Abstract
OBJECTIVE
11-β-hydroxysteroid dehydrogenase type 1 (11βHSD1) converts inactive cortisone into active cortisol, thereby amplifying intracellular glucocorticoid action. The efficacy and safety of the 11βHSD1 inhibitor INCB13739 were assessed when added to ongoing metformin monotherapy in patients with type 2 diabetes exhibiting inadequate glycemic control (A1C 7–11%).
RESEARCH DESIGN AND METHODS
This double-blind placebo-controlled paralleled study randomized 302 patients with type 2 diabetes (mean A1C 8.3%) on metformin monotherapy (mean 1.5 g/day) to receive one of five INCB13739 doses or placebo once daily for 12 weeks. The primary end point was the change in A1C at study end. Other end points included changes in fasting glucose, lipids, weight, adverse events, and safety.
RESULTS
After 12 weeks, 200 mg of INCB13739 resulted in significant reductions in A1C (−0.6%), fasting plasma glucose (−24 mg/dl), and homeostasis model assessment–insulin resistance (HOMA-IR) (−24%) compared with placebo. Total cholesterol, LDL cholesterol, and triglycerides were all significantly decreased in hyperlipidemic patients. Body weight decreased relative to placebo after INCB13739 therapy. A reversible dose-dependent elevation in adrenocorticotrophic hormone, generally within the normal reference range, was observed. Basal cortisol homeostasis, testosterone in men, and free androgen index in women were unchanged by INCB13739. Adverse events were similar across all treatment groups.
CONCLUSIONS
INCB13739 added to ongoing metformin therapy was efficacious and well tolerated in patients with type 2 diabetes who had inadequate glycemic control with metformin alone. 11βHSD1 inhibition offers a new potential approach to control glucose and cardiovascular risk factors in type 2 diabetes.
The phenotypic similarities between obesity, type 2 diabetes, and Cushing's syndrome have sparked considerable interest in the plausible role for endogenous glucocorticoids in the pathogenesis of type 2 diabetes. 11βHSD1 is an 11β-reductase that catalyzes the intracellular conversion of inactive cortisone into active cortisol (1). 11βHSD1 is expressed in specific tissues, most notably in liver, adipose, vasculature, brain, and macrophages (2,3), where it increases intracellular cortisol levels but does not participate in adrenal cortisol biosynthesis from cholesterol. 11βHSD1 activity is elevated in adipose tissue of obese rodents and humans (4,5). Mice engineered with similarly increased adipose tissue 11βHSD1 activity exhibit increased weight and visceral fat mass, insulin resistance, hyperlipidemia, hyperphagia, and hypertension (6,7). Reduction of intracellular glucocorticoid levels via 11βHSD1 gene deletion (8–10), inhibition (11), or ectopic expression of the cortisol-inactivating enzyme 11βHSD2 in adipose tissue (12) is sufficient to drive resistance to weight gain on a high-fat diet, improve glucose tolerance and insulin sensitivity, and attenuate dyslipidemia in rodents. These data suggest that 11βHSD1 inhibition may provide a novel treatment to reduce hyperglycemia and macrovascular disease risk in type 2 diabetes.
INCB13739 is an oral and selective 11βHSD1 inhibitor being developed to treat type 2 diabetes. We conducted a 12-week dose-ranging study of INCB13739 added to ongoing metformin monotherapy in patients with type 2 diabetes to evaluate the safety and efficacy of this compound.
RESEARCH DESIGN AND METHODS
This was a double-blind randomized paralleled trial conducted at 74 sites in the U.S. and six sites in Puerto Rico (NCT00698230). The study consisted of five periods: screening, metformin dose stabilization, 14-day placebo single-blind run-in, 12-week double-blind treatment, and 3-week off-treatment follow-up. The study was conducted pursuant to the Declaration of Helsinki and was approved by institutional review boards at participating sites. Patients provided informed consent before screening.
Patients (18–75 years) with type 2 diabetes, a BMI between 25 and 45 kg/m2, and A1C between 7–11% while taking metformin monotherapy at a stable dose for ≥10 weeks were eligible. Exclusion criteria included a medical history of disorders involving glucocorticoid, mineralocorticoid, or androgen excess; a history of type 1 diabetes or secondary forms of diabetes; previous insulin therapy; triglycerides >500 mg/dl; and treatment with any oral, systemic, topical, or inhaled glucocorticoids, thiazolidinediones, or exenatide within 3 months of screening. No inclusion criteria were specified for cholesterol or blood pressure and patients could enter the study on (and maintain) any hypolipidemic or antihypertensive regimen.
Patients were randomized equally to once-daily INCB13739 (5, 15, 50, 100, or 200 mg) or placebo. Dose selection was based on phase 1 pharmacokinetic and pharmacodynamic data, with the goal of evaluating regimens that achieve different degrees of inhibition, from <50 to >90%, with the duration of inhibition varying across the five dose levels. Patients with a fasting plasma glucose (FPG) >270 mg/dl through week 8 or >240 mg/dl subsequently were discontinued and offered rescue therapy.
