Abstract
OBJECTIVE
Restoration of insulin secretion is critical for the treatment of type 2 diabetes. Exercise and diet can alter glucose-induced insulin responses, but whether this is due to changes in β-cell function per se is not clear. The mechanisms by which lifestyle intervention may modify insulin secretion in type 2 diabetes have also not been examined but may involve the incretin axis.
RESEARCH DESIGN AND METHODS
Twenty-nine older, obese (aged 65 ± 1 years; BMI 33.6 ± 1.0 kg/m2) subjects, including individuals with newly diagnosed type 2 diabetes (obese-type 2 diabetic) and individuals with normal glucose tolerance (obese-NGT), underwent 3 months of nutritional counseling and exercise training. β-Cell function (oral glucose–induced insulin secretion corrected for insulin resistance assessed by hyperinsulinemic-euglycemic clamps) and the role of glucose-dependent insulinotropic polypeptide (GIP) were examined.
RESULTS
After exercise and diet-induced weight loss (−5.0 ± 0.7 kg), oral glucose–induced insulin secretion was increased in the obese-type 2 diabetic group and decreased in the obese-NGT group (both P < 0.05). When corrected for alterations in insulin resistance, the change in insulin secretion remained significant only in the obese-type 2 diabetic group (1.23 ± 0.26 vs. 2.04 ± 0.46 arbitrary units; P < 0.01). Changes in insulin secretion were directly related to the GIP responses to oral glucose (r = 0.64, P = 0.005), which were augmented in the obese-type 2 diabetic group and only moderately suppressed in the obese-NGT group.
CONCLUSIONS
After lifestyle-induced weight loss, improvements in oral glucose–induced insulin secretion in older, obese, nondiabetic subjects seem to be largely dependent on improved insulin sensitivity. However, in older obese diabetic patients, improved insulin secretion is a consequence of elevated β-cell function. We demonstrate for the first time that changes in insulin secretion after lifestyle intervention may be mediated via alterations in GIP secretion from intestinal K-cells.
Diet and exercise-based lifestyle interventions, such as the Diabetes Prevention Program, have been shown to successfully reduce the risk of developing diabetes (1). Insulin resistance is the major underlying defect driving hyperglycemia, the reversal of which is critical to reduce vascular complications and mortality (2). However, in addition to insulin resistance, progressive pancreatic β-cell dysfunction, marked by a decline in compensatory hyperinsulinemia across the glucose tolerance continuum, ultimately results in type 2 diabetes (3).
Previous work has highlighted the beneficial effects of lifestyle interventions on insulin secretion and β-cell function in obesity (4–7). However, it is rather well established that large improvements in insulin resistance occur after exercise and/or caloric restriction in obese nondiabetic and diabetic humans (8–10). Therefore, apparent changes in glucose tolerance and insulin secretion may be due to alterations in insulin resistance that expose the pancreas to less glucose, rather than intrinsic improvements in β-cell function.
Evidence suggests that postprandial insulin secretion may be partially controlled by nutrient-responsive incretin peptides released by intestinal cells. We recently reported that weight loss–induced reductions in insulin secretion in obese men and women with impaired glucose tolerance, are related to changes in secretion of the incretin hormone glucose-dependent insulinotropic polypeptide (GIP) (11). Indeed, bariatric surgery restores insulin secretory capacity in patients with type 2 diabetes via alterations in incretin secretion, including GIP (12). Further, exenatide-based incretin-mimetic pharmaceutical therapy also restores insulin secretion (13). These recent findings have indicated that the intravenous methods used previously to study insulin secretion, methods that bypass the incretin-releasing gastrointestinal system, may not be appropriate to study in vivo mechanisms of lifestyle-induced change in β-cell function.
