Abstract
OBJECTIVE
Sleep restriction results in decreased insulin sensitivity and glucose tolerance in healthy subjects. We hypothesized that sleep duration is also a determinant of insulin sensitivity in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
We studied seven patients (three men, four women) with type 1 diabetes: mean age 44 ± 7 years, BMI 23.5 ± 0.9 kg/m2, and A1C 7.6 ± 0.3%. They were studied once after a night of normal sleep duration and once after a night of only 4 h of sleep. Sleep characteristics were assessed by polysomnography. Insulin sensitivity was measured by hyperinsulinemic euglycemic clamp studies with an infusion of [6,6-2H2]glucose.
RESULTS
Sleep duration was shorter in the night with sleep restriction than in the unrestricted night (469 ± 8.5 vs. 222 ± 7.1 min, P = 0.02). Sleep restriction did not affect basal levels of glucose, nonesterified fatty acids (NEFAs), or endogenous glucose production. Endogenous glucose production during the hyperinsulinemic clamp was not altered during the night of sleep restriction compared with the night of unrestricted sleep (6.2 ± 0.8 vs. 6.9 ± 0.6 μmol · kg lean body mass−1 · min−1, NS). In contrast, sleep restriction decreased the glucose disposal rate during the clamp (25.5 ± 2.6 vs. 22.0 ± 2.1 μmol · kg lean body mass−1 · min−1, P = 0.04), reflecting decreased peripheral insulin sensitivity. Accordingly, sleep restriction decreased the rate of glucose infusion by ∼21% (P = 0.04). Sleep restriction did not alter plasma NEFA levels during the clamp (143 ± 29 vs. 133 ± 29 μmol/l, NS).
CONCLUSIONS
Partial sleep deprivation during a single night induces peripheral insulin resistance in these seven patients with type 1 diabetes. Therefore, sleep duration is a determinant of insulin sensitivity in patients with type 1 diabetes.
Intensive insulin therapy is essential for optimal glucoregulation in type 1 diabetes because the degree and duration of hyperglycemia determine the incidence of complications (1). However, glucoregulation cannot be normalized in patients with type 1 diabetes, as reflected by relatively large intra-individual variations of blood glucose levels. Subtle intra-individual variations in glucoregulation and insulin sensitivity in these patients depend on variations in physiological determinants such as dietary factors and exercise (2,3). In contrast to healthy subjects, however, patients with type 1 diabetes are unable to compensate for these physiological variations, other than by adaptations of the dose of exogenous insulin.
Normal glucose homeostasis shows a diurnal pattern with variations in glucose tolerance, in which sleep characteristics play a key role (4). In this respect, sleep duration may be particularly relevant because sleep curtailment is a common aspect of our modern 24-h society (5,6). A reduction in sleep duration impairs glucose tolerance in healthy subjects (7). The effects of reduction of sleep duration on insulin sensitivity have not been studied in patients with type 1 diabetes.
We hypothesized that partial sleep restriction decreases insulin sensitivity in patients with type 1 diabetes, which may contribute to intra-individual variations in glucoregulation. Therefore, we compared the effects of a single night of reduced sleep duration with those of a night of normal sleep duration on hepatic and peripheral insulin sensitivity, as assessed by hyperinsulinemic euglycemic clamp studies combined with tracer dilution of [6,6-2H2]glucose in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
We included nine patients (five men and four women) with type 1 diabetes on stable continuous subcutaneous insulin pump therapy. Diagnostic criteria for type 1 diabetes were past or present positive antibodies against GAD or islet cells and plasma free C-peptide levels <0.3 nmol/l. All participants were screened at our outpatient clinic prior to the study. Their weight had been stable for at least 3 months prior to participation in this study. A1C levels had been below 8.5% during the year prior to the start of the study. Exclusion criteria were a BMI >26 kg/m2, clinical signs of autonomic neuropathy, known sleep disorders, habitual sleep duration of less than 6 h or more than 9 h, psychiatric disorders, and use of sleep medication, β-blocking agents, or prokinetic drugs. All patients had normal blood pressure and serum creatinine levels and urinary microalbumin excretion rates below 30 mg/24 h.
