Abstract
OBJECTIVE
Intensive therapy targeting normal blood glucose increased mortality compared with standard treatment in a randomized clinical trial of 10,251 participants with type 2 diabetes at high-risk for cardiovascular disease (CVD) events. We evaluated whether the presence of cardiac autonomic neuropathy (CAN) at baseline modified the effect of intensive compared with standard glycemia treatment on mortality outcomes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial participants.
RESEARCH DESIGN AND METHODS
CAN was assessed by measures of heart rate variability (HRV) and QT index (QTI) computed from 10-s resting electrocardiograms in 8,135 ACCORD trial participants with valid measurements (mean age 63.0 years, 40% women). Prespecified CAN definitions included a composite of the lowest quartile of HRV and highest QTI quartile in the presence or absence of peripheral neuropathy. Outcomes were all-cause and CVD mortality. Associations between CAN and mortality were evaluated by proportional hazards analysis, adjusting for treatment group allocation, CVD history, and multiple prespecified baseline covariates.
RESULTS
During a mean 3.5 years follow-up, there were 329 deaths from all causes. In fully adjusted analyses, participants with baseline CAN were 1.55–2.14 times as likely to die as participants without CAN, depending on the CAN definition used (P < 0.02 for all). The effect of allocation to the intensive group on all-cause and CVD mortality was similar in participants with or without CAN at baseline (Pinteraction > 0.7).
CONCLUSIONS
Whereas CAN was associated with increased mortality in this high-risk type 2 diabetes cohort, these analyses indicate that participants with CAN at baseline had similar mortality outcomes from intensive compared with standard glycemia treatment in the ACCORD cohort.
It has been generally accepted that intensive glycemic control is paramount for preventing development and progression of chronic diabetes complications (1,2). The recently reported increased mortality associated with intensive control of hyperglycemia in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (3) has led to controversy about implementation of intensive glucose control in patients with type 2 diabetes.
There was a consistent effect on mortality from the intensive compared with standard treatment in the prespecified subgroup analyses (3). To date no adequate explanation for these findings of increased mortality with intensive glycemic control has been identified.
Cardiac autonomic neuropathy (CAN), which can be documented by abnormal heart rate variability (HRV), occurs commonly in patients with diabetes and is associated with silent myocardial ischemia (4) and increased mortality (5). In addition, peripheral neuropathy is a prevalent complication of diabetes, and emerging evidence links excess mortality in diabetes with the presence of diabetic peripheral neuropathy (DPN) (6,7).
It is also possible that individuals with baseline CAN may be more susceptible to mortality associated with hypoglycemia when glycemia therapy is intensified because of impaired hormonal and autonomic responses to subsequent hypoglycemia (8,9). To further investigate possible contributors to the higher mortality risk in the intensive glycemia arm of the ACCORD trial, we examined whether the presence of CAN at baseline with or without DPN may have contributed to this outcome.
RESEARCH DESIGN AND METHODS
The ACCORD trial design and patient population have been described elsewhere (10). In brief, 10,251 subjects with type 2 diabetes at high-risk for cardiovascular disease (CVD) events were enrolled in 77 clinical centers across the U.S. and Canada and randomly assigned to receive either comprehensive intensive glycemia therapy (INT) targeting a A1C level <6% or to receive standard glycemia therapy (STD) targeting an A1C level of 7–7.9%. The mean duration of the trial was expected to be 5 years. The INT arm of the study was discontinued after ∼3.5 years because of excess mortality in the INT group, and all participants were transitioned to the STD protocol (3). The ACCORD study and the consent forms were approved by institutional review boards at all participating institutions. The trial was funded by the National Institutes of Health. All participants provided written informed consent. Baseline clinical and laboratory investigations were obtained in the morning after an overnight fast as described (10).
