Abstract
OBJECTIVE
To assess the effect of a 4-week adjunctive therapy of exenatide (EXE) (5–10 μg b.i.d.) or sitagliptin (SITA) (100 mg once daily) in response to a standardized breakfast meal challenge in 48 men or women with type 2 diabetes receiving insulin glargine (GLAR) + metformin (MET).
RESEARCH DESIGN AND METHODS
This was a single-center, randomized, open-label, active comparator–controlled study with a three-arm parallel group design, consisting of: screening, 4- to 8-week run-in period, 4-week treatment period, and follow-up. In all three groups, the GLAR dose was titrated according to an algorithm (fasting blood glucose ≤100 mg/dl).
RESULTS
The unadjusted 6-h postprandial blood glucose excursion of both GLAR + MET + EXE and GLAR + MET + SITA was statistically significantly smaller than that of GLAR + MET (606 ± 104 vs. 612 ± 133 vs. 728 ± 132 mg/dl/h; P = 0.0036 and 0.0008). A1C significantly decreased in all three groups (P < 0.0001), with the greatest reduction of −1.9 ± 0.7 under GLAR + MET + EXE (GLAR + MET + SITA −1.5 ± 0.7; GLAR + MET −1.2 ± 0.5%-points; GLAR + MET + EXE vs. GLAR + MET P = 0.0154). The American Diabetes Association A1C target of <7.0% was reached by 80.0, 87.5, and 62.5% of subjects, respectively. GLAR + MET + EXE had the highest number (47) of adverse events, mostly gastrointestinal (56%) with one dropout. GLAR + MET or GLAR + MET + SITA only had 10 and 12 adverse events, respectively, and no dropouts. Hypoglycemia (blood glucose <50 mg/dl) rates were low and comparable among groups. Weight decreased with GLAR + MET + EXE (−0.9 ± 1.7 kg; P = 0.0396) and increased slightly with GLAR + MET (0.4 ± 1.5 kg; NS; GLAR + MET + EXE vs. GLAR + MET P = 0.0377).
CONCLUSIONS
EXE or SITA added to GLAR + MET further substantially reduced postprandial blood glucose excursions. Longer-term studies in a larger population are warranted to confirm these findings.
The UK Prospective Diabetes Study (UKPDS) demonstrated that good glycemic control in type 2 diabetes is associated with a reduced risk of diabetes complications (1). After lifestyle modifications (diet and exercise) and oral hypoglycemic agents (OHAs) the addition of basal insulin to OHAs is common practice (2), because this kind of regimen requires only a single injection in most cases and can improve glycemic control. Its use, however, may not adequately control postprandial hyperglycemia or may be associated with hypoglycemia and/or weight gain (3,4). Because obesity is frequently present in subjects with type 2 diabetes (5) and represents a factor contributing to insulin resistance (5) and cardiovascular risk (5), weight gain may be particularly undesirable.
A significant advance in basal insulin therapy was the introduction of insulin glargine, a long-acting insulin analog with an extended duration of action of ∼24 h without exhibiting a pronounced peak (6,7). In subjects with type 2 diabetes, insulin glargine was shown to confer glycemic control at least equivalent to that of NHP insulin with a lower incidence of hypoglycemia (3,8,9). However, insulin glargine still has the drawbacks of insulin treatment such as weight gain (3,8,9) and a lower effect on postprandial glucose excursions (8) than on fasting glucose values.
Exenatide is the first-in-class glucagon-like peptide 1 (GLP-1) receptor agonist (or incretin mimetic) approved in the U.S. and Europe (10). Compared with placebo, exenatide statistically reduced A1C, whereas there was no difference in A1C improvement between exenatide and insulin glargine or biphasic insulin aspart (11–14). However, postprandial glycemia as well as weight was further reduced with exenatide compared with insulin glargine or biphasic insulin, with a similar risk of hypoglycemia (12,13).