The primary end points were the change from baseline to week 12 compared with placebo in A1C, safety, and tolerability. Secondary end points included the change from baseline to week 12 compared with placebo in FPG and lipid profiles and the proportion of patients achieving an A1C ≤7% at week 12. Tertiary end points included the change from baseline in homeostasis model assessment–insulin resistance (HOMA-IR), weight, blood pressure, and the proportion of patients meeting rescue therapy criteria.
Study assessments
On-treatment study visits occurred at weeks 2, 4, 8, and 12 and a follow-up visit at week 15 off treatment. Fasting blood samples were collected after a minimum 10-h fast. Salivary samples were collected between 2200 and 2400. All assays were performed by Covance Central Labs. Monitoring for adverse events (AEs) (intensity, duration, outcome, and causality), physical examinations, vital signs, body weight and morphometrics, 12-lead electrocardiograms, and safety laboratory assessments including hematology, serum chemistry, and urinalysis were also performed.
Statistical analysis
There were 40 patients per group completing week 12 who provided 90% power to detect a mean 0.6% difference in A1C between the 200-mg group and placebo assuming an Emax dose-response model (13) with a half-maximal stimulation (ED50) of 30 mg and an SD in A1C of 1.2%. This Emax model is commonly used for phase 2 dose-ranging studies and was prespecified with the following optimal linear contrast: −0.45666 (placebo), −0.31381 (5 mg), −0.12333 (15 mg), 0.168336 (50 mg), 0.312566 (100 mg), and 0.412901 (200 mg) based on the half-maximal concentration (ED50) = 30 mg assumption. The study was powered for A1C alone and not for lipids or blood pressure. Two populations were prespecified: the evaluable analysis set was defined as all patients randomized who have completed the 12 weeks of study treatment with ≥80% compliance; and the full analysis set was defined as all patients randomized who have taken at least one dose of study drug with any missing week 12 data imputed by last observation carried forward. The A1C and FPG end points were prespecified to be analyzed using the evaluable analysis set; all other efficacy end points were prespecified to be analyzed using the full analysis set.
For all end points, treatment effect was assessed using a linear model with treatment as the model factor and baseline as a covariate. Changes from baseline were estimated with 90% CIs from the model.
RESULTS
The disposition of patients is in supplementary Fig. 1, found in an online appendix available at http://care.diabetesjournals.org/cgi/content/full/dc09-2315/DC1. Baseline assessments were performed in 302 patients who entered the treatment phase of the study, and 228 patients (75%) completed the 12-week treatment period. The most common reasons for discontinuation were loss to follow-up (5%), withdrawal of consent (5%), lack of efficacy (4%), noncompliance with study procedures/medication (4%), and adverse events (4%), none of which related to the dose level of study medication. The clinical characteristics of the population at baseline were similar between treatment groups (supplementary Table 1): the mean duration of diabetes was 6.2 years, BMI 32.4 kg/m2, A1C 8.3%, and FPG 173 mg/dl.
Efficacy
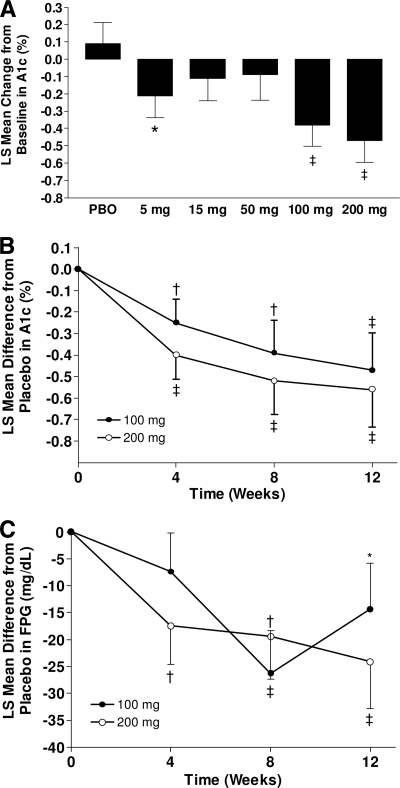
At week 12, treatment with INCB13739 resulted in a dose-dependent reduction in A1C (PEmax = 0.016; Table 1, Fig. 1A). The placebo-adjusted least-squares (LS) mean difference from baseline in A1C reached statistical significance for the 100-mg (−0.47%; P < 0.05) and 200-mg (−0.56%; P < 0.01) groups. A1C decreased compared with placebo in a time-dependent manner, reaching its maximum at week 12 (Fig. 1B). A greater proportion of patients (25%) randomized to 100 or 200 mg INCB13739 achieved an A1C <7% when compared with placebo (9.5%) at week 12. In a predefined subgroup analysis in patients with a baseline A1C ≥8%, the response to INCB13739 was more pronounced, with the 50-, 100-, and 200-mg groups achieving a significant (P < 0.05) change in A1C from baseline of −0.65 to −0.72%. The placebo-adjusted change in A1C for the 100- and 200-mg groups was greater in subjects with a baseline BMI >30 kg/m2 (−0.53% and −0.93%, respectively) than in subjects with a baseline BMI ≤30 kg/m2 (−0.35% and −0.17%, respectively). The number of patients requiring rescue therapy (12) did not differ significantly between treatment groups. FPG decreased in a dose- and time-dependent manner in the 100- and 200-mg treatment groups (Fig. 1C) and reached statistical significance (P < 0.01) from placebo in the 200-mg group with an LS mean difference of −24.1 mg/dl. A dose-dependent reduction in HOMA-IR was observed, reaching significance (P < 0.05) in the 200-mg group with an LS mean difference of −1.32 (−24%), suggesting an insulin-sensitizing mechanism of action.
