Abstract
OBJECTIVE
We assessed the association between A1C and cardiovascular diseases (CVDs) in an observational study of patients with type 1 diabetes followed for 5 years.
RESEARCH DESIGN AND METHODS
A total of 7,454 patients were studied from the Swedish National Diabetes Register (aged 20–65 years, diabetes duration 1–35 years, followed from 2002 to 2007).
RESULTS
Hazard ratios (HRs) for fatal/nonfatal coronary heart disease (CHD) per 1% unit increase in baseline or updated mean A1C at Cox regression analysis were 1.31 and 1.34 and 1.26 and 1.32, respectively, for fatal/nonfatal CVD (all P < 0.001 after adjustment for age, sex, diabetes duration, blood pressure, total and LDL cholesterol, triglycerides, BMI, smoking, and history of CVD). HRs were only slightly lower for CHD (P = 0.002) and CVD (P = 0.002–0.007) after also adjusting for albuminuria. Adjusted 5-year event rates of CHD and CVD increased progressively with higher A1C, ranging from 5 to 12%, as well as when subgrouped by shorter (1–20 years) or longer (21–35 years) duration of diabetes. A group of 4,186 patients with A1C 5–7.9% (mean 7.2) at baseline showed risk reductions of 41% (95% confidence intervals: 15–60) (P = 0.005) for fatal/nonfatal CHD and 37% (12–55) (P = 0.008) for CVD, compared with 3,268 patients with A1C 8–11.9% (mean 9.0), fully adjusted also for albuminuria.
CONCLUSIONS
This observational study of patients in modern everyday clinical practice demonstrates progressively increasing risks for CHD and CVD with higher A1C, independently of traditional risk factors, with no J-shaped risk curves. A baseline mean A1C of 7.2% showed considerably reduced risks of CHD and CVD compared with A1C 9.0%, emphasizing A1C as a strong independent risk factor in type 1 diabetes.
Patients with type 1 diabetes have long been considered to have increased risks of cardiovascular disease (CVD) and mortality (1,2), and this has recently been confirmed in two studies (3,4) from the General Practice Research Database in the U.K. Based on data from 1992 to 1999, risks of CVD and mortality were four to eight times higher in men and women with type 1 diabetes than nondiabetic individuals (3,4).
While the association between glycemia and microvascular complications is established (5,6), there have been no long-term randomized clinical studies satisfactorily examining the relationship with macrovascular complications in type 1 diabetes, and epidemiological studies have shown conflicting results (7–14). The Epidemiology of Diabetes Interventions and Complications (EDIC) Study showed that patients who had previously been subjected to intensive glucose control during the Diabetes Control and Complications Trial (DCCT) had a considerably lower risk of CVD than patients receiving standard treatment (1983–1993) (7). A small study from Finland on late-onset type 1 diabetic patients without albuminuria showed increased risk of coronary heart disease (CHD) with poor glycemic control (9), but the EURODIAB Prospective Complications Study (PCS), the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, and the Wisconsin Epidemiologic Study of Diabetic Retinopathy did not demonstrate a significant relationship between glycemia and CHD after controlling for other cardiovascular risk factors (10–13). However, a recent study (14) from the Pittsburgh EDC showed that change in A1C was related to coronary artery disease, whereas baseline A1C was not.
With this background, we assessed the association between A1C and CHD, stroke, and CVD in a large cohort of patients with type 1 diabetes, aged 20–65 years, treated in everyday clinical practice from 2002 to 2007. Data were used from the Swedish National Diabetes register (NDR), a quality-assurance tool in diabetes care with nationwide coverage with recently published reports regarding type 1 and type 2 diabetes (15–17).
RESEARCH DESIGN AND METHODS
The NDR was initiated in 1996, and annual reporting is carried out by trained physicians and nurses via the Internet or via transfer from clinical records databases, with information collected during patient visits at hospital outpatient clinics and primary-care clinics nationwide. All included patients have agreed by informed consent to be registered before inclusion, and all information is subsequently stored in a central database.
This observational study, approved by the regional ethics review board at the University of Gothenburg, consists of 7,454 female and male patients with type 1 diabetes, with an age range of 20–65 years, diabetes duration of 1–35 years, and data available in the NDR for all analyzed variables. Patients with a history of CVD were not excluded. Baseline clinical characteristics were estimated in 2002–2003, and the patients were followed for a maximum of 5 years, until 2007. The epidemiological definition of type 1 diabetes used here was treatment with insulin only and onset age of diabetes ≤30 years. Almost all participants in this study (97%) were treated in hospital clinics. An assessment of type of diabetes performed voluntarily by the reporting clinics, available in 75% of all participants, showed that 97% had type 1 diabetes.
