Abstract
OBJECTIVE
Although a clear link between diabetic peripheral neuropathy (DPN) and autonomic neuropathy is recognized, the relationship of autonomic neuropathy with subtypes of DPN is less clear. This study aimed to investigate the relationship of autonomic neuropathy with painless and painful DPN.
RESEARCH DESIGN AND METHODS
Eighty subjects (20 healthy volunteers, 20 with no DPN, 20 with painful DPN, 20 with painless DPN) underwent detailed neurophysiological investigations (including conventional autonomic function tests [AFTs]) and spectral analysis of short-term heart rate variability (HRV), which assesses sympathovagal modulation of the heart rate. Various frequency-domain (including low frequency [LF], high frequency [HF], and total power [TP]) and time-domain (standard deviation of all normal-to-normal R-R intervals [SDNN] and root mean square of successive differences [RMSSD]) parameters were assessed.
RESULTS
HRV analysis revealed significant differences across the groups in LF, HF, TP, SDNN, and RMSSD (ANOVA P < 0.001). Subgroup analysis showed that compared with painless DPN, painful DPN had significantly lower HF (3.59 ± 1.08 [means ± SD] vs. 2.67 ± 1.56), TP (5.73 ± 1.28 vs. 4.79 ± 1.51), and SDNN (2.91 ± 0.65 vs. 1.62 ± 3.5), P < 0.05. No significant differences were seen between painless DPN and painful DPN using an AFT.
CONCLUSIONS
This study shows that painful DPN is associated with significantly greater autonomic dysfunction than painless DPN. These changes are only detected using spectral analysis of HRV (a simple test based on a 5-min electrocardiogram recording), suggesting that it is a more sensitive tool to detect autonomic dysfunction, which is still under-detected in people with diabetes. The greater autonomic dysfunction seen in painful DPN may reflect more predominant small fiber involvement and adds to the growing evidence of its role in the pathophysiology of painful DPN.
Diabetic neuropathy is one of the most frequent complications of diabetes. The prevalence of some form of neuropathy has been reported to be as high as 66% in type 1 diabetes and 59% in type 2 diabetes (1). It is the source of great distress, disability, and premature death. It is the main initiating factor for foot ulceration and the most common cause of nontraumatic lower-limb amputation in the Western world (2). It is also one of the more poorly understood complications of diabetes.
Although a clear relationship between diabetic peripheral neuropathy (DPN) and cardiac autonomic neuropathy (CAN) has been recognized, the nature of the relationship of CAN with painless or painful neuropathy was less clear. Recently, there has been some evidence that at the level of the peripheral nerve, local autonomic dysfunction has an important role to play in the generation of pain (3). However, clinical studies looking to see if this translates into more generalized autonomic neuropathy have shown mixed and often opposite results (4,5). Part of the reason for this may be that all of these studies used conventional autonomic function tests (AFTs), which tend to detect autonomic dysfunction only at more advanced stages (6).
Over recent years, a number of different techniques have been developed that are more sensitive measures of autonomic function and are therefore able to detect subclinical abnormalities (7). One such technique is spectral analysis of heart rate variability (HRV). Short-term HRV analysis is relatively quick and simple to carry out because it is based on a 2- to 5-min resting electrocardiogram (ECG) recording. The recording is able to detect autonomic dysfunction in subjects in whom conventional AFTs are still normal (8).
The aim of this study was to determine if there are differences in autonomic function between painful and painless DPN using spectral analysis of HRV.
RESEARCH DESIGN AND METHODS
Subjects with type 1 diabetes were divided into three groups (no DPN, painless DPN, and painful DPN) with 20 subjects recruited in each group. In addition, 20 healthy volunteers were recruited. All subjects were between 18 and 70 years old. Subjects with nondiabetic neuropathies, a history of alcohol excess, and significant left ventricular dysfunction (≥ New York Heart Association Class III) or other cardiac problems that precluded HRV analysis were excluded. All subjects gave written, informed consent before participating in the study, which had prior approval by the South Sheffield Regional Ethics Committee.
