Abstract
OBJECTIVE
Growth deferentiation factor-15 (GDF-15) is involved in inflammation and apoptosis. Expression is induced in the heart in response to ischemia and in atherosclerotic plaques. The aim of this study was to investigate GDF-15 levels in relation to all-cause mortality, cardiovascular mortality and morbidity, decline in glomerular filtration rate (GFR), and progression toward end-stage renal disease (ESRD).
RESEARCH DESIGN AND METHODS
The study was a prospective observational follow-up study including 451 type 1 diabetic patients with diabetic nephropathy (274 men, aged 42.1 ± 0.5 years [means ± SD], diabetes duration 28.3 ± 8.9 years, GFR 76 ± 33 ml/min/1.73 m2) and a control group of 440 patients with longstanding type 1 diabetes and persistent normoalbuminuria (232 men, aged 45.4 ± 11.5 years, duration of diabetes 27.7 ± 10.1 years). The patients were followed for 8.1 (0.0–12.9) years (median [range]).
RESULTS
Among normoalbuminuric patients, GDF-15 above the median predicted an adjusted (age, systolic blood pressure [sBP], and estimated GFR) increased risk of all-cause mortality (hazard ratio [HR] 3.6 [95% CI 1.3–10.3]; P = 0.014). Among patients with diabetic nephropathy, higher (fourth quartile) versus lower (first quartile) GDF-15 levels predict all-cause mortality (covariate-adjusted [sex, age, smoking, blood pressure, A1C, cholesterol, GFR, N-terminal prohormone B-type natriuretic peptide, antihypertensive treatment, and previous cardiovascular events]; HR 4.86 [95% CI 1.37–17.30]) as well as fatal and nonfatal cardiovascular events (adjusted HR 5.59 [1.23–25.43] and 3.55 [1.08–11.64], respectively). In addition, higher GDF-15 levels predict faster decline in GFR (P < 0.001) but not development of ESRD.
CONCLUSIONS
Higher levels of GDF-15 are a predictor of all-cause and cardiovascular mortality and morbidity in patients with diabetic nephropathy. Furthermore, higher levels of GDF-15 are associated with faster deterioration of kidney function.
Diabetes is associated with accelerated atherosclerosis and an increased risk of cardiovascular disease (CVD), which has become the major cause of morbidity and mortality among patients with diabetic nephropathy (1). Left ventricular hypertrophy, hypertension, and diabetes are leading predictors for the development of heart failure and sudden death (2,3). In general, the hypertrophic growth of the myocardium is regulated by a number of pro- and antigrowth factors, e.g., angiotensin-II and B-type natriuretic peptide (BNP) related to the transforming growth factor-β superfamily (4–6).
Recently, growth differentiation factor-15 (GDF-15) has been identified as a novel anti-hypertrophic regulatory factor (7). GDF-15 is generated as a 40-kDa propeptide from which the NH2-terminus is cleaved and a 30-kDa protein secreted as the active form (8).
GDF-15 is induced in the hypertrophic and dilated cardiomyopathy following hypertension/volume overload, ischemia, and heart failure, possibly via proinflammatory cytokine and oxidative stress-dependent signaling pathways (9,10). GDF-15 is highly expressed in the infarcted myocardium in predominantly nondiabetic patients suffering an acute myocardial infarction (MI) (9) and in atherosclerotic plaques obtained from carotid artery surgery (11). In a nested case-control study, GDF-15 was shown to be associated with adverse cardiovascular outcomes in women (12). Furthermore, GDF-15 has been shown to predict mortality in patients with both ST-elevation MI (STEMI) and non–STEMI, independent of known biomarkers such as N-terminal prohormone B-type natriuretic peptide (NT-proBNP) (13,14).
Therefore, we investigated the predictive value of circulating GDF-15 levels on all-cause mortality, fatal and nonfatal CVD, decline in GFR, as well as progression to end-stage renal disease (ESRD) in a well-characterized population of type 1 diabetic patients with or without diabetic nephropathy.