Electrocardiogram-derived measures of HRV
We used baseline standard 12-lead digitized electrocardiograms (ECGs), recorded over 10 consecutive seconds with the patient resting supine after an overnight fast (GE MAC 1200 electrocardiograph system). The ECG recordings were transferred by analog phone line to the reading center and were analyzed and reviewed to determine their technical quality. Recordings that were missing (1,034) or demonstrated poor quality (362) and recordings from those with pacemakers (65), atrial fibrillation (108), premature beats/other arrhythmias (542), and atrioventricular conduction abnormalities (5) were excluded from these analyses, leaving a cohort of 8,135 participants assessed for HRV. The following time domain markers of cardiac autonomic tone were computed: heart rate and the SD of normally conducted R-R intervals (SDNN). From simultaneous lead recordings, QT intervals were measured, and the QT index (QTI) was calculated as observed/predicted QT duration where predicted value was based on Bazett's correction (QTc = QT/R − R1/2). Resting heart rate reflects both overall autonomic function and cardiorespiratory fitness (11), SDNN represents joint sympathetic/parasympathetic modulation of heart rate in the time domain (11,12), QT duration represents the time between the onset of ventricular activation and the end of repolarization, a process controlled in part by sympathetic input (13,14). Impaired HRV is an easily measured sensitive marker of CAN that may occur early in the course of diabetes (15).
Definitions of CAN and DPN
CAN was defined by measures of HRV and QTI. Lower HRV and higher resting heart rate and QTI indicate poorer autonomic function (11). These measures are a reliable estimate of CAN and are recommended for use in large population studies (12). DPN was documented by any pedal amputation or a score >2 on the clinical examination portion of the Michigan Neuropathy Screening Instrument, a validated tool for assessing DPN that evaluates abnormalities in foot appearance, ankle reflexes, and vibration at the great toe of both feet (16).
The following composite measures were computed to document the presence of CAN: 1) CAN1 defined as the lowest quartile of SDNN (<7.815 ms) and the highest quartile of QTI (>104.32%); 2) CAN2 as the lowest quartile of SDNN and the highest quartiles of QTI and resting heart rate; and 3) CAN3 as the lowest quartile of SDNN and the highest quartiles of QTI and heart rate, in the presence of DPN. Our rationale for using these composite prespecified definitions of CAN was that combined abnormalities in HRV and QT interval have demonstrated stronger predictive value for mortality than either abnormality alone in patients with diabetes (13,14). The presence of DPN was included in one of the composite measures because of prior evidence linking excess mortality to the presence of DPN (6,7).
Outcome measures
The outcomes were all-cause and CVD mortality (adjudicated by a blinded panel using predefined adjudication processes). Death from CVD included deaths from myocardial infarction, heart failure, arrhythmia, invasive cardiovascular interventions, cardiovascular causes after noncardiovascular surgery, stroke, unexpected death presumed to be from ischemic CVD occurring within 24 h after the onset of symptoms, and death from other vascular diseases.
Statistical analysis
We hypothesized that the increased all-cause and CVD mortality with INT observed in the ACCORD trial was due to a higher risk of mortality with INT in the subset of individuals with baseline CAN. We also assessed whether CAN was related to mortality risk independent of glycemia treatment and compared the strength of these relationships across the prespecified definitions of CAN.
These analyses are based on data collected on participants at the time of randomization and all-cause and CVD mortality data submitted to the coordinating center through 10 December 2007, the cutoff date used by the Data and Safety Monitoring Board to make its recommendation to stop intensive glycemia treatment. Baseline characteristics were compared between excluded and included participants and between CAN-positive and CAN-negative groups using χ2 and two sample t tests. Analysis of all-cause and CVD mortality was performed with time-to-event methods according to the intention-to-treat principle. Risk of these outcomes was evaluated through the use of hazard ratios (HR) and 95% CIs. Two-sided P values were obtained from Wald χ2 tests derived from Cox proportional hazards regression analysis. For both outcomes, we examined minimally adjusted models stratified for treatment allocation and history of CVD. We also fit fully adjusted models containing treatment allocation, history of CVD, and the following prespecified baseline covariates: age, sex, ethnicity, diabetes duration, A1C, BMI, systolic blood pressure (SBP) and diastolic blood pressure (DBP), LDL cholesterol, triglycerides, urinary microalbumin-to-creatinine ratio, and use of thiazolidinediones (TZDs), insulin, β-blockers, ACE inhibitors/angiotensin receptor blockers, statins, alcohol, and cigarettes. Participants with missing covariates (n = 235) were excluded from fully adjusted analyses. Because results were similar between models, only the fully adjusted models are presented here. We assessed the consistency of the effect of glycemia treatment allocation on all-cause and CVD mortality among prespecified subgroups using statistical tests of interaction between treatment allocation and each subgroup within the Cox model. Event rates are expressed as the percentage of events per follow-up year, taking into account censoring of follow-up data, with 95% Poisson CIs calculated using large sample methods. We have also examined CAN effects after adding events of severe hypoglycemia requiring medical assistance to the Cox regression models as a time-dependent covariate.