In obese, insulin-resistant individuals exhibiting basal and postprandial hyperinsulinemia, an improvement in β-cell function is regarded as a reduction of insulin hypersecretion. Conversely, in type 2 diabetes the compensatory postprandial hyperinsulinemia required to correct for the severe underlying insulin resistance is absent, and therefore an increase in insulin secretion would reflect an improvement in β-cell function. However, to demonstrate an alteration in β-cell function per se, one must account for changes in the β-cell exposure to glucose by assessing changes in insulin sensitivity. To date, the potential of nonsurgical and nonpharmacological lifestyle interventions to preserve β-cell function and increase insulin secretion in type 2 diabetes have not been fully explored. In addition, there is a paucity of data on the effects of exercise on incretin-mediated insulin secretion. In this investigation, we examined the effects of diet and exercise-induced weight loss on insulin resistance and insulin secretion in older obese type 2 diabetic individuals, compared with an age- and BMI-matched obese control group exhibiting normal glucose tolerance (NGT) and compensatory hyperinsulinemia. We hypothesized that in type 2 diabetes, in addition to relief from the underlying insulin resistance, β-cell insulin secretory function would be elevated in line with elevations in GIP secretion.
RESEARCH DESIGN AND METHODS
Older obese men and women (n = 29; aged 65 ± 1 years; BMI 33.6 ± 1.0 kg/m2) (Table 1) were recruited from the local community to participate in our ongoing obesity and diabetes studies. All participants were screened with a medical history and physical examination, blood and urine chemistry analyses, an oral glucose tolerance test (OGTT), and a resting and exercise stress test 12-lead electrocardiogram. Individuals were excluded from participation if they 1) smoked, 2) were weight unstable (>2 kg in previous 6 months), 3) undertook regular exercise (>30 min/day, >3 days/week), 4) had contraindications to elevated levels of physical activity as indicated by an electrocardiogram, 5) demonstrated any evidence of current or previous hematological, renal, hepatic, cardiovascular, or pulmonary disease, or 6) were taking medications known to affect our primary outcome variables. All participants also underwent resting metabolic rate testing by indirect calorimetry to calculate caloric requirements for the intervention. Participants were stratified into two age- and BMI-matched groups according to their oral glucose tolerance: those with NGT exhibiting insulin resistance with compensatory hyperinsulinemia (obese-NGT) and those with newly diagnosed type 2 diabetes (obese-type 2 diabetic) (2). Diabetic participants were only identified by our screening procedures, and their diabetes had not been previously diagnosed nor were they taking blood glucose-lowering medications. Signed informed consent was obtained before enrollment into the study, and all procedures were approved by our institutional review board.
Table 1.
Subject characteristics of each of the age- and BMI-matched groups
| Subject characteristics | Obese-NGT |
Obese-type 2 diabetic |
ANOVA |
|||
|---|---|---|---|---|---|---|
| Prestudy | Poststudy | Prestudy | Poststudy | Time | Time-group | |
| Age (years) | 63 ± 2 | 67 ± 2 | — | — | ||
| Sex (male/female) | 8/8 | 6/7 | — | — | ||
| Weight (kg) | 93.9 ± 3.2 | 88.8 ± 2.9 | 98.3 ± 4.6 | 93.4 ± 4.2 | <0.0001 | 0.90 |
| BMI (kg/m2) | 32.1 ± 1.1 | 30.4 ± 1.2 | 35.5 ± 1.5 | 33.7 ± 1.3 | <0.0001 | 0.78 |
| Fat (%) | 41.0 ± 1.6 | 39.0 ± 2.1 | 40.8 ± 2.0 | 38.5 ± 2.5 | 0.01 | 0.84 |
| VAT (cm2) | 187 ± 16 | 134 ± 15 | 202 ± 28 | 168 ± 27 | <0.0001 | 0.24 |
| Vo2max (l/min) | 2.11 ± 0.12 | 2.35 ± 0.16 | 1.99 ± 0.15 | 2.25 ± 0.15 | <0.0001 | 0.97 |
| Leptin (ng/ml) | 25.9 ± 4.8 | 19.9 ± 4.4 | 22.3 ± 4.7 | 18.5 ± 4.3 | 0.007 | 0.40 |
| TG (mg/dl) | 194 ± 28 | 136 ± 19 | 181 ± 17 | 156 ± 19 | 0.003 | 0.15 |
| Cholesterol (mg/dl) | 199 ± 9 | 178 ± 8 | 192 ± 10 | 182 ± 9 | 0.0006 | 0.24 |
| A1C (%) | 5.35 ± 0.09 | 5.51 ± 0.08 | 5.86 ± 0.29 | 5.16 ± 0.32 | 0.08 | 0.008 |
| FPG (mg/dl) | 97.9 ± 3.5 | 97.2 ± 3.0 | 129 ± 7* | 116 ± 6 | 0.03 | 0.04 |
| 2-h OGTT (mg/dl) | 124 ± 3 | 122 ± 7 | 225 ± 11* | 192 ± 11 | 0.03 | 0.05 |
| FPI (μU/ml) | 16.0 ± 2.0 | 13.7 ± 2.3 | 26.5 ± 7.1 | 17.4 ± 1.5 | 0.11 | 0.27 |
| AUC I (× 103 μU/ml · 0.3 h) | 12.9 ± 1.6 | 7.6 ± 1.6 | 10.1 ± 2.3 | 11.5 ± 1.9 | 0.01 | 0.13 |
| GDR (mg/kg/min) | 2.67 ± 0.26 | 3.91 ± 0.38 | 1.85 ± 0.37* | 2.34 ± 0.40 | <0.0001 | 0.03 |
Data are means ± SEM.
