Abstract
OBJECTIVE
Quick-release bromocriptine (bromocriptine-QR), a D2 dopamine receptor agonist, is indicated as a treatment for type 2 diabetes. The Cycloset Safety Trial, a 52-week, randomized, double-blind, multicenter trial, evaluated the overall safety and cardiovascular safety of this novel therapy for type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 3,095 patients with type 2 diabetes were randomized 2:1 to bromocriptine-QR or placebo in conjunction with the patient's usual diabetes therapy (diet controlled only or up to two antidiabetes medications, including insulin). The all-cause–safety end point was the occurrence of any serious adverse event (SAE), with a hazard ratio (HR) noninferiority margin of 1.5. In a prespecified analysis, the frequency of cardiovascular disease (CVD) events defined as a composite of myocardial infarction, stroke, coronary revascularization, and hospitalization for angina or congestive heart failure was evaluated using modified intent-to-treat analysis (clinicaltrials.gov, NCT00377676).
RESULTS
In the bromocriptine-QR group, 176 (8.6%) people reported SAEs compared with 98 (9.6%) in the placebo group (HR 1.02 [96% one-sided CI 1.27]). Fewer people reported a CVD end point in the bromocriptine-QR group versus the placebo group (37 [1.8%] vs. 32 [3.2%], respecively) (HR 0.60 [95% two-sided CI 0.35–0.96]). Nausea was the most commonly reported adverse event in the bromocriptine-QR group.
CONCLUSIONS
The frequency of SAEs was comparable between the treatment arms. Compared with patients in the placebo arm, fewer patients taking bromocriptine-QR experienced a cardiovascular end point.
Type 2 diabetes is a growing global pandemic that is estimated to afflict approximately 350 million people by the year 2030 (1,2). This growing threat to human health requires medical interventions to lessen the morbidity associated with type 2 diabetes. Results from several recent clinical trials have raised concerns about the cardiovascular safety of current therapies and therapeutic strategies (3–10). Therefore, the U.S. Food and Drug Administration has established cardiovascular safety standards that must be met for type 2 diabetes therapies prior to their marketing approval. The Cycloset Safety Trial was designed to assess the overall safety and specifically address cardiovascular safety for a novel treatment for type 2 diabetes, quick-release bromocriptine (bromocriptine-QR) (11).
Bromocriptine is a dopamine D2 receptor agonist, and bromocriptine-QR was designed to provide a short duration pulse of this dopamine agonist to centers in the brain (12,13) that regulate peripheral fuel metabolism (14). Bromocriptine-QR is administered in the morning, within 2 h of waking, to effectuate an increase in central dopaminergic tone at the time of day it normally peaks in healthy individuals (15). This circadian peak in central dopaminergic tone has been linked to preservation and/or induction of normal insulin sensitivity and glucose metabolism in several preclinical studies (14). Although bromocriptine-QR had demonstrated improvements in various metabolic parameters in patients with type 2 diabetes as well as improvements in many surrogate markers of cardiovascular disease, (16,17) data from these previous clinical studies were insufficiently powered to adequately assess cardiovascular safety. This article reports the results of a 1-year, double-blind, placebo-controlled, randomized clinical trial where the overall safety and specifically cardiovascular safety of bromocriptine-QR was the primary outcome.
RESEARCH DESIGN AND METHODS
The Cycloset Safety Trial study protocol has been previously published in abbreviated form (11). Patients were recruited from 74 centers across the U.S. and Puerto Rico, including 19 Veterans Affairs (VA) hospitals. Eligible patients had type 2 diabetes, as defined by the 2004 American Diabetes Association guidelines (18), were between the ages of 30 and 80 years, had a BMI of <43 kg/m2, and an A1C level ≤10.0%. Exclusion criteria were current chronic (greater than 10 days) use of prescription sympathomimetic drugs, ergot alkaloid derivatives, or abortive migraine medications, clinically significant comorbid conditions such as uncontrolled hypertension, New York Heart Classification (NYHC) III-IV congestive heart failure, renal failure, or cancer (other than nonmelanoma skin or nonmetastatic prostate cancer) within the past 5 years. Patients with NYHC I-II congestive heart failure were allowed in the trial as were those with significant cardiovascular disease, including a cardiovascular event prior to 6 months before screening.
