperglycemia, type 2 diabetes, and depressive symptoms: the British Whitehall II study. Diabetes Care 2009; 32: 1867–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F: Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006; 49: 837–845 [DOI] [PubMed] [Google Scholar]
- 6. Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, Henrichs M, Carnicke CL, Jr: The prevalence of psychiatric disorders among cancer patients. JAMA 1983; 249: 751–757 [DOI] [PubMed] [Google Scholar]
- 7. Sjösten N, Vahtera J, Salo P, Oksanen T, Saaresranta T, Virtanen M, Pentti J, Kivimäki M: Increased risk of lost workdays prior to the diagnosis of sleep apnea. Chest 2009; 136: 130–136 [DOI] [PubMed] [Google Scholar]
- 8. National Agency for Medicines. Finnish Statistics on Medicines 2000. Helsinki, National Agency for Medicines and Social Insurance Institution, 2001 [Google Scholar]
- 9. Jones LE, Doebbeling CC: Depression screening disparities among veterans with diabetes compared with the general veteran population. Diabetes Care 2007; 30: 2216–2221 [DOI] [PubMed] [Google Scholar]
- 10. Golden SH, Williams JE, Ford DE, Yeh HC, Paton Sanford C, Nieto FJ, Brancati FL: Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2004; 27: 429–435 [DOI] [PubMed] [Google Scholar]
- 11. Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torréns JI, Kravitz HM, Bromberger JT, Matthews KA: Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care 2004; 27: 2856–2862 [DOI] [PubMed] [Google Scholar]
- 12. Golden SH: A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diab Rev 2007; 3: 252–259 [DOI] [PubMed] [Google Scholar]
- 13. Ford DE, Erlinger TP: Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2004; 164: 1010–1014 [DOI] [PubMed] [Google Scholar]
- 14. Colman I, Ploubidis GB, Wadsworth ME, Jones PB, Croudace TJ: A longitudinal typology of symptoms of depression and anxiety over the life course. Biol Psychiatry 2007; 62: 1265–1271 [DOI] [PubMed] [Google Scholar]
- 15. de Lauzon-Guillain B, Balkau B, Charles MA, Romieu I, Boutron-Ruault MC, Clavel-Chapelon F: Birth weight, body silhouette over the life course, and incident diabetes in 91,453 middle-aged women from the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l'Education Nationale (E3N) cohort. Diabetes Care 2010; 33: 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas C, Hyppönen E, Power C: Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics 2008; 121: e1240–e1249 [DOI] [PubMed] [Google Scholar]
- 17. Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, Haffner SM, Price DW, Knowler WC. Diabetes Prevention Program Research Group. Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care 2008; 31: 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knol MJ, Geerlings MI, Egberts AC, Gorter KJ, Grobbee DE, Heerdink ER: No increased incidence of diabetes in antidepressant users. Int Clin Psychopharm 2007; 22: 382–386 [DOI] [PubMed] [Google Scholar]
- 19. Campayo A, de Jonge P, Roy JF, Saz P, de la Cámara C, Quintanilla MA, Marcos G, Santabárbara J, Lobo A. ZARADEMP Project. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry 2010; 167: 580–588 [DOI] [PubMed] [Google Scholar]
- 20. Aronne LJ, Segal KR: Weight gain in the treatment of mood disorders. J Clin Psychiatry 2003; 64(Suppl. 8): 22–29 [PubMed] [Google Scholar]
- 21. Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, Carney RM, McGill JB: Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med 1997; 59: 241–250 [DOI] [PubMed] [Google Scholar]
- 22. Demyttenaere K, Jaspers L: Review: Bupropion and SSRI-induced side effects. J Psychopharm 2008; 22: 792–804 [DOI] [PubMed] [Google Scholar]
- 23. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE: Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23: 934–942 [DOI] [PubMed] [Google Scholar]
- 24. de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ: Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001; 63: 619–630 [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(Suppl. 1): S12–S54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.