Abstract
OBJECTIVE
Because many patients with diabetic macular edema (DME) do not respond to focal/grid laser photocoagulation, the only currently approved treatment, alternatives are needed. Based on encouraging preliminary findings, we aimed to assess efficacy and safety of the anti–tumor necrosis factor (TNF) monoclonal antibody infliximab in this condition.
RESEARCH DESIGN AND METHODS
This was a single-center, double-blind, randomized, placebo-controlled, crossover study. Eleven patients with sight-threatening DME persisting after two sessions of laser photocoagulation received infliximab (5 mg/kg) intravenously at weeks 0, 2, 6, and 14, followed by placebo at weeks 16, 18, 22, and 30, or vice versa. Blinding was maintained to week 32, when the final assessments were performed. Best corrected visual acuity evaluated by a mixed-models approach for imbalanced crossover design using the percentage difference as the outcome variable was the primary study end point. Data were analyzed on an intention-to-treat basis.
RESULTS
Early Treatment of Diabetic Retinopathy Study (ETDRS) scores dropped from 31.6 ± 5.1 (mean ± SD) letters read at baseline to 28.8 ± 11.6 letters read at week 16 in six placebo-treated eyes and improved to 35.4 ± 11.2 letters read after infliximab. In contrast, visual acuity improved from 23.5 ± 10.3 at baseline to 30.4 ± 13.4 letters read at week 16 in eight infliximab-treated eyes and was sustained at completion of placebo treatment (31.4 ± 12.1 letters read). The excess visual acuity in infliximab-treated eyes was greater by 24.3% compared with that in placebo-treated eyes (95% CI 4.8–43.7; P = 0.017). Infliximab treatment was well tolerated.
CONCLUSIONS
The positive results of this small phase III study suggest that larger and longer term trials should be conducted to assess the efficacy of systemic or intravitreal anti-TNF agent administration for primary treatment of DME.
Diabetic macular edema (DME) is a serious complication of diabetes and a leading cause of vision loss in the working-age population of most developed countries (1,2). Data from the Wisconsin Epidemiological Study of Diabetic Retinopathy estimate that after 15 years of known duration of diabetes, the prevalence of DME is 20% in patients with type 1 diabetes, 25% in patients with type 2 diabetes who are treated with insulin, and 14% in the patients with type 2 diabetes who are not treated with insulin (3). A previous study has shown that 53% of the eyes with DME involving the center of the macula lost two or three lines of visual acuity over a 2-year period (4). Focal/grid laser photocoagulation (two sessions for optimal results) has been the standard for treatment for DME over the past two decades. However, this treatment effectively reduces the risk of vision loss in <50% of patients. Even among those patients who achieve an initial response, recurrences requiring ongoing treatment are common (1,5). Currently, there are no approved treatment options for eyes with DME refractory to laser photocoagulation (2,6).
Tumor necrosis factor (TNF) is a pleiotropic cytokine, central to the development and homeostasis of the immune system and a regulator of cell activation, differentiation, and death. In the past few decades, there has been an enormous scientific and clinical interest in understanding the function of TNF in physiology and disease, and a vast amount of data has accumulated at the biochemical, molecular, and cellular levels, establishing TNF as a prototype for in-depth understanding of physiological and pathogenic functions of a cytokine (7). This knowledge primed the successful development of anti-TNF therapies in the 1990s. Infliximab (Remicade) is a chimeric monoclonal antibody specific for human TNF that has shown efficacy in treatment of chronic inflammatory diseases affecting the joints, skin, and gut. Since its first launch in 1998, >1,100,000 patients worldwide have been treated with this drug for approved indications, including rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, and Crohn disease, including pediatric patients (8). Infliximab is given intravenously every 4–8 weeks at a dose ranging from 3 to 10 mg/kg and has an acceptable safety profile.
