Abstract
OBJECTIVE
Recent large randomized trials have linked adverse cardiovascular and cerebrovascular events with hypoglycemia. However, the integrated physiological and vascular biological mechanisms occurring during hypoglycemia have not been extensively examined. Therefore, the aim of this study was to determine whether 2 h of moderate clamped hypoglycemia could decrease fibrinolytic balance and activate pro-atherothrombotic mechanisms in individuals with type 1 diabetes and healthy individuals.
RESEARCH DESIGN AND METHODS
Thirty-five healthy volunteers (19 male and 16 female subjects age 32 ± 2 years, BMI 26 ± 2 kg/m2, A1C 5.1 ± 0.1%) and twenty-four with type 1 diabetes (12 male and 12 female subjects age 33 ± 3 years, BMI 24 ± 2 kg/m2, A1C 7.7 ± 0.2%) were studied during either a 2-h hyperinsulinemic (9 pmol · kg−1 · min−1) euglycemic or hypoglycemic (2.9 ± 0.1 mmol/l) clamp or both protocols. Plasma glucose levels were normalized overnight in type 1 diabetic subjects prior to each study.
RESULTS
Insulin levels were similar (602 ± 44 pmol/l) in all four protocols. Glycemia was equivalent in both euglycemic protocols (5.2 ± 0.1 mmol/l), and the level of hypoglycemia was also equivalent in both type 1 diabetic subjects and healthy control subjects (2.9 ± 0.1 mmol/l). Using repeated ANOVA, it was determined that plasminogen activator inhibitor (PAI-1), vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), E-selectin, P-selectin, interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and adiponectin responses were all significantly increased (P < 0.05) during the 2 h of hyperinsulinemic hypoglycemia as compared with euglycemia in healthy control subjects. All measures except PAI-1 were also found to be increased during hypoglycemia compared with euglycemia in type 1 diabetes.
CONCLUSIONS
In summary, moderate hypoglycemia acutely increases circulating levels of PAI-1, VEGF, vascular adhesion molecules (VCAM, ICAM, E-selectin), IL-6, and markers of platelet activation (P-selectin) in individuals with type 1 diabetes and healthy individuals. We conclude that acute hypoglycemia can result in complex vascular effects including activation of prothrombotic, proinflammatory, and pro-atherogenic mechanisms in individuals with type 1 diabetes and healthy individuals.
Hypoglycemia occurs very commonly in individuals with type 1 diabetes. Despite this, the effects of hypoglycemia on in vivo vascular biology in type 1 diabetes have not been extensively studied. Case reports and a recent study have linked hypoglycemia with angina, myocardial infarction, and acute cerebrovascular events (1,2). More recently, a large epidemiological study has determined that hypoglycemia results in an increased risk of cardiovascular disease and all-cause mortality (3). Additionally, a recent large randomized clinical trial in type 2 diabetes has highlighted an increased risk of death when glucose levels are intensively treated (4). Whereas the role played by hypoglycemia in directly causing the increased adverse events in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is not established, a much stronger link between severe hypoglycemia and vascular adverse events was indicated in the Veteran's Affairs Diabetes Trial (VADT) study (also in type 2 diabetes) (5). As patients with type 1 diabetes advance into middle age, they are also likely to become more susceptible to vascular disease. Hypoglycemia has been implicated with sudden death in type 1 diabetes, although the mechanism remains speculative (6). Offsetting this risk, a previous period of intensive glucose control combined with continued moderate glycemic levels (A1C ∼8.0%) has resulted in a reduction in cardiovascular disease in type 1 diabetes (7). However, information addressing any possible proinflammatory and resultant activation of pro-atherothrombotic responses in type 1 diabetes during hypoglycemia is scarce. To date, very few studies have investigated the effects of hypoglycemia on vascular physiology in type 1 diabetes (8–10). Complicating interpretation of this scarce data is the fact that insulin and glucose levels were not controlled. Thus, because it is now recognized that both insulin and hyperglycemia can have independent vascular biological effects (11), it becomes important to strictly control these two important variables. Therefore, this present study has tested the hypothesis that acute moderate hypoglycemia can activate proinflammatory mechanisms and reduce fibrinolytic balance in both individuals with type 1 diabetes and nondiabetic individuals. The hyperinsulinemic euglycemic and hypoglycemic clamp techniques were used so that insulin levels could be equated and glucose values could be controlled independently during each study.