RESEARCH DESIGN AND METHODS
From 1993 to 2000, adult Caucasian patients with type 1 diabetes and diabetic nephropathy attending the outpatient clinic at the Steno Diabetes Center were invited to participate in a study of genetic risk factors for the development of diabetic complications. Of these patients, 73% accepted. Type 1 diabetes was considered present if the age at onset of diabetes was <35 years and the time to definite insulin therapy was <1 year. In total, 458 patients with diabetic nephropathy defined by persistent albuminuria (>300 mg/24 h) in two out of three consecutive measurements, the presence of retinopathy, and the absence of other kidney or urinary tract disease were enrolled as case subjects.
The absence of diabetic nephropathy (control subjects) was defined as persistent normoalbuminuria (<30 mg/24 h) after >15 years of type 1 diabetes in patients not treated with ACE inhibitors or angiotensin-II receptor blockers. In total, 442 were included as control subjects.
Baseline clinical and laboratory investigations
All patients had blood samples and phenotypic characteristics collected as part of the European Rational Approach for the Genetics of Diabetic Complications (EURAGEDIC) project (15). Office blood pressure (BP) was measured twice using a sphygmomanometer after at least 10 min rest in the sitting position. The average value of three readings at two min apart was used for calculation.
From venous samples, A1C was measured by standard high-performance liquid chromatography (normal range 4.1–6.4%) (Tosoh automated glycohemoglobin analyzer; Tosoh Bioscience, Minato, Japan), and cholesterol concentrations were determined by standard methods (Hitachi 912 system; Roche Diagnostics). Urinary albumin excretion rate was measured in 24-h urine collections by an enzyme immunoassay. Serum creatinine concentration was determined by a modified Jaffe method.
GFR was measured annually in patients with diabetic nephropathy after a single injection of 3.7 MBq 51Cr-EDTA by determination of radioactivity in venous blood samples taken 180, 200, 220, and 240 min after injection (16). The results were standardized for 1.73 m2 body surface area using the patient's surface area at the start of the study. The mean day-to-day coefficient of variation is 4% in our laboratory. Linear regression analysis of the GFR determinations in each individual was used to estimate the rate of decline in kidney function.
In patients with normoalbuminuria, GFR at baseline was estimated by the four-variable Modification of Diet in Renal Disease (MDRD) equation (17). ESRD was defined as chronic dialysis or kidney transplantation. Diabetic retinopathy was assessed by fundus photography after pupillary dilatation and graded nil, simplex, and proliferative retinopathy. On the basis of standardized questionnaires, current smokers of one or more cigarettes/cigars/pipes per day were classified as smokers and all others as nonsmokers. Major CVD events were diagnosed as stroke, MI, coronary artery bypass graft, and/or percutanous coronary intervention.
Measurements of GDF-15, NT-proBNP, and CRP
Blood samples for determination of biomarkers were collected in EDTA tubes and centrifuged, and the plasma was stored at −80°C until analysis. Plasma GDF-15 was measured using a one-step enzyme immunoassay based on the electrochemiluminescence (ECLIA) technology (Elecsys 2010). This method is very comparable to the immunoradiometric assay previously described in detail (18), and the antibodies used for both assays are identical [capture antibody: PAb > GDF-15 > goat IgG(IS); detection antibody: Mab > GDF-15 > mouse-147627-IgG (R&D Systems)].
The NH2-terminal end of NT-proBNP was determined by sandwich immunoassay (Elecsys 2010), also as previously described (19). C-reactive protein (CRP) was analyzed with a chemiluminescent enzyme–labeled immunometric assay (Hitachi). All measurements of GDF-15, NT-proBNP, and CRP were performed by Roche Diagnostics (Penzberg, Germany) by investigators blinded to the characteristics and outcomes of the patients.
Follow-up data
In a prospective observational study design, the patients were followed until an end point was reached—to the last visit at the Steno Diabetes Center or until 1 September 2006. The end points were all-cause mortality, cardiovascular mortality, major cardiovascular events, decline in GFR, and development of ESRD.