The study was approved by the medical ethical committee of the Leiden University Medical Center, and written informed consent was obtained from all subjects prior to the study.
The subjects were studied on 3 days, separated by intervals of at least 3 weeks. Subjects kept a detailed diary of their diet and physical activity for 3 days prior to each study day and were asked to maintain a standardized schedule of bedtimes and mealtimes in accordance with their usual habits. Actigraphy (Actiwatch AW7; Cambridge Neurotechnology, Cambridge, U.K.) was performed to objectively assess patterns of habitual active and inactive (sleep) periods for 7 days prior to the actual study, including one weekend. In addition, self-reported sleep duration and sleep quality were assessed using validated questionnaires (Pittsburgh Sleep Quality Index, Epworth Sleepiness scale, and Berlin Questionnaire) (8–10).
Subjects were admitted to our clinical research center the night preceding each study day and spent 8.5 h in bed from 2300 h to 0730 h on all three occasions. Subjects fasted throughout these nights from 2200 h onwards. The first study day served to let subjects become accustomed to sleep conditions in a research setting. The optimal overnight infusion rate of insulin was determined in each subject prior to the start of the study. Subsequently, this same individualized infusion rate was used in both sleeping experiments. Subjects were instructed not to alter lifestyle habits during the study period. Premenopausal women were studied in the follicular phase of their menstrual cycle.
Subjects were randomly assigned to partial sleep deprivation on either the second (n = 4) or third (n = 5) study occasion. During the night of sleep restriction, subjects also spent 8.5 h in bed but were only allowed to sleep from 0100 h to 0500 h. They were allowed to read or watch movies in an upward position, and their wakefulness was monitored and assured if necessary.
Polysomnography
Sleep was visually scored for each of the three nights according to the guidelines of the American Association of Sleep Medicine (AASM) (11). In short, scoring of sleep stages depends on electroencephalography, eye movements, and submental muscle activity. To detect possible sleep disorders that might affect the study, respiratory movements were recorded by measurement of changes in nasal pressures and of truncal respiratory movements. Recordings were made using a portable polysomnography (PSG) recorder (Titanium; Embla Systems, Broomfield, CO). Sleep and wake stages were visually scored in consecutive epochs of 30 s, resulting in a list of epochs spent in wake, stages I (drowsiness), II, III, and rapid eye movement (REM) dream sleep. The times at which subjects went to bed and turned out the lights as well as times of getting out of bed were noted. The lists of stages were used to calculate the duration of time spent each night in the above-mentioned sleep and wake stages. These durations were also expressed as percentages of total sleep duration, defined as the summed duration of sleep stages I, II, III, and REM.
Hyperinsulinemic euglycemic clamp studies
Hyperinsulinemic euglycemic clamp studies were performed the day after the second and third study occasions. After an overnight fast, a catheter was inserted into an antecubital vein for infusion of isotopes, glucose, and insulin, and a sampling catheter was inserted into a dorsal hand vein of the contra lateral arm. For all blood samples, the heated hand technique was used to obtain arterialized blood (12). A primed (17.6 μmol/kg−1) continuous (0.22 μmol · kg−1 · min−1) infusion of [6,6-2H2]glucose (Cambridge Isotope Laboratory, Andover, MA) was started at 0830 h, after basal blood samples had been taken for determination of background glucose enrichment. Labeled glucose was infused by a Pilot C syringe pump (Fresenius Vial, Brezins, France). Blood samples were obtained after 160, 170, and 180 min of [6,6-2H2]glucose infusion for assessment of glucose kinetics in the basal state and concentrations of glucose and plasma nonesterified fatty acids (NEFAs).