RESEARCH DESIGN AND METHODS
Thirty-five healthy subjects (19 male and16 female subjects, age 32 years ± 2, BMI 26 ± 2 kg/m2, A1C 5.1 ± 0.1) and twenty-four with type 1 diabetes (12 male and12 female subjects, age 33 ± 3 years, BMI 24 ± 2 kg/m2, A1C 7.7 ± 0.2 [normal range 4–6.5%], diabetes duration 17 ± 8 years) were studied (data can be found in online appendix Table 1, available at http://care.diabetesjournals.org/cgi/content/full/dc09-0354/DC1). Individuals with type 1 diabetes had normal bedside tests of autonomic function (12) and did not have hypoglycemia unawareness based on the methods of Gold, et al. (13). Type 1 diabetic individuals had no major macro or micro complications of diabetes. Type 1 diabetic subjects were excluded from the study if they had a history of hypoglycemia induced convulsions or a major episode of hypoglycemia in the preceding 2 years. Type 1 diabetes individuals were treated with multiple daily insulin injections or with an insulin pump. All subjects were nonsmokers and had a normal blood count, plasma lipids, plasma electrolytes, liver, and renal function, and were normotensive. No subject was taking medications known to affect neuroendocrine responses to hypoglycemia (specifically, fluoxetine) or influence platelet, clotting, or fibrinolytic balance. Some of the individuals included in the present study had served as control subjects to determine usual baseline counterregulatory responses to hypoglycemia in previous studies (14,15). Studies were approved by the Vanderbilt University human subjects Institutional Review Board, and all participants gave written informed consent.
Subjects participated in either a single hyperinsulinemic euglycemic or hypoglycemic clamp or both sets of experiments. The number of individuals participating in each arm of the study were: type 1 diabetes euglycemia (n = 14), type 1 diabetes hypoglycemia (n = 17), healthy control euglycemia (n = 22), healthy control hypoglycemia (n = 25). Ten healthy control subjects and seven individuals with type 1 diabetes participated in both euglycemic and hypoglycemic protocols in a randomized fashion. Results from this subgroup are conceptually similar to the entire cohort (data are available in online appendix Table 3, Fig. A1 and A2). All study patients were asked to avoid any exercise and consume their usual weight-maintaining diet for 3 days before each experiment. All type 1 diabetic patients were asked to perform intensive home blood glucose monitoring (i.e., at least four glucose tests per day) and to avoid hypoglycemia for at least 5 days before a study. On the day prior to a study, intermediate or long-acting insulin was discontinued and replaced by injections of regular insulin before breakfast and lunch. Each subject was admitted to the Vanderbilt General Clinical Research Center (CRC) at ∼5:00 p.m. the evening before an experiment. At this time in the individuals with type 1 diabetes, two intravenous cannulae were inserted under 1% lidocaine local anesthesia. One cannula was placed in a retrograde fashion into a vein on the back of the hand. This hand was placed in a heated box (55–60°C) during the study so that arterialized blood could be obtained. The other cannula was placed in the contralateral arm for infusions. All subjects received an evening meal, and individuals with type 1 diabetes received a continuous low-dose infusion of insulin to normalize plasma glucose. The insulin infusion was adjusted overnight to maintain blood glucose between 4.4 and 7.2 mmol/l.
Hypoglycemia experiments
All subjects were studied after an overnight, 10-h fast. Venous cannulae as described above were placed in the healthy control subjects. After this, a period of 120 min was allowed to elapse followed by a 120-min hyperinsulinemic hypoglycemic experimental period. At time 120 min, a primed constant (9.0 pmol · kg−1 · min−1) infusion of insulin (Human Regular Insulin; Eli Lilly, Indianapolis, IN) was started and continued until 240 min. The rate of fall of glucose was controlled (∼0.08 mmol/min), and the glucose nadir (2.9 mmol/l) was achieved using a modification of the glucose clamp technique. During the clamp period, plasma glucose was measured every 5 min and a 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 2.9 ± 0.1 mmol/l (16). Potassium chloride (20 mmol/l) was infused during the clamp to reduce insulin-induced hypokalemia.
Euglycemia experiments
These experiments followed the same format as the above described hypoglycemia experiments with the exception that euglycemia (5.2 ± 0.1 mmol/l) was maintained during each study.