All patients were traced through the National Death Register and if a patient died before 1 September 2006, the date of death was recorded and information on the cause of death was obtained. Two observers reviewed all death certificates independently and the primary cause of death was recorded. Additional available information from necropsy reports was included. All deaths were classified as cardiovascular deaths unless an unequivocal noncardiovascular cause was established. Information about CVD events and ESRD was obtained from patient records or discharge letters from other hospitals. In total, plasma GDF-15 levels were determined and follow-up data were available for 451 (98.5%) patients with diabetic nephropathy and 440 (99.5%) control subjects. The study was performed in accordance with the Helsinki Declaration, with the local ethics committee's approval, and all patients gave their informed consent.
Statistical analysis
Normally distributed variables are means ± SD, whereas non-normally distributed variables were log10 transformed before analysis and are median (range). Comparisons between groups were performed by unpaired Student t test, ANOVA, or linear regression when appropriate. The Chi2 test was used to compare noncontinuous variables. A two-tailed P value of ≤0.05 was considered statistically significant.
Multiple linear regression analysis was used to determine the influence of baseline level of GDF-15 on decline in GFR. All time-to-end point variables were analyzed using a log-rank test and displayed in Kaplan–Meier plots. The relations between baseline GDF-15 levels and end points during follow-up were assessed using Cox regression enter model (all-cause mortality, fatal and nonfatal CVD, and ESRD) by univariable and multivariable analyses displayed as unadjusted and adjusted HRs, respectively. HRs and 95% CIs for risk factors and significance level for χ2 (likelihood ratio test) are given. Levels of NT-proBNP and GDF-15 were normalized by log10 transformation and thus the corresponding HRs refers to a 10-fold rise in the variables. In addition, the predictive accuracy of GDF-15 were compared by generating receiver operating characteristic (ROC) curves for all-cause mortality and CVD mortality, respectively, and the areas under the curves (AUCs) were calculated for patients with diabetic nephropathy. All calculations were performed using a commercially available program (version 14.0; SPSS, Chicago, IL).
RESULTS
Baseline characteristics
The study population included two groups: 451 patients with type 1 diabetes and diabetic nephropathy and 440 control subjects with type 1 diabetes for >15 years and persistent normoalbuminuria. Baseline clinical and laboratory characteristics of the 891 patients are shown in Table 1. As expected, the patients with diabetic nephropathy received more antihypertensive medication and had higher A1C, BP, S-creatinine, and total cholesterol than patients with normoalbuminuria (P < 0.05). In parallel, the plasma GDF-15 concentration was higher in patients with diabetic nephropathy than in normoalbuminuric patients (median [range] 1,322 ng/l [443–17,735] vs. 749 ng/l [158–13,933], respectively; P < 0.001). On average, GFR was well preserved among the patients with diabetic nephropathy (GFR 76 ± 33 [ml/min/1.73 m2]).
Table 1.
Baseline clinical and laboratory characteristics of 891 type 1 diabetic patients followed for 8.1 (0.0–12.9) years according to nephropathy status
| Characteristic | Nephropathy | Normoalbuminuria | P |
|---|---|---|---|
| n | 451 | 440 | |
| Sex (men/women) | 274/177 | 232/208 | 0.018 |
| Age (years) | 42.1 ± 10.5 | 45.4 ± 11.5 | <0.001 |
| Duration of diabetes (years) | 28.3 ± 8.9 | 27.7 ± 10.1 | 0.37 |
| BMI (kg/m2) | 24.3 ± 3.3 | 24.2 ± 3.1 | 0.77 |
| A1C (%) | 9.4 ± 1.5 | 8.4 ± 1.1 | <0.001 |
| Antihypertensive treatment (%)* | 77.0 | 16.6 | <0.001 |
| Systolic BP (mmHg) | 144 ± 22 | 134 ± 19 | <0.001 |
| Diastolic BP (mmHg) | 82 ± 12 | 76 ± 10 | <0.001 |
| Urinary albumin excretion rate (mg/24 h) | 593 (3–14,545)† | 9 (1–30) | — |
| Serum creatinine (μmol/l) | 102 (52–706) | 80 (53–134) | <0.001 |
| GFR (ml/min per 1.73 m2) | 76 ± 33 | — | — |
| Serum cholesterol (mmol/l) | 5.6 ± 1.2 | 4.8 ± 1.0 | <0.001 |
| Smoking (%) | 46.3 | 39.8 | 0.05 |
| Retinopathy (0/SR/PR) | 6/139/306 | 159/162/119 | <0.001 |
| History of MI (%)‡ | 4.2 | 2.1 | 0.05 |
| History of stroke (%)‡ | 6.9 | 1.6 | <0.001 |
| NT-proBNP (ng/l) | 89.1 (5–19,394) | 42.8 (5–1,552) | <0.001 |
| GDF-15 (ng/l) | 1,322 (443–17,735) | 749 (158–13,933) | <0.001 |
Data are n, means ± SD, or median (range).