Subsequently, administration of subcutaneous insulin was stopped and infusion of intravenous insulin was started, using the method of DeFronzo et al. (13). Briefly, this consisted of a primed (80 mU · m−2 · min−1 for 5 min and subsequently 40 mU · m−2 · min−1 for 5 min), followed by continuous (20 mU · m−2 · min−1) infusion of insulin (Actrapid, Novo Nordisk, Alphen a/day Rijn, the Netherlands), dissolved in sterile NaCl 0.9%, using a Pilot C syringe pump. A variable infusion of glucose 20% enriched with 3% [6,6-2H2]glucose was started 4 min after the start of insulin infusion. Plasma glucose concentrations were measured in intervals of 5 min with a bedside calibrated glucose analyzer (Accu-Chek; Roche, Mannheim, Germany), and the infusion rate of glucose 20% was adjusted in order to keep the plasma glucose levels constant at 5.0 mmol/l during the clamp study. Blood samples were obtained after 150, 160, 170, and 180 min of combined insulin and [6,6-2H2]glucose infusion for assessment of glucose kinetics and of concentrations of glucose, insulin, and plasma NEFA.
Biochemical analysis
Serum concentrations of glucose were measured using a fully automated Modular P 800 analyzer (Roche/Hitachi; Mannheim, Germany) with intra-assay variations of 1%. Serum insulin concentrations were measured by enzyme labeled chemiluminescent immunometric assay (Immulite 2500; Siemens, Bad Nauheim, Germany) with an intra-assay coefficient of variation (CV) of 4%. NEFA levels were determined spectrophotometrically by enzymatic colorimetric acyl-CoA synthase/acyl-CoA oxidase assay (WAKO Chemicals, Neuss, Germany) with intra-assay CV of 2.7%. Enrichment of plasma [6,6-2H2]glucose was determined in a single analytical run, using gas chromatography coupled to mass spectrometry, as described previously (14). All isotope enrichments were measured on a gas chromatograph mass spectrometer (model 6890/5973; Hewlett-Packard, Palo Alto, CA).
Calculations
Isotopic steady state was achieved during the final 30 min of the basal period and the final 30 min of the hyperinsulinemic clamp study. Therefore, the rates of appearance (Ra) and disappearance (Rd) of glucose were calculated as the tracer infusion rates divided by the tracer-to-tracee ratios. Endogenous glucose production (EGP) during the basal steady state is equal to Ra of glucose, whereas EGP during the hyperinsulinemic clamp study was calculated as the difference between Ra and the glucose infusion rates.
Statistical analysis
Data are presented as mean ± SEM. Differences between the effects of the night of normal sleep duration and the night of partial sleep restriction were analyzed by the Wilcoxon signed rank test for paired samples. All analyses were performed using SPSS for Windows, version 16.0 (SPSS, Chicago, IL). Significance was accepted at P < 0.05.
RESULTS
Clinical characteristics
The results of two of the nine patients were excluded because of nocturnal hypoglycemia and subsequent nocturnal hyperglycemia during the study (n = 1), and because of the presence of a previously undetected sleep apnea syndrome (n = 1). Therefore, the analyses included the data of seven patients (three men) for analysis. Mean age of these seven subjects was 44.3 ± 6.6 years, mean weight 72.0 ± 4.0 kg, mean height 175 ± 3 cm, and mean BMI 23.5 ± 0.9 kg/m2. Mean A1C of the patients was 7.6 ± 0.3%, and mean duration of diabetes was 23 ± 3.5 years. All subjects had normal results on validated sleep questionnaires. Self-reported sleep duration and recorded habitual sleep duration by actigraphy were not different (475 ± 8 vs. 490 ± 7 min, P = 0.12).
Effects of partial sleep restriction on polysomnographic parameters
Sleep duration was considerably shorter in the night with partial sleep restriction, compared with the night with normal sleep duration (P = 0.02) (Table 1). Sleep in the sleep-deprived night showed a higher proportion of stage III sleep (P = 0.02) and a lower proportion of REM sleep (P = 0.04). The proportions of time spent in other stages did not differ between the conditions.
Table 1.