Table 1.
Efficacy assessments
| Placebo | 5 mg | 15 mg | 50 mg | 100 mg | 200 mg | |
|---|---|---|---|---|---|---|
| Baseline A1C (%) | 8.3 ± 1 | 8.2 ± 1 | 8.3 ± 1 | 8.3 ± 1 | 8.2 ± 1 | 8.2 ± 1 |
| A1C (%) | 0.09 ± 0.1 | −0.21 ± 0.1*‖ | −0.11 ± 0.1 | −0.09 ± 0.2 | −0.38 ± 0.1†‖ | −0.47 ± 0.1‡# |
| A1C ≥8% | −0.10 ± 0.2 | −0.39 ± 0.2‖ | −0.24 ± 0.2 | −0.65 ± 0.3*‖ | −0.72 ± 0.2†‖ | −0.65 ± 0.2*# |
| Subgroup n | 23 | 23 | 18 | 11 | 16 | 19 |
| BMI >30 kg/m2 | 0.17 ± 0.1 | −0.24 ± 0.2*§ | −0.10 ± 0.2 | −0.25 ± 0.2* | −0.36 ± 0.2† | −0.76 ± 0.2‡# |
| Subgroup n | 29 | 23 | 26 | 15 | 26 | 18 |
| Baseline FPG (mg/dl) | 179 ± 51 | 172 ± 41 | 175 ± 44 | 178 ± 53 | 170 ± 64 | 165 ± 41 |
| FPG (mg/dl) | 12.6 ± 6.1 | 6.0 ± 6.3 | 2.3 ± 6.4 | −4.7 ± 7.2* | −1.6 ± 6.1* | −11.5 ± 6.2‡§ |
| C-peptide (pmol/l) | −9.48 ± 40 | −9.84 ± 41 | −14.0 ± 41 | −39.6 ± 45 | −32.2 ± 39 | −47.4 ± 40 |
| HOMA-IR | 0.25 ± 0.4 | −0.29 ± 0.4 | 0.33 ± 0.4 | −0.42 ± 0.5 | −0.51 ± 0.4 | −1.06 ± 0.4†‖ |
| HOMA-B | −3.9 ± 4.1 | −3.92 ± 4.2 | 4.78 ± 4.2 | 6.67 ± 4.6* | −2.35 ± 4.1 | 2.58 ± 4.1 |
| Cholesterol (mg/dl) | 1.2 ± 4 | −0.7 ± 4 | −1.2 ± 4 | −3.9 ± 4 | −6.6 ± 4* | −7.3 ± 4* |
| ≥200 mg/dl | −10.0 ± 6 | −11.6 ± 5 | −12.4 ± 7 | 1.5 ± 7 | −16.2 ± 5‖ | −18.5 ± 6§ |
| Subgroup n | 19 | 22 | 14 | 12 | 28 | 20 |
| LDL cholesterol (mg/dl) | 2.3 ± 4 | −1.2 ± 4 | 0.4 ± 4 | −7.0 ± 4§ | −4.6 ± 3§ | −4.3 ± 3 |
| ≥130 mg/dl | −8.5 ± 8 | −19.3 ± 8 | −9.7 ± 9 | −8.5 ± 13 | −17.0 ± 6‖ | −14.3 ± 8 |
| Subgroup n | 12 | 10 | 9 | 6 | 18 | 12 |
| HDL cholesterol (mg/dl) | 0.8 ± 1.3 | −0.4 ± 1.2 | 1.2 ± 1.3 | 1.2 ± 1.4 | 0.4 ± 1.2 | 0.8 ± 1.2 |
| <40 mg/dl | 3.5 ± 1.9§ | 0.8 ± 2.0 | 2.7 ± 1.6 | 2.7 ± 1.7 | 5.0 ± 2.0 | 1.9 ± 1.8 |
| Subgroup n | 13 | 12 | 21 | 17 | 14 | 14 |
| Triglyceride (mg/dl) | 0.0 ± 12 | −4.4 ± 12 | −27.4 ± 5 | −12.4 ± 13 | −11.5 ± 12 | −10.6 ± 12 |
| ≥200 mg/dl | −19.5 ± 28 | −3.5 ± 28 | −105.3 ± 31 | −57.5 ± 29 | −74.3 ± 27‖ | −55.8 ± 29 |
| Subgroup n | 16 | 17 | 13 | 15 | 18 | 15 |
| FFA (mmol/l) | 0.0 ± 0.03 | −0.03 ± 0.03 | 0.02 ± 0.03 | −0.01 ± 0.03 | −0.03 ± 0.03 | 0.0 ± 0.03 |
| Systolic blood pressure (mmHg) | 0.9 ± 1.6 | −0.3 ± 1.5 | 0.17 ± 1.6 | 1.2 ± 1.6 | 0.5 ± 1.5 | −0.4 ± 1.5 |
| Diastolic blood pressure (mmHg) | 1.2 ± 1.0 | 0.0 ± 1.0 | 1.4 ± 1.0 | −0.2 ± 1.1 | −0.5 ± 1.0 | −0.8 ± 1.0 |
| Weight (kg) | −0.2 ± 0.3 | −0.5 ± 0.3§ | −0.6 ± 0.4‖ | 0.0 ± 0.4 | −1.1 ± 0.3*‖ | −0.9 ± 0.3# |
| Waist-to-hip ratio | −0.01 ± 0.01 | 0.02 ± 0.01* | 0.0 ± 0.01 | −0.01 ± 0.01 | −0.02 ± 0.01 | −0.01 ± 0.01 |
Data are LS mean change from baseline ± SEM unless noted.
*P < 0.1,
†P < 0.05,
‡P < 0.01, active vs. PBO.
§P < 0.1,
‖P < 0.05,
#P < 0.01, week 12 vs. baseline.
Figure 1.