RESULTS
For the current analyses, we included 8,135 ACCORD trial participants with complete data, including 4,050 (79%) randomly assigned to the INT arm and 4,085 (80%) to the STD arm (supplementary Fig. A1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0125/DC1). The main reason for missing data was failure of the site to capture and transmit an electronic ECG record (n = 1,034). Other reasons for exclusion, including arrhythmias (n = 542), were described in the research design and methods.
The baseline characteristics of these participants, comparing those with (included) and without (excluded) available CAN measures are shown in supplementary Table A1 (available in an online appendix). This was a cohort with long diabetes duration, elevated A1C levels, and multiple associated CVD risk factors as prespecified by the ACCORD trial design. Participants excluded from this analysis due to inadequate ECG data were older (P < 0.0001), were more likely to have had a previous cardiovascular event (P = 0.0002), and had a longer diabetes duration (P = 0.045). Sex, triglyceride levels, and β-blocker use also showed significant differences (supplementary Table A1).
Participants with CAN at baseline (Table 1) consistently had higher A1C, BMI, DBP, and triglycerides (P ≤ 0.01 in all cases) and were more likely to use insulin and to be female (P < 0.01 for all three definitions of CAN) than those without CAN. Minority status, prior CVD, diabetes duration, SBP, urinary albumin-to-creatinine ratio, smoking history, and TZD, statin, and β-blocker use showed inconsistent differences across the three definitions of CAN.
Table 1.
Baseline characteristics in the analyzed cohort expressed as a function of CAN
| Characteristic | CAN1 |
CAN2 |
CAN3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P | No | Yes | P | No | Yes | P | |
| n | 7,563 | 572 | 7,853 | 282 | 7,994 | 141 | |||
| Age (years) | 61.9 ± 6.7 | 62.5 ± 6.7 | 0.05 | 62 ± 6.7 | 61.8 ± 6.5 | 0.66 | 62 ± 6.7 | 62.5 ± 6.7 | 0.33 |
| Women | 39.1 | 49.7 | <0.0001 | 39.3 | 55 | <0.0001 | 39.6 | 51.8 | <0.01 |
| Minorities | 36.1 | 31.8 | 0.04 | 35.8 | 34.4 | 0.62 | 35.9 | 29.1 | 0.09 |
| Prior CVD | 33.7 | 41.8 | <0.0001 | 34.3 | 34 | 0.93 | 34.3 | 33.3 | 0.81 |
| Diabetes duration (years) | 10.7 ± 7.6 | 12 ± 8.2 | <0.0001 | 10.8 ± 7.7 | 10.8 ± 7.6 | 0.88 | 10.7 ± 7.7 | 12.5 ± 8 | <0.01 |
| A1C (%) | 8.3 ± 1 | 8.5 ± 1.2 | <0.0001 | 8.3 ± 1 | 8.5 ± 1.2 | <0.001 | 8.3 ± 1.1 | 8.6 ± 1.1 | <0.0001 |
