Abstract
Substance abuse and addiction are well recognized public health concerns, with 2 NIH institutes (the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism) specifically targeting this societal problem. As such, this is an important area of research for which animal experiments play a critical role. This overview presents the importance of substance abuse and addiction in society; reviews the development and refinement of animal models that address crucial areas of biology, pathophysiology, clinical treatments, and drug screening for abuse liability; and discusses some of the unique veterinary, husbandry, and IACUC challenges associated with these models.
Substance abuse and addiction are enormous public health concerns that affect society and public policy in multiple arenas, including health care, education, worker productivity, criminal law, and prison systems. The World Health Organization reported that 76.3 million persons worldwide have alcohol use disorders and at least 15.3 million have drug use disorders.112 Use of injected drugs has been reported in 136 countries, of which 93 report HIV infection among this population.112 In 2007, an estimated 19.9 million Americans 12 y or older were current (past month) illicit drug users. This estimate represents 8% of the population 12 y of age or older.97
The costs of this situation to society are substantial. The National Institute on Drug Abuse estimated the total economic cost of alcohol and drug abuse at $245.7 billion for 1992. Of this, $98 billion was due to drug abuse (not including nicotine). By 1998 the societal cost of drug abuse was $143.4 billion.73 In addition to health care costs and the costs of lost productivity, costs are incurred by the criminal justice system, efforts to reduce the supply of drugs, and provision of social welfare. Crime-related costs alone account for 59% of total societal costs.21 Government spending related to smoking and the abuse of alcohol and illegal drugs reached $468 billion in 2005, accounting for more than 10% of combined federal, state, and local expenditures for all purposes. Despite the fact that for every dollar invested in drug treatment 7 dollars are saved in health and social cost, just over 2% of the total government expenditures went to prevention, treatment, and addiction research.69 According to the NIH Office of Budget, research awards supported by the National Institute on Drug Abuse in fiscal year 2008 totaled just over $750 million (http://officeofbudget.od.nih.gov/spending_hist.html).
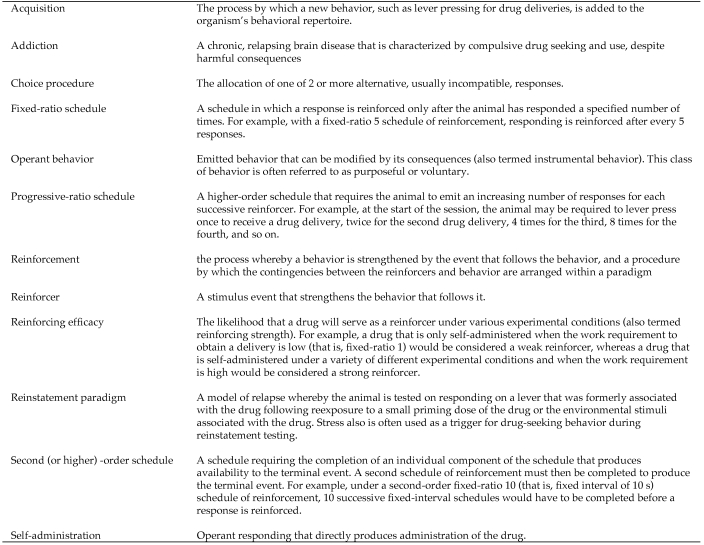
Substance related disorders are divided into 2 groups: the substance-use disorders, which include substance dependence and substance abuse, and the substance-induced disorders, which include intoxication, withdrawal, substance-induced delirium, persisting dementia, and a variety of other substance-induced effects (see Figure 1 for a glossary of terms). The common feature of substance dependence is a group of behavioral and physiologic symptoms indicating continued use of the substance despite significant problems resulting from such use. Drug addiction exists when drug procurement and use begins to govern the subject's (human or animal) behavior and the drug appears to control the subject's motivational status.11 Drug addiction has been described as a chronic, relapsing brain disease that is characterized by compulsive drug seeking and use, despite harmful consequences.71 Substance dependence is often linked to other mental health illnesses. According to the 2007 National Survey on Drug Use and Health survey, serious psychologic distress in the past year often was associated with past year substance dependence or abuse. Among adults 18 y or older with serious psychologic distress, 22.1% were dependent on or abused illicit drugs or alcohol, compared with 7.6% among adults without serious psychologic distress.97
Figure 1.
A glossary of some terms used in studying drug reinforcement. Based on information in reference 71.
Development of dependence on prescription opioids such as oxycodone, and addiction resulting from nonmedical use, are also growing societal problems. Pain lasting more than 24 h affects more than 25% of Americans each year22 and costs approximately $100 billion in lost productivity, lost income, and health care.96 Unfortunately, treatment of chronic pain with addictive medications and the spillover abuse of prescription drugs by persons without medical indications have led to widespread prescription drug abuse.