Neuropathy assessment
Subjects underwent detailed neurophysiological assessment to determine the presence and severity of neuropathy. The presence of painful symptoms was established using the McGill pain questionnaire (9). Detailed neurological examination was graded by defined criteria using the standard Neuropathy Impairment Score (NIS) questionnaire (10). Sensory function was assessed by measuring vibration and cooling detection thresholds acquired from the dorsal aspect of the right foot using the Computer-Assisted Sensory Evaluation IV system (CASE IV) (W.R. Electronics, Stillwater, MN) and employing standard techniques (11,12). In addition, nerve conduction studies were carried out at a stable skin temperature of 31°C and a room temperature of 24°C using a Medelec electrophysiological system (Synergy Oxford Instruments, Oxford, U.K.). The following nerve attributes were measured: 1) sural sensory nerve action potentials and conduction velocities and 2) common peroneal and tibial motor nerve distal latency, compound muscle action potential, and conduction velocity. Subjects also underwent conventional cardiac AFT performed with a computer-assisted technique and evaluated using standard cardiovascular reflex tests based on O'Brien's criteria: heart rate responses (R-R variation) 1) at rest, 2) during deep breathing, 3) during Valsalva maneuver, and 4) on standing (13).
Based on these clinical and neurophysiological assessments, diabetic subjects were divided into three groups: 1) no DPN, consisting of asymptomatic subjects with normal clinical and neurophysiological assessments; 2) painless DPN, comprising subjects with both clinical and neurophysiological abnormalities (at least two abnormalities of neurophysiologic assessment) but no painful symptoms; and 3) painful DPN, with similar clinical and neurophysiological abnormalities and painful symptoms for at least 6 months. A Neuropathy Composite Score (NCS) derived from the assessments described above (NIS of the lower limbs plus seven tests of nerve function) was calculated, a full description of which has been published previously (14,15). This scoring system takes into account the neurological examination and neurophysiological assessments: the higher the NCS, the more severe the neuropathy.
Spectral analysis of HRV
HRV describes the timing variation between consecutive heartbeats (measured using the R-R interval on an ECG). The regulation of HRV originates from both sympathetic and parasympathetic nervous systems, and thus HRV can be used as a quantitative marker of autonomic function (16,17). Spectral analysis of HRV provides the basic information of how power of R-R variation distributes as a function of frequency. Three main components are distinguished in a spectrum calculated from an R-R time series derived from short-term ECG recordings: very low frequency (VLF), low frequency (LF), and high frequency (HF) components. In addition, various time-domain measures can be calculated based on the intervals between successive normal complexes, the so-called normal-to-normal (NN) intervals. The most common measures used include the standard deviation of the NN interval (SDNN) and the root mean square of successive differences of the NN interval (RMSSD) (16,18).