| BMI (kg/m2) | 32.2 ± 5.4 | 32.8 ± 5.4 | <0.01 | 32.2 ± 5.4 | 33.2 ± 5.5 | <0.01 | 32.2 ± 5.4 | 34.4 ± 5.3 | <0.0001 |
| SBP (mmHg) | 136.1 ± 17 | 137.6 ± 17.3 | 0.04 | 136.2 ± 17 | 137.8 ± 17 | 0.13 | 136.2 ± 17 | 137.4 ± 18.5 | 0.42 |
| DBP (mmHg) | 74.9 ± 10.5 | 76 ± 10.9 | 0.01 | 74.8 ± 10.5 | 78.9 ± 10.8 | <0.0001 | 74.9 ± 10.5 | 78.3 ± 11.4 | <0.001 |
| Peripheral neuropathy | 41.8 | 49 | <0.001 | 42 | 50 | <0.01 | 41.3 | 100 | <0.0001 |
| Urinary albumin-to-creatinine ratio (mg/g) | 89.5 ± 358.6 | 135 ± 343.1 | <0.01 | 91.3 ± 357.9 | 132.3 ± 349 | 0.06 | 92 ± 358.9 | 133.9 ± 272.8 | 0.17 |
| LDL cholesterol (mg/dl) | 105.2 ± 33.8 | 104 ± 34.4 | 0.44 | 105 ± 33.8 | 108.8 ± 36.5 | 0.06 | 105 ± 33.9 | 107.8 ± 34.6 | 0.33 |
| Triglycerides (mg/dl) | 191.3 ± 151.4 | 208.5 ± 166 | <0.01 | 191.3 ± 151.6 | 224.1 ± 174.7 | <0.001 | 191.8 ± 152.1 | 230.3 ± 173.2 | <0.01 |
| Insulin users | 33.6 | 46.7 | <0.0001 | 34.1 | 46.5 | <0.0001 | 34.3 | 50.4 | <0.0001 |
| TZD users | 19.6 | 19.2 | 0.84 | 19.7 | 16 | 0.12 | 19.7 | 12.1 | 0.02 |
| β-Blocker users | 28.2 | 33.2 | <0.01 | 28.8 | 20.9 | <0.01 | 28.7 | 17.7 | <0.01 |
| ACE inhibitor/ARB users | 52.7 | 53.3 | 0.78 | 52.9 | 49.3 | 0.24 | 52.8 | 48.2 | 0.28 |
| Statin users | 62.1 | 62.9 | 0.68 | 62.3 | 56.4 | 0.04 | 62.3 | 53.2 | 0.03 |
| Alcohol (drinks/week) | 1 ± 2.9 | 1.1 ± 4 | 0.53 | 1 ± 3 | 0.8 ± 3.4 | 0.32 | 1 ± 3 | 0.7 ± 2.2 | 0.23 |
| Current smokers | 13.7 | 15.2 | 0.32 | 13.6 | 19.5 | <0.01 | 13.6 | 24.8 | <0.001 |
| Former smokers | 43.8 | 42.7 | 0.61 | 44 | 36.2 | <0.01 | 43.9 | 34 | 0.02 |
Values shown are percentages for dichotomous variables and means (standard deviations) for continuous measures. HbA1c: hemoglobin A1c, BMI: body mass index, SBP: systolic blood pressure, DPB: diastolic blood pressure, DPN: diabetic peripheral neuropathy diagnosed by any pedal amputation or score > 2 on the Michigan Neuropathy Screening Instrument, LDLc: low-density lipoprotein cholesterol, ACEIs: angiotensin- converting enzyme inhibitors, ARBs: angiotensin receptor blockers, TZD: thiazolidinediones. CAN1 was defined as the lowest quartile of SDNN and the highest quartile of QTI; CAN2 as the lowest quartile of SDNN, the highest quartile of QTI and the highest quartile of heart rate; CAN3 as the lowest quartile of SDNN and the highest quartiles of QTI and heart rate in the presence of DPN.