Several lines of evidence suggest an inflammatory basis for DME (9). Along this line, treatment modalities have been tried with variable success. Such treatments include pharmacological therapy with oral protein kinase C inhibitors (10), antibodies targeted to vascular endothelial growth factor (VEGF) (11), intravitreal injections of corticosteroids (12,13), and high doses of nonsteroidal anti-inflammatory drugs that lower retinal expression of TNF (14). According to our previously published preliminary results, a clinically meaningful recovery of useful vision was achieved after two infliximab infusions in four of six eyes with severe diffuse DME (15). Comparable beneficial results have been obtained in patients with severe, chronic cystoid macular edema complicating intermediate uveitis, Adamantiades-Behçet disease, or adult-type vascular pseudotumor (16). Repeated treatment in one diabetic patient produced a further significant improvement of DME (15), suggesting that the clinical response to anti-TNF dosing regimens is individualized, as observed in patients with arthritis (8) or in patients with uveitic macular edema (16).
Based on the evidence for anti-TNF treatment in DME and the limitations of current treatments, we undertook this phase III study to prospectively investigate the efficacy and safety of infliximab in the treatment of patients who were in danger of vision loss due to DME refractory to laser photocoagulation.
RESEARCH DESIGN AND METHODS
This is an investigator-initiated phase III double-blind, randomized, placebo-controlled, two-arm crossover clinical study. The study adhered to the guidelines of the Declaration of Helsinki, and the protocol and consent form were approved by the local investigational review board, the National Ethics Committee, and the Ministry of Health. Each patient provided written informed consent.
Patient eligibility and exclusion criteria
Patients (aged >18 years) with type 1 or type 2 diabetes and DME resulting in best corrected visual acuity (BCVA) of ≤0.4 were eligible if they had at least two previous sessions of laser photocoagulation >6 months before enrollment or if they had leaking microaneurysms within the foveal avascular zone, making laser photocoagulation unsafe for the central vision. In addition to standard inclusion and exclusion criteria for phase III studies of infliximab, patients were excluded if they had 1) vitreoretinal traction, 2) retinal detachment, 3) proliferative diabetic retinopathy requiring immediate panretinal photocoagulation, 4) any previous eye surgery 6 months before the study, including any intravitreal infusions, 5) macular edema of the ischemic type or caused by retinal conditions other than diabetes, 6) cataract or media opacities of a degree that precluded accurate retinal photographs or optical coherence tomography (OCT) measurement, 7) hard exudates under the fovea, or 8) uncontrolled arterial hypertension (blood pressure >180/110 mmHg), a major change in glycemic control (e.g., 2% change in A1C) within the last 6 months, or a change in daily number of insulin injections.
Study protocol
Consenting patients were screened for the study within 2 weeks before random assignment with a medical history, physical examination, electrocardiogram, purified protein derivative test, chest X-ray, and laboratory tests including hemoglobin, A1C, platelet count, white blood cell count and differential, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transferase, alkaline phosphatase, total and conjugated bilirubin, lactate dehydrogenase, plasma lipids (total cholesterol, HDL cholesterol, and triglycerides), creatinine phosphokinase, renal function (urea and creatinine), sodium, potassium, calcium, phosphate, and serological tests for hepatitis and HIV infection. In addition, an experienced examiner obtained ophthalmic/DME history and performed, in both eyes, measurements of BCVA, OCT, stereoscopic fundus photographs (seven fields), applanation tonometry, and fluorescein angiography.
Patients were randomly allocated 1:1 to receive placebo or infliximab in a two-armed crossover, double-blind design according to the permuted block randomization list generated in SAS. Patients received placebo at weeks 0, 2, 6, and 14, followed by infliximab at weeks 16, 18, 22, and 30 (group A), or vice versa (group B) in addition to standard therapy for diabetes, hypertension, and dyslipidemia, which remained unchanged during the study. All study drugs were administered via a 2-h intravenous infusion at a dose of 5 mg/kg body wt on the scheduled visits at weeks 0, 2, 6, 14, 16, 18, 22, and 30. Blinding was maintained to week 32, when the final clinical, laboratory, and ophthalmic evaluation was performed in all patients. Finally, adverse event reporting and a complete physical examination were performed at week 56 (long-term follow-up visit).