Sitagliptin is an approved once-daily, potent, and highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor (15). When added to metformin, sitagliptin, given at a dose of 100 mg once daily over 24 weeks, led to significant reductions in A1C, fasting, and 2-h postprandial plasma glucose and was weight-neutral (16).
With this background, a therapy controlling both fasting blood glucose (FBG) and postprandial glucose excursions seems to be a promising approach for subjects with type 2 diabetes (17–21). Therefore, in the present study we investigated the influence of a 4-week adjunctive therapy of either a GLP-1 receptor agonist (exenatide) or a DPP-4 inhibitor (sitagliptin) to titrated basal insulin (insulin glargine) plus metformin versus the continuation with titrated insulin glargine plus metformin alone as active comparator in subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Enrolled subjects were men or women aged 35–70 years, with type 2 diabetes for >6 months and <10 years, treated either with a stable dose of metformin with or without sulfonylureas or treated solely with a long- or intermediate-acting insulin formulation (insulin glargine, insulin detemir, or NPH insulin) with or without a stable dose of metformin for at least 3 months. Inclusion criteria included BMI between 21.0 and 39.9 kg/m2 inclusive, A1C ≥7.0 and ≤10.0%, and stable antihypertensive or lipid-lowering therapy for at least 3 months, if applicable. Subjects were excluded, if they had a history or evidence of any other clinically relevant medical conditions or had used a rapid-acting insulin or a mixed insulin formulation or any other oral antidiabetic agent except for metformin or metformin plus sulfonylurea during the previous 3 months. Exclusion criteria further included abnormal laboratory test results, in particular, alanine aminotransferase and/or aspartate aminotransferase ≥3 times the laboratory's upper limit of reference range or serum creatinine ≥1.6 mg/dl for men and ≥1.4 mg/dl for women, uncontrolled hypertension, anemia, recurrent hypoglycemia, or any systemic or topical treatment with drugs known to influence glucose metabolism.
This single-center, randomized, open-label, active comparator–controlled study with a three-arm parallel group design was performed between 9 January and 29 September 2008. It was conducted in accordance with the principles of the Declaration of Helsinki (22) and approved by the local ethics committee and regulatory authorities. All subjects provided written informed consent.
Study protocol and treatment
After a screening visit (visit 0) to assess a subject's eligibility for study participation, a 4- or 8-week run-in period, depending on the subject's preexisting antidiabetic therapy, with insulin glargine at bedtime followed, starting at visit 1:4 weeks run-in for a subject who already had an insulin pretreatment or 8 weeks for a subject who had oral antidiabetes treatment only (OHA). Preexisting treatment with metformin was continued for the entire study, whereas sulfonylurea treatment was stopped at the beginning of the run-in period. All subjects received insulin glargine at bedtime, at a starting dose of 10 units for those subjects new to insulin. During the whole course of the study each subject's insulin glargine dose was titrated to a FBG target of ≤5.6 mmol/l (≤100 mg/dl) using a predefined treatment algorithm which, with slight modifications, corresponds to that used by Riddle et al. (8) in the treat-to-target trial for the introduction of insulin glargine to oral antidiabetes drug–treated patients with type 2 diabetes. During the run-in period telephone contacts (two per week) and weekly outpatient visits (visits 2–4 or 2–4 d for the 8-week run-in) at the study site were performed. At the end of the run-in period, 2 days before allocation to one of the three treatment arms, the insulin glargine dose was reduced by 20% to avoid hypoglycemia. At visit 5 (baseline), subjects in an open-label fashion were randomly assigned to either the addition of exenatide or sitagliptin to insulin glargine plus metformin (GLAR + MET + EXE or GLAR + MET + SITA, respectively) or to the continuation of insulin glargine plus metformin alone (GLAR + MET) for 4 weeks. Exenatide was to be administered subcutaneously at a dose of 5 μg in the morning and evening (b.i.d.) for 2 weeks followed by 10 μg b.i.d. for the second 2 weeks. Sitagliptin was taken orally at a dose of 100 mg once daily in the morning. During the 4-week-treatment period, weekly visits (visits 6–8) at the study site plus twice weekly telephone contacts were continued to further titrate blood glucose to the FBG target of ≤5.6 mmol/l (≤100 mg/dl). The 4-week treatment period ended with a 24-h in-house visit, during which efficacy assessments were performed (visit 9). The study was completed with a final visit (visit 10) 2–10 days after the in-house stay.