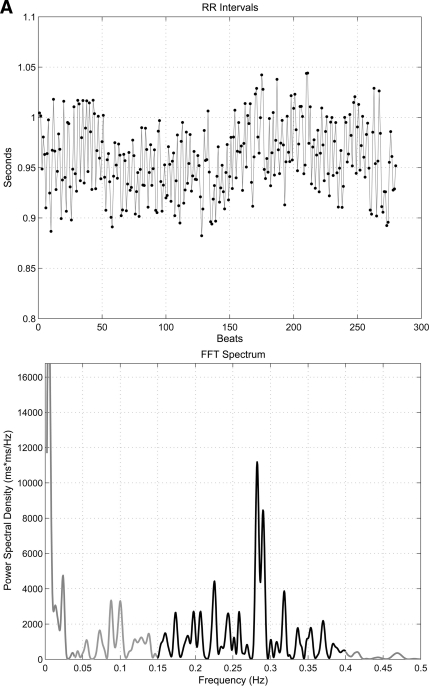
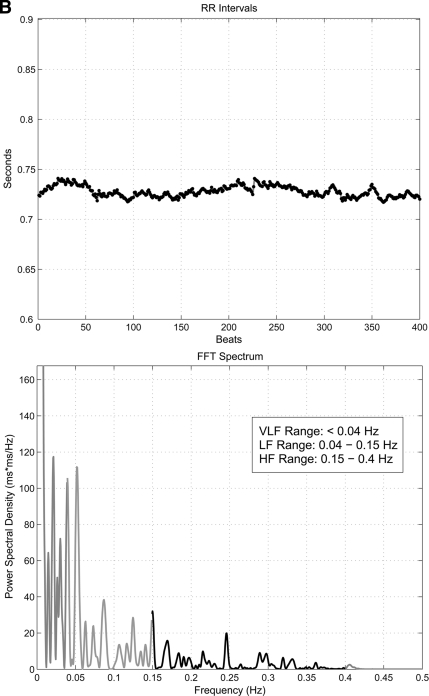
HRV analysis was based on the recording of 5 min of ECG with the patient resting in a supine position after a period of adaptation. Subjects were advised to refrain from any caffeine or alcohol for 12 h prior to the examination, and any subjects with hypoglycemia in the preceding 24 h had their examination postponed. All examinations took place in the morning. The equipment required consisted of a standard ECG amplifier, a device to convert the analog ECG signals into a digital format, and a laptop computer to process and analyze the ECG using in-house developed software for HRV analysis (19). The software and digital signal processing algorithms followed established guidelines for HRV analysis (16). Frequency-domain parameters of HRV analyzed were power in the VLF range (<0.04 Hz), LF range (0.04–0.15 Hz), HF range (0.15–0.4 Hz), LF-to-HF ratio, and total power (TP) of the spectrum (0.003–0.4 Hz). Time-domain parameters of HRV analyzed were SDNN and RMSSD. Figure 1 shows examples of the frequency-domain analysis in a diabetic subject with no CAN and one with severe CAN.
Figure 1.
Examples of spectral analysis of HRV showing the R-R variability tracing (top panel) and frequency domain plot in a diabetic subject with no CAN (A) and severe CAN (B) (note the virtual abolition of peak within the LF and HF range in severe CAN). FFT, fast Fourier transformation.
Statistical analysis
All analyses were performed using the statistical package SPSS 14. All values are described as means ± SD for continuous data and as percentages for categorical data. HRV parameters were logarithmically transformed to adjust for skewness. One-way ANOVA was used to compare baseline characteristics between groups, and least significant difference post hoc testing was used to compare differences between groups. As age is recognized to be a significant influence on HRV, one-way ANCOVA, with age as a covariate, was used to assess the significance of any differences between the groups in the AFT results. Pearson's correlation was used to study the linear relationship between HRV measurements and NCS.
RESULTS
Table 1 shows the baseline characteristics of the groups. As would be expected, subjects with no DPN had better glycemic control, had better systolic blood pressure, had fewer other complications of diabetes, and were on less drug therapy than both DPN groups. Importantly, there was no significant difference in the mean age of the groups, which is recognized as a major confounder of HRV. Subgroup analysis showed that there were no significant differences between painful and painless DPN. In particular, there was no difference between them in terms of the severity of neuropathy as assessed by NCS. In addition, apart from tibial motor nerve distal latency, which was significantly higher in painless DPN (6.4 ± 1.4 vs. 4.9 ± 2.2 m/s, P = 0.008), no significant differences were detected in individual neurophysiological parameters between painless and painful DPN.
Table 1.