Patients were required to be on a stable antidiabetes regimen consisting of either diet, oral hypoglycemic agents (no more than two), or insulin (alone or with no more than one oral hypoglycemic agent) for at least 30 days prior to randomization after a two-week lead-in period. Patients were randomized in a 2:1 ratio to their usual antidiabetes regimen plus bromocriptine-QR or placebo taken with their morning meal. During the first 6 weeks of the study, the daily dose of the study drug was titrated up by one tablet (0.8 mg) per day on a weekly basis until a maximal dose of up to six tablets (4.8 mg) per day was achieved or until the patient could not tolerate a higher dose.
Patients were required to continue their usual antidiabetes regimen during the first 3 months of the study but were allowed to alter the dosages of these medications to optimize blood glucose control as deemed appropriate by the site investigator (19). After 3 months, alterations to antidiabetes medications (elimination or addition of an agent) were allowed when necessary, but if a medication was added the final regimen was limited to no more than two oral antidiabetes agents, or no more than one antidiabetes agent if taking insulin.
During the first 6 weeks of the study, patients were called weekly, had office visits at weeks 3 and 6, and then visits every 3 months until the study end (week 52) or early termination. Patients were contacted 30 days after stopping a study drug to record any adverse events that occurred after cessation. Physical exams and laboratory assessments (blood chemistries, hematology, and urine analyses) were obtained at baseline, week 24, and week 52 or study termination. The study protocol was approved by each VA hospital institutional review board and a central or academic-affiliated institutional review board for non-VA centers.
Outcome measures
There were two main objectives of the Cycloset Safety Trial: 1) assessment of overall safety of bromocriptine-QR by measuring the frequency of serious adverse events (SAEs) among patients taking bromocriptine-QR and placebo, and 2) cardiovascular safety assessed by determining the frequency of major cardiovascular events, defined as a composite of first myocardial infarction, stroke, coronary revascularization, or hospitalization for angina or congestive heart failure that occurred after randomization. An independent adverse event adjudication committee (AEAC) consisting of two cardiologists and an endocrinologist, blinded to treatment assignment and Medical Dictionary for Regulatory Activities (MedDRA) coding of events by the study team, made the final SAE system organ class (SOC) classifications and assignment of an SAE as a cardiovascular end point.
Additional safety measures included laboratory measures (blood chemistries, hematology, and urine analyses) at weeks 0, 24, and 52 of the study and evaluation of electrocardiograms (ECGs) at weeks 0, 24, 52 or early termination.
Statistical analysis
An analysis of noninferiority tested the hypothesis that usual antidiabetes therapy (UADT) plus bromocriptine-QR is not inferior to that of UADT plus placebo in terms of the occurrence of all-cause SAEs using a one-sided α = 0.05 level. In order to test the primary hypothesis at a one-sided α = 0.05 and with a power of one −β = 0.90 when the noninferiority margin is 1.5, the total number of patients with all-cause SAEs needed during the study was calculated to be 235. All randomized patients that took at least one dose of study drug were included in the all-cause SAE analysis. SAEs that occurred on a study drug or within 30 days of stopping a study drug were considered as treatment emergent events. Statistical testing between the two groups was conducted using the two-sided 91.9% CI for the rate ratio of all-cause SAEs because this provides the same confidence boundaries as a one-sided 96% CI. The final analysis takes into account one interim testing. The CI was estimated by the corresponding estimate for the hazard ratio (HR) with Cox regression analysis with consideration for possible center interaction.
The analysis of the composite cardiovascular endpoint used the modified intent-to-treat population of patients receiving at least one dose of a study drug. Superiority or noninferiority between bromocriptine-QR and placebo for the composite CVD end point was defined as the upper bound of the two-sided 95% confidence limit being <0 and <1.5, respectively. Assuming a CVD composite event rate of 3.4%, this sample size provided a power of 75% to demonstrate CVD noninferiority. Upon confirming noninferiority, a sequential superiority analysis of CVD end point based on the Cox proportional-hazards regression with two-sided P values was then calculated. No interim analysis was conducted on the CVD end point. Analysis of the CVD end point was conducted adjusting for baseline covariates including history of stroke and cardiovascular revascularization and center.