All participants were divided at baseline into two groups. One group included 4,186 patients and was defined to have A1C within the interval 5.0–7.9% at baseline of the study. The other group of 3,268 patients had A1C within the interval 8.0–11.9%. Both groups were followed-up for CVDs during 5 years.
Examinations at baseline
Clinical characteristics evaluated at baseline were sex, age (years), diabetes duration (years), A1C (%), weight (kg), height (m), systolic blood pressure (mmHg), total cholesterol (mmol/l), LDL cholesterol (mmol/l), triglycerides (mmol/l), smoking, microalbuminuria (μg/min), and macroalbuminuria (μg/min). BMI (kg/m2) was calculated as weight in kilograms divided by the square of height meters. The Swedish standard for blood pressure recording, used in the NDR, was the mean value of two supine readings (Korotkoff 1–5) with a cuff of appropriate size after at least 5 min of rest. A smoker was defined as a patient smoking one or more cigarettes per day, or smoking tobacco using a pipe, or who had stopped smoking within the past 3 months.
Laboratory analyses of A1C, blood lipids, and albuminuria values were carried out at local laboratories. A1C analyses are quality assured nationwide by regular calibration with the high-performance liquid chromatography Mono-S method. In this study, all A1C values were converted to the DCCT standard values using the following formula: A1C (DCCT) = 0.923 × A1C (Mono-S) + 1.345 (R2 = 0.998) (18). A1C was measured at baseline. A1C was also measured over time as an updated mean of annual measurements, calculated for each individual from baseline to each year of follow-up, with the last observation carried forward for missing data. In case of an event during follow-up, the period for estimating updated mean A1C was from baseline to the year before this event occurred. Otherwise, this period was from baseline to the censor year.
LDL cholesterol values were calculated using the Friedewald formula (LDL cholesterol = total cholesterol − HDL cholesterol − [0.45 × triglycerides]), if triglycerides were <4.0 mmol/l (19). Albuminuria was defined as micro- or macroalbuminuria. Microalbuminuria was defined as urine albumin excretion 20–200 μg/min in two of three consecutive tests and macroalbuminuria as a urine albumin excretion of >200 μg/min.
Follow-up and definition of end points
All patients were followed from baseline until a cardiovascular event or death or otherwise until censor date 31 December 2007. Mean follow-up was 4.95 years. The following end points were used: fatal/nonfatal CHD, fatal/nonfatal stroke, fatal/nonfatal CVD and total mortality. Fatal CHD was defined as ICD-10 codes I20–I25 (available at http://www.who.int/classifications/icd/en/). A nonfatal CHD event was defined as nonfatal myocardial infarction (ICD-10 code I21), unstable angina (ICD-10 code I20.0), percutaneous coronary intervention, and/or coronary artery bypass graft. Fatal/nonfatal stroke was defined as fatal or nonfatal intracerebral hemorrhage, cerebral infarction, or unspecified stroke (ICD-10 codes I61, I63, I64, and I67.9). Fatal/nonfatal CVD was fatal/nonfatal CHD or stroke, whichever came first.
All end point events were retrieved by data linkage with the Swedish Cause of Death and Hospital Discharge Registers (National Board of Health and Welfare, Sweden), which is a reliable validated alternative to revised hospital discharge records and death certificates (20,21).
Statistical methods
Mean values with 1 SD and SE and frequencies (%) with SE of baseline characteristics, and numbers and incidence rates (events per 1,000 person-years) of outcomes, are given in Table 1. Significance of differences was estimated with Student t test for mean values, χ2 test for frequencies, and log-rank test at survival analysis for outcome incidences.
Table 1.