During both the run-in and the treatment period, subjects self-measured and recorded their FBG (and any potential voluntary preprandial and/or postprandial blood glucose values) on a daily basis using a commercial glucose meter (Accu-Chek® Aviva; Roche Diagnostics, Mannheim, Germany). Once per week they performed 7-point blood glucose profiles at the following times: before and 2 h after breakfast, lunch, and dinner and before bedtime (11:00 p.m.). All results and times of self-measurements of blood glucose as well as insulin doses were entered by the subjects into a study diary. If at any time during the study (except at screening and the final visit) self-measured FBG values on 2 consecutive days of >13.3 mmol/l (>240 mg/dl) were confirmed at the site by means of a validated glucose oxidase method (Super-GL glucose analyzer; Hitado Diagnostic Systems, Möhnesee, Germany), the subject had to be withdrawn from the study. The same procedure applied for hypoglycemic blood glucose values occurring at 2 consecutive days. Hypoglycemia was classified as major if a subject was not able to treat the episode himself or herself, and as minor if the subject was able to treat the episode himself or herself and blood glucose was <2.8 mmol/l (<50 mg/dl) or if no blood glucose value was available or if blood glucose was ≥2.8 mmol/l (≥50 mg/dl) with symptoms only.
In addition to a thorough review of the subject's blood glucose values in the study diary and adjustments of the insulin glargine dose, the following assessments were performed at the weekly visits at the study site: vital signs, i.e., blood pressure and heart rate; adverse events and hypoglycemic episodes (at each visit); physical examination and safety laboratory (at screening, before random assignment at visit 5 and at the final visit); pregnancy test for women of child-bearing potential (at screening, at visits 5 and 9, and at the final visit); electrocardiogram (ECG) (at screening and at the final visit), FBG (at screening and at visits 5, 7, and 9 [on both days of the 24-h in-house stay]); and fasting lipid profile (at visits 5 and 9). At visit 9, during the 24-h in-house stay the following was assessed on day 1: dosing of either exenatide or sitagliptin 60 min before a standardized 618.2 kcal breakfast consisting of 99.4 g carbohydrates, 11.9 g lipids, and 26.2 g protein, a 7-point 24-h blood glucose profile, postprandial blood glucose excursions after the standardized breakfast (16 blood glucose samples from −30 to 360 min). On day 2 of the in-house stay, after completion of all assessments including A1C, the subjects were instructed on how to continue with their prestudy antidiabetes medication and were asked to measure at least FBG and document these measurements as well as any additional blood glucose values in the diary provided.
Study end points
The primary outcome measure was the unadjusted 6-h postprandial blood glucose excursion (AUC BG0–6 h) after ingestion of the standardized breakfast, assessed at the end of the 4-week treatment period. Secondary end points included the following: mean daily blood glucose as assessed by means of the 7-point 24-h blood glucose profile at the end of the 4-week treatment period, the subjects' self-measurements of FBG and the 7-point blood glucose profiles throughout the run-in and the treatment period, the percentage of subjects achieving American Diabetes Association treatment goals, i.e., A1C <7.0%, at the end of the treatment period, fasting lipid profile, body weight, each as assessed at the end of the treatment period, number and severity of hypoglycemic episodes, and general safety parameters (adverse events, physical examination, vital signs, safety laboratory parameters, and ECG recordings).