Analytical methods
The collection of blood samples has been described elsewhere (14). Plasma glucose concentrations were measured in triplicate using the glucose oxidase method with a glucose analyzer (Beckman, Fullerton, CA). Insulin was measured as previously described with an interassay coefficient of variation (CV) of 9% (14). Catecholamines were determined by high-performacne liquid chromatography with an interassay CV of 12% for epinephrine and 8% for norepinephrine (14). Cortisol was assayed using the clinical assays γ-coat radioimmunoassay kit with an interassay CV of 6% (14). Nonesterified fatty acids (NEFAs) were measured using the WAKO kit with an interassay CV of 7%.
Blood was drawn every 60 min for soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), E-selectin, P-selectin, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), plasminogen activator inhibitor 1 (PAI-1), tissue plasminogen activator (tPA), and adiponectin was drawn every 60 min and every 30 min for catecholamines, cortisol, and NEFA during the experimental period. Vascular adhesion molecules and adiponectin were assayed using LINCO Research Kits (St. Charles, Missouri) with interassay CVs of 8.5% for sVCAM, 9.7% for sICAM, 13.4% for sE-selectin, 15.9% for adiponectin), 9.02% for IL-6, 9.98% for TNF-α, and 8.2% for VEGF. P-selectin (CV 9.9%), Meso scale discovery (Gaithersburg, MD), PAI-1, and tPA antigen were determined by TintElize PAI-1 Kit with interassay CV of 3.3%.
Statistical analysis
Data are expressed as means ± SE and were analyzed using standard, parametric, one and two-way analysis of variance (ANOVA) with repeated measures where appropriate (SPSS, Chicago, IL). Data were also analyzed with paired and unpaired two tailed t test (Graph Pad Software, San Diego, CA). In all cases, a P < 0.05 was accepted as statistically significant. Individual peak and nadir values during the final 60 min (at time 180 or 240 min) of hypoglycemia and euglycemia were compared for vascular biological parameters.
Glucose and insulin
Plasma glucose was maintained equivalently (5.2 ± 0.1 mmol/l) during the euglycemic clamps. Plasma glucose reached steady state by 150 min, and equivalent hypoglycemia (2.9 ± 0.1 mmol/l) was maintained during all hypoglycemia clamp procedures (online appendix Fig. A3). Insulin levels (602 ± 24 pmol/l) for both healthy and type 1 diabetes groups were similar during all four protocol clamp studies (online appendix Fig. A3).
Neuroendocrine counterregulatory hormones
Epinephrine responses were significantly higher (P < 0.001) during the final 30 min of hypoglycemia (5.6 ± 0.7 nmol/l, 3.14 ± 0.5 nmol/l) as compared with euglycemia (0.19 ± 0.03 nmol/l, 0.27 ± 0.04 nmol/l) in both the healthy and type 1 diabetic groups, respectively. Epinephrine values were higher during hypoglycemia in healthy control subjects (P = 0.008) as compared with type 1 diabetic subjects. Norepinephrine levels were also significantly higher (P < 0.001) during the final 30 min of hypoglycemia (1.9 ± 0.3 nmol/l, 1.8 ± 0.02 nmol/l) as compared with euglycemia (0.9 ± 0.2 nmol/l, 1.1 ± 0.01 nmol/l) in healthy control subjects and type 1 diabetic subjects, respectively. Norepinephrine levels were not different during hypoglycemia in healthy control subjects and type 1 diabetic subjects. There was a trend for plasma cortisol levels to be higher in healthy control subjects as compared with type 1 diabetic subjects (861 ± 42 vs. 725 ± 46 nmol/l, P = 0.09). Cortisol values were higher (P <0.001) during hypoglycemia as compared with euglycemia in both healthy control subjects (861 ± 42 vs. 357 ± 24 nmol/l) and type 1 diabetic subjects (725 ± 46 vs. 302 ± 66 nmol/l), respectively.
Intermediary metabolism
Blood NEFA levels fell significantly (P < 0.0001) during hypoglycemic and euglycemic clamps. However, there were significantly higher levels (P <0.05) of NEFA during the final 30 min of hypoglycemia as compared with euglycemia in both healthy control subjects (97 ± 11 vs. 68 ± 18 μmol/l) and type 1 diabetic patients (100 ± 18 vs. 50 ± 4 μmol/l), respectively.