Changes in laboratory measures were assessed using the Wilcoxon rank sum test except for A1C, which was based on a general linear model with baseline and treatment as fixed effects. The significant level was set at P < 0.05.
All statistical analyses were conducted by Everest Inc. (Toronto, Canada) using SAS software version 8.2 (Cary, NC).
RESULTS
A total of 4,074 patients were screened and 879 patients were excluded for not meeting eligibility criteria, withdrawal of consent, or other reasons. Of the 3,070 patients that received a study drug, 47% stopped treatment on bromocriptine-QR and 32% stopped treatment on placebo prior to the final study visit. The on treatment exposure time was 1,643 person-years and 914 person-years for bromocriptine-QR and placebo, respectively, which represents 77 and 86% of the expected person-time, respectively. Additionally, 82% of the study patients had final assessments, which accounts for 91% of the expected person time for this trial (2,906 of 3,193 possible total person-years) (Appendix IV, Figure A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2009/DC1).
Demographics
The study included a diverse population of patients with type 2 diabetes (Table 1) with the majority of patients receiving cardioprotective medications. On average for the study population, the baseline A1C, lipid, and blood pressure values indicated reasonably good control of hyperglycemia, dyslipidemia, and hypertension, respectively; however, the presence of cardiovascular disease was still prevalent. Overall, there were no differences in baseline characteristics between the groups that would not be expected by chance.
Table 1.
Baseline demographics
| Bromocriptine-QR | Placebo | |
|---|---|---|
| n | 2,054 | 1,016 |
| Mean age (years) | 59.5 ± 10.2 | 60.2 ± 9.97 |
| Duration of diabetes diagnosis (years) | 7.9 ± 7.42 | 8.0 ± 7.41 |
| BMI (kg/m2) | 32.4 ± 5.08 | 32.3 ± 5.07 |
| Waist circumference (inches) | 41.8 ± 5.11 | 42.0 ± 5.52 |
| Male sex | 1,141 (56) | 598 (59) |
| Caucasian | 1,381 (67) | 698 (69) |
| African American | 348 (17) | 168 (16.5) |
| Hispanic | 277 (13.5) | 131 (13) |
| Asian | 4 (1.1) | 3 (1.6) |
| Health risks | ||
| Hypertension | 1,548 (75) | 767 (75.5) |
| Angina pectoris | 214 (10) | 101 (10) |
| Myocardial infarction | 186 (9.1) | 106 (10) |
| Revascularization surgery | 204 (10) | 128 (13) |
| Stroke | 87 (4.2) | 65 (6.3) |
| Hypercholesterolemia* | 1,575 (77) | 767 (75.5) |
| Hypertriglyceridemia | 853 (41.5) | 422 (41.5) |
| Current smoker | 306 (15) | 133 (13) |
| Former smoker | 802 (39) | 419 (41) |
| Diabetes treatment | ||
| Diet only | 257 (12) | 114 (11) |
| One oral hypoglycemic agent | 806 (39) | 403 (40) |
| Two oral hypoglycemic agents | 686 (33) | 323 (32) |
| Oral agent plus insulin | 171 (8) | 98 (10) |
| Insulin only | 133 (6) | 78 (8) |
| Not reported | 1 | 0 |
| Antidiabetes medications | ||
| Insulin | 309 (15) | 176 (17) |
| Metformin | 1,209 (59) | 581 (57) |
| Rosiglitazone | 233 (11) | 111 (11) |
| Pioglitazone | 161 (8) | 83 (8) |
| Sulfonylurea/glinide | 759 (37) | 392 (39) |
| Cardio-protective medications | ||
| ACE inhibitors | 994 (48) | 477 (47) |
| Angiotensin II receptor inhibitors | 271 (13) | 135 (13) |
| Beta blockers | 452 (22) | 247 (24) |
| Diuretics, thiazide | 445 (22) | 233 (23) |
| Sulfamides, loop diuretics | 166 (8) | 89 (9) |
| Other diuretic† | 75 (4) | 49 (5) |
| Calcium channel blockers‡ | 362 (18) | 202 (20) |
| Hmg CoA reductase inhibitor | 1,165 (57) | 594 (58) |
| Fibrate | 157 (8) | 78 (8) |
| Platelet aggregation inhibitors | 943 (46) | 500 (49) |
Data are means ± SD or n (%).