*Indicates significant difference vs. obese-NGT, P < 0.05. AUC I, area under the insulin response curve to the OGTT; FPI, fasting plasma insulin; GDR, glucose disposal rate during the hyperinsulinemic-euglycemic clamp; TG, triglycerides; VAT, intra-abdominal visceral adipose tissue.
Lifestyle intervention.
All participants were entered into a 3-month diet and exercise-induced weight loss intervention. These approaches are routine in our laboratory and have been described in detail previously (8).
Diet counseling.
Prestudy nutritional habits were assessed using 3-day diet records, and subjects underwent weekly counseling with a registered dietitian. Dietary habits were continuously assessed throughout the intervention. The intent of the counseling was to moderately reduce total caloric intake (−300 kcal/day) and to optimize the macronutrient composition. Nutritional analysis was performed using Nutritionist Pro (Axxya Systems, Stafford, TX).
Exercise training.
Subjects also partook in fully supervised aerobic treadmill-walking exercise that was conducted for 1 h/day, 5 days/week. Initial sessions were completed at 60–65% of maximum heart rate; however, by week 4, intensity was increased and maintained at 80–85% maximum heart rate. Exercise intensity was calculated from data collected during maximal aerobic exercise tests conducted at biweekly intervals throughout the intervention (described below).
Prestudy/poststudy control period.
Metabolic testing was conducted during a 3-day inpatient stay in the Clinical Research Unit. During this period, isocaloric (based on resting metabolic rate multiplied by 1.2) meals (55% carbohydrate, 30% fat, and 15% protein) were provided. Compliance with these meals was estimated by food weigh backs. Nonhabitual physical activity was also restricted during the prestudy inpatient period.
Prestudy/poststudy metabolic measures.
To determine body composition, weight and height were measured by standard techniques. Whole-body fat percentage was determined using dual-energy X-ray absorptiometry (iDXA; Lunar, Madison, WI). Computed tomography scanning (Picker PQ6000 scanner; Marconi/Picker, Highland Heights, OH) was used to measure cross-sectional visceral abdominal adiposity at the fourth lumbar vertebral body, as described previously (8).
Aerobic fitness.
Vo2max (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA) measured during exhaustive exercise was used as a marker of aerobic fitness. These measurements were repeated at biweekly intervals to adjust training intensity in relation to changes in aerobic fitness. These procedures have been fully described elsewhere (8).
Oral glucose–induced insulin secretion
A 3-h 75-g OGTT was administered at 8:00 a.m., after an overnight fast. Blood samples were obtained from an intravenous antecubital line at 30-min intervals. Incremental metabolite responses (area under the curve) during the OGTT were calculated using the trapezoidal rule. Oral glucose–induced insulin secretion (referred to throughout as ΔC-Pep/ΔG) was calculated as incremental plasma C-peptide (picomoles per liter) during the first 30 min of the OGTT divided by incremental plasma glucose (millimoles per liter) during the first 30 min of the OGTT. This is a slight modification of a previous model presented by Abdul-Ghani et al. (14), based on the principle that changes in C-peptide more accurately reflect insulin secretion rates. In addition, early-phase GIP secretion was estimated as the incremental peptide response during the first 30 min of the OGTT (ΔGIP0–30).