Statistical analysis
A sample size of 16 subjects per treatment arm (in a total of 48 subjects) was estimated to detect significant differences between treatments (23). SAS (version 9.1; SAS Institute, Cary, NC) was used to conduct all statistical analyses. ANCOVA models, allowing the estimation of least squares means with corresponding 95% confidence limits, were used on untransformed Δ body weight, Δ lipids, and Δ A1C and on natural logarithm–transformed AUC BG(0–6 h), and 7-point blood glucose profile, with baseline assessments as confounders. ANCOVA models with repeated measures on the factor day were applied to test for differences in logarithm-transformed FBG and self-measured 7-point blood glucose. For log-transformed data, the calculated 95% CIs were back-transformed to derive the appropriate confidence limits for the geometric mean ratios of the pairwise treatment comparisons. Paired t tests were used to test within-group changes, and for between-group comparisons unpaired t tests were applied. χ2 tests were used to compare the frequency of subjects achieving ADA treatment goals (A1C <7.0%) and of subjects achieving International Diabetes Federation treatment goals (A1C <6.5%) at the end of the treatment period. Descriptive statistics on demographics, safety, and glycemic end points were provided for all randomly assigned subjects who received at least one dose of investigational medication. The areas under the blood glucose curve, extending from the meal intake to 6 h after ingestion, were calculated using the trapezoid rule. Δ A1C was determined as the difference of visit 9 A1C minus screening A1C. Δ body weight as well as Δ lipid were calculated as visit 9 values minus visit 5 values. The statistical significance level was set at P < 0.05. Data are means ± SD if not otherwise stated.
RESULTS
Subject disposition and clinical characteristics
A total of 48 subjects with type 2 diabetes (60.4% men, aged [mean ± SD] 57 ± 7 years, with BMI 31.7 ± 3.4 kg/m2, diabetes duration 6 ± 1 years, and A1C 8.1 ± 0.7%) were randomly assigned to three arms: GLAR + MET + EXE (up to 10 μg b.i.d.; n = 16), GLAR + MET + SITA (100 mg q.d.; n = 16), or continuation with GLAR + MET (control; n = 16) for 4 weeks (Fig. 1). Of the subjects, 47 completed the study according to the protocol. One subject in the GLAR + MET + EXE group was withdrawn after the first 2 weeks of the 4-week treatment period (because of a loss of appetite deemed to be possibly related to the study treatment). Baseline A1C was 7.9% in both the GLAR + MET + SITA and GLAR + MET groups, whereas it was slightly higher (8.4%) in the GLAR + MET + EXE group. This difference, however, was not of statistical significance. All subjects were receiving MET at the study start; in addition, 7 subjects had preexisting sulfonylurea therapy and 9 subjects had been treated with insulin.
Figure 1.
Subject disposition. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate transferase.
Primary and secondary end points
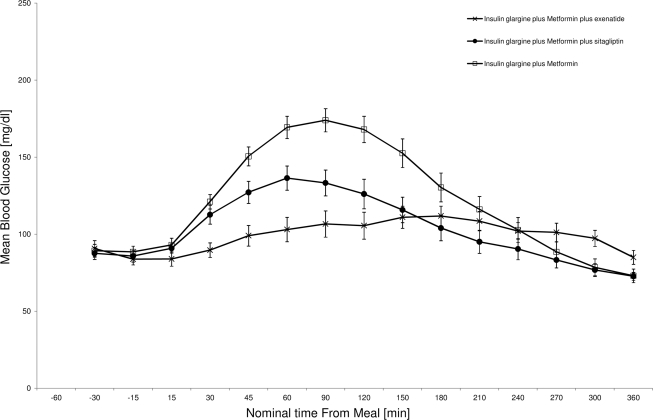
After 4 weeks of treatment the AUC BG0–6 h values of both GLAR + MET + EXE and GLAR + MET + SITA were significantly smaller (EXE 17% reduction, P = 0.0036; SITA 20% reduction, P = 0.0008) than that of GLAR + MET, whereas values for the two combination treatments were not significantly different from each other (P = 0.5734) (Table 1, Fig. 2).
Table 1.