Association between CAN and mortality
During a mean follow-up of 3.5 years, there were 329 deaths from all causes in this sample of 8,351 participants. In unadjusted analyses, there was a significant increase in all-cause mortality (HR 1.61 [1.14–2.27], P = 0.007 for CAN1, 2.22 [1.45–3.39], P = 0.0002 for CAN2, and 2.72 [1.56–4.74], P = 0.0004 for CAN3) (Fig. A2, available in an online appendix) and in CVD mortality (1.93 [1.22–3.07], P = 0.005 for CAN1, 2.55 [1.41–4.60, P = 0.002 for CAN2, and 3.39 [1.59–7.26], P = 0.0002 for CAN3) compared with those without CAN.
Table 2 shows fully adjusted HRs (95% CI) for all-cause and CVD mortality for participants with CAN1, CAN2, and CAN3 compared with participants without CAN. All-cause mortality remained significantly higher in participants with CAN after adjustment for treatment arm allocation, CVD history, and all other covariates listed in research design and methods (1.55 [1.09–2.21], P = 0.016 for CAN1, 2.14 [1.37–3.37], P = 0.0009 for CAN2, and 2.07 [1.14–3.76], P = 0.02 for CAN3). Similar results were observed for CVD mortality (1.94 [1.20–3.12], P = 0.007 for CAN1, 2.62 [1.4–4.91], P = 0.003 for CAN2, and 2.95 [1.33–6.53], P = 0.008 for CAN3) (Table 2).
Table 2.
HR (95% CI) for all-cause and CVD mortality in participants with CAN compared with participants without CAN
| Measure | All-cause mortality* |
CVD mortality* |
||
|---|---|---|---|---|
| HR (95% CI): CAN+/CAN− | P | HR (95% CI): CAN+/CAN− | P | |
| CAN1 | 1.55 (1.09–2.21) | 0.016 | 1.94 (1.20–3.12) | 0.007 |
| CAN2 | 2.14 (1.37–3.37) | 0.0009 | 2.62 (1.40–4.91) | 0.003 |
| CAN3 | 2.07 (1.14–3.76) | 0.02 | 2.95 (1.33–6.53) | 0.008 |
*Adjusted for treatment allocation, CVD history, and other prespecified covariates including baseline age, sex, ethnicity, diabetes duration, A1C, BMI, SBP and DBP, LDL cholesterol, triglycerides, microalbumin-to-creatinine ratio, and use of TZDs, insulin, β-blockers, ACE inhibitors/angiotensin-receptor blockers, statins, alcohol, and cigarettes.
Effects of glycemia treatment and CAN on mortality
After adjustment for covariates, the overall HR for all-cause mortality for INT versus STD arm in the 8,135 participants analyzed herein was 1.17(95% CI 0.94–1.46, P = 0.17). The HR for mortality in the INT versus STD arm for participants with missing ECG data (1.25 [0.88–1.78]) was not significantly different from that of participants included in this analysis (Pinteraction = 0.77), although participants with missing ECG data were more likely to die (1.72 vs. 1.15% per year for included participants) regardless of glycemia treatment group.
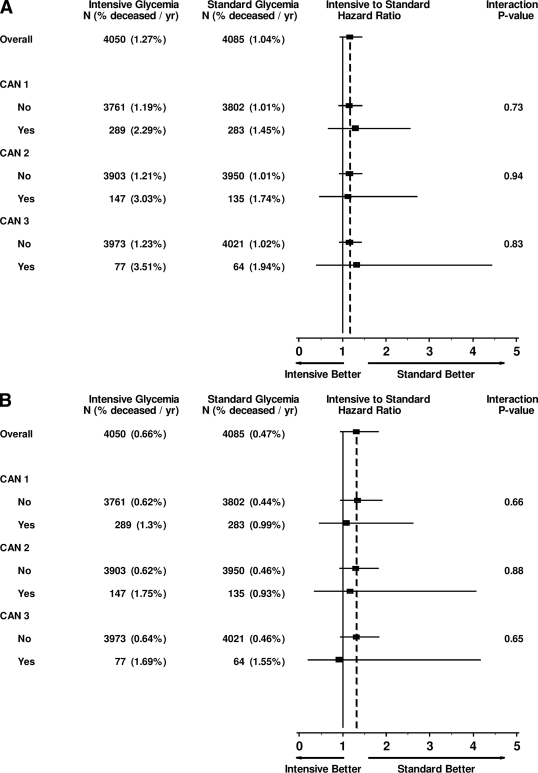
Figure 1A shows the event rate for all-cause mortality by treatment group, INT versus STD, as a function of CAN in the analyzed cohort, using the three prespecified CAN definitions. After adjustment for all covariates, there was no significant difference in all-cause mortality among any of the subgroups defined by the presence of CAN1, CAN2, and CAN3 (Pheterogeneity > 0.7 for all).