*Based on history as assessed by study site investigator.
†Other diuretics include aldosterone inhibitors, low ceiling diuretics.
‡Calcium channel blockers include dihydropryidine, pheny-alkylamine, benozothiazepine.
Clinical outcomes
SAEs.
There were 374 SAEs among 274 people. All SAEs were grouped by SOC (Table 2). SAEs occurred among 9.6% of placebo-treated patients versus 8.6% of bromocriptine-QR–treated patients. Aside from the cardiac SOC class, the frequency of all remaining SAEs grouped by SOC was similar between bromocriptine-QR and placebo. It should be noted that the cardiac SOC includes a much broader definition comprising thirty-seven preferred terms (i.e., arrhythmia, chest pain, syncope) as opposed to the prespecified cardiovascular end point.
Table 2.
SAEs by SOC and all-cause safety and composite cardiovascular end point
| Bromocriptine-QR | Placebo | |
|---|---|---|
| n | 2,054 | 1,016 |
| SAEs by SOC* | ||
| Cardiac** | 51 (2.5) | 37 (3.6) |
| Infections and infestations | 27 (1.3) | 13 (1.3) |
| Nervous system disorders | 26 (1.3) | 14 (1.4) |
| General disorders | 14 (0.7) | 9 (0.9) |
| Gastrointestinal disorders | 13 (0.6) | 9 (0.9) |
| Vascular disorders | 10 (0.5) | 8 (0.8) |
| Respiratory, thoracic and mediastinal | 13 (0.6) | 3 (0.3) |
| Injury, poisoning, and procedural complications | 12 (0.6) | 3 (0.3) |
| Musculoskeletal and connective tissue | 11 (0.5) | 4 (0.4) |
| Neoplasm benign, malignant, and unspecified | 8 (0.4) | 2 (0.2) |
| Endocrine | 5 (0.2) | 4 (0.4) |
| Hypoglycemia† | 4 (0.2) | 4 (0.4) |
| Metabolism and nutrition disorders | 7 (0.3) | 2 (0.2) |
| Renal and urinary disorders | 6 (0.3) | 3 (0.3) |
| Hepatobiliary disorders | 4 (0.2) | 1 (0.1) |
| Psychiatric disorders | 4 (0.2) | 1 (0.1) |
| Reproductive system and breast disorders | 4 (0.2) | 0 |
| Eye disorders | 0 | 2 (0.2) |
| Any SAE | 176 (8.6) | 98 (9.6) |
| Hazard ratio and 96% one-sided confidence limit | 1.02 (—, 1.27) | |
| Prespecified adjudicated composite cardiovascular endpoint | ||
| Time to first composite CVD event | 37 (1.8%) | 32 (3.1%) |
| Hazard ratio and 95% confidence limit | 0.60 (0.37–0.96) | |
| Composite CVD end point by each component‡ | ||
| Myocardial infarction | 7 (0.3) | 9 (0.9) |
| Stroke | 5 (0.2) | 6 (0.6) |
| Hospitalization for unstable angina | 9 (0.4) | 9 (0.9) |
| Hospitalization for congestive heart failure | 9 (0.4) | 6 (0.4) |
| Coronary revascularization | 11 (0.5) | 8 (0.8) |
Data are n (%).
*SOC as determined by MedDRA coding of preferred term provided by study investigator. Patients may appear in more than one system organ category if they experienced multiple AEs belonging to different SOCs. All SAEs were adjudicated by an independent AEAC.