Baseline clinical characteristics in all 7,454 patients with type 1 diabetes, aged 20–65 years, and outcomes when followed-up for 5 years
| All patients | Group at baseline | Group at baseline | P value | |
|---|---|---|---|---|
| A1C 5.0–7.9% | A1C 8.0–11.9% | |||
| n | 7,454 | 4,186 | 3,268 | |
| A1C (%) | 8.0 (1.2–0.01) | 7.2 (0.6–0.01) | 9.0 (0.8–0.01) | <0.001 |
| Age (years) | 36.9 (10.0–0.12) | 36.4 (9.8–0.15) | 37.4 (10.2–0.18) | <0.001 |
| Diabetes duration (years) | 19.9 (9.1–0.11) | 19.1 (9.3–0.14) | 20.9 (8.9–0.15) | <0.001 |
| Systolic blood pressure (mmHg) | 125.3 (14.9–0.17) | 124.2 (14.3–0.22) | 126.7 (15.5–0.27) | <0.001 |
| Total cholesterol(mmol/l) | 4.82 (0.9–0.01) | 4.72 (0.9–0.01) | 4.95 (0.9–0.02) | <0.001 |
| LDL cholesterol (mmol/l) | 2.75 (0.8–0.01) | 2.67 (0.8–0.01) | 2.85 (0.8–0.01) | <0.001 |
| Triglycerides (mmol/l) | 1.09 (0.6–0.01) | 1.0 (0.5–0.01) | 1.21 (0.6–0.01) | <0.001 |
| BMI (kg/m2) | 25.3 (3.7–0.04) | 25.1 (3.5–0.06) | 25.5 (3.8–0.07) | <0.001 |
| Male sex | 55.8 (0.58) | 55.9 (0.77) | 55.6 (0.87) | n.s. |
| Smoker | 13.5 (0.40) | 10.0 (0.46) | 18.0 (0.67) | <0.001 |
| Albuminuria | 19.6 (0.46) | 14.1 (0.54) | 26.7 (0.7) | <0.001 |
| Microalbuminuria | 12.2 (0.38) | 9.3 (0.45) | 15.9 (0.64) | <0.001 |
| Macroalbuminuria | 7.4 (0.30) | 4.7 (0.33) | 10.8 (0.54) | <0.001 |
| History of CVD | 2.6 (0.18) | 2.0 (0.22) | 3.2 (0.31) | <0.01 |
| Antihypertensive drugs | 24.4 (0.50) | 20.2 (0.62) | 29.8 (0.8) | <0.001 |
| Lipid-lowering drugs | 16.6 (0.43) | 12.4 (0.51) | 21.8 (0.72) | <0.001 |
| Acetylsalicylic acid | 6.8 (0.29) | 5.7 (0.36) | 8.0 (0.47) | <0.001 |
| Outcomes [n (events per 1,000 person years)] | ||||
| Fatal CVD | 36 | 17 | 19 | |
| Fatal CHD | 34 | 17 | 17 | |
| Fatal stroke | 4 | 0 | 4 | |
| Non-fatal CVD | 118 | 38 | 80 | |
| Non-fatal CHD | 97 | 28 | 69 | |
| Non-fatal stroke | 33 | 14 | 19 | |
| Fatal/non-fatal CHD | 131 (4.0) | 45 (2.4) | 86 (6.0) | <0.001 |
| Fatal/nonfatal stroke | 37 (1.1) | 14 (0.7) | 23 (1.6) | <0.05 |
| Fatal/nonfatal CVD | 154 (4.7) | 55 (3.0) | 99 (6.9) | <0.001 |
| Total mortality | 94 (2.8) | 50 (2.7) | 44 (3.0) | n.s. |
| Non-CVD mortality | 58 | 33 | 25 |
Data for baseline characteristics are means with standard deviation and standard error (SD-SE) or percent frequencies with standard error (SE). Data for outcomes are numbers, with crude events per 1000 person years in parenthesis. Two groups at baseline are also shown, defined to have an A1C within a lower or a higher interval at baseline of the study. Albuminuria: microalbuminuria (urine albumin excretion 20–200 μg/min) or macroalbuminuria (>200 μg/min).
Cox regression analysis was used to estimate hazard ratios (HRs) with 95% CIs for A1C as a continuous variable or for a lower A1C interval versus a higher interval and the outcomes (Table 2) adjusted for covariates as given in the table. The updated mean A1C value was treated as a strictly time-dependent variable in the Cox regression analysis to evaluate glycemic exposure during follow-up, allowing the use of the most recent value of updated mean A1C at each specific point of time in the modeling process. In case of an event during follow-up, the period for estimating updated mean A1C was from baseline to the year before this event occurred. Otherwise, this period was from baseline to the censor year. The proportional hazards assumption was confirmed for all covariates with the Kolmogorov-type supremum test using resampling and with the test of all time-dependent covariates simultaneously. Maximum likelihood estimation was used to evaluate interaction between A1C and all covariates, with no significant interaction found.
Table 2.