Baseline characteristics of study subjects
| Healthy volunteers | No DPN | Painless DPN | Painful DPN | P (ANOVA) | |
|---|---|---|---|---|---|
| n | 20 | 20 | 20 | 20 | |
| Age (years) | 46.6 ± 14.7 | 45.7 ± 10 | 52 ± 9.7 | 52.1 ± 11.1 | NS |
| BMI | 27.9 ± 9.2 | 28 ± 8.3 | 28.9 ± 4.5 | 29.1 ± 6.8 | NS |
| Duration of diabetes (years) | — | 23.3 ± 13 | 27.1 ± 11.4 | 20.3 ± 9.4 | NS |
| A1C (%) | — | 7.7 ± 1.3 | 8.8 ± 1.5 | 8.6 ± 1.5 | 0.042 |
| Total cholesterol (mmol/l) | 4.7 ± 0.6 | 4.5 ± 0.7 | 4.5 ± 0.9 | 4.1 ± 0.8 | NS |
| Systolic blood pressure (mmHg) | 131 ± 21 | 135 ± 14 | 151 ± 20 | 141 ± 19 | 0.007 |
| Diastolic blood pressure (mmHg) | 80 ± 7 | 77 ± 9 | 79 ± 4 | 77 ± 8 | NS |
| Retinopathy (%) | — | 35 | 90 | 75 | 0.001 |
| Albuminuria (%) | — | 10 | 44 | 47 | 0.02 |
| Severity of neuropathy (Dyck's score) | 0.8 ± 0.8 | 1.3 ± 1.1 | 18.5 ± 7.9 | 21.8 ± 10.2 | <0.001 |
| Cardiovascular disease (%) | 5 | 5 | 10 | 20 | NS |
| Hypertension (%) | 20 | 30 | 85 | 70 | <0.001 |
| β-Blocker (%) | 5 | 0 | 16 | 20 | NS |
| ACE/angiotensin receptor blocker (%) | 0 | 27 | 67 | 55 | <0.001 |
| Statin (%) | 10 | 50 | 75 | 85 | <0.001 |
Data are means ± SD, unless otherwise indicated. NS, not significant.
Table 2 shows the results of conventional AFT and HRV analysis in the different groups. On conventional autonomic function testing, a significant difference was seen across the groups for all R-R variability results. Post hoc subgroup analysis, however, showed no significant differences could be detected between painless and painful DPN. Not surprisingly, the majority of significant differences were between healthy volunteers or no DPN with the two DPN groups.
Table 2.
Autonomic function test results using conventional methods and HRV analysis
| Healthy volunteers | No DN | Painless DPN | Painful DPN | P (ANOVA) | |
|---|---|---|---|---|---|
| n | 20 | 20 | 20 | 20 | |
| Conventional autonomic function tests | |||||
| Resting heart rate (bpm) | 65.4 ± 14.9 | 68.6 ± 13.6 | 67.3 ± 8.8 | 70.3 ± 8.6 | NS |
| R-R variation (rest) | 1.21 ± 0.09 | 1.21 ± 0.09 | 1.14 ± 0.08 | 1.10 ± 0.05 | <0.001 |
| R-R variation (deep breathing) | 1.47 ± 0.27 | 1.38 ± 0.26 | 1.24 ± 0.16 | 1.16 ± 0.14 | <0.001 |
| R-R variation (Valsalva) | 1.49 ± 0.24 | 1.71 ± 0.32 | 1.37 ± 0.16 | 1.37 ± 0.14 | <0.001 |
| R-R variation (standing) | 1.46 ± 0.25 | 1.38 ± 0.22 | 1.27 ± 0.25 | 1.21 ± 0.19 | 0.02 |
| HRV frequency domain analysis (log-transformed values) | |||||
| VLF | 4.23 ± 3.35 | 3.55 ± 3.6 | 2.60 ± 3.82 | 1.62 ± 3.5 | NS |
| LF | 5.59 ± 1.14 | 5.23 ± 1.32 | 4.12 ± 1.67 | 3.31 ± 1.49 | <0.001 |
| HF | 5.45 ± 1.29 | 4.41 ± 1.57 | 3.59 ± 1.08 | 2.67 ± 1.56* | <0.001 |
| LF/HF | 1.06 ± 0.28 | 1.26 ± 0.39 | 1.19 ± 0.48 | 1.57 ± 0.97 | NS |
| TP | 7.11 ± 0.93 | 6.55 ± 1.14 | 5.73 ± 1.28 | 4.79 ± 1.51* | <0.001 |
| HRV time-domain analysis (log-transformed values) | |||||
| SDNN | 3.52 ± 0.43 | 3.31 ± 0.55 | 2.91 ± 0.65 | 1.62 ± 3.5* | <0.001 |
| RMSSD | 3.39 ± 0.54 | 2.99 ± 0.72 | 2.58 ± 0.69 | 2.16 ± 0.71 | <0.001 |
Data are means ± SD.