Atherogenic vascular adhesion molecules
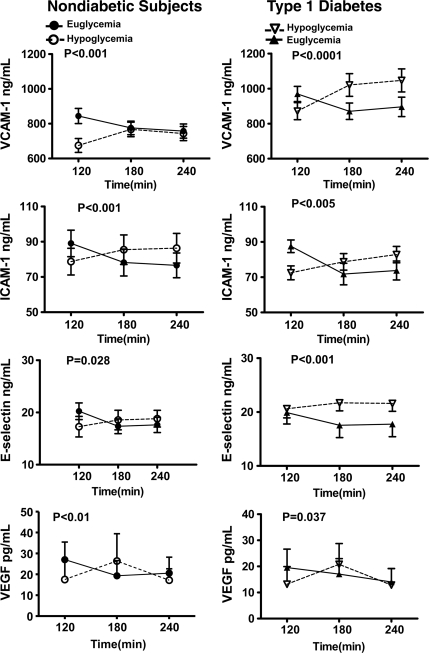
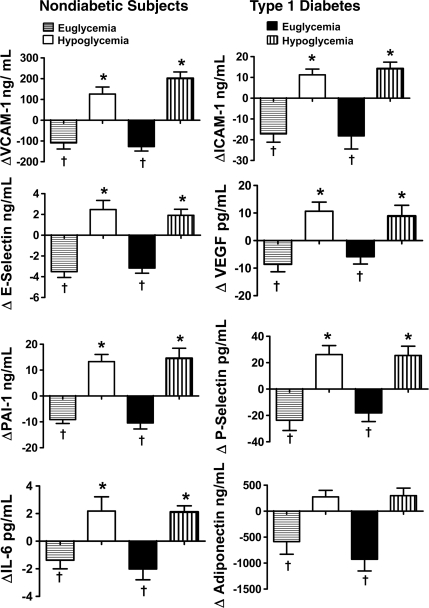
With the exception of VCAM-1 in healthy control subjects and ICAM-1 in subjects with type 1 diabetes, baseline values were similar at the start of euglycemic and hypoglycemic clamps (online appendix Tables 2 and 3). Plasma VCAM, ICAM, and E-selectin responses during the 120-min clamp studies were significantly different (P < 0.05–P < 0.0001 ANOVA) during hypoglycemia as compared with euglycemia in both healthy control subjects and type 1 diabetic subjects (Fig. 1). Individual peak values for VCAM, ICAM, and E-selectin were increased significantly from baseline (P < 0.05) during hypoglycemia (Fig. 3); mean individual nadir values of VCAM, ICAM, and E-selectin were significantly (P < 0.05) decreased from baseline during euglycemia in both type 1 diabetic subjects and healthy control subjects (Fig. 3). The magnitude of responses did not differ in healthy control subjects and type 1 diabetic subjects.
Figure 1.
Effects of hyperinsulinemic euglycemia and hypoglycemia (2.9 mmol/l) in overnight-fasted healthy control subjects (n = 35) and individuals with type 1 diabetes (n = 24) that participated in either one or both of the studies on vascular biological markers. Response of VCAM-1, ICAM-1, E-selectin, and VEGF are significantly increased during hypoglycemia as compared with euglycemia in both healthy control subjects and type 1 diabetic subjects. Statistical difference with two-way ANOVA during the 120-min clamp experiments is marked in each graph panel.
Figure 3.
*Mean individual peak responses of VCAM-1, ICAM-1, E-selectin, VEGF, PAI-1, IL-6, P-selectin, and adiponectin in both healthy control subjects (n = 35) and subjects with type 1 diabetes (n = 24) that participated in either one or both of the studies are significantly increased (P <0.05) compared with baseline. †Mean individual nadir responses of VCAM-1, ICAM-1, E-selectin, VEGF, PAI-1, P-selectin, and adiponectin during hyperinsulinemic euglycemia in both healthy control subjects and type 1 diabetic subjects are significantly decreased (P <0.05) compared with baseline.