*In 2002, the recommendations at the Steno Diabetes Center were extended to include statins and low-dose aspirin for all patients with diabetic nephropathy.
†Some patients with previously persistent macroalbuminuria receiving antihypertensive treatment had values <300 mg/24 h at the time of investigation.
‡Presence of previous CVD is defined as either MI or stroke. SR, simplex retinopathy; PR, proliferative retinopathy.
Plasma concentration of GDF-15 did not differ significantly between type 1 diabetic men and women (P = 0.99), but correlated positively with age (r = 0.06, P < 0.001) and sBP (r = 0.15, P < 0.001) and inversely related to increased estimated GFR (eGFR) (r = −0.42, P < 0.001).
Follow-up data
This study was a prospective observational follow-up study with a median follow-up time until end point or last visit of 8.1 (0.0–12.9) years. Because of the low number of events in the normoalbuminuric group, the analyses are restricted to the median (749 ng/l) level of GDF-15 among these patients. During the follow-up period, five patients (2%) with GDF-15 levels below the median and 29 (13%) with GDF-15 levels above the median died. The log-rank test demonstrated a significant difference between the groups (P < 0.001). This corresponded to a sixfold increased risk for all-cause mortality (HR 6.3 [95% CI 2.4–16.3], P < 0.001) in a Cox regression analysis. This relationship persisted after adjusting for age, sBP, and eGFR (3.6 [1.3–10.3], P = 0.014).
Similarly, 12 normoalbuminuric patients (3%) with GDF-15 levels below the median versus 36 (8%) above the median suffered a fatal or nonfatal cardiovascular event during follow-up (HR 3.5 [95% CI 1.8–6.6], P < 0.001). However when adjusting for age, sBP, and eGFR, this was no longer significant (covariate-adjusted HR 1.8 [0.8–3.6], P = 0.16).
The 451 patients with diabetic nephropathy were divided into quartiles according to GDF-15 levels (≤969; 970–1,327; 1,328–2,172; and ≥2,173 ng/l, respectively). Six patients (5%) with GDF-15 levels in the first quartile, 26 (23%) in the second, 47 (42%) in the third, and 61 patients (54%) in the fourth quartile died during follow-up (P < 0.001). A 15-fold increased risk of all-cause mortality was found for high (fourth quartile) versus low (first quartile) GDF-15 levels (HR 15.84 [95% CI 6.84–36.69], P < 0.001). High GDF-15 levels remained independently predictive of all-cause mortality in the Cox regression model (covariate-adjusted [sex, age, smoking, BP, A1C, cholesterol, GFR, NT-proBNP, antihypertensive treatment, and previous cardiovascular events] 4.86 [1.37–17.30], P = 0.015) (Table 2).
Table 2.