Primary and secondary end points
| Parameter | Time point* | GLAR+MET+EXE | GLAR+MET+SITA | GLAR+MET |
|---|---|---|---|---|
| n | 15 | 16 | 16 | |
| AUCBG0–6 h (mg/dl/h) | EOT | 606 ± 104† | 612 ± 133† | 728 ± 132 |
| BG0–6 h (mg/dl)‡ | EOT | 97§ | 96§ | 116 |
| Mean BG (mg/dl) | EOT | 97 ± 17† | 100 ± 18† | 121 ± 19 |
| FBG (mg/dl) | Baseline | 94 | 96 | 94 |
| EOT | 82‖ | 84‖ | 89 | |
| Mean from self-measured 7-point BG profiles (mg/dl) | Baseline–EOT | 109† | 109† | 118 |
| Self-measured FBG (mg/dl) | Baseline–EOT | 93 | 93 | 96 |
| A1C (%) | Screen | 8.39 ± 0.98 | 7.89 ± 0.48 | 7.91 ± 0.57 |
| EOT | 6.53 ± 0.59 | 6.41 ± 0.50 | 6.73 ± 0.42 | |
| Change | −1.80†‖ | −1.49‖ | −1.23‖ | |
| A1C <7.0% (% patients) | EOT | 80¶ | 88¶ | 63 |
| Changes in total cholesterol (mmol/l) | Baseline–EOT | −0.24 ± 0.48§ | −0.27 ± 0.61§ | 0.30 ± 0.52‖ |
| Changes in HDL cholesterol (mmol/l) | Baseline–EOT | −0.04 ± 0.14 | −0.04 ± 0.21 | 0.08 ± 0.17 |
| Changes in LDL cholesterol (mmol/l) | Baseline–EOT | −0.30 ± 0.46†‖ | −0.28 ± 0.42†‖ | 0.09 ± 0.36 |
| Changes in body weight (kg) | Baseline–EOT | −0.9 ± 1.7†‖ | 0.1 ± 1.6 | 0.4 ± 1.5 |
| Hypoglycemic episodes (events per subject year) | Major | — | — | — |
| BG <50 mg/dl | 2 (1.68) | 3 (2.45) | 2 (1.62) | |
| Symptoms only | 10 | 1 | 4 | |
| Adverse events | Total | 47 (62.5) | 12 (43.8) | 10 (25.0) |
| Gastrointestinal disorders | 28 (56.3) | 4 (18.8) | 1 (6.3) |
Data are means ± SD, geometric means (least squares means) for comparisons, or n (%) unless otherwise indicated. AUCBG0–6 h, area under the blood glucose curve 0–6 h after a standard breakfast; BG, blood glucose.
*Screen, before washout of oral agents except for metformin and run-in period; Baseline, before randomization; EOT, end of treatment.
†P < 0.05 vs. GLAR.
‡Post hoc analysis (repeated-measures ANCOVA).
§P < 0.01 vs. GLAR.
‖P < 0.05 vs. Screen/Baseline;
¶P < 0.05 (χ2 test) vs. A1C <7.0%.
Figure 2.
Mean (SEM) meal BG profiles.
A1C significantly declined in all three groups (least squares means GLAR + MET + EXE −1.8, GLAR + MET + SITA −1.5, and GLAR + MET −1.2; P < 0.0001 each) with GLAR + MET + EXE leading to a significantly higher A1C decrease than GLAR + MET (least squares mean −0.6, P = 0.0154). Metabolic control on average was excellent: the ADA A1C target of <7% was achieved by 80% of subjects in the GLAR + MET + EXE group, 88% of subjects in the GLAR + MET + SITA group, and 63% of subjects in the GLAR + MET group.
With GLAR + MET + EXE and GLAR + MET + SITA, the 7-point 24-h blood glucose profiles after 4 weeks of treatment were significantly lower than those under GLAR + MET. The weekly self-measured 7-point blood glucose profiles were also lower during the treatment period.