Platelet activation and fibrinolytic balance
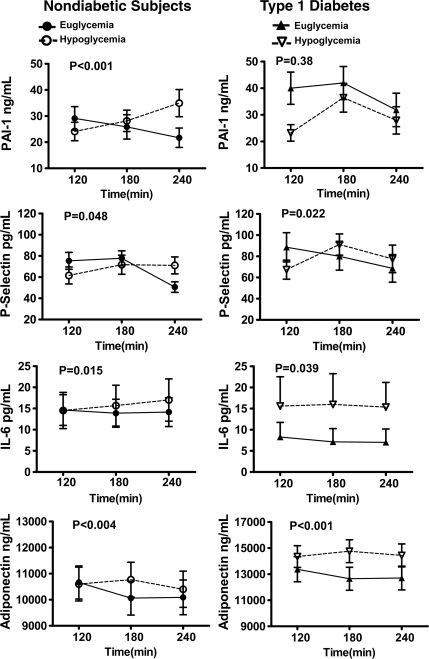
Plasma P-selectin responses were significantly different (P < 0.05 ANOVA) during the hypoglycemic as compared with euglycemic clamp studies in both healthy control subjects and type 1 diabetic subjects (Fig. 2). Baseline PAI-1 levels were significantly different (P < 0.05) in the type 1 diabetes group (online appendix Table 3). Plasma PAI-1 values were significantly different during hypoglycemia as compared with euglycemia in healthy control subjects but not type 1 diabetic subjects (Fig. 2). Mean individual peak values from baseline for P-selectin and PAI-1 responses were increased (P < 0.05) during hypoglycemia in both healthy control subjects and type 1 diabetic subjects (Fig. 3). Nadir P-selectin and PAI-1 values were significantly (P < 0.01) lower from baseline during hyperinsulinemic euglycemia in both type 1 diabetic subjects and healthy control subjects (Fig. 3). Plasma concentrations of tPA did not alter during either euglycemic or hypoglycemic clamps in both normal subjects and individuals with type 1 diabetes. Responses of P-selectin, tPA and PAI-1 were similar in healthy control subjects and type 1 diabetic subjects.
Figure 2.
Effects of hyperinsulinemic euglycemia and hypoglycemia (2.9 ± 0.1 mmol/l) in overnight-fasted healthy control subjects (n = 35) and subjects with type 1 diabetes (n = 24) that participated in either one or both of the studies on vascular biological markers. Responses of PAI-1, P-selectin, IL-6, and adiponectin are significantly increased during hypoglycemia as compared with euglycemia in healthy control subjects. Responses of P-selectin, IL-6, and adiponectin are significantly increased during hypoglycemia as compared with euglycemia in type 1 diabetes. Statistical difference with two-way ANOVA during the 120-min clamp experiments is marked in each graph panel.
Adiponectin, VEGF, and inflammatory cytokines
Baseline values for adiponectin, VEGF, IL-6, and TNFα were similar at the start of both sets of glucose clamps (online appendix Tables 2 and 3). Plasma adiponectin responses were significantly different (P <0.01 ANOVA) during the 120-min hypoglycemic and euglycemic clamps in healthy control subjects and type 1 diabetic subjects (Fig. 3). Mean individual nadir values for adiponectin were significantly (P <0.05) reduced from baseline during hyperinsulinemic euglycemia in both subjects with type 1 diabetes and healthy control subjects (Fig. 3). There was a trend (P = 0.06 ANOVA) for peak adiponectin levels to increase from baseline during hypoglycemia in type 1 diabetes (Fig. 3). VEGF and IL-6 responses were significantly different (P <0.04–P = 0.0004 ANOVA) during hypoglycemia and euglycemia in type 1 diabetic subjects and healthy control subjects (Figs. 1 and 2). Nadir values for VEGF and IL-6 were significantly decreased (P < 0.04 – P <0.01) from baseline during hyperinsulinemic euglycemia in both subjects type 1 diabetes and healthy control subjects (Fig. 3). Mean individual peak values during hypoglycemia were increased from baseline (P <0.05) in both type 1 diabetic subjects and healthy control subjects. Peak values for TNF-α increased from baseline (P <0.05) during hypoglycemia in type 1 diabetic subjects and nadir values were also significantly decreased (P <0.05) from baseline during hyperinsulinemic euglycemia in healthy control subjects. Adiponectin, VEGF, TNF-α and IL-6 responded similarly during hypoglycemia in healthy control subjects and type 1 diabetic subjects (Fig. 3).