Physical examination and BCVA measurements of the number of letters a patient was able to read from the Early Treatment Diabetic Retinopathy study (ETDRS) charts with correction for individual refractive errors were performed at every visit. Foveal thickness measurements by third-generation OCT (Stratus OCT III), using the fast macular thickness scan, stereoscopic fundus photographs (seven fields), and intraocular pressure measurements using a Goldman applanation tonometer were performed at weeks 8, 16, 24, and 32. Hematological/biochemical tests and fluorescein angiograms were performed at weeks 16 and 32. Study physicians were blinded to the subject's treatment (infliximab or placebo) as well as to the subject's previous visual acuity assessments.
Outcome measures and statistical analysis
The primary end point of the study was to assess the efficacy and safety of four infusions of infliximab on BCVA, evaluated by a mixed-models approach for imbalanced crossover design using the percent difference between infliximab and placebo groups as an outcome variable. The secondary end points were 1) the effect of infliximab on the anatomic change of DME, assessed by OCT and 2) the effect of infliximab on diabetic retinopathy, assessed by fundus photographs and fluoroangiographic studies. Data were analyzed on an intention-to-treat basis. The treatment effect of infliximab versus placebo in macular thickness and fundus photographs results was also evaluated by a mixed-models approach for imbalanced crossover design using the percent difference as an outcome variable. The carryover effect was also tested in this model. The residual maximum likelihood technique was used for estimating variance components.
The planned sample size of 26 patients was based on the expected reduction of BCVA after treatment with infliximab (16). It was estimated that 22 evaluable eyes (11 per study arm) would provide 90% power to detect a mean difference in log minimum angle of resolution (logMAR) BCVA of 0.67 (equivalent to 22 letters read in the EDTRS chart) at a 0.001 level of statistical significance. Under the assumption of a 15% dropout rate, it was decided to recruit 13 patients for each treatment sequence. However, the study was terminated after enrollment of the first 12 patients because of inability to recruit additional patients who had not any intravitreal infusion within the prior 6 months (exclusion criterion 4, as described above). Statistical analysis was performed by SAS (version 9.1.3) statistical software.
RESULTS
Demographic and disease characteristics of the 11 treated patients are shown in Table 1. There were three women and eight men, aged between 40 and 73 years, with diabetes duration ranging between 3 and 20 years (1 patient with type 1 diabetes and 10 patients with type 2 diabetes). The additional enrolled patient, aged 72, was randomly assigned to initially receive placebo (group A), but had an acute myocardial infarction 2 days before the first scheduled injection and withdrew from the study. One patient from group A withdrew consent at week 18 after receiving four placebo injections and the first infliximab injection. In total 14 eyes were eligible for analysis (6 eyes in group A, including this patient's response to placebo treatment, and 8 eyes in group B) (Table 1).
Table 1.