*P < 0.05 compared with painless DPN group. NS, not significant.
HRV analysis revealed significant differences across the groups for various parameters using both time- and frequency-domain analysis (Table 2). Post hoc subgroup analysis showed the group with painful DPN had significantly lower values compared with painless DPN for HF (means ± SD, 2.67 ± 1.56 vs. 3.59 ± 1.08, P = 0.04), TP (4.79 ± 1.51 vs. 3.59 ± 1.08, P = 0.02), and SDNN (1.62 ± 3.5 vs. 2.91 ± 0.65, P = 0.04). In addition, there were trends toward lower values in painful DPN compared with painless DPN for LF (3.31 ± 1.49 vs. 4.12 ± 1.67, P = 0.07) and RMSSD (2.16 ± 0.71 vs. 2.58 ± 0.69, P = 0.06). Subjects with no DPN also had significantly lower HF values compared with healthy volunteers (4.41 ± 1.57 vs. 5.45 ± 1.29, P = 0.02).
A significant negative correlation was noted between NCS and LF across all the groups (r = −0.64), HF (r = −0.55), TP (r = −0.68), SDNN (r = −0.64), and RMSSD (r = −0.54), P < 0.001.
CONCLUSIONS
This study shows that subjects with painful DPN have significantly greater autonomic dysfunction than subjects with painless DPN in a carefully matched and well-characterized population. These differences are only detected using spectral analysis of HRV. No significant differences were detected using conventional AFT. To our knowledge, HRV analysis has not previously been used to assess differences in autonomic function in subtypes of DPN.
The detection of greater autonomic dysfunction in painful DPN compared with painless DPN has biological plausibility. Both pain sensation and autonomic function are mediated by small poorly myelinated and unmyelinated fibers (whereas the larger fibers tend to transmit sensations such as vibration and touch) and are therefore more likely to be vulnerable to the pathological processes that occur in diabetic neuropathy. Sorensen et al. (20) demonstrated that painful DPN is associated with abnormalities in these small fibers when they showed it was associated with lower intra-epidermal nerve fiber density. In addition, there is a variety of evidence that local autonomic dysfunction is leading to changes in epineural blood flow and may have an important role to play in pain generation. We have previously shown that patients with acute painful neuropathy, so called “insulin neuritis,” have abnormal epineural vessel anatomy and increased arterio-venous shunting (21). More recently, Eaton et al. (22) demonstrated increases in sural nerve epineurial blood flow in painful DPN. Quattrini et al. (3) subsequently showed impairment of acetylcholine-induced foot skin vasodilator response in painful DPN. What is less clear is whether this local autonomic dysfunction is a reflection of a more generalized autonomic neuropathy given that previous studies have been inconsistent. Young et al. (4) showed that subjects with painful DPN had a higher ratio of autonomic to electrophysiological abnormality compared with painless DPN. In contrast, Veves et al. (5) showed no difference in autonomic function between painful and painless DPN.
One of the reasons why previous studies looking at generalized autonomic function in painful DPN may have shown mixed results is that they have generally used conventional tests of autonomic function (cardiovascular reflex tests), which only pick up abnormalities in the more advanced stages. Current recommendations for the diagnosis of CAN are based on a consensus statement from the San Antonio Conference on Diabetic Neuropathy in 1988 and involve a battery of detailed cardiovascular reflex tests (23). They recommend three tests of heart rate control, which were heart rate response to 1) deep breathing, 2) standing, and 3) the Valsalva maneuver. Two tests of blood pressure control were also recommended by testing blood pressure response to standing/passive tilting and sustained handgrip. The majority of these tests require specialist equipment and training and are time-consuming to perform. An additional drawback is that they require active subject participation and compliance.