CONCLUSIONS
This study has determined the effects of 2 h of clamped hyperinsulinemic euglycemia and moderate hypoglycemia on proinflammatory mechanisms and fibrinolytic balance in individuals with type 1 diabetes and healthy individuals. Our results demonstrate that hypoglycemia can have acute and widespread effects on vascular biology. Hypoglycemia can 1) activate proinflammatory mechanisms (ICAM, VCAM, E-selectin, VEGF, IL-6), 2) increase platelet activation (P-selectin), and 3) simultaneously decrease systemic fibrinolytic balance (increase in PAI-1, no change in tPA) in both individuals type 1 diabetes and healthy individuals. These effects were of a similar magnitude in the healthy control subjects and type 1 diabetic subjects and were in contrast to the reduced responses of the above vascular biological mechanisms that occurred during clamped euglycemia with equivalent hyperinsulinemia.
In this present study, the effects of hypoglycemia and hyperinsulinemia on different proinflammatory and potentially pro-atherothrombotic mechanisms have been determined. ICAM-1, VCAM-1 and E-selectin are cell surface proteins that are upregulated during inflammation and increase the adhesion of leukocytes to injured arterial endothelial cells, which is a primary step in plaque formation and subsequent atherosclerosis. Analysis of the responses during the hyperinsulinemic clamps demonstrates a significant effect of hypoglycemia to increase VCAM, ICAM, E-selectin, and VEGF relative to euglycemia in both healthy control subjects and type 1 diabetic subjects individuals. VEGF has been reported to be pro-atherosclerotic in animals (17). In addition, VEGF can potently increase proliferation of the endothelium leading to new blood vessels. Hypoglycemia also had similar effects, increasing plasma levels of P-selectin in both individuals with type 1 diabetes and nondiabetic individuals. P-selectin is an adhesion molecule that is activated by inflammation (18) and is expressed by both endothelial cells and platelets. Recent work has demonstrated that platelets are the major source of P-selectin, and thus this molecule has been recognized as a significant marker of platelet activation and increased thrombotic mechanisms (18). PAI-1 was increased during hypoglycemia relative to euglycemia in healthy control subjects but not subjects with type 1 diabetes. However, individual peak values of PAI-1 were increased during hypoglycemia in both healthy control subjects and subjects with type 1 diabetes, demonstrating that hypoglycemia of 2.9 mmol/l can be a stimulus for increased values of PAI-1.
Our present results demonstrate that hypoglycemia decreases systemic fibrinolytic balance by increasing PAI-1 levels while maintaining tPA values. Thus, at least two separate mechanisms for increasing thrombosis are activated by hypoglycemia in individuals with type 1 diabetes and healthy individuals. The potential in vivo causes responsible for the reduced fibrinolytic balance and increased proinflammatory mechanisms activated by hypoglycemia are not known. During hypoglycemia, a wide spectrum of physiologic responses are activated that could have potential vascular biological effects. To date, the role played by catecholamines, the sympathetic nervous system, and neuroendocrine hormones on activating adhesion molecules and influencing fibrinolytic balance is incompletely understood. One study using cultured human adipose tissue has indicated that PAI-1 levels may be suppressed by catecholamines, but increased by high dose glucocorticoids (19). Other work (20) has reported that epinephrine can play a role via α-2 adrenoceptor mechanisms in increasing platelet activation during hypoglycemia in both individuals with type 2 diabetes and healthy individuals. Therefore, it is possible that both increases in the hypothalamic-pituitary axis and the sympathetic nervous system could have effects to activate proinflammatory and pro-thrombotic mechanisms during hypoglycemia.
Circulating triglycerides and NEFA are known to decrease endothelial function and increase cellular insulin resistance via nuclear factor-κB (21). NEFAs were increased in both type 1 diabetic subjects and healthy control subjects during hypoglycemia as compared with the euglycemia studies. This occurs principally due to the increased levels of catecholamines and sympathetic nervous system drive during hypoglycemia and the unopposed suppressive effects of insulin on lipolysis during the euglycemic experiments. Thus, the elevated NEFA levels remain a possible mechanism for our findings. The cytokines, IL-6 and TNF-α, had differential responses during hypoglycemia and euglycemia in both the type 1 diabetic subjects and healthy control subjects. IL-6 was increased during hypoglycemia relative to euglycemia in both groups whereas TNF-α was increased during hypoglycemia in type 1 diabetes. The magnitude of the increase in IL-6 during hypoglycemia in the healthy control subjects was similar to that reported in two recent studies by Dotson et al. (22) and Razavi Nematollahi et al. (23) Thus, it would appear that similar to hyperglycemia, acute hypoglycemia can also mediate its pro-atherothrombotic effects via TNF-α or IL-6 pathways (21). Nitric oxide (NO) has also been implicated in the regulation of both endothelial and platelet-derived adhesion molecules (24). Although not specifically addressed in the present study, we have preliminary data indicating that hypoglycemia impairs endothelial NO function. We would therefore suggest that NO may also be a significant molecular mechanism involved in hypoglycemia's vascular effects.