Demographic and disease characteristics of patients with DME and individual BCVA values of eligible eyes at baseline (week −2), end of study treatment 1 (week 16), and end of study treatment 2 (week 32)
| Patient's sex, age (years), diabetes type, years of diabetes, A1C (%) | Eye | No. of previous laser treatments, months since last session | Treatment* | Period† | BCVA baseline | BCVA | BCVA Change (%) |
|---|---|---|---|---|---|---|---|
| M, 40, 1, 4, 6.9 | 01 | 2, 7 | A | 1 | 33 | 40 | 21.2 |
| 01 | B | 2 | 40 | 47 | 17.5 | ||
| M, 67, 2, 27, 7.1 | 02 | 0 | A | 1 | 40 | 34 | −15.0 |
| 02 | B | 2 | 34 | 40 | 17.6 | ||
| F, 71, 2, 20, 7.0 | 03 | 3, 13 | A | 1 | 29 | 34 | 17.2 |
| 03 | B | 2 | 34 | 35 | 2.9 | ||
| F, 56, 2, 19, 6.9 | 04 | 4, 6 | A | 1 | 28 | 10 | −64.3 |
| 04 | B | 2 | 10 | 17 | 70.0 | ||
| 05 | 4, 6 | A | 1 | 28 | 26 | −7.1 | |
| 05 | B | 2 | 26 | 38 | 46.2 | ||
| M, 64, 2, 4, 5.5 | 06 | 4, 12 | A | 1 | 40 | 40 | 0.0 |
| M, 73, 2, 18, 9.3 | 07 | 0 | B | 1 | 27 | 28 | 3.7 |
| 07 | A | 2 | 28 | 26 | −7.1 | ||
| F, 63, 2, 19, 7.9 | 08 | 2, 12 | B | 1 | 6 | 9 | 50.0 |
| 08 | A | 2 | 9 | 13 | 44.4 | ||
| 09 | 2, 8 | B | 1 | 10 | 12 | 20.0 | |
| 09 | A | 2 | 12 | 15 | 25.0 | ||
| F, 40, 2, 3, 5.4 | 10 | 2, 6 | B | 1 | 28 | 31 | 10.7 |
| 10 | A | 2 | 31 | 42 | 35.5 | ||
| 11 | 2, 9 | B | 1 | 24 | 39 | 62.5 | |
| 11 | A | 2 | 39 | 40 | 2.6 | ||
| F, 57, 2, 10, 5.6 | 12 | 8, 14 | B | 1 | 25 | 40 | 60.0 |
| 12 | A | 2 | 40 | 33 | −17.5 | ||
| M, 71, 2, 11, 8.3 | 13 | 2, 6 | B | 1 | 35 | 39 | 11.4 |
| 13 | A | 2 | 39 | 39 | 0.0 | ||
| M, 73, 2, 27, 6.8 | 14 | 3, 10 | B | 1 | 33 | 45 | 36.4 |
| 14 | A | 2 | 45 | 43 | −4.4 |
*A denotes placebo; B denotes infliximab.
†Study treatment 1: from baseline to week 16; study treatment 2: from week 16 to week 32. F, female; M, male.
Primary study objective: changes in best corrected visual acuity
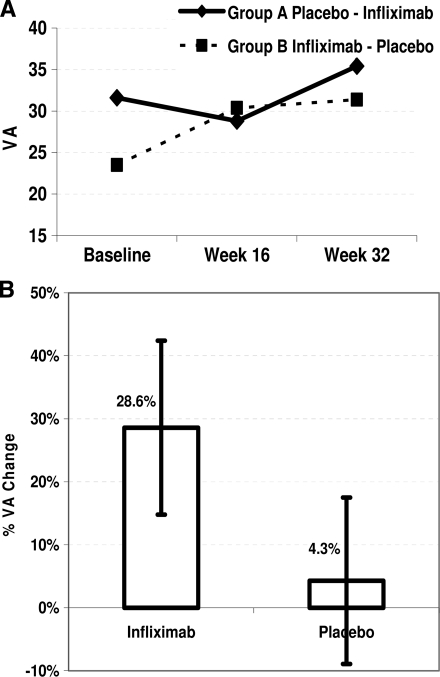
Individual values of BCVA at baseline, week 16 (end of the first study treatment), and week 32 (final evaluation after the second study treatment) are shown in Table 1. Baseline BCVA was not different between groups (31.6 ± 5.1 vs. 23.5 ± 10.3 letters read; t = 1.7; P = 0.10). As shown in Fig. 1A, BCVA decreased from 31.6 ± 5.1 at baseline to 28.8 ± 11.6 letters read at week 16 in eyes treated initially with placebo and subsequently increased to 35.4 ± 11.2 letters read at completion of infliximab treatment (week 32). On the other hand, BCVA increased from 23.5 ± 10.3 at baseline to 30.4 ± 13.4 letters read at week 16 in eyes treated initially with infliximab and remained essentially unchanged at completion of placebo treatment (31.4 ± 12.1 letters read, week 32).