HR of long-term cumulative mortality, cardiovascular mortality, and morbidity after adjustment for confounding factors among the 451 patients with diabetic nephropathy
| Parameter | HR | 95% CI | P |
|---|---|---|---|
| All-cause mortality (n = 140) | |||
| Second vs. first quartile | 1.90 | 0.60–6.03 | 0. 28 |
| Third vs. first quartile | 3.75 | 1.22–11.57 | 0.022 |
| Fourth vs. first quartile | 4.86 | 1.37–17.30 | 0.015 |
| Cardiovascular mortality (n = 89) | |||
| Second vs. first quartile | 1.76 | 0.44–6.97 | 0.42 |
| Third vs. first quartile | 3.99 | 1.06–14.99 | 0.040 |
| Fourth vs. first quartile | 5.59 | 1.23–25.43 | 0.026 |
| Non-fatal cardiovascular event (n = 99) | |||
| Second vs. first quartile | 2.36 | 0.91–6.13 | 0.079 |
| Third vs. first quartile | 2.04 | 0.75–5.56 | 0.16 |
| Fourth vs. first quartile | 3.55 | 1.08–11.64 | 0.036 |
All primary end points are adjusted for the following confounding factors: sex, age, smoking, A1C, systolic BP, cholesterol, GFR, NT-proBNP, antihypertensive treatment, and a history of cardiovascular events at baseline.
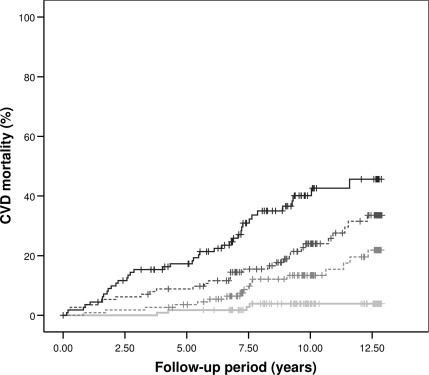
Regarding risk of fatal cardiovascular events, four patients (4%) with GDF-15 levels in the first quartile, 17 (15%) in the second quartile, 29 (26%) in the third quartile, and 39 (35%) in the fourth quartile died of CVD during follow-up. Figure 1 shows the Kaplan–Meier curve for cardiovascular mortality (P < 0.001). High (fourth quartile) versus low (first quartile) GDF-15 levels predicted an increased risk of cardiovascular mortality (HR 14.64 [95% CI 5.22–41.03], P < 0.001), which persisted following covariate-adjustment (5.59 [1.23–25.43], P = 0.026) (Table 2). A similar independent predictive value of GDF-15 was seen for nonfatal cardiovascular events (n = 99). High levels (fourth quartile) versus low levels (first quartile) increased the risk of nonfatal cardiovascular events when adjusting for confounding factors (3.55 [1.08–11.64], P = 0.036) (Table 2). GDF-15 concentrations correlated with the cardiovascular risk factors NT-proBNP (r = 0.42, P < 0.001) and more weakly with CRP (r = 0.01, P = 0.016).
Figure 1.
Unadjusted Kaplan–Meier curves for CVD mortality among the 451 patients with diabetic nephropathy according to quartiles of GDF-15 levels (≤969; 970–1,327; 1,328–2,172; and ≥2,173 ng/l, respectively). First quartile (light gray line); second quartile (light gray dotted line); third quartile (dark gray dotted line); and fourth quartile (black line). Log-rank test resulted in P < 0.001.
The overall prognostic value of GDF-15 levels and other risk markers for all-cause mortality were evaluated by ROC curves. For GDF-15, the mean AUC was HR 0.79 (95% CI 0.74–0.84) as compared with NT-proBNP (0.75 [0.69–0.81]) and GFR (0.71 [0.64–0.77]). In comparison, the AUCs for a combined risk score model—including the previously mentioned covariates with and without GDF-15—were 0.84 (0.80–0.88) and 0.83 (0.78–0.87), respectively. Regarding CVD mortality, similar AUCs for the predictive value of GDF-15 levels with and without covariates were found (data not shown). Evaluating GDF-15 as a continuous variable introduced only minor changes in the statistical outputs. Finally, when performing the survival analyses for all patients combined (n = 891), adjusting for the previously mentioned covariates and nephropathy status, GDF-15 continued to be associated with all-cause and CVD mortality (HR 4.61 [95% CI 1.93–11.00], P = 0.001 and 5.04 [1.71–14.85], P = 0.003, respectively).