Self-measured daily FBG values (mean of all measurements in treatment period) were comparable among the three treatments after significant decreases in FBG had been reached already during the run-in period. However, when FBG at randomization (visit 5) was compared with that at the end of the 4-week treatment (visit 9), a significant reduction was observed for each of the combination treatments (P = 0.0018 for GLAR + MET + EXE and P = 0.0016 for GLAR + MET + SITA), whereas this was not the case for GLAR + MET (P = 0.2084).
Both adjunctive therapies showed a comparable lowering effect on serum cholesterol (total and LDL). This effect was not seen in the control group.
Body weight was stable with GLAR + MET and GLAR + MET + SITA (0.4 ± 1.5 and 0.1 ± 1.6 kg, respectively) and slightly decreased with GLAR + MET + EXE (−0.9 ± 1.7 kg), which was statistically significant versus GLAR + MET (P = 0.0377).
Hypoglycemic episodes and general safety
Over the 4-week treatment period no major hypoglycemic episode occurred in any of the three groups. Thirteen subjects experienced in total 22 hypoglycemic episodes: 5 subjects in the GLAR + MET + EXE group experienced 12 hypoglycemic episodes (10.1 events per subject year), 2 of these were minor (1.7 events per subject year) and 10 were symptoms only (GLAR + MET + SITA group: 2 subjects, 4 hypoglycemic episodes, 3.3 events per subject year, 3 were minor = 2.5 events per subject year; GLAR + MET: 6 subjects, 6 hypoglycemic episodes, 2 were minor = 1.6 events per subject year). Two subjects in the GLAR + MET + EXE group had 4 hypoglycemic episodes each. There was no nocturnal hypoglycemia (between 11:00 p.m. and 6:00 a.m.) in any of the three groups. Eight of the 12 hypoglycemic episodes in the GLAR + MET + EXE group occurred between 6:00 and 7:00 p.m., whereas all hypoglycemic episodes in the GLAR + MET group took place between 10 a.m. and 2:30 p.m.
The insulin glargine dose did not decrease with EXE or SITA, whereas it increased in the control group. Mean insulin doses at randomization and at the end of treatment were 40.3 IU (0.60 ± 0.25 IU/kg) and 41.1 IU (0.42 ± 0.18 IU/kg) in the GLAR + MET + EXE group (GLAR + MET + SITA: 33.4 IU [0.50 ± 0.20 IU/kg] and 35.0 IU [0.36 ± 0.17 IU/kg]; GLAR + MET: 32.3 IU [0.50 ± 0.21 IU/kg] and 37.9 IU [0.42 ± 0.20 IU/kg]), with a mean increase of 5.6 IU in the GLAR group.
Ten of the 16 subjects (62.5%) in the GLAR + MET + EXE group experienced in total 47 adverse events, 56% being due to gastrointestinal disorders and causing 1 dropout after 2 weeks (in the GLAR + MET + SITA group 7 subjects had 12 adverse events and in the GLAR + MET group 4 subjects had 10 adverse events; gastrointestinal disorders were 19 and 16%, respectively). All gastrointestinal adverse events were of mild or moderate intensity. There was no serious adverse event in any of the groups and only one severe adverse event, not related to trial drug, in the GLAR + MET + SITA group.
No clinically relevant findings or changes were seen in safety laboratory tests, physical examination results, ECG recordings, and vital sign measurements.
CONCLUSIONS
To our knowledge this is the first study investigating a 4-week adjunctive therapy of either a GLP-1 analog or a DPP-4 inhibitor added to titrated insulin glargine plus metformin, compared with insulin glargine plus metformin alone acting as active control in subjects with type 2 diabetes. The addition of exenatide or sitagliptin to insulin glargine plus metformin led to statistically significant improvements in nearly all parameters of metabolic control: postprandial glucose excursions as well as A1C, 7-point blood glucose profiles, FBG, and lipids were all lower with the combination therapy compared with the control therapy, which is in line with previous data (17,19,20). Overall glycemic control in all groups was excellent, as shown by >60% of all patients reaching the ADA A1C target of <7%. Body weight decreased with exenatide and was stable in the other two groups, indicating that the weight-lowering activity of GLP-1 receptor stimulation (10,20) persists even with concomitant insulin treatment. This is an important novel finding. The incidence of hypoglycemic episodes (blood glucose <50 mg/dl) was comparable among groups and in the expected range for insulin-treated patients (24). Gastrointestinal side effects, however, were more frequent with exenatide, which is consistent with the adverse event profile seen during phase III (10).