Insulin sensitivity
A 2-h hyperinsulinemic euglycemic (90 mg/dl) clamp was performed after an overnight fast, as described previously (15). In brief, a primed continuous 40 mU · m−2 · min−1 infusion of insulin (Humulin R U-100; Eli Lilly, Indianapolis, IN) was administered via an antecubital intravenous line, while a variable rate glucose (20% w/v) infusion was simultaneously administered to titrate fluctuations in plasma glucose. Arterialized plasma samples were obtained every 5 min from a retrograde intravenous dorsal line in a hand warmed to ∼60°C. Alterations to the glucose infusion rate were calculated as described previously (15). Peripheral tissue insulin sensitivity was estimated as the mean space-corrected glucose disposal rate over the last 30 min of the clamp.
β-Cell function
Because of the hyperbolic relationship between insulin secretion and sensitivity across the glucose tolerance continuum (3), the magnitude of the insulin response to oral glucose is influenced by the underlying state of insulin resistance. Thus, to estimate β-cell function, we corrected our measurements of insulin secretion (ΔC-Pep/ΔG) for the prevailing insulin resistance (IR) to derive an insulin secretion-to-insulin resistance index (ΔC-Pep/ΔG ÷ IR) equivalent to the disposition index of Gastaldelli et al. (16). Insulin resistance was calculated as the inverse of glucose disposal rate (micromoles per kilogram fat-free mass per minute) divided by the late-phase plasma insulin (picomoles per liter) response during the hyperinsulinemic-euglycemic clamp. In our subjects, we confirmed that baseline insulin resistance was indeed related to insulin secretion (r = 0.78, P < 0.001). In addition, hepatic insulin extraction was estimated as the incremental (area under the curve) plasma C-peptide response during the first 30 min of the OGTT divided by the incremental plasma insulin response during the first 30 min of the OGTT (17).
Analytical chemistry
Plasma glucose was measured using a glucose oxidase assay (YSI 2300 STAT Plus; YSI, Yellow Springs, OH). Plasma insulin and leptin concentrations were determined by radioimmunoassay (Millipore, Billerica, MA). Plasma triglycerides and total cholesterol were analyzed on an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Total plasma GIP (n = 10 patients with NGT; n = 8 patients with type 2 diabetes) and C-peptide were assayed using an ELISA (Linco Research, St. Charles, MO). A1C was measured using nonporous ion-exchange high-pressure liquid chromatography (G7 HPLC analyzer; Tosoh Bioscience, San Francisco, CA).
Statistics
Statistical analyses were performed using Statview (SAS Institute, Cary, NC), and all data are expressed as means ± SEM. To examine differences in prestudy variables between groups, one-way ANOVA was used to compare means. Between-group (obese-NGT vs. obese-type 2 diabetic) changes for all variables were analyzed using two-way repeated-measures ANOVA. Bonferroni post hoc tests were applied to significant group × time interactions to identify specific statistical differences between means. The addition of sex as a covariate did not reveal any group × sex × time interactions. Potential relationships between variables were analyzed using linear regression models. Statistical significance was accepted when P < 0.05.
RESULTS
Twenty-nine older obese adults were successfully screened into this study: n = 16 obese-NGT (8 men and 8 women) and n = 13 obese-type 2 diabetic (6 men and 7 women) individuals (Table 1). Attendance at exercise training sessions was 96.9 ± 0.6%.
Body composition and aerobic fitness
Both obese-NGT and obese-type 2 diabetic groups demonstrated significant weight loss and reductions in whole body and visceral fat (P < 0.05) (Table 1). Vo2max also improved (P < 0.05) (Table 1), demonstrating excellent compliance and response to the exercise training program. No group differences were observed in body composition or aerobic fitness.
Blood chemistry
All subjects exhibited significant reductions in plasma leptin, triglycerides, and cholesterol after the intervention (P < 0.05) (Table 1). A1C showed a nonsignificant fall in the obese-type 2 diabetic group.