Adiponectin is released from adipose tissue and is known to have powerful insulin sensitizing and antiatherogenic properties. To our knowledge, adiponectin responses have not been investigated during hypoglycemia in type 1 diabetes or healthy volunteers. We were intrigued to discover that hypoglycemia also increased (relative to euglycemia) adiponectin levels in type 1 diabetic subjects and healthy control subjects. The significance of the finding is unclear and requires further study.
We would also like to indicate that in this present study, insulin, per se, had significant, beneficial effects on vascular physiology. It is notable that in every case where hypoglycemia resulted in an activation of a pathologic process, there was an equal and opposite beneficial effect during the hyperinsulinemic euglycemic control studies in both type 1 diabetes and healthy individuals. Thus, our present results would support previous work demonstrating that insulin has anti-inflammatory and antiatherogenic properties (10,21,24,25). There are some limitations to this study. There are sizeable inter individual variations in inflammatory and pro-atherothrombotic vascular biomarkers. Thus, baseline values for VCAM-1 in healthy control subjects and ICAM-1 and PAI-1 in subjects with type 1 diabetes were significantly different at the start of the euglycemic and hypoglycemic clamps. We do not know the reason for these baseline differences. One possible explanation is that not everyone in the study participated in both euglycemic and hypoglycemic clamps. We believe this is unlikely to be the cause as there was also a significant difference in baseline PAI-1 values in the type 1 diabetic subjects who participated in both series of glucose clamps. The magnitude of the changes of the vascular biomarkers during hypoglycemia (and euglycemia) are relatively modest. Thus, maximal changes in vascular biomarkers during hypoglycemia of 50–75% is greatly reduced compared with the multiple fold increases of neuroendocrine hormones occurring during identical hypoglycemia. However, the ability to compare the increases of the vascular biomarkers during hyperinsulinemic hypoglycemia with the similar decreases occurring during hyperinsulinemic euglycemia does demonstrate the independent effects of hypoglycemia. The clinical significance of the magnitude of the changes in the differing proinflammatory and pro-atherothrombotic vascular biomarkers is yet to be established. We only studied one level of hypoglycemia. Thus, we cannot comment on whether deeper hypoglycemia (i.e., less than 2.9 mmol/l) would have induced greater changes in vascular biological markers. Additionally, the present study was designed to study vascular physiologic changes during prolonged hypoglycemia of 2 h. Therefore, we cannot determine the effects of shorter durations of hypoglycemia (i.e., 30–45 min) on study end points.
In summary, this present study has demonstrated the complex effects of acute moderate hypoglycemia on fibrinolytic balance and proinflammatory mechanisms in type 1 diabetic subjects and healthy control subjects. Using glucose clamps to equate insulin levels, this study has demonstrated that hypoglycemia results in significant increases in proinflammatory and potentially pro-atherogenic adhesion molecules (ICAM, VCAM, E-selectin, IL-6, VEGF) platelet activation (P-selectin), and reduced fibrinolytic balance (increased PAI-1). We conclude that 1) hypoglycemia can similarly activate a broad spectrum of vascular biological mechanisms in both type 1 diabetic subjects and age- and weight-matched nondiabetic control subjects, and 2) the potentially deleterious long-term effects of hypoglycemia on pro-atherothrombotic mechanisms needs further study.
Supplementary Material
Acknowledgments
This work was supported by the following National Institutes of Health Grants: P50-HL-081009, R01-DK-069803, MO1-RR-000095, P01-HL-056693, and P60-DK-020593.
N.G.J. has received a fellowship award from Takeda Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
The authors thank Wanda Snead, Eric Allen, and the Vanderbilt Hormone Assay Core laboratory for their excellent technical assistance, and the nursing staff of the Vanderbilt Clinical Research Center for their excellent care. The authors are grateful to Jan Hicks, Vanderbilt University, Department of Medicine, for her superb editorial assistance.