**The cardiac SOC includes 37 different preferred terms such as arrhythmia, myocardial infarction, chest pain, and coronary artery disease; however the composite cardiovascular end point comprised events that met the prespecified end point criteria set forth by an independent AEAC that was blinded to treatment assignment.
†Hypoglycemia is a subcategory of the endocrine SOC.
‡Patients may appear in more than one component of the composite cardiovascular end point if they experienced multiple cardiovascular AEs.
In the analysis of time to first all-cause SAE, the and upper bound of the one-sided confidence limit support noninferiority between bromocriptine-QR and placebo, (HR 1.02 [96% one-sided CI 1.27]). The treatment effect did not change appreciably with the addition of the baseline covariates of age, duration of diabetes, insulin usage, sex and race. The center by treatment interaction term was also nonsignificant.
CVD.
The composite CVD end point occurred in 37 bromocriptine-QR–treated (1.8%) and 32 placebo-treated (3.2%) patients resulting in a 40% CVD relative risk reduction (HR 0.60 [95% two-sided CI 0.37–0.96]; Table 2). The observed CVD risk reduction did not change appreciably with the addition of the baseline covariates of age, baseline A1C level, sex, race, and prior history of stroke or prior history of coronary revascularization.
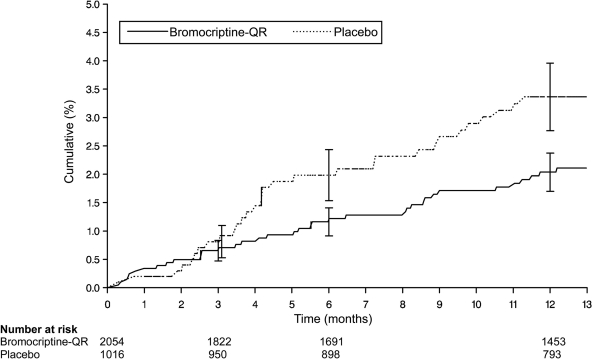
Figure 1 shows the Kaplan-Meier estimates of the proportion of patients by treatment that experienced an event within the composite end point and demonstrates a clear separation in the curves that begins after 3 months of treatment.
Figure 1.
Kaplan-Meier curve of the occurrence of the prespecified composite cardiovascular end point among patients randomized to bromocriptine-QR or placebo. The composite cardiovascular end point consisted of the time to first myocardial infarction, stroke, coronary revascularization, hospitalization for unstable angina, or hospitalization for congestive heart failure that occurred after randomization. All cardiovascular events were independently adjudicated. The hazard ratio of bromocriptine-QR versus placebo for the occurrence of the composite cardiovascular end point was 0.60 (95% CI 0.37–0.96). The effect of treatment was estimated from the unadjusted Cox proportional-hazard model that used all the available data.
Deaths.
The frequency of deaths that occurred while on a study drug or within 30 days of stopping a study drug was similar between the treatment groups (0.19% bromocriptine-QR, 0.20% placebo). Five patients randomized to bromocriptine-QR and one randomized to placebo died in excess of 30 days after stopping the study drug.
Overall adverse events summary
Adverse events (AEs) occurred in 89% of bromocriptine-QR–treated and 83% of placebo-treated patients. AEs were not commonly reported as severe in either treatment group (17% events reported as severe on bromocriptine-QR compared with 14% on placebo). More patients discontinued bromocriptine-QR than placebo due to an adverse event (24% vs. 11%, respectively). The most commonly reported adverse event among patients who discontinued bromocriptine-QR was nausea (7.6% bromocriptine-QR vs. 1% placebo). The only AEs that occurred at a frequency rate of at least 5% and were numerically greater in the bromocriptine-QR arm were nausea, vomiting, dizziness, headache, and diarrhea. Nausea was the most common adverse event (32.2% bromocriptine-QR vs. 7.6% placebo). None of the events of nausea were reported as serious. The majority of the commonly occurring adverse events attributed to bromocriptine-QR occurred during the initial titration phase were dose related and transient in nature (mean duration of <14 days). After the initial titration phase, commonly occurring adverse events were reported at a frequency similar to that observed in the placebo treated arm.