Figure 1.
Changes in visual acuity (VA) measured by the number of letters that a patient was able to read from the ETDRS chart from baseline to study end. Eyes of group A and group B were treated initially with placebo followed by infliximab or vice versa, respectively (A). The improvement of visual acuity in infliximab-treated eyes is significantly greater by 24.3% compared with that of placebo-treated eyes, as evaluated by a mixed-models approach for imbalanced crossover design (B).
Collectively, four infusions of infliximab resulted in an increase in BCVA, from mean ± SD 25.5 ± 10.7 (range 6–40) to 32.3 ± 12.4 (range 9–47) letters read (n = 13). In contrast, BCVA remained essentially unchanged in placebo-treated eyes (n = 14), from 31.5 ± 10.5 (range 9–45) to 31.1 ± 11.3 (range 10–43) letters read. Least squares means indicated that infliximab administration resulted in 28.6% and placebo resulted in 4.3% improvement in visual acuity. A possible carryover effect of infliximab in the second part of the study was tested in this model and was found to be nonsignificant. Overall, the improvement in visual acuity in the infliximab-treated eyes was significantly greater by 24.3% compared with that in placebo-treated eyes (95% CI 4.8–43.7; P = 0.0167) (Fig. 1B).
Secondary anatomic and vision-related objectives
A similar analysis failed to reveal a significant effect of infliximab over placebo in the secondary end points of the study. Least squares means indicated that central macular thickness assessed by OCT decreased by 3.7% with infliximab and increased by 1.3% with placebo (P > 0.5). Moreover, no significant difference between infliximab and placebo could be demonstrated in the scores of fundus photographs graded according to the ETDRS protocol.
Baseline versus 32-week evaluation measurements
As shown in Table 2, the following changes from baseline (−2 week) to the end of the study (32 weeks) were evident in our 10 patients (13 eyes) who, either in the first or second part of the study, received four infliximab infusions: BCVA improved by at least one line (5 letters in the EDTRS chart) in 10 of 14 eyes (77%), whereas 5 eyes (38%) gained two or more lines, 2 eyes remained stable, and 1 eye worsened by 5 letters. Foveal thickness decreased by >10% in 5 eyes (38%), remained stable in 5 eyes, and increased by >10% in 3 eyes. Finally, as documented by both fundus photographs and fluoroangiography, the status of diabetic retinopathy improved in 3 eyes, remained stable in 5 eyes, and deteriorated in 1 eye. Fundus photographs and fluoroangiography yielded conflicting results in the remaining 4 eyes.
Table 2.
Changes from baseline to 32 weeks in BCVA, DME, and retinopathy status after infliximab, given either during study treatment 1 (eyes 01–05) or study treatment 2 (eyes 07–14)
| Eye | Difference in letters read (% BCVA change) | % DME thickness change | Fundus photographs* | Fluoroangiography |
|---|---|---|---|---|
| 01 | +13 (42) | −30 | 35 to 35 | Worst |
| 02 | 0 (0) | −7 | 35 to 35 | Stable |
| 03 | +6 (21) | 15 | 20 to 35 | Stable |
| 04 | −9 (−39) | −7 | 53 to 53 | Stable |
| 05 | +10 (36) | −14 | 43 to 43 | Stable |
| 07 | −1 (−4) | 16 | 35 to 20 | Improved |
| 08 | +7 (117) | −45 | 53 to 53 | Stable |
| 09 | +5 (50) | 6 | 47 to 47 | Stable |
| 10 | +14 (50) | −20 | 43 to 35 | Improved |
| 11 | +14 (67) | −15 | 35 to 35 | Improved |
| 12 | +8 (32) | 6 | 53 to 43 | Worst |
| 13 | +4 (11) | −2 | 35 to 47 | Worst |
| 14 | +10 (30) | 20 | 35 to 20 | Improved |
*Grading according to the ETDRS protocol: 20: macular edema only; 35, 43, 47, and 53: mild, moderate, moderately severe, and severe nonproliferative diabetic retinopathy, respectively.