Glycemic efficacy. A: LS mean (SE) change from baseline in A1C at week 12. B: LS mean difference (SE) from placebo in A1C from baseline to week 12 in the 100-mg (●) and 200-mg (○) treatment groups. C: LS mean difference (SE) from placebo in FPG from baseline to week 12 in the 100- and 200-mg treatment groups. *P < 0.1, †P < 0.05, ‡P < 0.01, active vs. placebo (PBO).
Body weight decreased with INCB13739 treatment, with statistical significance from baseline (P < 0.05) achieved in the 15 (−0.6 kg), 100 (−1.1 kg), and 200 mg (−0.9 kg) treatment groups (Table 1). Waist-to-hip ratio did not change with treatment.
Plasma lipids and blood pressure were generally well controlled at baseline (supplementary Table 1). Treatment with INCB13739 resulted in a modest dose-dependent (Ptrend = 0.026) decrease in total cholesterol, reaching a maximum of −7 mg/dl (−3%) from baseline in the 200-mg group (Table 1). In a prespecified analysis, patients with Adult Treatment Panel (ATP) III defined hyper-lipidemia (total cholesterol >200 mg/dl; LDL cholesterol >130 mg/dl) or hyper-triglyceridemia (>200 mg/dl) at baseline exhibited a greater improvement, reaching statistical significance (P < 0.05) in the 100-mg group for all three lipid categories (cholesterol −16 mg/dl, −6%; LDL −17 mg/dl, −10%; triglycerides −74 mg/dl, −16%). Similar responses were observed in the 200-mg group, but these did not reach significance, possibly because of the smaller size of the subgroups. Changes in HDL and free fatty acids were not significantly different between the treatment groups. Systolic and diastolic blood pressure did not change appreciably during the study.
Safety
Treatment with INCB13739 was well tolerated and AEs were reported at similar frequencies across all treatment groups (Table 2). No drug-related serious AEs occurred in the trial. One death occurred in the 200-mg group because of complications after a serious AE of acute ischemia of the lower extremities. This AE occurred ∼2 weeks after the last dose of study medication in a subject with preexisting congestive heart failure and aortic valvular disease. The death was due to cardiac arrest immediately after induction of anesthesia before bilateral iliofemoral embolectomy. The AE was judged by the investigator as unrelated to study medication. No hypoglycemic events were reported during the treatment phase of the trial. The most frequent AEs reported were typical for this population and did not exhibit dose dependence. There were four reports of nausea in the 200-mg group (compared with one in the placebo group); however, all of these resolved during continued dosing and three were categorized by the investigator as unrelated to study medication. There were no clinically relevant differences between treatment groups in electrocardiograms, hematology, serum chemistry, or urinalysis.