Other AEs
Somnolence (4.3 bromocriptine-QR vs. 1.3% placebo) and hypoesthesia (1.4 bromocriptine-QR vs. 1.1% placebo) were the only AEs within the nervous system organ class that were reported at a rate of <5% and ≥1% and that occurred at a numerically greater frequency among bromocriptine-QR-treated patients. Additionally, 15 (0.7%) of patients on bromocriptine-QR and 14 (1.4%) on placebo reported depression or depressed mood, and 13 (0.6%) patients on bromocriptine-QR and 8 (0.8%) on placebo reported anxiety. Hypoglycemic AEs occurred infrequently, with 6.9% bromocriptine-QR patients reporting an event versus 5.3% in the placebo-treated arm.
Laboratory measures, vital signs, and electrocardiograms
There were no significant differences between the treatment arms in changes from baseline in hematology or chemistry laboratory values. There were minimal changes from baseline in lipid measures and blood pressure in both groups (online appendix IV, Table B). Compared with those on placebo, heart rate among patients randomized to bromocriptine-QR decreased ∼1 bpm from a baseline study population mean heart rate of 68 bpm at week 52 (P = 0.02). The corrected QT interval decreased, albeit nonsignificantly, from baseline to week 52 among bromocriptine-QR–treated patients by 3.2 ms (baseline average 418 ms) and by 1.9 ms for the placebo arm (baseline average 420 ms). There was no difference in the mean change in body weight from baseline to week 52 for either bromocriptine-QR (+0.2 kg) or placebo (+0.1 kg).
CONCLUSIONS
The present studyis the first to assess, as a primary objective and end point, the overall and cardiovascular safety of a new oral antidiabetes therapy in a large population of patients with type 2 diabetes. The findings from this trial indicate that morning bromocriptine-QR therapy is noninferior to placebo for overall safety. The overall frequency of all-cause SAEs and the proportion of SAEs observed in each SOC among patients taking bromocriptine-QR was noninferior to the frequency among patients in the placebo arm. Furthermore, the frequency of the composite cardiovascular end point was statistically significantly reduced in the bromocriptine-QR group compared with the placebo group. The Kaplan-Meier estimates indicate that among 1,000 patients allocated to bromocriptine-QR, 13 first myocardial infarctions, strokes, coronary revascularizations, hospitalizations for congestive heart failure, or hospitalizations for unstable angina would be avoided over 1 year. Simply stated, 79 patients would need to be treated for 1 year to avoid one first important CVD event. It should be appreciated that the reduction in CVD events occurred among a study population where the majority of the patients were receiving appropriate cardioprotective medications and where blood pressure, glucose and lipids on average were optimally controlled. Furthermore, these results were demonstrated among patients with an average duration of type 2 diabetes of 8 years and in which a third had preexisting cardiovascular disease.
Bromocriptine-QR was developed to provide a discrete and brief daily interval of circulating bromocriptine at a particular time of day (morning) and thereby provide a timed pulse of dopamine activity centrally. This formulation/treatment feature differentiates bromocriptine-QR bioavailability and dosing from the multiple-times per day and higher overall dosing of traditional formulations of bromocriptine for other indications such as Parkinson's disease or acromegaly, which may deliver higher circulating drug levels throughout the day and as a consequence prolonged dopamine stimulation centrally.