Several factors should be considered when interpreting our results. First, the number of patients (16 in each group) is relatively small; thus, further studies both in a larger number of patients and of longer duration are warranted. Second, mean duration of type 2 diabetes in this population was only 6 ± 1 years, and mean baseline A1C was 8.1 ± 0.7%. Patients with longer disease duration and/or worse metabolic control might be less likely to benefit to the extent seen in the study population, especially when the focus is on postprandial blood glucose (25). For the present subject group, one could also conclude that it is more cost-effective to just continue metformin plus basal insulin glargine, because overall glucose control at the end of the study has significantly improved in all three groups, with a slightly further improvement only seen for the addition of exenatide given to titrated insulin glargine plus metformin. Moreover, the present subjects might not be the ones requiring the combination of metformin with insulin and sitagliptin/exenatide, because excellent glucose control is achieved with insulin and metformin alone and would most likely also be achieved with metformin plus an incretin. Nevertheless, this study provides a proof of concept. Besides, given the increasing incidence of type 2 diabetes and obesity (5), there is a need for further effective, weight-focused, convenient, and safe therapies including incretins (21). Future research will be necessary to clarify whether there are clinical benefits in choosing exenatide or sitagliptin versus another commonly used agent to lower postprandial blood glucose. Another limitation of the study was a statistically nonsignificant, slight difference, however, in baseline A1C values among the three groups, which should be taken into account when the results are interpreted. Furthermore, the duration of the study was too short to see the full effect on A1C. The open-label design also represents a certain limitation. A double-blind double-dummy design, however, would have put a lot of effort into the conduct of the trial and a major additional restriction to the study subjects.
In summary, this study demonstrates that insulin glargine/metformin provides excellent fasting glycemic control and that additional exenatide and, to a lesser degree, sitagliptin, provide additional postprandial glucose control, without the necessity for a major change in insulin dose because of hypoglycemia. In addition, the weight-reducing ability of exenatide persists despite concomitant insulin administration. Longer-term studies are warranted to further explore the benefits of this novel treatment approach.
Acknowledgments
The study was funded by sanofi-aventis, Paris, France. M.A.N. has received research grants from Bayer Vital Pharma, Leverkusen, Germany, Eli Lilly & Co., Indianapolis, Indiana, Menarini/Berlin-Chemie, Berlin, Germany, Merck, Sharp Dohme, Munich, Germany, Novartis Pharma, Basel, Switzerland, and Novo Nordisk, Copenhagen, Denmark; he has accepted honoraria for membership in advisory boards and consulting and has received honoraria for speaking on incretin-based antidiabetic medications from Amylin Pharmaceuticals San Diego, California, AstraZeneca, Mjölndal, Sweden, Bayer Vital Pharma, Leverkusen, Germany, Berlin Chemie/Menarini, Berlin, Germany, Biovitrum, Stockholm, Sweden, Eli Lilly & Co., GlaxoSmithKline, Munich, Germany, Hoffman La Roche, Basel, Switzerland, Novartis Pharma, Basel, Switzerland/Nürnberg, Germany, Novo Nordisk, sanofi-aventis Pharma, Bad Soden/Taunus, Germany, and Takeda, Deerfield, Illinois. C.K. is a shareholder of Profil Institut für Stoffwechselforschung, which received research grants from several pharmaceutical companies, including the insulin-producing companies Eli Lilly, sanofi-aventis (the manufacturer of insulin glulisine), and Novo Nordisk (the manufacturer of insulin aspart). No other potential conflicts of interest relevant to this article were reported.
We thank the clinical staff at Profil Institut für Stoffwechselforschung, Neuss, Germany, for their dedicated work.