Figure 1.
A: Effects of CAN and glycemia intervention on all-cause mortality: HRs adjusted for treatment allocation and baseline age, sex, ethnicity, diabetes duration, A1C, BMI, SBP and DBP, LDL cholesterol, triglycerides, microalbumin-to-creatinine ratio, CVD history, and use of TZDs, insulin, β-blockers, ACE inhibitors/angiotensin receptor blockers, statins, alcohol, and cigarettes. B: Effects of CAN and glycemia intervention on CVD mortality: HRs adjusted for treatment allocation and baseline age, sex, ethnicity, diabetes duration, A1C, BMI, SBP and DBP, LDL cholesterol, triglycerides, microalbumin-to-creatinine ratio, CVD history, and use of TZDs, insulin, ACE inhibitors/angiotensin-receptor blockers, β-blockers, statins, alcohol, and cigarettes. CAN1 was defined as the lowest quartile of SDNN, and the highest quartile of QTI, CAN2 as the lowest quartile of SDNN, the highest quartile of QTI, and the highest quartile of heart rate, and CAN3 as the lowest quartile of SDNN and the highest quartiles of QTI and heart rate in the presence of DPN.
The overall HR for CVD mortality (INT versus STD) in the subgroup of individuals analyzed herein was 1.30 (95% CI 0.93–1.82). As with all-cause mortality, in fully adjusted analyses, the HR for the treatment difference in CVD mortality was not affected significantly by CAN status for any of the definitions examined (Fig. 1B). We have also adjusted for the presence of severe hypoglycemia during the trial in the individuals with baseline CAN. The lack of a significant effect of CAN on all-cause mortality in the INT arm compared with the STD arm persisted after controlling for the presence of severe hypoglycemia in the model (Pinteraction > 0.25 for all three definitions of CAN).
CONCLUSIONS
These data confirm in one of the largest and most carefully characterized cohorts of patients with type 2 diabetes that the presence of CAN strongly predicts all-cause and CVD mortality independently of baseline CVD, diabetes duration, and multiple other important CVD risk factors. However, the increased risk of either all-cause or CVD mortality in individuals with CAN compared with those without CAN at baseline was similar in the INT versus the STD glycemia treatment group. These findings add substantial epidemiological evidence regarding the prognostic importance of CAN.
CAN is frequently observed in patients with diabetes. Associations between measures of CAN and mortality, including sudden death, have been described previously (5,17,18). In a recent large meta-analysis, Maser et al. (5) reported that the presence of CAN was associated with a greater than threefold increase in mortality and sudden death. Silent ischemic heart disease or cardiac arrhythmias have both been invoked as contributors to sudden death. In the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study of 1,123 patients with type 2 diabetes, CAN was a strong predictor of silent ischemia and subsequent cardiovascular events (4).
Because CAN is associated with multiple factors including duration of diabetes, severity of hyperglycemia, and the presence of coronary artery disease, the exact contribution of CAN to the increased mortality risk has been difficult to quantify in prior studies. The present analysis in the ACCORD cohort provided a unique opportunity to evaluate and confirm the independent effect of CAN on all-cause and CVD mortality.