The mechanism by which such timed bromocriptine-QR may reduce cardiovascular outcomes is not clear. In this trial, treatment with bromocriptine-QR was associated with reductions in fasting triglyceride levels, blood pressure, and heart rate but not to the extent one would expect to explain the observed CVD risk reduction. Improvements in a host of CVD risk factors in addition to glycemic control are likely necessary to truly attenuate the excessive risk of CVD events experienced by patients with type 2 diabetes (20). The peripheral cardiometabolic effects of bromocriptine-QR on cardiovascular risk may be in part the consequence of its attenuation of central nervous system and hypothalamic functions potentiating sympathetic nervous system over-activity to the vasculature, visceral adipose, and liver as well as attenuation of increased hypothalamic-pituitary-adrenal axis activity, (14) which are known to increase CVD risk if overactive (21,22). Improvements in postprandial hyperglycemia and hyperlipidemia (16) have been observed with this treatment and may have contributed to the CVD event reduction in this trial. Moreover, recent studies in animal models of insulin resistance have demonstrated marked improvements in both liver inflammatory pathways potentiating vascular damage and endothelial dysfunction during treatment with a parental formulation of bromocriptine (A.C., unpublished data). Although increased sympathetic tone (23), postprandial dysmetabolism (24), endothelial dysfunction and inflammation (25) are important CVD risk factors that have been attenuated with bromocriptine therapy in previous clinical and preclinical studies, the impact of bromocriptine-QR on these measures was not assessed in this study. Much more work is required to completely understand the complex biochemical and physiological mechanisms by which timed bromocriptine-QR produces its potential cardiovascular effects. A study of longer duration and sufficient size to provide a greater number of cardiovascular outcomes could allay any concerns that the results in this trial are a chance finding while further quantifying the potential long-term reduction in cardiovascular events.
Nausea was the chief limiting adverse event in this trial. For the majority of patients experiencing nausea, the symptoms occurred during the initial titration of the drug and lasted less than 2 weeks. The gastrointestinal side effects associated with bromocriptine-QR are dose related and the “forced” titration scheme (rechallenge with study drug after an AE at a given dose and attempt to continue to uptitrate to achieve a maximum tolerated dose of 4.8 mg) likely contributed to a higher than expected discontinuation rate. The incidence rates of hypoglycemia were low and similar between bromocriptine-QR and placebo groups. Importantly, the lack of hypoglycemic AEs was observed even when bromocriptine-QR was used as add-on therapy to a variety of antidiabetes agents including insulin. This is most likely because bromocriptine-QR does not increase insulin levels and primarily reduces postprandial glucose by way of improving the body's responsiveness to insulin (16).
Bromocriptine-QR represents a new treatment modality for type 2 diabetes. In patients with type 2 diabetes, the addition of bromocriptine-QR to routine standard of care therapies was shown to be safe and associated with fewer cardiovascular outcomes. This first-in-class therapy may provide a new approach to addressing the comorbidities associated with type 2 diabetes.
Supplementary Material
Acknowledgments
J.M.G. has received investigator-initiated federal funding from National Institutes of Health (National Cancer Institute; National Heart, Lung, and Blood Institute; National Institute on Aging; National Eye Institute) and the Veteran's Affairs Cooperative Studies Program.
The study was initially sponsored by Pliva, Inc. (Zagreb, Croatia) in collaboration with VeroScience, LLC (Tiverton, RI), and subsequently by VeroScience, LLC unilaterally. The sponsors were involved in the study design and statistical analysis plan. The writing of this report and the decision to publish was the responsibility of all the study authors. J.M.G. has received nonfederal investigator initiated funding from Amgen and Pliva; has received research support in the form of pills and/or packaging from Wyeth Pharmaceuticals; and has received honoraria from Bayer and McNeil Consumer Products for speaking engagements. A.H.C. and R.E.S. are officers at VeroScience, LLC. C.M.O. served as paid chairman of the Adverse Adjudication Committee for VeroScience, LLC. M.E. is employed by VeroScience, LLC. Z.J.M. has been a paid consultant for VeroScience, LLC. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 67th Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 22–26 June 2007.
The authors thank the study patients for their important contribution in furthering the scientific community's understanding of this treatment for type 2 diabetes and all of the site investigators and coordinators for their excellent work on this trial. A complete list of site investigators is available in the online appendix.