Dietary intake
Compared with subjects' prestudy diet records, during the intervention total caloric intake was significantly reduced (−342.9 ± 90.3 kcal/day; P < 0.05) as was fat intake (−4.7 ± 1.2% kcal; P < 0.05). No differences were noted in dietary habits between groups.
Insulin action and β-cell function
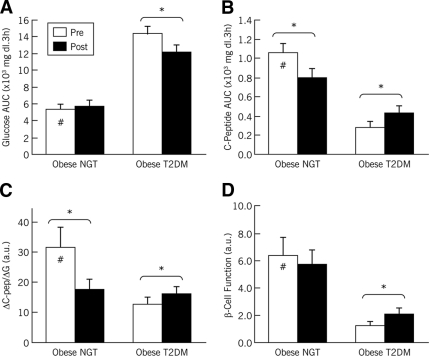
Participants' fasting plasma glucose (FPG) and 2-h plasma glucose during the OGTT (2-h OGTT) are shown in Table 1. At baseline, the obese-type 2 diabetic group exhibited fasting and postprandial hyperglycemia compared with the obese-NGT group (P < 0.05). After the intervention, there were significant decreases in FPG and 2-h OGTT in the obese-type 2 diabetic group (P < 0.05). These improvements were reflected in the changes in plasma glucose responses to the OGTT (area under the glucose curve) (Fig. 1A). Baseline plasma C-peptide responses to OGTT were markedly lower in the obese-type 2 diabetic group than in the obese-NGT group (P < 0.05) (Fig. 1B). Postintervention, C-peptide responses were significantly increased in the obese-type 2 diabetic group and decreased in the obese-NGT group (both P < 0.05) (Fig. 1B). At baseline, hepatic insulin extraction was decreased in the obese-type 2 diabetic group versus the obese-NGT group (8.96 ± 1.34 vs. 2.77 ± 0.62; P < 0.05). Insulin extraction was unaffected by the intervention. Changes in oral glucose–induced insulin secretion (ΔC-Pep/ΔG) are depicted in Fig. 1C. At baseline, insulin secretion was significantly lower in the obese-type 2 diabetic group (vs. the obese-NGT group; P < 0.05). After the study, there was a significant increase in insulin secretion in the obese-type 2 diabetic group, whereas the obese-NGT group showed a significant decrease (both P < 0.05). Insulin-stimulated glucose disposal was lower in the obese-type 2 diabetic group than in the obese-NGT group, but the intervention induced significant elevations in both groups (Table 1). β-Cell function (ΔC-Pep/ΔG ÷ IR) is shown in Fig. 1D. Baseline β-cell function was significantly impaired in the obese-type 2 diabetic group compared with the obese-NGT group (P < 0.05). After the lifestyle intervention, β-cell function was significantly increased in the obese-type 2 diabetic group (P < 0.05), showing no alteration in the obese-NGT group (P > 0.05).
Figure 1.
Oral glucose–induced insulin secretion and β-cell function. Older obese men and women participated in a 3-month caloric restriction and exercise training–induced weight loss intervention. Participants were stratified by oral glucose tolerance: obese-NGT and obese-type 2 diabetic groups. Plasma glucose (A) and C-peptide (B) responses to OGTT were determined. After the intervention, glucose responses were reduced in the obese-type 2 diabetic group; C-peptide responses were increased in the obese-type 2 diabetic group, whereas they were reduced in the obese-NGT group. Changes in oral glucose–induced insulin secretion (ΔC-Pep/ΔG) (C) and insulin secretion corrected for the underlying insulin resistance (β-cell function) (D) were also assessed before and after the study. Insulin secretion was significantly increased in the obese-type 2 diabetic group and decreased in the obese-NGT group, whereas β-cell function significantly increased in the obese-type 2 diabetes group only, showing no change in the obese-NGT group. □, mean prestudy data; ■, mean poststudy data; errors bars represent S.E.M. *Significant prestudy vs. poststudy differences (P < 0.05). #Prestudy differences between groups (P < 0.05). a.u., arbitrary units; AUC, area under the curve.