The effects of a night of normal sleep duration versus a night of sleep duration restricted to 4 h on sleep parameters assessed by PSG, basal and insulin-stimulated glucose, and fatty acid metabolism in seven patients with type 1 diabetes
| Normal sleep | Partial sleep deprivation | P | |
|---|---|---|---|
| Sleep parameters | |||
| TST (min) | 469 ± 8.5 | 222 ± 7.1 | 0.02 |
| Stage 1 (% of TST) | 10.6 ± 1.0 | 10.2 ± 2.1 | 0.49 |
| Stage 2 (% of TST) | 44.4 ± 2.8 | 40.4 ± 2.4 | 0.13 |
| Stage 3 (% of TST) | 22.7 ± 3.4 | 35.3 ± 4.4 | 0.02 |
| REM sleep (% of TST) | 22.2 ± 1.7 | 13.7 ± 2.3 | 0.04 |
| Wake time during sleep (% of total sleep period) | 4.2 ± 0.7 | 5.4 ± 2.3 | 0.87 |
| Basal values | |||
| Glucose (mmol/l) | 7.2 ± 1.0 | 6.7 ± 1.4 | 0.87 |
| NEFA (μmol/l) | 673 ± 125 | 591 ± 99 | 0.35 |
| EGP (μmol · kg LBM−1 · min−1) | 17.6 ± 0.9 | 16.3 ± 1.0 | 0.31 |
| Hyperinsulinemic euglycemic clamp study | |||
| Glucose (mmol/l) | 5.1 ± 0.2 | 5.2 ± 0.1 | 0.23 |
| Insulin (mU/l) | 24.0 ± 2.2 | 24.9 ± 1.5 | 0.40 |
| NEFA (μmol/l) | 143 ± 29 | 133 ± 29 | 0.69 |
| EGP (μmol · kg LBM−1 · min−1) | 6.2 ± 0.8 | 6.9 ± 0.6 | 0.74 |
| Glucose Rd (μmol · kg LBM−1 · min−1) | 25.5 ± 2.6 | 22.0 ± 2.1 | 0.04 |
| Glucose infusion rate (μmol · kg LBM−1 · min−1) | 19.0 ± 2.9 | 14.9 ± 2.1 | 0.04 |
Data are means ± SEM. Data in bold represent statistical significance. LBM, lean body mass; TST, total sleep time.
Effects of partial sleep restriction on basal metabolic parameters
The mean overnight rate of subcutaneous infusion of insulin was 0.7 IE/h and was identical in both conditions (Table 1). Compared with normal sleep duration, partial sleep deprivation did not alter basal levels of glucose or NEFA measured the following morning. In addition, partial sleep restriction did not affect basal EGP assessed by primed, continuous infusion of [6,6-2H2]glucose.
Effects of partial sleep restriction on metabolic parameters during hyperinsulinemic euglycemic clamp conditions
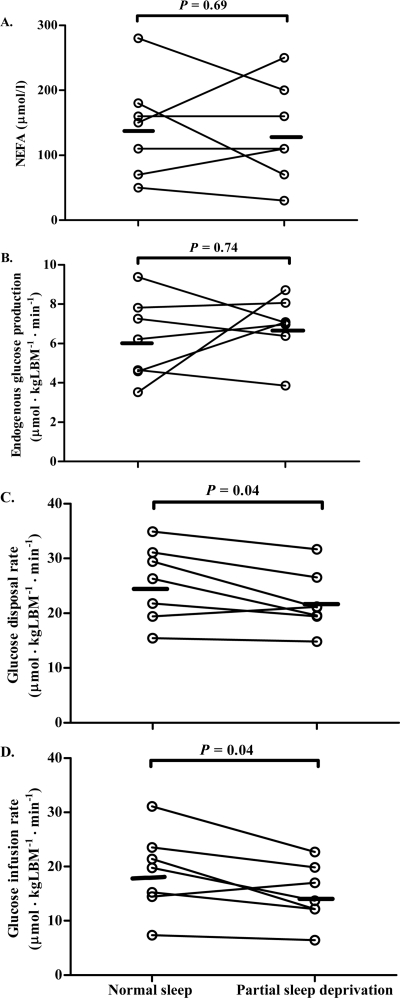
Steady state glucose and insulin levels did not differ between the two clamp studies (Table 1 and Fig. 1). Sleep restriction did not affect EGP during the clamp conditions. However, sleep restriction decreased the rate of glucose disposal (Rd) during the clamp by ∼14% (P = 0.04). Accordingly, the rate of infusion of glucose necessary to maintain constant plasma glucose levels during the hyperinsulinemic clamp study was ∼21% lower after the night of reduced sleep duration than after the night of normal sleep duration (P = 0.04), reflecting decreased peripheral insulin sensitivity. There was no significant effect of partial sleep restriction on plasma NEFA levels.