Footnotes
Clinical trial reg. no. NCT00377676, clinicaltrials.gov
A complete list of site investigators and coordinators is available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2009/DC1.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H: The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005; 28: 2130–2135 [DOI] [PubMed] [Google Scholar]
- 2. Diabetes Facts and Figures. World Health Ogranization. Available from http://www.who.int/diabetes/facts/en/. Accessed
- 3. Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457–2471 [DOI] [PubMed] [Google Scholar]
- 4. Olsson J, Lindberg G, Gottsäter M, Lindwall K, Sjöstrand A, Tisell A, Melander A: Increased mortality in Type II diabetic patients using sulphonylurea and metformin in combination: a population-based observational study. Diabetologia 2000; 43: 558–560 [DOI] [PubMed] [Google Scholar]
- 5. Walker AM, McAfee AT, Koro C: Studies of diabetes, thiazolidinediones, and coronary heart disease. Pharmacoepidemiol Drug Saf 2007; 16: 1313–1314 [DOI] [PubMed] [Google Scholar]
- 6. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R: Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004; 27: 256–263 [DOI] [PubMed] [Google Scholar]
- 7. Johnson JA, Majumdar SR, Simpson SH, Toth EL: Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002; 25: 2244–2248 [DOI] [PubMed] [Google Scholar]
- 8. Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes–an interim analysis. N Engl J Med 2007; 357: 28–38 [DOI] [PubMed] [Google Scholar]
- 9. Skyler JS: PROactive: A sad tale of inappropiate analysis and unjustified interpretation. Clinical Diabetes 2006; 24: 63–65 [Google Scholar]
- 10. Dluhy RG, McMahon GT: Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med 2008; 358: 2630–2633 [DOI] [PubMed] [Google Scholar]
- 11. Scranton RE, Gaziano JM, Rutty D, Ezrokhi M, Cincotta A: A randomized, double-blind, placebo-controlled trial to assess safety and tolerability during treatment of type 2 diabetes with usual diabetes therapy and either Cycloset or placebo. BMC Endocr Disord 2007; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo S, Liang Y, Cincotta AH: Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose-intolerant state in hamsters. Neuroendocrinology 1999; 69: 160–166 [DOI] [PubMed] [Google Scholar]
- 13. Luo S, Luo J, Meier AH, Cincotta AH: Dopaminergic neurotoxin administration to the area of the suprachiasmatic nuclei induces insulin resistance. Neuroreport 1997; 8: 3495–3499 [DOI] [PubMed] [Google Scholar]
- 14. Cincotta A: Hypothalamic Role in the Insulin Resistance Syndrome. In Reistance and Insulin Resistance Syndrome. Hansen B, Shaffrir E. Eds. London, Taylor and Francis, 2002, p. 271–312 [Google Scholar]
- 15. Monti JM, Monti D: The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev 2007; 11: 113–133 [DOI] [PubMed] [Google Scholar]
- 16. Cincotta AH, Meier AH, Cincotta M, Jr: Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8: 1683–1707 [DOI] [PubMed] [Google Scholar]
- 17. Cincotta AH, Meier AH: Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care 1996; 19: 667–670 [DOI] [PubMed] [Google Scholar]
- 18. American Diabetes Association. Diagnosis and classification of diabetes mellitus (Position Statement). Diabetes Care 2004; 27(Suppl. 1): 5–10 [Google Scholar]
- 19. American Diabetes Association. Standards of Medical Care in Diabetes (Position Statement). Diabetes Care 2004; 27(Suppl. 1): 15–35 [DOI] [PubMed] [Google Scholar]
- 20. Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. American Diabetes Association, American College of Cardiology Foundation, American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009; 53: 298–304 [DOI] [PubMed] [Google Scholar]
- 21. Grassi G: Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertens Res 2006; 29: 839–847 [DOI] [PubMed] [Google Scholar]
- 22. Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, Epaminonda P, Masserini B, Beck-Peccoz P, Orsi E, Ambrosi B, Arosio M: Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 2007; 30: 83–88 [DOI] [PubMed] [Google Scholar]
- 23. Grassi G: Role of the sympathetic nervous system in human hypertension. J Hypertens 1998; 16: 1979–1987 [DOI] [PubMed] [Google Scholar]
- 24. O'Keefe JH, Bell DS: Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 2007; 100: 899–904 [DOI] [PubMed] [Google Scholar]
- 25. Sjöholm A, Nyström T: Endothelial inflammation in insulin resistance. Lancet 2005; 365: 610–612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.