Table 2.
End point endocrine assessments and safety summary
| Reference range | Placebo | 5 mg | 15 mg | 50 mg | 100 mg | 200 mg | |
|---|---|---|---|---|---|---|---|
| Endocrinology | |||||||
| ACTH | 1.6–13.9 pmol/l | 4.9 ± 0.9 | 8.3 ± 0.9‡ | 7.1 ± 0.9 | 9.2 ± 1.0‡ | 9.4 ± 0.9‡ | 11.2 ± 0.9‡ |
| Aldosterone | 111–859 pmol/l | 218 ± 23 | 198 ± 24 | 208 ± 25 | 204 ± 28 | 204 ± 23 | 276 ± 24 |
| Renin | 3.5–65.6 pg/ml | 24.9 ± 7.6 | 26.0 ± 7.5 | 38.1 ± 7.8 | 19.8 ± 8.7 | 18.7 ± 7.3 | 28.0 ± 7.5 |
| DHEAS, ♂ | 0.14–18.73 μmol/l | 4.1 ± 0.6 | 3.7 ± 0.6 | 5.2 ± 0.6 | 5.0 ± 0.7 | 5.4 ± 0.6 | 6.6 ± 0.7‡ |
| DHEAS, ♀ | 0.19–10.61 μmol/l | 2.3 ± 0.6 | 3.5 ± 0.7 | 4.2 ± 0.6† | 3.4 ± 0.7 | 3.5 ± 0.6 | 4.0 ± 0.6† |
| A4, ♂ | 0.8–2.9 ng/ml | 1.7 ± 0.2 | 1.5 ± 0.1 | 2.1 ± 0.2 | 1.7 ± 0.2 | 2.1 ± 0.2 | 2.6 ± 0.2‡ |
| A4, ♀ | <1.0–4.3 ng/ml | 1.1 ± 0.2 | 1.6 ± 0.3 | 1.9 ± 0.3† | 2.2 ± 0.3‡ | 1.6 ± 0.2 | 1.8 ± 0.2† |
| T, ♂ | 6.1–27.1 nmol/l | 12.7 ± 0.9 | 11.5 ± 0.8 | 10.4 ± 0.9 | 12.0 ± 1.0 | 11.3 ± 0.9 | 13.9 ± 0.9 |
| Ta, ♀ | <0.4–2.6 nmol/l | 1.3 ± 0.4 | 1.5 ± 0.3 | 1.7 ± 0.8† | 1.6 ± 0.5 | 1.6 ± 0.6 | 1.8 ± 0.8† |
| SHBG, ♂ | 7–70 nmol/l | 25.9 ± 3.2 | 29.9 ± 2.8 | 20.6 ± 3.1 | 23.5 ± 3.7 | 20.8 ± 3.1 | 29.7 ± 3.4 |
| SHBG, ♀ | 15–120 nmol/l | 23.0 ± 5.1 | 27.1 ± 6.1 | 30.8 ± 5.8 | 39.9 ± 6.4† | 40.0 ± 5.1† | 24.9 ± 5.1 |
| FAI, ♂ | NA | 63.9 ± 5.8 | 43.5 ± 5.0 | 60.2 ± 5.5 | 55.8 ± 6.6 | 62.7 ± 5.7 | 53.3 ± 6.2 |
| FAIa, ♀ | NA | 6.9 ± 1.1 | 7.9 ± 1.3 | 8.2 ± 1.2 | 5.7 ± 1.4 | 7.2 ± 1.1 | 7.9 ± 1.1 |
| Safety and tolerability | |||||||
| ≥1 AE | 23 (46) | 25 (49) | 22 (44) | 27 (57) | 25 (47) | 20 (39) | |
| Rx-related* | 3 (6) | 8 (16) | 8 (16) | 9 (19) | 4 (8) | 5 (10) | |
| ≥1 SAE | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | |
| Rx-related* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| d/c for AE | 2 (4) | 2 (4) | 2 (4) | 3 (6) | 1 (2) | 1 (2) | |
| AEs occurring in ≥3% | |||||||
| Nasopharyngitis | 1 (2) | 4 (8) | 3 (6) | 5 (11) | 3 (6) | 1 (2) | |
| Diarrhea | 3 (6) | 3 (6) | 1 (2) | 3 (6) | 3 (6) | 1 (2) | |
| Upper respiratory tract infection | 3 (6) | 3 (6) | 2 (4) | 2 (4) | 2 (4) | 1 (2) | |
| Headache | 3 (6) | 2 (4) | 5 (10) | 1 (2) | 1 (2) | 0 (0) | |
| Arthralgia | 0 (0) | 7 (14) | 1 (2) | 2 (4) | 0 (0) | 0 (0) | |
| Cough | 0 (0) | 1 (2) | 2 (4) | 1 (2) | 3 (6) | 2 (4) | |
| Nausea | 1 (2) | 2 (4) | 0 (0) | 1 (2) | 1 (2) | 4 (8) |
Endocrine data are week 12 LS mean ±SEM unless otherwise noted. Androgens and their precursors are categorized by sex. Central lab normal reference ranges are provided. Treatment emergent AE data are n (%) for all AEs or for those occurring in at least 3% of patients. A4, androstenedione; d/c, discontinuation; FAI, free androgen index; SAE, serious adverse event; T, testosterone.