Progression of nephropathy
The mean (SD) rate of decline in GFR was 3.0 (2.9), 3.4 (3.6), 4.8 (4.0), and 6.3 (4.8) ml/min/1.73 m2 /year (P < 0.001), respectively, when dividing the patients according to quartiles of GDF-15. Furthermore, when including the baseline variables sex, age, A1C, systolic BP, GFR, cholesterol, and antihypertensive treatment as covariates in the analysis, the GDF-15 levels still predicted a faster rate of decline in GFR during follow-up (P < 0.001).
In addition, progression to chronic dialysis or transplantation (ESRD) during the follow-up period was seen in three patients in the first quartile (3%), four patients in the second quartile (4%), 21 patients in the third quartile (19%), and 45 patients in the fourth quartile (47%) (P < 0.001). Cox regression analysis revealed an increased risk (40 times) of ESRD in patients with the highest GDF-15 levels (fourth quartile) compared with the lowest levels (first quartile) (HR 39.9 [95% CI 12.33–129.0], P < 0.001). However this association disappeared when adjusting for confounding factors (2.08 [0.46–9.40], P = 0.34).
CONCLUSIONS
In the prospective observational analysis, including 8 years of follow-up, GDF-15 levels were independently predictive of all-cause mortality among the type 1 diabetic patients with and without diabetic nephropathy. In addition, among the 451 patients with overt diabetic nephropathy, elevated levels of GDF-15 independently predicted CVD mortality and morbidity as well as deterioration of renal function (dGFR) after adjustment for conventional CVD and renal risk factors including GFR and NT-proBNP in multivariable models.
The elevated levels of GDF-15 may reflect several underlying conditions including acute/or chronic ischemia associated with adverse cardiovascular outcomes or may merely indicate reduced renal excretion of GDF-15 due to renal dysfunction. In particular, renal dysfunction appears to mediate part of the prognostic impact of GDF-15 given the strong inverse relationship between baseline levels of GDF-15 and eGFR in our study. Thus, among the several confounding factors, impaired renal function may be particularly important since a reduced GFR represents a cardiovascular risk factor by itself and raises the possibility of residual confounding by decreased renal clearance. Therefore, we adjusted for several conventional CVD covariates in our study including GFR at baseline. The remaining strong independent predictive value of GDF-15 suggests mechanisms other than renal dysfunction to account for the correlation between CVD risk and GDF-15 levels. The possibility remains that elevated levels of GDF-15 may reflect a compensatory production in individuals suffering more severe CVD.
Circulating GDF-15 levels have previously been shown to be an independent risk factor for adverse cardiovascular events including stroke and acute myocardial infarction among women (12). At the cellular level, GDF-15 mRNA and propeptide expression is strongly induced by both permanent and transient ischemia in mice and in patients who died after an MI (9). In addition, the gdf-15 gene–targeted mice have virtually normal hearts under nonstressed conditions. In contrast, they develop greater infarct sizes and greater extent of cardiomyocyte cell death following the ischemia/reperfusion injury that could be prevented by treatment with recombinant GDF-15 (9). Thus, GDF-15 may function as a necessary protective factor to the heart in association with immediate and long-term alterations in the hypertrophic response antagonizing the onset or severity of heart failure, which is analogous to BNP.
Secretion of BNP and NT-proBNP is induced from the heart following acute and chronic stimulation associated with cardiac injury and long-standing disease that signals a protective and anti-hypertrophic response (6,20). Circulating levels of NT-proBNP have previously been shown to be associated with increased mortality due to chronic heart failure and acute coronary syndromes in various disease states such as hypertension (21), ESRD (22), and of excess all-cause and cardiovascular mortality in both type 1 (19) and type 2 (23) diabetic patients with diabetic nephropathy. Thus, we also included NT-proBNP levels in the multivariate analysis. Still, elevated GDF-15 levels remained independently associated with CVD in an analogous manner to circulating NT-proBNP levels and as such may offer future therapeutic targets for the treatment of hypertrophic and dilated cardiomyopathy. However, studies showing that GDF-15 levels are modified by intervention are needed.