Spectral analysis of HRV, in contrast, has several advantages over conventional techniques. In addition to being a more sensitive measure of autonomic dysfunction, it 1) is easy to perform with limited specialist training, 2) does not require expensive and cumbersome equipment, 3) is very quick to carry out, and 4) is not affected by subject variability.
Although the results of this study are consistent with the hypothesis of autonomic dysfunction being involved in the pathophysiology of painful DPN, the cross-sectional design means that whether it has a direct role or is simply a para-phenomenon is not yet clear. It seems unlikely that this association is a reflection of the severity of neuropathy given that there were no significant differences between painful and painless DPN in either NCS or the majority of individual parameters of nerve function. In addition, as there were no subjects in the painful DPN group without objective neuropathic impairment (i.e., those most likely to have isolated small fiber involvement), no conclusions can be drawn about differences in autonomic dysfunction in this subgroup. Perhaps, however, the most important message of this study for clinical practice is that the presence of painful symptoms should increase the physician's vigilance for the presence of autonomic neuropathy, which still often goes undetected.
One possible confounder to these results is the greater use of drugs that may interfere with HRV in the DPN groups. Pharmacological agents such as β-blockers, ACE inhibitors, and calcium channel blockers generally result in increases in HRV (24). Therefore, if anything, their usage would lead to an underestimation of the differences seen between the groups. This is also true of drugs such as gabapentin and selective serotonin re-uptake inhibitors; tricyclic antidepressants on the other hand can result in lower HRV (25). Although five subjects in the painful DPN group were on a tricyclic antidepressant, removing them from the analysis did not substantially alter the results. None of the subjects were on any other sympathomimetics.
This study also demonstrated subjects with no evidence of DPN had lower HF compared with healthy volunteers. No differences were detected between these two groups using the conventional tests. HF is thought to be a marker of parasympathetic function, and these findings suggest that it can be detected early before any manifestation of symptoms and when conventional tests are still normal. It also suggests that parasympathetic autonomic dysfunction is the first abnormality to arise in the development of CAN. This is consistent with previous findings (8).
One of the unresolved mysteries in regard to the pathogenesis of diabetic complications remains the puzzle of why some people develop severe chronic pain while others have no pain or symptoms. The assumption has been that DPN is not a unitary condition but develops as a result of different disturbances within the peripheral nervous system. The paradox is that many studies have failed to detect consistent differences between painful and painless DPN at the level of the peripheral nerve (5). This was once again demonstrated in this study, where no major differences were detected between painful and painless DPN on detailed neurophysiological testing. This study has clearly demonstrated that spectral analysis of HRV, based only on a 5-min ECG recording at rest, is a highly sensitive marker of autonomic dysfunction. It has detected differences in autonomic dysfunction between painful and painless DPN, which cannot be detected by cruder conventional AFTs. Whether this greater autonomic dysfunction has a direct role to play in the generation or persistence of pain in DPN now needs to be the subject of larger prospective studies.