GIP responses
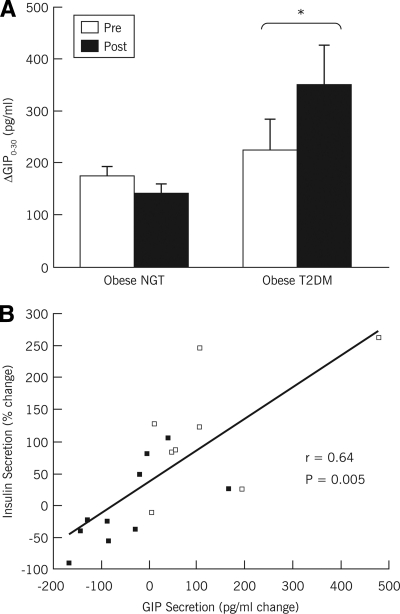
Figure 2A illustrates the changes in plasma GIP secretory responses to OGTT (ΔGIP0–30). Baseline ΔGIP0–30 was not different between the obese-type 2 diabetic and obese-NGT groups (P > 0.05). After the intervention, ΔGIP0–30 increased in the obese-type 2 diabetic group (P < 0.05) and was nonsignificantly reduced in the obese-NGT group (P = 0.07). Further analysis demonstrated that ΔGIP0–30 was not related to changes in body weight or composition in these subjects (all P = 0.05), whereas a significant relationship was identified between the changes in ΔGIP0–30 and the changes in insulin secretion (r = 0.64, P = 0.005) (Fig. 2B).
Figure 2.
Changes in plasma GIP secretion. A: Changes in total plasma GIP secretory responses to OGTT (ΔGIP0–30) (obese-NGT group n = 10; obese-type 2 diabetic [T2DM] group n = 8) were assessed before and after the 3-month weight loss intervention. □, mean data before the intervention; ■, mean data after weight loss; error bars represent S.E.M. ΔGIP0–30 was significantly increased in the obese-type 2 diabetic group. B: Linear regression analyses revealed a significant correlation between the changes in plasma GIP responses to oral glucose and oral glucose-induced insulin secretion (r = 0.64, P = 0.005). ■, obese-NGT group; ▵, obese-type 2 diabetic group. *Significantly increased in obese-T2DM compared to obese-NGT (P < 0.05).
CONCLUSIONS
After 3 months of diet- and exercise-induced weight loss, oral glucose–induced insulin secretion was increased in older obese type 2 diabetic individuals. In older obese individuals with NGT, the compensatory postprandial hyperinsulinemia was suppressed after the intervention. These changes were found to be directly related to the lifestyle-induced changes in oral glucose–induced GIP responses. When insulin secretion was corrected for the decrement in the underlying insulin resistance, it became apparent that the improvement in insulin secretion in obese individuals with NGT was driven by reduced insulin resistance. Yet, in the obese type 2 diabetic individuals, the improvement in insulin secretion appears to be a result of increased β-cell function. For the first time, using a physiological, incretin-related measure of insulin secretion corrected for the underlying rates of insulin-stimulated glucose disposal, we have demonstrated that nonsurgical and nonpharmacological weight loss can promote the restoration of insulin secretion in older obese individuals with type 2 diabetes via a mechanism related to increments in GIP secretion from intestinal K-cells.