HRs for CVDs or total mortality and baseline or updated mean A1C in 7,454 patients with type 1 diabetes followed for 5 years
| Patients n/events n/event rate (%)* | Baseline A1C as predictor |
Updated mean A1C as predictor |
|||
|---|---|---|---|---|---|
| HR (95% CI)† | HR (95% CI)‡ | HR (95% CI)† | HR (95% CI)‡ | ||
| A1C per 1% unit increase | |||||
| Fatal/nonfatal CHD | |||||
| All patients | 7,454/131/2.0 | 1.31 (1.12–1.52)§ | 1.28 (1.09–1.49)‖ | 1.34 (1.14–1.58)§ | 1.30 (1.10–1.53)‖ |
| Duration 1–20 years | 3,763/25/0.8 | 1.49 (1.08–2.05)¶ | 1.46 (1.06–2.01)¶ | 1.45 (1.03–2.03)¶ | 1.41 (1.01–1.97)¶ |
| Duration 21–35 years | 3,691/106/3.2 | 1.30 (1.10–1.54)‖ | 1.27 (1.07–1.50)‖ | 1.38 (1.15–1.67)§ | 1.34 (1.11–1.62)‖ |
| Fatal/nonfatal stroke | |||||
| All patients | 7,454/37/0.6 | 1.12 (0.83–1.51) | 1.08 (0.80–1.47) | 1.24 (0.89–1.72) | 1.19 (0.86–1.66) |
| Fatal/nonfatal CVD | |||||
| All patients | 7,454/154/2.4 | 1.26 (1.09–1.45)§ | 1.22 (1.06–1.40)‖ | 1.32 (1.14–1.54)§ | 1.27 (1.09–1.80)‖ |
| Duration 1–20 years | 3,763/26/0.8 | 1.49 (1.09–2.04)¶ | 1.46 (1.07–2.00)¶ | 1.48 (1.06–2.07)¶ | 1.44 (1.04–2.01)¶ |
| Duration 21–35 years | 3,691/128/4.0 | 1.23 (1.06–1.44)‖ | 1.19 (1.02–1.39)¶ | 1.34 (1.13–1.59)§ | 1.29 (1.08–1.53)‖ |
| Total mortality | |||||
| All patients | 7,454/94/1.4 | 0.97 (0.80–1.17) | 0.92 (0.76–1.11) | 1.04 (0.85–1.28) | 0.98 (0.80–1.20) |
| A1C intervals (mean) at baseline in two patient groups | |||||
| Fatal/non-fatal CHD | |||||
| 5.0–7.9% (7.2) | 4,186/45/1.2 | 1.0 | 1.0 | ||
| 8.0–11.9% (9.0) | 3,268/86/2.9§ | 1.80 (1.24–2.60)‖ | 1.71 (1.18–2.48)‖ | ||
| Fatal/non-fatal stroke | |||||
| 5.0–7.9% (7.2) | 4,186/14/0.5 | 1.0 | 1.0 | ||
| 8.0–11.9% (9.0) | 3,268/23/0.8§ | 1.51 (0.76–2.98) | 1.40 (0.70–2.79) | ||
| Fatal/non-fatal CVD | |||||
| 5.0–7.9% (7.2) | 4,186/55/1.6 | 1.0 | 1.0 | ||
| 8.0–11.9% (9.0) | 3,268/99/3.3§ | 1.70 (1.21–2.38)‖ | 1.59 (1.13–2.24)‖ | ||
HRs for CVDs per 1% unit increase in A1C, or with a patient group with A1C at baseline 8–11.9%, compared with another group with A1C at baseline 5–7.9%, when followed for 5 years.
*Mean event rates in a Cox model adjusted as in model 2.
†Model 1: adjusted for age, sex, diabetes duration, systolic blood pressure, total cholesterol, LDL cholesterol, triglycerides, BMI, smoking, and a history of CVD.
‡Model 2: adjusted as in model 1 and also for albuminuria (>20 μg/min). Significance level for the difference between two mean log event rates and for HRs.
§P < 0.001;
‖P < 0.01;
¶P < 0.05.
A Cox regression model was also used to estimate 5-year event rates (1 − survival rate) for CVDs (Fig. 1), in which model output was the adjusted 5-year event rate in each participant, adjusted for covariates as given in Table 2. Stratification (SAS Phreg; Strata) was performed to achieve adjusted mean event rates by groups or deciles of lower and higher A1C (Table 2; Fig. 1). Subgrouping of event rates by sample intervals has also been used in the Framingham Study (22). The significance of the difference in mean event rates between lower and higher A1C groups was estimated with Student t test, after logarithmic transformation of event rates to achieve a normal distribution. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute). P < 0.05 was considered statistically significant.