Figure 1.
Individual values obtained during steady state of the hyperinsulinemic euglycemic clamp studies of NEFAs (A), EGP (B), glucose disposal rate (C), and the glucose infusion rate (D) after a night of normal sleep duration versus after a night of partial sleep deprivation in patients with type 1 diabetes (n = 7). Black horizontal lines represent the mean of the values of seven subjects.
CONCLUSIONS
In this study, we assessed the effects of a single night of partial sleep restriction on insulin sensitivity in patients with type 1 diabetes. The results indicate that a single night of partial sleep restriction reduces insulin sensitivity of insulin-stimulated glucose uptake by 14–21%. Partial sleep restriction did not significantly affect basal glucose metabolism. We conclude that sleep duration is a determinant of peripheral insulin sensitivity in patients with type 1 diabetes.
In the current study we included the data of only seven patients with type 1 diabetes. The strictly controlled design of this pathophysiological study in combination with the fact that each subject served as his/her own control enabled us to establish subtle effects of partial sleep deprivation on parameters of insulin sensitivity. Nonetheless, larger numbers of subjects are required to assess the involvement of relevant patient characteristics such as sex, age, and antecedent glucoregulation on the effects of sleep restriction on insulin sensitivity.
This is the first study that documents an adverse effect of partial sleep restriction on insulin sensitivity in patients with type 1 diabetes. In healthy subjects, sleep restriction induces insulin resistance and reduces glucose tolerance (7,15). By analogy, it can be expected that sleep restriction increases postprandial glucose levels in patients with type 1 diabetes in the absence of concurrent adaptations of the dose of exogenous insulin.
Insulin sensitivity is not static but varies over time within individuals. This is also reflected by the current study in patients with type 1 diabetes. Several epidemiological studies documented an association between chronic partial sleep restriction and development of insulin resistance and type 2 diabetes (5,16,17). Therefore, exposure to chronic sleep restriction might contribute to insulin resistance in patients with type 1 diabetes. In turn, insulin resistance is associated with an increased risk for microvascular and macrovascular complications in type 1 diabetes (18).
Unfortunately, the current study was not designed to elucidate the mechanisms involved in the induction of insulin resistance by partial sleep deprivation. A single night of partial sleep restriction to 4.5 h does not cause endocrine changes that simply explain the induction of insulin resistance (19). Subsequent nights of partial sleep deprivation induce subtle changes in cortisol and catecholamine secretion (7,15,20). However, the relations between these effects of sleep deprivation on endocrine homeostasis and glucose tolerance are uncertain. Partial sleep deprivation for a single and subsequent nights increased the sympathetic tone based on recordings of heart rate variability after sleep deprivation (21,22). However, the relationship between elevated sympathovagal balance at the level of the heart and the sympathetic outflow to liver, muscles, and adipose tissue is uncertain (21).
Interestingly, in addition to sleep duration, the composition of sleep in terms of sleep stages is also a determinant of insulin sensitivity. Selective suppression of slow-wave sleep, without a change in total sleep duration, decreased glucose tolerance in healthy subjects (23). The differential effects of altered sleep composition versus decreased total sleep duration on insulin sensitivity awaits further study.
Data on sleep physiology and sleep disturbances in patients with type 1 diabetes are rare. Jauch-Chara et al. (24) reported alterations in neuroendocrine sleep architecture and a trend toward less slow-wave sleep in 14 patients with type 1 diabetes. Children with type 1 diabetes have a more disrupted sleep than healthy children (25). If type 1 diabetes indeed causes disruption of sleep patterns, this may in turn impair glucose regulation, creating a vicious circle.