*Determined by the investigator to be possibly, probably, or definitely drug related.
†P < 0.05;
‡P < 0.01, active vs. PBO. aT♀ and FAI♀ reflects week 8 concentrations.
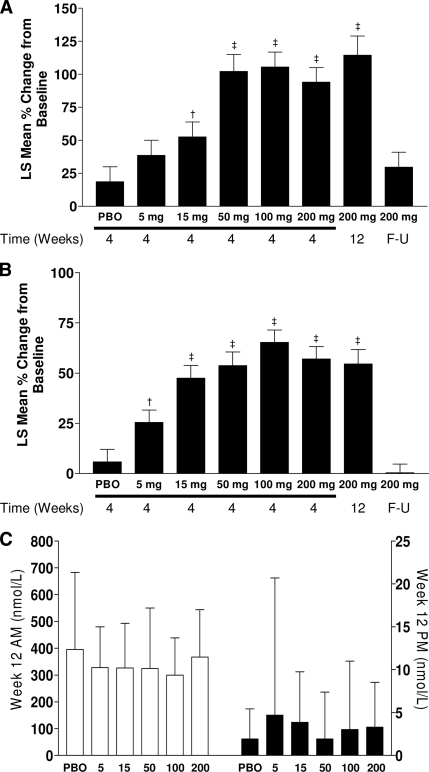
The anticipated compensatory activation of the hypothalamic-pituitary-adrenal axis to overcome reduced cortisol regeneration on 11βHSD1 inhibition was evaluated. INCB13739 caused a dose-related increase in morning plasma ACTH and the ACTH-sensitive adrenocorticosteroid dehydroepiandrosterone sulfate (DHEAS) levels, although mean concentrations of both hormones remained within laboratory reference ranges (Table 2). ACTH and DHEAS rises after INCB13739 reached a plateau at week 4 (+102 and +54%, respectively, versus +19 and +6% in the placebo group); did not exhibit a further increase at week 12, even in the 200-mg treatment group (+114% and +55%, respectively); and returned to baseline levels by the 3-week follow-up visit (Fig. 2A and B). Morning plasma cortisol and evening salivary cortisol levels were unaltered by INCB13739 at any dose (Fig. 2C), suggesting that the rise in ACTH was a compensatory response.
Figure 2.
Change in ACTH, DHEAS, and cortisol. A: LS mean percent (%) change (SE) from baseline in ACTH by treatment group and time on therapy. B: LS mean percent (%) change (SE) from baseline in DHEAS by treatment group and time on therapy. C: LS mean (SE) cortisol concentrations at week 12 in the morning (plasma concentrations, □, left axis) or at night (salivary concentrations, ■, right axis). F-U, follow-up. †P < 0.05, ‡P < 0.01, active vs. placebo (PBO).
DHEAS is a precursor for androgen biosynthesis. INCB13739 treatment resulted in a dose-related increase in morning fasting serum androstenedione (A4), although mean concentrations remained within the laboratory reference range (Table 2). In males, there were no differences between treatment groups in total testosterone, sex hormone–binding globulin (SHBG), or free androgen index (FAI). In females, total testosterone (available at baseline and week 8) increased in a dose-dependent manner with mean concentrations within the laboratory reference range. Maximal concentrations were observed in the 200-mg group (1.8 vs. 1.3 nmol/l in the placebo group; P < 0.05). These changes occurred alongside modest increases in SHBG (assessed at week 12), apparent in the 50- and 100-mg groups (P < 0.05), but not the 200-mg group. Importantly, there were no significant differences between treatment groups in calculated FAI in females (placebo = 6.9; INCB13739 range = 5.7–8.2).
CONCLUSIONS
The results from this study indicate, for the first time, that decreasing local cortisol exposure through 11βHSD1 inhibition improves hyperglycemia over 12 weeks in patients with type 2 diabetes. The addition of once-daily INCB13739 in patients inadequately controlled with metformin significantly reduced A1C, FPG, and HOMA-IR. These effects were dose dependent, and the greatest improvements were achieved at the highest dose administered (200 mg), with evidence for a more profound A1C reduction in subjects with a BMI >30 kg/m2, compatible with elevated 11βHSD1 in adipose tissue in obesity. Preliminary data from pharmacokinetic analyses (data not shown) indicate that the 100- and 200-mg groups achieved, 4 h after administration, mean free drug exposures that reached 100 mg or exceeded 200 mg, the concentrations required to inhibit 90% of the enzyme activity in cellular assays; however, only the 200-mg group retained such a mean exposure at the end of the dosing interval. Thus, glycemic efficacy may be associated with a high degree of enzyme inhibition, and it is possible that greater glycemic improvement might be achieved with increased dose levels or frequency of administration.