Acknowledgments
This study was funded in part by a generous grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O'Brien PC, Melton LJ, 3rd, Service FJ: The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817–824 [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Kirsner RS, Vileikyte L: Clinical practice: neuropathic diabetic foot ulcers. N Engl J Med 2004; 351: 48–55 [DOI] [PubMed] [Google Scholar]
- 3. Quattrini C, Harris ND, Malik RA, Tesfaye S: Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care 2007; 30: 655–659 [DOI] [PubMed] [Google Scholar]
- 4. Young RJ, Zhou YQ, Rodriguez E, Prescott RJ, Ewing DJ, Clarke BF: Variable relationship between peripheral somatic and autonomic neuropathy in patients with different syndromes of diabetic polyneuropathy. Diabetes 1986; 35: 192–197 [DOI] [PubMed] [Google Scholar]
- 5. Veves A, Young MJ, Manes C, Boulton AJ: Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy: a clinical study. Diabetes Care 1994; 17: 1200–1202 [DOI] [PubMed] [Google Scholar]
- 6. Vinik AI, Ziegler D: Diabetic cardiovascular autonomic neuropathy. Circulation 2007; 115: 387–397 [DOI] [PubMed] [Google Scholar]
- 7. Freeman R: Autonomic peripheral neuropathy. Lancet 2005; 365: 1259–1270 [DOI] [PubMed] [Google Scholar]
- 8. Weston PJ, James MA, Panerai RB, McNally PG, Potter JF, Thurston H: Evidence of defective cardiovascular regulation in insulin-dependent diabetic patients without clinical autonomic dysfunction. Diabetes Res Clin Pract 1998; 42: 141–148 [DOI] [PubMed] [Google Scholar]
- 9. Melzack R: The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975; 1: 277–299 [DOI] [PubMed] [Google Scholar]
- 10. Grant I, O'Brien P, Dyck P: Neuropathy tests and normative results. In Diabetic Neuropathy. 2nd ed. Philadelphia, PA, W.B. Saunders, 1999, p. 123–141 [Google Scholar]
- 11. Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL: A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology 1993; 43: 1508–1512 [DOI] [PubMed] [Google Scholar]
- 12. Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O'Brien PC: Cool, warm, and heat-pain detection thresholds: testing methods and inferences about anatomic distribution of receptors. Neurology 1993; 43: 1500–1508 [DOI] [PubMed] [Google Scholar]
- 13. Pfeifer M: Cardiovascular assessment. In Diabetic Neuropathy. 2nd ed. Philadelphia, PA, W.B. Saunders, 1999, p. 171–183 [Google Scholar]
- 14. Dyck PJ, Davies JL, Litchy WJ, O'Brien PC: Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 1997; 49: 229–239 [DOI] [PubMed] [Google Scholar]
- 15. Dyck PJ, Litchy WJ, Daube JR, Harper CM, Davies J, O'Brien PC: Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve 2003; 27: 202–210 [DOI] [PubMed] [Google Scholar]
- 16. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043–1065 [PubMed] [Google Scholar]
- 17. Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A: Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90: 1826–1831 [DOI] [PubMed] [Google Scholar]
- 18. Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA: Software for advanced HRV analysis. Comput Methods Programs Biomed 2004; 76: 73–81 [DOI] [PubMed] [Google Scholar]
- 19. Petry D, Palodeto V, Suzuki DOH, Marques JLB: System for ECG signals variability analysis: heart rate variability and QT interval variability. IFMBE Proceedings 2007; 14: 1160–1163 [Google Scholar]
- 20. Sorensen L, Molyneaux L, Yue DK: The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care 2006; 29: 883–887 [DOI] [PubMed] [Google Scholar]
- 21. Tesfaye S, Malik R, Harris N, Jakubowski JJ, Mody C, Rennie IG, Ward JD: Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis). Diabetologia 1996; 39: 329–335 [DOI] [PubMed] [Google Scholar]
- 22. Eaton SE, Harris ND, Ibrahim S, Patel KA, Selmi F, Radatz M, Ward JD, Tesfaye S: Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia 2003; 46: 934–939 [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association and American Academy of Neurology. Report and recommendations of the San Antonio conference on diabetic neuropathy. Consensus statement. Diabetes 1988; 37: 1000–1004 [DOI] [PubMed] [Google Scholar]
- 24. Nolan RP, Jong P, Barry-Bianchi SM, Tanaka TH, Floras JS: Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: a systematic review. Eur J Cardiovasc Prev Rehabil 2008; 15: 386–396 [DOI] [PubMed] [Google Scholar]
- 25. Gorman JM, Sloan RP: Heart rate variability in depressive and anxiety disorders. Am Heart J 2000; 140: 77–83 [DOI] [PubMed] [Google Scholar]