Study strengths were the large sample size of high-risk participants analyzed, the balanced sex randomization, diverse ethnic participation, high rate of follow-up, and rigorous CVD end point adjudication procedures. These data document that the presence of CAN strongly predicts all-cause and CVD mortality independently of multiple important CVD risk factors in this high-risk cohort with type 2 diabetes. These observations suggest that documenting CAN in individuals with type 2 diabetes identifies a subset at higher risk for CVD events and that CAN may explain at least in part the increased risk of CVD events observed in type 2 diabetes. Furthermore, these data indicate that CAN should be considered in models of CVD and mortality risk in type 2 diabetes cohorts.
Although clinical symptoms of autonomic dysfunction usually occur late in the course of diabetes, subclinical CAN, manifested as impaired HRV, may be detected within 1 year of diagnosis in type 2 diabetes and within 2 years of diagnosis in type 1 diabetes (15). Traditionally, the diagnosis of CAN involves a number of tests, including the R-R response to deep breathing, postural changes and during Valsalva maneuver, or 24-h ECG recordings, which may be cumbersome to perform uniformly, especially in large multicenter trials such as ACCORD. This study demonstrates that using a combination of HRV and QTI measurements derived from a standard 10-s ECG can identify subsets of patients at increased mortality risk independently of traditional CVD risk factors. HRV and QT abnormalities have different origins, as reflected by a weak correlation between the two parameters demonstrated by this study (data not shown) and by others (13). Decreased HRV is an early marker of cardiovascular parasympathetic dysfunction. The QT interval abnormalities have a different pathophysiological background, representing the consequences of sympathetic tone on cardiac depolarization and repolarization (13). The ACCORD findings in type 2 diabetes are in line with another recent report in patients with type 1 diabetes showing an increased mortality risk with combined abnormalities in HRV and QT interval (13). This finding has important implications because these measures obtained from a standard ECG can be used as a noninvasive and objective method for assessing CAN in other large trials as well as in clinical practice.
Despite the significant increase in the mortality risk in all subgroups of participants with CAN, we did not find that the presence of CAN at baseline contributed significantly to the increased mortality observed with the intensive versus standard treatment of glycemia in this cohort. This finding may have practical implications for diabetes care. Control of blood glucose is a cornerstone of diabetes management because more intensive glycemic control decreases the incidence and progression of diabetic microvascular (1,2) and, in some studies, macrovascular complications (19). The reported excess mortality in the intensive arm of the ACCORD trial (3) has led to controversy about implementation of intensive glucose control in patients with type 2 diabetes although two other major trials, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) and Veterans Affairs Diabetes Trial (VADT), reported no increase in mortality with intensive treatment compared with standard treatment in type 2 diabetes (20,21). Furthermore, the long-term follow-up of the UKPDS cohort reported risk reductions for myocardial infarction and death from any cause associated with intensive glucose control (22).
One of the most feared consequences of rigorous glycemic control is an increased incidence of hypoglycemia (1,2). Prior and more recent reports have shown strong associations between hypoglycemia and increased mortality (23,24). Hypoglycemia can impair hormonal and autonomic responses to subsequent hypoglycemia (8), and hypoglycemia may promote a reduced threshold for malignant arrhythmias and subsequent sudden cardiac death. Likewise, a recent study reported that exposure to hypoglycemia leads to impaired cardiovascular autonomic function in healthy volunteers (9). In the ACCORD trial, even though participants with severe hypoglycemia were at higher risk of death, this risk was not explained by CAN; after adjusting for the effects of postrandomization hypoglycemia, we could not document that CAN was an independent determinant of the higher mortality associated with intensive glycemia treatment. Therefore, our data imply that type 2 diabetic patients with CAN are not necessarily at increased risk with intensive versus standard glucose management.
There are several limitations to our analysis. HRV measures were limited by the short duration of the standard ECG recordings and by the absence of control for respiration. The prevalence of CAN may be higher in the excluded subset because of their older age, longer diabetes duration, and higher prevalence of CVD. Although the large sample of participants analyzed and their uniform characterization probably provides a reasonable estimate of CAN prevalence, it is possible that we underestimated the true effect of CAN on mortality in this cohort. There was limited statistical power to detect an interaction between subgroups defined by the presence or absence of CAN because the study was not designed for this purpose. There remains a theoretical possibility that CAN was worsened by intensive treatment during the trial and did account for the increased mortality with intensive treatment. We believe that this possibility is unlikely, considering that recent evidence showed a beneficial effect of intensive glucose treatment on CAN in type 1 diabetes (25).