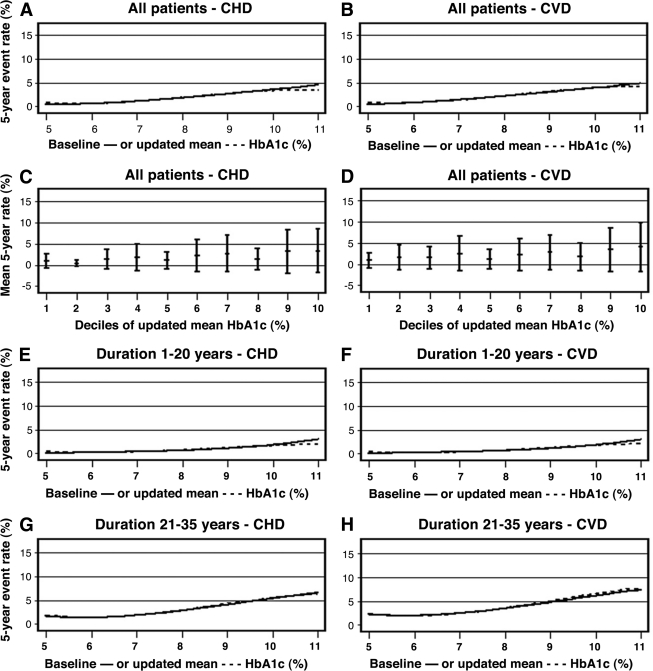
Figure 1.
Five-year rates (%) of fatal/nonfatal CHD and CVD in a Cox regression model, fully adjusted as in model 2 of Table 2. A and B: Splines for event rates in all 7,454 patients, as a cubic function of baseline A1C (solid line) or updated mean A1C (dashed line). C and D: Adjusted mean (SD) rates by deciles of updated mean A1C, with a solid line for the linear association by deciles. E–H: Splines for event rates in subgroups by median duration 20 years.
RESULTS
Baseline characteristics
Table 1 shows baseline characteristics in all 7,454 patients. The age range was 20–65 years (mean 37), the range of diabetes duration was 1–35 years (mean 20), and mean A1C was 8.0%. Albuminuria was present in 20% of subjects and a history of CVD at baseline in 3% of subjects. Two patient groups are also shown: 4,186 patients with A1C at baseline of the study within the interval 5.0–7.9% (mean 7.2) and 3,268 patients within 8.0–11.9% (mean 9.0). The group with higher A1C had somewhat higher mean values of most risk factors and higher frequencies of smoking and albuminuria.
Events
In total, 154 CVD events occurred, of which 36 were fatal and 118 nonfatal (Table 1). Events per 1,000 person-years for fatal/nonfatal CHD and CVD in all patients were 4.0 and 4.7, respectively (based on 32,931 person-years), higher in the group with higher A1C at baseline than in those with lower A1C at baseline. The incidence of total mortality was similar in the two A1C groups, and considerably more cases were non-CVD mortality than fatal CVD.
A1C in all patients
Analyzing baseline A1C as a continuous variable, Table 2 shows increased HRs (95% CI) per 1% unit increase in A1C for fatal/nonfatal CHD and CVD (1.31 [1.12–1.52] and 1.26 [1.09–1.45], P < 0.001) after adjustment for age, sex, diabetes duration, systolic blood pressure, smoking, total and LDL cholesterol, triglycerides, BMI, and a history of CVD. These HRs (95% CI) for CHD and CVD were only somewhat attenuated after adjusting also for albuminuria (1.28 [1.09–1.49;], P = 0.002, and 1.22 [1.06–1.40], P = 0.007). HRs for fatal/nonfatal stroke and total mortality were nonsignificant.
Using updated mean A1C, similar strong associations with CHD and CVD were found. HR for CHD decreased slightly from 1.34 (95% CI 1.14–1.58; P < 0.001) to 1.30 (1.10–1.53; P = 0.002) when adjusting also for albuminuria, and HR for CVD decreased from 1.32 (1.14–1.54; P < 0.001) to 1.27 (1.09–1.80; P = 0.003). Adjusted 5-year event rates of CHD and CVD by baseline A1C or updated mean A1C ranging from 5 to 12% are presented in Fig. 1A and B, showing progressively increasing event rates with higher A1C. No elevated risk was seen at the lowest A1C levels, as also verified with mean CHD and CVD rates by deciles of updated mean A1C (Fig. 1C and D).