Safety issues
Infliximab was well tolerated and no safety issues emerged from hematologic monitoring, urinalysis, or ophthalmic assessments, including intraocular pressure or cataract formation during the study. Moreover, no significant impact of placebo or infliximab on glycemic control was noted. One male patient (aged 64, with diabetes type 2 for 4 years; Table 1) had a diagnosis of breast cancer 5 months after the baseline evaluation. This condition was considered to be unrelated to infliximab treatment, because a slightly palpable mass leading to the final diagnosis was revealed in a physical examination at week 18, only 14 days after the first infliximab injection. Another male patient (aged 73, with diabetes type 2 for 18 years; Table 1) developed an upper respiratory tract infection that was treated successfully with antibiotics at week 29 while receiving placebo. Finally, one male patient (aged 71, with diabetes type 2 for 11 years; Table 1) developed a neuro-ischemic foot ulcer at week 51 (18 weeks after receiving the eighth study injection of placebo).
CONCLUSIONS
Evidence suggests that altered local expression of TNF may play an important role in the pathogenesis of DME (17,18) and that low-grade subclinical inflammation is responsible for many of the signature vascular lesions of diabetic retinopathy (9). Moreover, studies in patients with arthritis have shown that anti-TNF therapy negatively affects vascular permeability and angiogenesis by decreasing VEGF (19), which has been implicated directly in the pathogenesis of DME and diabetic retinopathy (2,9,11). Although studies have shown the possible benefits of intravitreal corticosteroids and anti-VEGF antibodies in the treatment of DME, focal/grid laser photocoagulation continues to be the only proven safe and effective treatment (2). Still, this treatment targets only advanced stages of the disease. It is noteworthy that there are no previous randomized placebo-controlled phase III studies for any treatment option in DME.
The present study included patients with sight-threatening DME that was unmanageable by laser photocoagulation. Of the 14 evaluable eyes, 12 had previously received at least two laser sessions (maximum eight sessions, eye 12) (Table 1). The two remaining eyes had leaking microaneurysms within the foveal avascular zone, making laser photocoagulation unsafe for the central vision. In view of our previously published encouraging preliminary results with infliximab (15), the crossover design of this phase III study was chosen to allow all participants with sight-threatening DME to receive infliximab and to enhance the statistical power of the study.
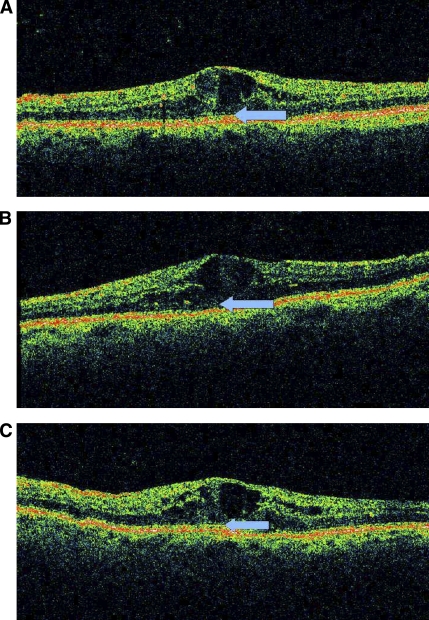
Because of the strict study exclusion criteria and because intravitreal administration of anti-VEGF agents has been increasingly used over the past 2 years in Greece, we were able to recruit only 12 patients. Thus, the main limitation of the present study is the small sample size, limiting statistical analysis and not ensuring that randomization balanced all known and unknown risk factors between groups. However, the mean duration of diabetes, as well as the mean number of previous laser treatments and length of time since the last session were similar (Table 1), whereas baseline BCVA was also not different between groups. Despite the small sample size, our short crossover trial of a conventional dose of infliximab demonstrated a significant improvement over placebo on the severely impaired visual acuity of these patients. Infliximab, either as a first or second agent resulted in almost similar increases in BCVA (6.9 and 6.6 mean letters read, respectively). Thus, this