Recent data suggest that patients with non-ST–elevation acute coronary syndrome with elevated GDF-15 levels benefit from early revascularization whereas patients with lower levels of GDF-15 do not (24). Thus, future studies should elucidate whether GDF-15 could be informative as part of a multimarker strategy identifying high-risk patients. The ROC curve analysis further illustrated GDF-15 as a strong marker of mortality risk with an AUC of 0.79 as compared with NT-proBNP (HR 0.75 [95% CI 0.69–0.81]) and GFR (0.71 [0.64–0.77]). However, in a combined risk score model including the previously mentioned covariates, GDF-15 only slightly improved the predictive accuracy of the model. This could be due to the fact that the combined model without GDF-15 has an AUC of 0.83 and, hence, the predictive accuracy is difficult to increase. Still, combining markers of renal dysfunction, inflammation, and natriuretic peptides enhance risk stratification of type 1 diabetic patients with and without diabetic nephropathy and may provide additional insight into pathophysiological mechanisms.
Although the cellular sources, upstream regulators, and functional effects of GDF-15 in the cardiovascular system remain to be fully elucidated, several details are emerging. Basal GDF-15 expression has so far only been detected in liver and placenta tissue. However, studies of cultured human kidney cells showed a rapid rise in the secretion of mature GDF-15 following injury, hypoxia, or cytokine/growth factor stimulation due to a dramatic induction of expression of GDF-15 and not the release of stored GDF-15 (8,11).
GDF-15 has been suggested to provide endogenous protection against cardiomyocyte apoptosis possibly via a PI3K-Akt–dependent pathway (9). In addition, GDF-15 treatment also transiently activated Akt and ERK1/2 signaling, both of which are thought to be cardioprotective pathways (10). Other studies indicate that bone marrow–derived GDF-15 protects against macrophage accumulation within atherosclerotic lesions and promotes lesion stabilization possibly due to inhibition of adhesion molecules (25). Finally, the potential heterogeneity in the mechanism of GDF-15 activin in different tissues or cell types may be attributable to differential expression of the type I and type II transforming growth factor-β activin receptors with affinity for GDF-15. Thus, GDF-15 might not only be a marker of ongoing tissue damage but actively influence multiple downstream signaling effectors as part of a cardioprotective mechanism. However, a suggestive role for GDF-15 therapy in improving treatment and prevention of complications among diabetic patients remains to be investigated further in both experimental and clinical settings.
There are some limitations that should be mentioned. Firstly, the end point fatal CVD is based on death certificates in contrast to nonfatal CVD events that are based on information obtained from the patients' medical records. However, on a positive note, the number of events may be underestimated and hence the predictive value of GDF-15 could be regarded as a conservative estimate.
Secondly, the stability of GDF-15 after 15+ years of storage is not known. However, all samples of this study were treated and stored at −80°C under the same conditions. Furthermore, GDF-15 has been shown to be stable at room temperature for 48 h and resistant to at least four freeze-thaw cycles and, equally important, the choice of anticoagulant matrix had no influence on the measurements (18).
In conclusion, higher levels of GDF-15 independently predicted all-cause and cardiovascular mortality and morbidity in patients with diabetic nephropathy beyond conventional cardiovascular risk factors including NT-proBNP and GFR. Furthermore, higher levels of GDF-15 were predictive of deterioration of kidney function. Therefore, GDF-15 may be considered as an additional marker of inflammation and atherosclerosis involved in the pathogenesis of ischemic heart disease.
Acknowledgments
The EURAGEDIC study was supported by the European Commission (contract QLG2-CT-2001–01669).
No potential conflicts of interest relevant to this article were reported.