Last, because the ACCORD trial was not designed to ascertain interactions between CAN and a rapid lowering of A1C or/and hypoglycemia and considering the post hoc nature of our analysis, we cannot draw more specific conclusions regarding the precise mechanisms involved by which CAN increases mortality. Incomplete understanding of the role of CAN in the pathogenesis of CVD and the precise mechanisms underlying its associations with mortality are areas deserving further research.
In summary, although CAN was associated with increased all-cause and CVD mortality in the ACCORD trial, these analyses indicate that among the subset with CAN, assignment to intensive compared with standard glycemia treatment was associated with similar mortality outcomes. Considering the vast array of variables that could have contributed to the increased mortality in the INT arm, it will be difficult and perhaps impossible to sort out the true determinants of this outcome. However, the presence of CAN, defined by simple, easily derived resting ECG measures, identified a subset of type 2 diabetic patients at higher all-cause and CVD mortality risk independent of multiple traditional CVD risk factors.
Supplementary Material
Acknowledgments
This work was supported by contracts and interagency agreements (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute; by other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, sanofi-aventis, and Schering-Plough.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
Clinical trials reg. no., NCT00000620, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 2. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 3. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE: Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301: 1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maser RE, Mitchell BD, Vinik AI, Freeman R: The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003; 26: 1895–1901 [DOI] [PubMed] [Google Scholar]
- 6. Cusick M, Meleth AD, Agrón E, Fisher MR, Reed GF, Knatterud GL, Barton FB, Davis MD, Ferris FL, 3rd, Chew EY: Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care 2005; 28: 617–625 [DOI] [PubMed] [Google Scholar]
- 7. Coppini DV, Bowtell PA, Weng C, Young PJ, Sönksen PH: Showing neuropathy is related to increased mortality in diabetic patients—a survival analysis using an accelerated failure time model. J Clin Epidemiol 2000; 53: 519–523 [DOI] [PubMed] [Google Scholar]
- 8. Cryer PE: Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab 2001; 281: E1115–E1121 [DOI] [PubMed] [Google Scholar]
- 9. Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R: Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009; 58: 360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD: Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99: 21i–33i [DOI] [PubMed] [Google Scholar]
- 11. Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME: The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care 2006; 29: 914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93: 1043–1065 [PubMed] [Google Scholar]
- 13. Lykke JA, Tarnow L, Parving HH, Hilsted J: A combined abnormality in heart rate variation and QT corrected interval is a strong predictor of cardiovascular death in type 1 diabetes. Scand J Clin Lab Invest 2008; 1–6 [DOI] [PubMed] [Google Scholar]
- 14. Ziegler D, Zentai CP, Perz S, Rathmann W, Haastert B, Döring A, Meisinger C: Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 2008; 31: 556–561 [DOI] [PubMed] [Google Scholar]
- 15. Pfeifer MA, Weinberg CR, Cook DL, Reenan A, Halter JB, Ensinck JW, Porte D, Jr: Autonomic neural dysfunction in recently diagnosed diabetic subjects Diabetes Care 1984; 7: 447–753 [DOI] [PubMed] [Google Scholar]
- 16. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA: A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281–1289 [DOI] [PubMed] [Google Scholar]
- 17. O'Brien IA, McFadden JP, Corrall RJ: The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med 1991; 79: 495–502 [PubMed] [Google Scholar]
- 18. Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD: Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care 2001; 24: 1793–1798 [DOI] [PubMed] [Google Scholar]
- 19. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD: Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [DOI] [PubMed] [Google Scholar]
- 21. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 22. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 23. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ: Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–1297 [DOI] [PubMed] [Google Scholar]
- 24. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R: Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354: 449–461 [DOI] [PubMed] [Google Scholar]
- 25. Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH: Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009; 119: 2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.