infliximab-induced mean observed improvement of almost 7 letters read in the EDTRS chart is comparable to the mean gain in BCVA at 6 months in patients with DME treated with four intravitreal injections of the anti-VEGF agent ranibizumab (11). Moreover, at the end of the study BCVA improved by at least one line in 77% and by at least two lines in 38% of infliximab-treated eyes. These results are considered clinically important, given the fact that patients included in this study were unsuitable for all available approved treatment options. It seems that improvement of BCVA was not correlated with the secondary anatomic and vision-related end points, because neither an anatomic improvement of DME by OCT nor a decrease in fundus photographs grading by the ETDRS protocol could be demonstrated. A recent study of 323 eyes from a randomized clinical trial of two methods of laser photocoagulation for DME found that the OCT-based assessment of the extensiveness of DME neither explains additional variation in baseline visual acuity above that explained by other known important variables nor predicts changes in macular thickness or visual acuity after laser photocoagulation (20). It is possible that other infliximab-related changes may account for our findings. For example, local TNF neutralization by infliximab could have exerted a beneficial effect on photoreceptor function, explaining in part the improvement in BCVA despite the persistence of macular edema in patients throughout our study. Interestingly, as shown in Fig. 2, the photoreceptor inner/outer segment line, which was invisible at baseline or after the placebo treatment, partially reappeared after infliximab treatment. Whether such changes underlie the infliximab-induced BCVA improvement remains to be seen, because the photoreceptor inner/outer segment junction line was not identifiable by OCT at any time in all other patients.
Figure 2.
Sequential OCT images at baseline (A), at completion of placebo treatment (B), and at completion of infliximab treatment (C). The photoreceptor inner/outer segment junction line at the foveola is highly disrupted at week −2 (A, arrow), becomes almost absent at week 16 (B, arrow), and appears partially restored at week 32 (C, arrow).
The key safety considerations that emerged during the first years of clinical use of infliximab included infections, autoimmune disease, demyelinating disease, malignancies, and congestive heart failure (8). Overall rates of these conditions in randomized controlled trials were not significantly increased during treatment compared with placebo. Postmarketing surveillance data in thousands of patients have clearly shown that the safety profile of infliximab is excellent, provided that it is not used to treat patients with active infection, malignancy, preexisting demyelinating conditions, and heart failure and that precautions are taken for reactivation of latent tuberculosis. No other particular safety signals in patients with diabetes have emerged (8). Overall, infliximab was well tolerated in our study.
To summarize, a short-term treatment with infliximab significantly improved BCVA in eyes with advanced-stage sight-threatening DME refractory to standard treatment, further suggesting an important role for TNF-mediated pathogenetic mechanisms in this condition. This positive result also suggests that larger and longer term placebo-controlled trials are warranted to assess the efficacy and safety of systemic TNF blockade and/or of local delivery of anti-TNF antibodies by intravitreal injection (21–24) for the primary treatment of DME.
Acknowledgments
Funding for this study was provided by Centocor (Malvern, PA).