When subgrouped by median duration, patients with longer duration (21–35 years [mean 28]) had a higher adjusted mean rate of CVD (4.0%) than those with shorter duration (1–20 years [mean 12]; adjusted mean rate of CVD 0.8%) (see Table 2).
Figure 1E–H shows progressively increasing rates of CHD and CVD with higher A1C in both subgroups. HRs for these outcomes per 1% unit increase in A1C were significant in both subgroups.
Groups at baseline by intervals of A1C
HRs for fatal/nonfatal CHD and CVD, fully adjusted also for albuminuria, were 1.71 (95% CI 1.18–2.48; P = 0.005) and 1.59 (1.13–2.24; P = 0.008) for a group of 3,268 patients with higher A1C at baseline (8.0–11.9% [mean 9.0]) compared with a group of 4,186 patients with A1C 5.0–7.9% (mean 7.2), when followed for 5 years. Correspondingly, the group with lower A1C showed risk reductions of 41% for CHD and 37% for CVD. These findings were consistent when patients with a history of CVD were excluded from Cox regression analysis (HRs for CHD and CVD with mean A1C 9.0% compared with mean A1C 7.2% were 1.63 [1.08–2.45] and 1.72 [1.18–2.52] when unadjusted for albuminuria and 1.54 [1.02–2.33] and 1.61 [1.10–2.36] when adjusted for albuminuria).
Other risk factors at baseline
A comparison between 154 patients who developed CVD during follow-up and those 7,300 patients with no CVD showed that patients with CVD were older ([means ± SD] 48 ± 9 vs. 37 ± 10 years); had longer diabetes duration (28 ± 7 vs. 20 ± 9 years); had higher mean systolic blood pressure (136 ± 18 vs. 125 ± 15 mmHg), LDL cholesterol (3.1 ± 1.1 vs. 2.7 ± 0.8 mmol/l), and triglycerides (1.4 ± 0.7 vs. 1.1 ± 0.6 mmol/l) (all differences P < 0.001); and had a higher mean BMI (26.1 ± 4.3 vs. 25.3 ± 3.6 kg/m2, P < 0.01). Those with CVD events had higher frequencies of smokers (29 vs. 13%), history of CVD before baseline (20 vs. 2%), and albuminuria (49 vs. 19%)—microalbuminuria (21 vs. 12%) and macroalbuminuria (28 vs. 7%)—all P < 0.001.
CONCLUSIONS
This large observational study of younger and middle-aged patients (aged 20–65 years) with type 1 diabetes of varying duration, treated in everyday clinical practice, demonstrates a strong association between A1C and both CHD and CVD. Each 1% unit increase in baseline A1C or updated mean A1C was associated with risk increases of 31–34% for CHD and 26–32% for CVD, after adjustment for age, sex, diabetes duration, and traditional cardiovascular risk factors. These increases in risk remained significant but were slightly attenuated to 28–30% and 22–27%, respectively, after adjustment also for albuminuria. Fully adjusted 5-year event rates of CHD and CVD increased progressively with higher A1C levels, and no J-shaped risk curves were seen. The group with mean A1C 7.2% at baseline had risk reductions of 41% for CHD and 37% for CVD compared with the group with mean 9.0%, when followed for 5 years.
The most previous comparable epidemiological studies have not demonstrated clear associations between glycemic control and risk of CHD (10,11,13,14). The EURODIAB PCS (11) and the Pittsburgh EDC trials (10,14) were smaller with respect to the numbers of participating patients, although the follow-up periods were longer. The mean duration of diabetes was shorter in the EURODIAB PCS, while they were similar to our study in the Pittsburgh EDC. In our study, two subgroups by the median duration are presented, with 1–20 years and 21–35 years of diabetes duration. Although those with longer diabetes duration had higher mean rates of CHD and CVD, the event rate curve increased progressively with higher A1C in both subgroups, verified by significant HRs for these outcomes per unit A1C increase. Interestingly, there was no increase in risk at low A1C levels even with longer duration, a possibility that has been discussed in generally older patients with type 2 diabetes (23).
The DCCT/EDIC study provides the most convincing evidence regarding glycemic control and risk of CVD in type 1 diabetes (7). Patients were randomized to intensive or standard treatment and followed for 6 years during the DCCT. In the subsequent observational EDIC study of 1,182 patients, two groups with mean A1C 7.4 and 9.1% (mean age 34 years, mean diabetes duration 12 years, and albuminuria 7 vs. 13%) were followed for another 11 years regarding CVD outcomes, and, in total, 144 CVD events occurred in 83 patients. Risk reductions of 42% for any first-incident CVD and 57% for fatal/nonfatal CVD with lower A1C was found at univariate survival analysis (P = 0.02), unadjusted for albuminuria. We found a risk reduction of 41% for CVD, unadjusted for albuminuria, in the group with mean A1C 7.2% compared with those with mean 9.0% (P = 0.002).