Plasma lipids were generally well controlled in this population, and 30% of patients were receiving lipid-lowering medications. INCB13739 treatment resulted in a dose-dependent reduction in total cholesterol, and while of modest magnitude, these changes also associated with directional beneficial trends in LDL cholesterol and triglycerides. Of interest, patients who met ATP III criteria for “borderline high” LDL cholesterol (>130 mg/dl), total cholesterol (>200 mg/dl), or “hyper-triglyceridemia” (>200 mg/dl) exhibited a larger improvement in all three lipid parameters. The magnitude of effect was equivalent in the 100- and 200-mg groups, reaching statistical significance for the 100-mg group, which had the largest subgroup size.
INCB13739 treatment resulted in a dose-dependent modest decrease in body weight of ∼1 kg at the highest dose studied. This change was time dependent and did not plateau over the 12-week treatment period (data not shown). The thiazolidinedione insulin sensitizers increase body weight through adipocyte differentiation (14,15). As cortisol can drive adipocyte differentiation and expansion (16), it is possible that attenuating cortisol signaling in adipose may decrease adipocyte size. This has been reported in preclinical models with an 11βHSD1 inhibitor (17) and suggests the potential for positive effects of INCB13739 on total body weight and/or regional adiposity with longer exposure.
INCB13739 was well tolerated at all dose levels, and there were no differences in AE frequency relative to placebo nor were there any apparent dose-dependent changes in AEs.
While 11βHSD1 is not involved in adrenal cortisol biosynthesis, 11βHSD1 activity within the splanchnic bed does contribute ∼25% of total cortisol production (18). An expected consequence of 11βHSD1 inhibition is increased clearance of cortisol and compensatory hypothalamic-pituitary-adrenal axis activation to maintain blood cortisol concentrations. INCB13739 treatment did result in a dose-related increase in ACTH levels that was generally within the normal reference range. The ACTH response reached a plateau with the 50-mg dose at week 4, suggesting that the maximal response to INCB13739 had been realized. This plateau in ACTH and its rapid return to baseline levels after cessation of therapy are consistent with an adaptive endocrine process driven by reversible 11βHSD1 inhibition. Importantly, cortisol levels and circadian rhythm were unaltered by INCB13739 treatment. These data indicate normal hypothalamic-pituitary-adrenal axis function after 12 weeks of INCB13739 therapy that adjusted appropriately to the inhibition of 11βHSD1 activity to maintain basal cortisol homeostasis. The leftward shift in the ACTH dose relationship relative to efficacy might reflect a greater contribution of hepatic 11βHSD1 inhibition to splanchnic cortisol reactivation.
Aldosterone and renin were unaltered by INCB13739 treatment (Table 2), and serum electrolytes were unchanged (supplementary Table 2). Modest elevations in the androgenic precursors DHEAS and A4 paralleled changes in ACTH. Like ACTH, these changes were generally within the reference range, plateaued with respect to both dose and time, and were reversed at follow-up. The highest concentration of DHEAS observed in this study (13.2 μmol/l in males and females) is equivalent to levels observed after 50 mg/day dehydroepiandrosterone supplement use (19). In men, there was no change in plasma testosterone, SHBG, or FAI after INCB13739 treatment, consistent with the testes being the main source of androgens. In females, a modest rise in total testosterone at week 8 was observed that was paralleled by a rise in SHBG such that the resulting FAI calculation was not significantly different in any INCB13739 group compared with placebo or baseline levels. SHBG is known to increase in response to improved insulin sensitivity (20), and whether the changes observed in this study reflect this or result from more complex endocrine adaptation to small changes in total testosterone are unknown. Importantly, FAI is an accepted surrogate in clinical practice for free testosterone and a marker of biologic androgen activity in women (21). No signs or symptoms of androgen excess were observed, and longer-term studies will be required to ascertain the clinical relevance of the small androgen changes observed.
In summary, in patients with type 2 diabetes who had inadequate glycemic control with metformin alone, the addition of once-daily INCB13739 was well tolerated and resulted in significant improvements in A1C, FPG, and HOMA-IR. INCB13739 treatment decreased body weight and improved cholesterol and triglycerides in patients with hyper-lipidemia at baseline. 11βHSD1 inhibition offers a new potential approach to control glucose and cardiovascular risk factors in type 2 diabetes. Further clinical characterization of INCB13739 with long-term controlled studies is warranted.