In conclusion, the present study indicates that partial sleep restriction decreases insulin sensitivity of insulin-mediated glucose uptake in patients with type 1 diabetes. It is important to further assess the relationship between sleep physiology and glucoregulation in patients with type 1 diabetes. Sleep duration might become another therapeutic target to improve glucoregulation in type 1 diabetes.
Acknowledgments
This study was supported by the European Foundation for the Study of Diabetes and the Dutch Diabetes Research Foundation.
No conflicts of interest relevant to this article were reported.
We thank all patients for participating in this study. We also thank Trea Streefland and all sleep technicians for their help.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 2. Rosenfalck AM, Almdal T, Viggers L, Madsbad S, Hilsted J: A low-fat diet improves peripheral insulin sensitivity in patients with Type 1 diabetes. Diabet Med 2006; 23: 384–392 [DOI] [PubMed] [Google Scholar]
- 3. Landt KW, Campaigne BN, James FW, Sperling MA: Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care 1985; 8: 461–465 [DOI] [PubMed] [Google Scholar]
- 4. Scheen AJ, Van Cauter E: The roles of time of day and sleep quality in modulating glucose regulation: clinical implications. Horm Res 1998; 49: 191–201 [DOI] [PubMed] [Google Scholar]
- 5. Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB: A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003; 26: 380–384 [DOI] [PubMed] [Google Scholar]
- 6. Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF: American time use survey: sleep time and its relationship to waking activities. Sleep 2007; 30: 1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spiegel K, Leproult R, Van Cauter E: Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354: 1435–1439 [DOI] [PubMed] [Google Scholar]
- 8. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213 [DOI] [PubMed] [Google Scholar]
- 9. Johns MW: A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545 [DOI] [PubMed] [Google Scholar]
- 10. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP: Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131: 485–491 [DOI] [PubMed] [Google Scholar]
- 11. Iber C: The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, Illinois, American Academy of Sleep Medicine, 2007 [Google Scholar]
- 12. Copeland KC, Kenney FA, Nair KS: Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol 1992; 263: E1010–E1014 [DOI] [PubMed] [Google Scholar]
- 13. DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223 [DOI] [PubMed] [Google Scholar]
- 14. Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F, Meijer AJ, Sauerwein HP, Romijn JA: Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am J Clin Nutr 2001; 73: 554–559 [DOI] [PubMed] [Google Scholar]
- 15. Nedeltcheva AV, Kessler L, Imperial J, Penev PD: Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009; 94: 3242–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ: Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005; 165: 863–867 [DOI] [PubMed] [Google Scholar]
- 17. Yaggi HK, Araujo AB, McKinlay JB: Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006; 29: 657–661 [DOI] [PubMed] [Google Scholar]
- 18. Chillarón JJ, Goday A, Flores-Le-Roux JA, Benaiges D, Carrera MJ, Puig J, Cano-Pérez JF, Pedro-Botet J: Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab 2009; 94: 3530–3534 [DOI] [PubMed] [Google Scholar]
- 19. Schmid SM, Jauch-Chara K, Hallschmid M, Schultes B: Mild sleep restriction acutely reduces plasma glucagon levels in healthy men. J Clin Endocrinol Metab 2009; 94: 5169–5173 [DOI] [PubMed] [Google Scholar]
- 20. Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M: Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab 1999; 84: 1979–1985 [DOI] [PubMed] [Google Scholar]
- 21. Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E: Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89: 5762–5771 [DOI] [PubMed] [Google Scholar]
- 22. Tochikubo O, Ikeda A, Miyajima E, Ishii M: Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension 1996; 27: 1318–1324 [DOI] [PubMed] [Google Scholar]
- 23. Tasali E, Leproult R, Ehrmann DA, Van Cauter E: Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008; 105: 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B: Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care 2008; 31: 1183–1188 [DOI] [PubMed] [Google Scholar]
- 25. Matyka KA, Crawford C, Wiggs L, Dunger DB, Stores G: Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: relationship to nocturnal hypoglycemia. J Pediatr 2000; 137: 233–238 [DOI] [PubMed] [Google Scholar]