No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00505947, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Ciulla TA, Amador AG, Zinman B: Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003; 9: 2653–2664 [DOI] [PubMed] [Google Scholar]
- 2. Simó R, Hernández C: Advances in the medical treatment of diabetic retinopathy. Diabetes Care 2009; 32: 1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IV. Diabetic macular edema. Ophthalmology 1984; 91: 1464–1474 [DOI] [PubMed] [Google Scholar]
- 4. Ferris FL, 3rd, Patz A: Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 1984; 28(Suppl.): 452–461 [DOI] [PubMed] [Google Scholar]
- 5. Antcliff RJ, Marshall J: The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol 1999; 14: 223–232 [DOI] [PubMed] [Google Scholar]
- 6. O'Doherty M, Dooley I, Hickey-Dwyer M: Interventions for diabetic macular oedema: a systematic review of the literature. Br J Ophthalmol 2008; 92: 1581–1590 [DOI] [PubMed] [Google Scholar]
- 7. Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP: Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 1999; 17: 331–367 [DOI] [PubMed] [Google Scholar]
- 8. Sfikakis PP: The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun 2010; 11: 180–210 [DOI] [PubMed] [Google Scholar]
- 9. Adamis AP, Berman AJ: Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol 2008; 30: 65–84 [DOI] [PubMed] [Google Scholar]
- 10. Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M: Activation of nuclear factor-κB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002; 51: 2241–2248 [DOI] [PubMed] [Google Scholar]
- 11. Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PA. READ-2 Study Group. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 2009; 116: 2175–2181.e1 [DOI] [PubMed] [Google Scholar]
- 12. Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, Lavaque AJ, Larson RJ: Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology 2009; 116: 902–911; quiz 912–913 [DOI] [PubMed] [Google Scholar]
- 13. Grover D, Li TJ, Chong CC: Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev 2008; 23: CD005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Döhmen S, Adamis AP: Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-α suppression. FASEB J 2002; 16: 438–440 [DOI] [PubMed] [Google Scholar]
- 15. Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG: Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care 2005; 28: 445–447 [DOI] [PubMed] [Google Scholar]
- 16. Theodossiadis PG, Markomichelakis NN, Sfikakis PP: Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007; 27: 399–413 [DOI] [PubMed] [Google Scholar]
- 17. Limb GA, Webster L, Soomro H, Janikoun S, Shilling J: Platelet expression of tumour necrosis factor-α (TNF-α), TNF receptors and intercellular adhesion molecule-1 (ICAM-1) in patients with proliferative diabetic retinopathy. Clin Exp Immunol 1999; 118: 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Limb GA, Hollifield RD, Webster L, Charteris DG, Chignell AH: Soluble TNF receptors in vitreoretinal proliferative disease. Invest Ophthalmol Vis Sci 2001; 42: 1586–1591 [PubMed] [Google Scholar]
- 19. Cañete JD, Pablos JL, Sanmartí R, Mallofré C, Marsal S, Maymó J, Gratacós J, Mezquita J, Mezquita C, Cid MC: Antiangiogenic effects of anti-tumor necrosis factor α therapy with infliximab in psoriatic arthritis. Arthritis Rheum 2004; 50: 1636–1641 [DOI] [PubMed] [Google Scholar]
- 20. Browning DJ, Apte RS, Bressler SB, Chalam KV, Danis RP, Davis MD, Kollman C, Qin H, Sadda S, Scott IU. Diabetic Retinopathy Clinical Research Network. Association of the extent of diabetic macular edema as assessed by optical coherence tomography with visual acuity and retinal outcome variables. Retina 2009; 29: 300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Theodossiadis PG, Liarakos VS, Sfikakis PP, Charonis A, Agrogiannis G, Kavantzas N, Vergados IA: Intravitreal administration of the anti-TNF monoclonal antibody infliximab in the rabbit. Graefes Arch Clin Exp Ophthalmol 2009; 247: 273–281 [DOI] [PubMed] [Google Scholar]
- 22. Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP: Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol 2009; 147: 825–830.e1 [DOI] [PubMed] [Google Scholar]
- 23. Hosseini H, Safaei A, Khalili MR, Nowroozizadeh B, Eghtedari M, Farvardin M, Nowroozizadeh S, Tolide-Ie HR: Intravitreal infliximab in experimental endotoxin-induced uveitis. Eur J Ophthalmol 2009; 19: 818–823 [DOI] [PubMed] [Google Scholar]
- 24. Giansanti F, Ramazzotti M, Giuntoli M, Virgili G, Vannozzi L, Degl'Innocenti D, Menchini U: Intravitreal infliximab clearance in a rabbit model: different sampling methods and assay techniques. Invest Ophthalmol Vis Sci 2009; 50: 5328–5335 [DOI] [PubMed] [Google Scholar]