Supplementary Material
Acknowledgments
This study was supported by Incyte Corporation. J.R., V.A.F., S.E.I., and J.R.S. received consulting fees from Incyte Corporation as part of their participation in the study's design and data analyses. W.S., G.H., R.F, R.L., W.Y., W.V.W., and R.H. hold stock in Incyte Corporation. W.S., W.Y., G.H., R.F., W.V.W., R.H., and R.L. are employees of Incyte Corporation. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009, and the 44th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 27 September to 2 October 2009.
Footnotes
Clinical trial registry no. NCT00698230, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Seckl JR, Walker BR: Minireview: 11beta-Hydroxysteroid dehydrogenase type 1: a tissue-specific amplifier of glucocorticoid action. Endocrinology 2001; 142: 1371–1376 [DOI] [PubMed] [Google Scholar]
- 2. Stewart PM, Krozowski ZS: 11 beta-Hydroxysteroid dehydrogenase. Vitam Horm 1999; 57: 249–324 [PubMed] [Google Scholar]
- 3. Thieringer R, Le Grand CB, Carbin L, Cai TQ, Wong B, Wright SD, Hermanowski-Vosatka A: 11 Beta-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J Immunol 2001; 167: 30–35 [DOI] [PubMed] [Google Scholar]
- 4. Kannisto K, Pietiläinen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Järvinen H: Overexpression of 11β-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab 2004; 89: 4414–4421 [DOI] [PubMed] [Google Scholar]
- 5. Rask E, Olsson T, Söderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR: Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 2001; 86: 1418–1421 [DOI] [PubMed] [Google Scholar]
- 6. Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS: A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294: 2166–2170 [DOI] [PubMed] [Google Scholar]
- 7. Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, Shinyama H, Sharp MGF, Fleming S, Mullins JJ, Seckl JR, Flier JS: Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest 2003; 112: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ: 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci U S A 1997; 94: 14924–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morton NM, Holmes MC, Fiévet C, Staels B, Tailleux A, Mullins JJ, Seckl JR: Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11beta-hydroxysteroid dehydrogenase type 1 null mice. J Biol Chem 2001; 276: 41293–41300 [DOI] [PubMed] [Google Scholar]
- 10. Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR: Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1–deficient mice. Diabetes 2004; 53: 931–938 [DOI] [PubMed] [Google Scholar]
- 11. Hermanowski-Vosatka A, Balkovec JM, Cheng K, Chen HY, Hernandez M, Koo GC, Le Grand CB, Li Z, Metzger JM, Mundt SS, Noonan H, Nunes CN, Olson SH, Pikounis B, Ren N, Robertson N, Schaeffer JM, Shah K, Springer MS, Strack AM, Strowski M, Wu K, Wu T, Xiao J, Zhang BB, Wright SD, Thieringer R: 11beta-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J Exp Med 2005; 202: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS: Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes 2005; 54: 1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bretz F, Pinheiro JC, Branson M: Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics 2005; 61: 738–748 [DOI] [PubMed] [Google Scholar]
- 14. Kletzien RF, Clarke SD, Ulrich RG: Enhancement of adipocyte differentiation by an insulin-sensitizing agent. Mol Pharmacol 1992; 41: 393–398 [PubMed] [Google Scholar]
- 15. Fonseca V: Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med 2003; 115(Suppl. 8A): 42S–48S [DOI] [PubMed] [Google Scholar]
- 16. Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF: Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 1989; 84: 1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berthiaume M, Laplante M, Festuccia W, Gélinas Y, Poulin S, Lalonde J, Joanisse DR, Thieringer R, Deshaies Y: Depot-specific modulation of rat intraabdominal adipose tissue lipid metabolism by pharmacological inhibition of 11beta-hydroxysteroid dehydrogenase type 1. Endocrinology 2007; 148: 2391–2397 [DOI] [PubMed] [Google Scholar]
- 18. Basu R, Singh RJ, Basu A, Chittilapilly EG, Johnson MC, Toffolo G, Cobelli C, Rizza RA: Obesity and type 2 diabetes do not alter splanchnic cortisol production in humans. J Clin Endocrinol Metab 2005; 90: 3919–3926 [DOI] [PubMed] [Google Scholar]
- 19. Tummala S, Svec F: Correlation between the administered dose of DHEA and serum levels of DHEA and DHEA-S in human volunteers: analysis of published data. Clin Biochem 1999; 32: 355–361 [DOI] [PubMed] [Google Scholar]
- 20. Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P: Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 2007; 92: 41289–41295 [DOI] [PubMed] [Google Scholar]
- 21. Mueller A, Dittrich R, Cupisti S, Beckmann MW, Binder H: Is it necessary to measure free testosterone to assess hyperandrogenemia in women? The role of calculated free and bioavailable testosterone. Exp Clin Endocrinol Diabetes 2006; 114: 182–187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.