Footnotes
Clinical trial reg. no. NCT00574340, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Gold AE, Marshall SM: Cortical blindness and cerebral infarction associated with severe hypoglycemia. Diabetes Care 1996; 19: 1001–1003 [DOI] [PubMed] [Google Scholar]
- 2. Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V: Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003; 26: 1485–1489 [DOI] [PubMed] [Google Scholar]
- 3. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Stern MP, Blair SN: Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation 2000; 101: 2047–2052 [DOI] [PubMed] [Google Scholar]
- 4. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [DOI] [PubMed] [Google Scholar]
- 6. Campbell I: Dead in bed syndrome: a new manifestation of nocturnal hypoglycemia? Diabet Med 1991; 8: 3–4 [DOI] [PubMed] [Google Scholar]
- 7. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright RJ, Frier BM: Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes/Metabolism Research and Reviews 2008; 24: 353–363 [DOI] [PubMed] [Google Scholar]
- 9. Wieczorek I, Pell AC, McIver B, MacGregor IR, Ludlam CA, Frier BM: Coagulation and fibrinolytic systems in type 1 diabetes: effects of venous occlusion and insulin induced hypoglycaemia. Clin Sci (Lond) 1993; 84: 79–86 [DOI] [PubMed] [Google Scholar]
- 10. Ibbotson SH, Catto A, Davies JA, Grant PJ: The effect of insulin-induced hypoglycaemia on factor VIII:C concentrations and thrombin activity in subjects with type 1 (insulin-dependent) diabetes. Thromb Haemost 1995; 73: 243–246 [PubMed] [Google Scholar]
- 11. Dandona P, Chaudhuri A, Ghanim H, Mohanty P: Effect of hyperglycemia and insulin in acute coronary syndromes. Am J Cardiol 2007; 99: 12H–18H [DOI] [PubMed] [Google Scholar]
- 12. Ewing DJ, Martyn CN, Young RJ, Clarke BF: The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985; 8: 491–498 [DOI] [PubMed] [Google Scholar]
- 13. Gold AE, MacLeod KM, Frier BM: Frequency of severe hypoglycemia in patients with type 1 diabetes and impaired awareness of hypoglycemia. Diabetes Care 1994; 17: 697–703 [DOI] [PubMed] [Google Scholar]
- 14. Briscoe VJ, Ertl AC, Tate DB, Dawling S, Davis SN: Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes 2008; 57: 2453–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Briscoe VJ, Ertl AC, Tate DB, Davis SN: Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes 2008; 57: 3315–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS: Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987; 316: 1376–1383 [DOI] [PubMed] [Google Scholar]
- 17. Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD: Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 2001; 4: 425–429 [DOI] [PubMed] [Google Scholar]
- 18. Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, Ciatti F, Guadagni F. Soluble P-selectin as a marker of in vivo platelet activation. Clin Chim Acta 2009; 399: 88–91 [DOI] [PubMed] [Google Scholar]
- 19. Halleux CM, Declerck PJ, Tran SL, Detry R, Brichard SM: Hormonal control of plasminogen activator inhibitor-1 gene expression and production in human adipose tissue: stimulation by glucocorticoids and inhibition by catecholamines. J Clin Endocrinol Metab 1999; 84: 4097–4105 [DOI] [PubMed] [Google Scholar]
- 20. Trovati M, Anfossi G, Cavalot F, Vitali S, Massucco P, Mularoni E, Schinco P, Tamponi G, Emanuelli G: Studies on mechanisms involved in hypoglycemia-induced platelet activation. Diabetes 1986; 35: 818–825 [DOI] [PubMed] [Google Scholar]
- 21. Dandona P, Mohanty P, Chaudhuri A, Garg R, Aljada A: Insulin infusion in acute illness. J Clin Invest 2005; 115: 2069–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dotson S, Freeman R, Failing HJ, Adler GK: Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care 2008; 31: 1222–1223 [DOI] [PubMed] [Google Scholar]
- 23. Razavi Nematollahi L, Kitabchi AE, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, Gozashti MH, Omidfar K, Taheri E: Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism Clinical and Experimental 2009; 58: 443–448 [DOI] [PubMed] [Google Scholar]
- 24. Dandona P: Endothelium, inflammation, and diabetes. Curr Diab Rep 2002; 2: 311–315 [DOI] [PubMed] [Google Scholar]
- 25. Dandona P, Aljada A, Mohanty P, Ghanim H, Bandyopadhyay A, Chaudhuri A: Insulin suppresses plasma concentration of vascular endothelial growth factor and matrix metalloproteinase-9. Diabetes Care 2003; 26: 3310–3314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.