Both studies showed similar difference in baseline mean A1C of 1.7–1.8% between the groups, similar mean ages, and generally quite comparable baseline risk factor levels. Mean duration and frequency of albuminuria were slightly higher in this study. The confounding effect of the inclusion of 3% of all patients with a history of CVD in this study, reasonably also present in the normal patient population treated in clinical practice, was adjusted for at the Cox regression analysis. Our study on data from 2002 to 2007 provides strong support to the results of the EDIC study conducted from 1994 to 2005. Furthermore, a significant risk reduction for CHD with improved glycemic control was demonstrated here but not in the DCCT/EDIC study.
The present study also emphasizes the role of albuminuria as a risk factor for CVD in type 1 diabetes. It has been suggested that glycemia is a more potent risk factor of macrovascular disease in the absence of albuminuria (9,14,24,25). In our study, the HRs for CHD and CVD were 1.31 and 1.26, respectively, per 1% unit increase in baseline A1C, when adjusted for important CVD risk factors except albuminuria (P < 0.001), and were attenuated to 1.28 and 1.22, respectively, when adjusted also for albuminuria (P = 0.002–0.007). The HR for CVD with the group with lower A1C was 0.59 when unadjusted, but it attenuated to 0.63 when adjusted for albuminuria. In the Cox regression analysis of the DCCT/EDIC study, the HR for the intensive treatment group effect on CVD was 0.53 when adjusted only for risk factors at DCCT baseline (P = 0.005) but attenuated to 0.58–0.62 when adjusted also for in-treatment differences in albuminuria between the groups during the EDIC study follow-up (P = 0.02–0.04). Thus, both risk factors are important, and, furthermore, their effects on risk seem to be additive as we found no interaction between them.
Although observational studies generally are regarded as evidence of lower degree than randomized controlled trials, such trials often use strict inclusion and exclusion criteria, which may limit their application to the normal patient population. This study allowed for an analysis of patients with daily treatment at hospitals nationwide during recent years with no exclusion criteria regarding risk factors or history of CVD. A major strength of this study was the large number of patients and person-years, and it has not as yet been possible to perform randomized trials of tight glycemic control in type 1 diabetes with a larger number of participants included. It is important to adjust for confounding variables in an observational study, and such adjustments were made for age, sex, duration, and several traditional cardiovascular risk factors, including albuminuria representing microangiopathy, as well as history of CVD. Analysis of interactions are also important for reliable results, and we could exclude heterogeneity as there were no significant interactions between A1C and all included covariates. The capture of data on the outcomes was based on reliable and validated national registers of morbidity and mortality.
There were also limitations. Although substantial adjustments were made for confounding variables, unmeasured confounding may exist because of unknown and not included covariates. Data from participating centers may vary slightly in accuracy, although increased use of computer software with direct transfer of data are reducing this problem. Laboratory analyses are carried out in local laboratories, but there is a nationwide program to allow calibration of A1C to a standard, and there have been only very marginal changes in the cross-sectional mean A1C values during the study period. There is also currently no information on autonomic neuropathy, frequency or severity of hypoglycemia, or detailed information on types of insulin used or doses in the NDR.
In conclusion, this observational study in a large number of type 1 diabetic patients in modern everyday clinical practice demonstrates a strong independent effect of increased baseline or updated mean A1C values on risks of CHD and CVD, with no sign of a J-shaped curve at lower A1C values even with longer diabetes duration. A risk reduction of ∼40% for CHD and CVD was found when a group with A1C mean 7.2% at baseline and followed for 5 years was compared with a group with baseline A1C mean 9.0%. This emphasizes the role of A1C as a strong independent risk factor in type 1 diabetes.
Acknowledgments
The patient organization Swedish Diabetes Association and the Swedish Society of Diabetology support the NDR. The Swedish Association of Local Authorities and Regions funds the NDR.
No potential conflicts of interest relevant to this article were reported.
K.E.O. and J.C. participated in study design, data gathering, statistical analysis, interpretation, and writing the report. B.E. participated in study design, interpretation, and writing the report. S.G. participated in study design, data gathering, interpretation, and critical review. B.Z. and P.M.N. were involved in study design, interpretation, and critical review. A.-M.S. was involved in data gathering and critical review of the report.