Caloric restriction studies have previously demonstrated the potential to preserve β-cell function in diabetic individuals (4,17–19). Bogardus et al. (20) additionally found elevated insulin responses to OGTT after caloric restriction and exercise-induced weight loss, and, more recently, Dela et al. (21) and Slentz et al. (4) provided strong evidence of exercise training–induced increases in β-cell function in response to intravenous glucose in type 2 diabetic and/or obese patients. However, the nature of intravenous techniques used in several previous studies has not allowed for physiological mechanistic insight into incretin-mediated changes in insulin secretion. The assessment of insulin secretory responses to oral glucose ingestion (OGTT) is related to the incretin axis and allows clinical scientists to study β-cell function during a physiological postprandial perturbation. However, a change in the insulin secretory response may not necessarily be due to an alteration in β-cell glucose sensitivity or β-cell insulin secretory capacity per se, it may also be driven by a change in insulin sensitivity that would consequently alter the exposure of the β-cell to circulating glucose. In this respect, we applied the work of Gastaldelli et al. (16) to correct insulin secretion for the underlying peripheral tissue insulin resistance. We previously demonstrated that insulin secretion is reduced in obese impaired glucose-tolerant individuals after a similar lifestyle intervention (11). Here we show that in subjects exhibiting insulin resistance yet normal glucose tolerance, weight loss can dramatically reverse the state of compensatory hyperinsulinemia. Although our findings do not demonstrate any alterations in β-cell function per se in such individuals, it is interesting to note that the potential progression toward impaired glucose tolerance that may occur if this group were left untreated has probably been reversed. At the other end of the glucose tolerance continuum, we demonstrate potential for exercise- and diet-induced weight loss to augment insulin secretion in type 2 diabetic patients via improvements in β-cell function, in addition to elevated rates of insulin-stimulated glucose disposal and reduced fasting and postprandial hyperglycemia. The restoration of insulin secretion in the diabetic state is crucial for achieving optimal glycemic control, without which uncontrolled hyperglycemia would advance vascular inflammation and oxidative stress, ultimately leading to microvascular endothelial dysfunction and macrovascular disease (3).
The dichotomy of “improved insulin secretion” in diabetic versus nondiabetic individuals is highlighted by this current study: reversal of inadequate postprandial insulinemia in diabetic individuals versus reversal of compensatory hyperinsulinemia in nondiabetic individuals. Exposure of the β-cell to chronic hyperglycemia leads to functional impairment or glucotoxicity (22). Thus, relief of extreme hyperglycemia and therefore toxicity in our diabetic population may explain the augmentation of postprandial insulin secretion. However, our diabetic subjects also demonstrate an increase in GIP secretion after the intervention. The mechanisms behind these changes are not understood, but our correlation analyses negate the effects of changes in body composition; however, it has been demonstrated that chronic hyperglycemia can downregulate pancreatic GIP receptor expression (23) and also increase GIP glycation, thus rendering the peptide dysfunctional with respect to insulinogenic capacity (24). This process may translate to the chronic hyperglycemic state in diabetes, where glucotoxicity is systemic and may therefore have the potential to impair GIP functional capacity or indeed intestinal K-cell secretion. Although caution must be used when correlative interpretations are made with low numbers of subjects, our finding that weight loss–induced changes in GIP are related to changes in insulin secretion warrants further attention. Future researchers should also examine more thoroughly the incretin axis to include the insulinogenic protein, GLP-1.
It is also interesting to note that after 3 months of lifestyle intervention neither hyperglycemia nor insulin secretion is normalized. Perhaps alternative treatment modalities are sensible. In recent years, surgical techniques and exenatide compounds have emerged (13) and received attention for their potential to initiate insulin secretion in severely obese diabetic patients. Recent data show that Roux-en-Y gastric bypass can increase pancreatic β-cell insulin secretory capacity via alteration of incretin signaling and can normalize hyperglycemia in up to 83% of diabetic patients (12,25). Thus, the relative impact of lifestyle intervention on β-cell function may seem small. However, more prolonged interventions demonstrate even more dramatic improvements (4), and indeed the goal of clinical care is on-going dietary monitoring and physical activity. In the U.S. health care system, pharmaceutical and surgical options are a financial burden and not accessible to all, leaving low-cost lifestyle programs as a universal and valuable option.
In summary, we have furthered the work of prior investigations with intravenous methods by using an oral glucose technique to assess incretin-related insulin secretion and account for β-cell glucose exposure. We demonstrate that lifestyle intervention not only relieves the underlying insulin resistance in obesity but in older obese type 2 diabetic individuals it also has the potential to reverse β-cell dysfunction and therefore help reduce the risk of vascular disease. We also present new data demonstrating that elevations in in vivo insulin secretion in response to a physiological orally ingested glucose load is related to changes in postprandial GIP responses. This information highlights a potential mechanism to show that modification of intestinal incretin secretion after 3 months of mild caloric restriction and aerobic exercise training may explain the improvement in β-cell function in type 2 diabetic patients. A relationship between the changes in incretin secretion and β-cell function after lifestyle intervention has never before been demonstrated in type 2 diabetes.