Footnotes
Clinical trial reg. no. NCT00971659, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 2. Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006; 29: 1963–1972 [DOI] [PubMed] [Google Scholar]
- 3. van Avendonk MJ, Rutten GE: Insulin therapy in type 2 diabetes: what is the evidence? Diabetes Obes Metab 2009; 11: 415–432 [DOI] [PubMed] [Google Scholar]
- 4. Mäkimattila S, Nikkilä K, Yki-Järvinen H: Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999; 42: 406–412 [DOI] [PubMed] [Google Scholar]
- 5. Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG: Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27: 2067–2073 [DOI] [PubMed] [Google Scholar]
- 6. Owens DR, Bolli GB: Beyond the era of NPH insulin—long-acting insulin analogs: chemistry comparative pharmacology, and clinical application. Diabetes Technol Ther 2008; 10: 333–349 [DOI] [PubMed] [Google Scholar]
- 7. sanofi-aventis Deutschland. Lantus. Summary of product characteristics [article online], 2008. Available from http://www.fachinfo.de. Accessed 10 June 2009
- 8. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients Diabetes Care 2003; 26: 3080–3086 [DOI] [PubMed] [Google Scholar]
- 9. Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V: Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med 2008; 25: 924–932 [DOI] [PubMed] [Google Scholar]
- 10. Lilly Deutschland. Byetta. Summary of product characteristics, [article online], 2009. Available from http://www.fachinfo.de. Accessed 10 June 2009
- 11. Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 12. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes. Ann Intern Med 2005; 143: 559–569 [DOI] [PubMed] [Google Scholar]
- 13. Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M: A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50: 259–267 [DOI] [PubMed] [Google Scholar]
- 14. Amori RE, Lau J, Pittas AG: Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298: 194–206 [DOI] [PubMed] [Google Scholar]
- 15. MSD Sharp & Dohme. Januvia. Summary of product characteristics, [article online], 2008. Available from http://www.fachinfo.de. Accessed 10 June 2009
- 16. Krentz AJ, Patel MB, Bailey CJ: New drugs for type 2 diabetes mellitus. What is their place in therapy? Drugs 2008; 68: 2131–2162 [DOI] [PubMed] [Google Scholar]
- 17. Barnett AH: New treatments in type 2 diabetes: a focus on the incretin-based therapies. Clin Endocrinol 2009; 70: 343–353 [DOI] [PubMed] [Google Scholar]
- 18. Nauck MA: Unraveling the science of incretin biology. Am J Med 2009; 122: S3–S10 [DOI] [PubMed] [Google Scholar]
- 19. Gilbert MP, Pratley RE: Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med 2009; 122: S11–S24 [DOI] [PubMed] [Google Scholar]
- 20. Mudaliar S, Henry RR: Incretin therapies: effects beyond glycemic control. Am J Med 2009; 122: S25–S36 [DOI] [PubMed] [Google Scholar]
- 21. Kendall DM, Cuddihy RM, Bergenstal RM: Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med 2009; 122: S37–S50 [DOI] [PubMed] [Google Scholar]
- 22. World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 52nd WMA General Assembly, Edinburgh, Scotland, October 2000. Last amended with Note of Clarification on Paragraph 29 by the WMA general assembly, Washington 2002, and the Note of Clarification on Paragraph 30 by the WMA general assembly, Tokyo 2004 [Google Scholar]
- 23. Linnebjerg H, Kothare PA, Skrivanek Z, de la Peña A, Atkins M, Ernest CS, Trautmann ME: Exenatide: effect of injection time on postprandial glucose in patients with type 2 diabetes. Diabet Med 2006; 23: 240–245 [DOI] [PubMed] [Google Scholar]
- 24. Amiel SA, Dixon T, Mann R, Jameson K: Hypoglycaemia in type 2 diabetes. Diabet Med 2008; 25: 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monnier L, Colette C, Dunseath GJ, Owens DR: The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007; 30: 263–269 [DOI] [PubMed] [Google Scholar]