We thank all regional NDR coordinators, contributing nurses, physicians, and patients.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR: Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol 1987; 59: 750–755 [DOI] [PubMed] [Google Scholar]
- 2. Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC: Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003; 46: 760–765 [DOI] [PubMed] [Google Scholar]
- 3. Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM: All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia 2006; 49: 660–666 [DOI] [PubMed] [Google Scholar]
- 4. Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM: High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 2006; 29: 798–804 [DOI] [PubMed] [Google Scholar]
- 5. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 6. Reichard P, Pihl M, Rosenqvist U, Sule J: Complications in IDDM are caused by elevated blood glucose level: the Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up. Diabetologia 1996; 39: 1483–1488 [DOI] [PubMed] [Google Scholar]
- 7. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995; 75: 894–903 [DOI] [PubMed] [Google Scholar]
- 9. Lehto S, Ronnemaa T, Pyorala K, Laakso M: Poor glycemic control predicts coronary heart disease events in patients with type 1 diabetes without nephropathy. Arterioscler Thromb Vasc Biol 1999; 19: 1014–1019 [DOI] [PubMed] [Google Scholar]
- 10. Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ: Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003; 26: 1374–1379 [DOI] [PubMed] [Google Scholar]
- 11. Soedamah-Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, Manes C, Fuller JH: Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care 2004; 27: 530–537 [DOI] [PubMed] [Google Scholar]
- 12. Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH: Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008; 31: 1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein BE, Klein R, McBride PE, Cruickshanks KJ, Palta M, Knudtson MD, Moss SE, Reinke JO: Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med 2004; 164: 1917–1924 [DOI] [PubMed] [Google Scholar]
- 14. Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ: Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia 2007; 50: 2280–2288 [DOI] [PubMed] [Google Scholar]
- 15. Cederholm J, Zethelius B, Nilsson PM, Eeg-Olofsson K, Eliasson B, Gudbjornsdottir S: Effect of tight control of HbA1c and blood pressure on cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Res Clin Pract 2009; 86: 74–81 [DOI] [PubMed] [Google Scholar]
- 16. Eeg-Olofsson K, Cederholm J, Nilsson PM, Gudbjornsdottir S, Eliasson B: Glycemic and risk factor control in type 1 diabetes: results from 13,612 patients in a national diabetes register. Diabetes Care 2007; 30: 496–502 [DOI] [PubMed] [Google Scholar]
- 17. Gudbjornsdottir S, Cederholm J, Nilsson PM, Eliasson B: The National Diabetes Register in Sweden: an implementation of the St. Vincent Declaration for Quality Improvement in Diabetes Care. Diabetes Care 2003; 26: 1270–1276 [DOI] [PubMed] [Google Scholar]
- 18. Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, Hoshino T, John WG, Kobold U, Little R, Mosca A, Mauri P, Paroni R, Susanto F, Takei I, Thienpont L, Umemoto M, Wiedmeyer HM: IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 2004; 50: 166–174 [DOI] [PubMed] [Google Scholar]
- 19. Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502 [PubMed] [Google Scholar]
- 20. Merlo J, Lindblad U, Pessah-Rasmussen H, Hedblad B, Rastam J, Isacsson SO, Janzon L, Rastam L: Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two Swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol 2000; 16: 235–243 [DOI] [PubMed] [Google Scholar]
- 21. Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A: Myocardial infarction and coronary deaths in the World Health Organization MONICA Project: registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90: 583–612 [DOI] [PubMed] [Google Scholar]
- 22. Ingelsson E, Sullivan LM, Fox CS, Murabito JM, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O'Donnell CJ, Wang TJ, D'Agostino RB, Wolf PA, Vasan RS: Burden and prognostic importance of subclinical cardiovascular disease in overweight and obese individuals. Circulation 2007; 116: 375–384 [DOI] [PubMed] [Google Scholar]
- 23. Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS: Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation 2009; 119: 351–357 [DOI] [PubMed] [Google Scholar]
- 24. Orchard TJ, Costacou T, Kretowski A, Nesto RW: Type 1 diabetes and coronary artery disease. Diabetes Care 2006; 29: 2528–2538 [DOI] [PubMed] [Google Scholar]
- 25. Retnakaran R, Zinman B: Type 1 diabetes, hyperglycaemia, and the heart. Lancet 2008; 371: 1790–1799 [DOI] [PubMed] [Google Scholar]