The authors acknowledge the technical assistance of Tina R. Juhl, Anne G. Lundgaard, Berit R. Jensen, and Ulla M. Smidt. In addition, the authors thank Georg Hess and Dietmar Zdunek from Roche Diagnostics for supplying the GDF-15, NT-proBNP, and CRP measurements.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Rossing P, Hougaard P, Borch-Johnsen K, Parving HH: Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 1996; 313: 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kannel WB: Incidence and epidemiology of heart failure. Heart Fail Rev 2000; 5: 167–173 [DOI] [PubMed] [Google Scholar]
- 3. Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106: 3068–3072 [DOI] [PubMed] [Google Scholar]
- 4. Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G: Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc Res 2004; 63: 373–380 [DOI] [PubMed] [Google Scholar]
- 5. Azhar M, Schultz Jel J, Grupp I, Dorn GW, 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T: Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev 2003; 14: 391–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molkentin JD: A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest 2003; 111: 1275–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strelau J, Böttner M, Lingor P, Suter-Crazzolara C, Galter D, Jaszai J, Sullivan A, Schober A, Krieglstein K, Unsicker K: GDF-15/MIC-1: a novel member of the TGF-beta superfamily. J Neural Transm 2000;( Suppl. 2): 273–276 [DOI] [PubMed] [Google Scholar]
- 8. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN: MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A 1997; 94: 11514–11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC: The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 2006; 98: 351–360 [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD: GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res 2006; 98: 342–350 [DOI] [PubMed] [Google Scholar]
- 11. Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R: Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res 2004; 318: 325–333 [DOI] [PubMed] [Google Scholar]
- 12. Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM: Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet 2002; 359: 2159–2163 [DOI] [PubMed] [Google Scholar]
- 13. Kempf T, Björklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, Tongers J, Wollert KC, Wallentin L: Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J 2007; 28: 2858–2865 [DOI] [PubMed] [Google Scholar]
- 14. Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L: Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation 2007; 115: 962–971 [DOI] [PubMed] [Google Scholar]
- 15. Tarnow L, Groop PH, Hadjadj S, Kazeem G, Cambien F, Marre M, Forsblom C, Parving HH, Trégouët D, Thévard A, Farrall M, Gut I, Gauguier D, Cox R, Matsuda F, Lathrop M, Vionnet N. EURAGEDIC Consortium. European rational approach for the genetics of diabetic complications–EURAGEDIC: patient populations and strategy. Nephrol Dial Transplant 2008; 23: 161–168 [DOI] [PubMed] [Google Scholar]
- 16. Bröchner-Mortensen J, Rödbro P: Selection of routine method for determination of glomerular filtration rate in adult patients. Scand J Clin Lab Invest 1976; 36: 35–43 [DOI] [PubMed] [Google Scholar]
- 17. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266 [PubMed] [Google Scholar]
- 18. Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC: Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem 2007; 53: 284–291 [DOI] [PubMed] [Google Scholar]
- 19. Tarnow L, Hildebrandt P, Hansen BV, Borch-Johnsen K, Parving HH: Plasma N-terminal pro-brain natriuretic peptide as an independent predictor of mortality in diabetic nephropathy. Diabetologia 2005; 48: 149–155 [DOI] [PubMed] [Google Scholar]
- 20. Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N: Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A 1997; 94: 14730–14735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olsen MH, Wachtell K, Tuxen C, Fossum E, Bang LE, Hall C, Ibsen H, Rokkedal J, Devereux RB, Hildebrandt P: N-terminal pro-brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study. J Hypertens 2004; 22: 1597–1604 [DOI] [PubMed] [Google Scholar]
- 22. Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D: N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int 2007; 71: 548–554 [DOI] [PubMed] [Google Scholar]
- 23. Gaede P, Hildebrandt P, Hess G, Parving HH, Pedersen O: Plasma N-terminal pro-brain natriuretic peptide as a major risk marker for cardiovascular disease in patients with type 2 diabetes and microalbuminuria. Diabetologia 2005; 48: 156–163 [DOI] [PubMed] [Google Scholar]
- 24. Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H, Wallentin L: Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation 2007; 116: 1540–1548 [DOI] [PubMed] [Google Scholar]
- 25. Preusch MR, Strelau J, Baeuerle M, Blessing E, Bischof M, Kinscherf R, Unsicker K, Katus HA, Bea F: Bone marrow derived growth differentiation factor-15 (GDF-15) protects from macrophage accumulation and effects atherosclerotic lesion stabilisation in low-density lipoprotein receptor-null mice. Circulation 2008; 118: S511–S512 [Google Scholar]