Acknowledgments
This study was funded by National Institutes of Health (NIH) grant R01-AG-12834 (to J.P.K.), General Clinical Research Center grants M01-RR-10732, RR-00080, and RR-018390, and NIH National Center for Research Resources CTSA 1UL1-RR-024989, Cleveland, Ohio.
No potential conflicts of interest relevant to this article were reported.
We thank the research volunteers for their dedication to the lifestyle intervention. We also thank the nursing staff of the Clinical Research Unit for their contributions to this work.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 1691.
References
- 1. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32(Suppl. 1): S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeFronzo RA: Lilly Lecture 1987: The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687 [DOI] [PubMed] [Google Scholar]
- 4. Slentz CA, Tanner CJ, Bateman LA, Durheim MT, Huffman KM, Houmard JA, Kraus WE: Effects of exercise training intensity on pancreatic β-cell function. Diabetes Care 2009; 32: 1807–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bloem CJ, Chang AM: Short-term exercise improves β-cell function and insulin resistance in older people with impaired glucose tolerance. J Clin Endocrinol Metab 2008; 93: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kahn SE, Larson VG, Beard JC, Cain KC, Fellingham GW, Schwartz RS, Veith RC, Stratton JR, Cerqueira MD, Abrass IB: Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol 1990; 258: E937–E943 [DOI] [PubMed] [Google Scholar]
- 7. Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS: Diet-induced weight loss is associated with an improvement in β-cell function in older men. J Clin Endocrinol Metab 2004; 89: 2704–2710 [DOI] [PubMed] [Google Scholar]
- 8. O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP: Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 2006; 100: 1584–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE: Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 2007; 56: 2142–2147 [DOI] [PubMed] [Google Scholar]
- 10. Bruce CR, Kriketos AD, Cooney GJ, Hawley JA: Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia 2004; 47: 23–30 [DOI] [PubMed] [Google Scholar]
- 11. Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP: The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab 2009; 296: E1269–E1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kashyap SR, Daud S, Kelly KR, Win H, Gastaldelli A, Kirwan JP, Brethuaer S, Schauer PS: Acute effects of gastric bypass vs. gastric restriction on pancreatic β-cell function and insulinotropic hormones in patients with type 2 diabetes. Int J Obes (Lond) 2010; 34: 462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ: One-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32: 762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdul-Ghani MA, Williams K, DeFronzo R, Stern M: Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006; 29: 1613–1618 [DOI] [PubMed] [Google Scholar]
- 15. DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223 [DOI] [PubMed] [Google Scholar]
- 16. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: β-Cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31–39 [DOI] [PubMed] [Google Scholar]
- 17. Henry RR, Brechtel G, Griver K: Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1988; 66: 979–986 [DOI] [PubMed] [Google Scholar]
- 18. Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M: Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993; 77: 1287–1293 [DOI] [PubMed] [Google Scholar]
- 19. Gumbiner B, Polonsky KS, Beltz WF, Griver K, Wallace P, Brechtel G, Henry RR: Effects of weight loss and reduced hyperglycemia on the kinetics of insulin secretion in obese non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab 1990; 70: 1594–1602 [DOI] [PubMed] [Google Scholar]
- 20. Bogardus C, Ravussin E, Robbins DC, Wolfe RR, Horton ES, Sims EA: Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin-dependent diabetes mellitus. Diabetes 1984; 33: 311–318 [DOI] [PubMed] [Google Scholar]
- 21. Dela F, von Linstow ME, Mikines KJ, Galbo H: Physical training may enhance β-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 2004; 287: E1024–E1031 [DOI] [PubMed] [Google Scholar]
- 22. Rossetti L, Shulman GI, Zawalich W, DeFronzo RA: Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987; 80: 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S: Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 2007; 56: 1551–1558 [DOI] [PubMed] [Google Scholar]
- 24. Mooney MH, Abdel-Wahab YH, Morgan LM, O'Harte FP, Flatt PR: Detection of glycated gastric inhibitory polypeptide within the intestines of diabetic obese (ob/ob) mice. Endocrine 2001; 16: 167–171 [DOI] [PubMed] [Google Scholar]
- 25. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D: Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238: 467–484; discussion 484–485 [DOI] [PMC free article] [PubMed] [Google Scholar]