Animal studies have been crucial in understanding the biology and pathophysiology of drug addiction and substance abuse. In contrast to clinical studies, the subject population can be controlled for variables more easily. Animal models often focus on the ability of the drugs to directly control the animal's behavior, an outcome that is consistent with the behavioral definition of addiction. As mentioned elsewhere in this overview, animal studies have demonstrated that the rewarding effect is not dependent on preexisting conditions; that is, exposure to the drug is sufficient to motivate drug-taking behavior. Self-administration by laboratory animals of drugs abused by humans also supports the concept that drugs act as universal reinforcers. In other words, some of the typical behaviors associated with drug abuse in humans are not necessary for drug reinforcement to occur; rather they involve biologic processes common to mammalian species.11
Despite the considerable value of past and ongoing research in this area, research on drug addiction and substance abuse is often controversial, and researchers who study these topics are often the target of animal rights activities. Without a clear understanding of the biologic basis of drug dependence and addiction, the public may not perceive an equivalent value from this area of research as compared with better understood medical issues such as heart disease, diabetes, and cancer. In this overview, we aim to present important aspects of substance abuse research, describe various animal models that have been developed to study specific aspects of drug addiction and substance abuse disorders, and delve into important veterinary, husbandry, and ethical (IACUC) issues associated with substance abuse research.
Animal Models of Substance Abuse and Addiction
The reinforcing effects of drugs of abuse are believed to play a key role in substance abuse and addiction. Early demonstrations that drugs could serve as reinforcers maintaining operant behavior in laboratory animals led to the development of a model of human drug abuse. In this section, we provide an overview of the paradigms used for establishing drugs as reinforcers in animals, focusing on drug self-administration, conditioned place preference, and drug discrimination paradigms. Recently, the conceptual framework for modeling addiction in animals has focused on modeling different phases of the addiction process, such as drug initiation or acquisition, and on the factors that affect or predict vulnerability to drug addiction. In addition, interest is increasing in developing more comprehensive models that take into consideration more of the features that are characteristic of human drug abuse, that is, compulsive and binge patterns of use, loss of control over drug use, and chronic relapse.
Drug self-administration paradigm.
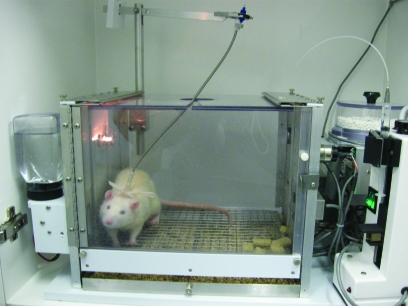
The traditional animal models of drug abuse are framed by the behaviorist view that emphasizes the action of drugs as positive reinforcers, much like food, water, and other ‘natural’ reinforcers. The fundamental principle is that aspects of behavior are controlled by their consequences. A drug is said to be functioning as a reinforcer if responding for it is maintained above responding for saline or other control conditions. The traditional model entails training an animal to self-administer a drug during a short daily session, typically 1 to 3 h. Figure 2 shows a rat in a typical operant chamber with an intravenous catheter for chronic self-administration. Although rodents are most often used in these studies, this model has been used with a variety of species including nonhuman primates, dogs, and cats. In rodents, a low-ratio requirement typically is used, such as a fixed-ratio 1, where each operant response produces a drug delivery. In addition, a variety of operant responses have been used. Typically they depend on the species studied (for example, a lever press or a nose poke response typically is used for rodents, whereas a panel press response typically is used for nonhuman primates).
Figure 2.
Use of an operant chamber for a rodent intravenous drug self-administration paradigm. Rats are implanted with chronic, indwelling catheters in the jugular vein. The catheter exits the rat on the dorsum, where it is connected to a tether-and-tubing system that is attached to a drug-loaded syringe. Responding on the active lever leads to infusions of the drug.
The most common routes of administration are intravenous and oral, but intracerebroventricular, intracranial, inhalation, intragastric, and intramuscular routes have also been used; some of these other routes (for example, smoked) are used relatively infrequently, because of practical and logistical difficulties. Generally, these studies use the route of administration that is most similar to the route used in humans for that particular drug. For example, animal studies with alcohol typically use an oral route of administration, whereas an intravenous route is used for drugs like cocaine, heroin, and nicotine, to mimic the rapid onset produced by intravenous or inhalational administration in humans.19 Taste factors must often be considered with the oral route, given that these often limit consumption of pharmacologically active doses; however, use of intragastric self-administration or sweetening an alcohol solution with saccharin are 2 methods used to avoid the influence of taste. Results from animal drug self-administration studies have revealed that drugs can serve as positive reinforcers, and there appears to be good correspondence between humans and animals in terms of drugs that are self-administered and patterns of drug intake. For example, drugs that are abused by humans generally maintain responding in animals, whereas drugs that do not maintain responding in animals typically are not abused by humans.23,37,42 In addition, similar patterns of drug intake have been reported in humans and animals for ethanol, opioids, nicotine, and cocaine self-administration.36 These parallel results between the human and animal drug literature validate the animal model of drug abuse and suggest that the use of this model may lead to a better understanding of human drug-taking behavior.
The traditional self-administration procedure has been instrumental in characterizing the brain regions and signaling pathways that are responsible for rewarding behaviors. This ‘reward pathway’ is comprised of several brain regions, the most prominent being the ventral tegmental area, nucleus accumbens, and prefrontal cortex. The ventral tegmental area has dopaminergic projections to both the nucleus accumbens and prefrontal cortex. These projections are critically involved in mediating rewarding behaviors, although other brain regions and signaling pathways are involved also.47,111 All drugs of abuse are known to increase dopaminergic signaling in the reward pathway, particularly in the nucleus accumbens, and if dopamine release is prevented (for example, by pharmacologic blockage or lesions), drug reinforcement is blocked. Importantly, this pathway is known to be activated in response to drugs of abuse as well as natural rewards, such as food and sex.
Reinforcement or the rewarding effects of drugs of abuse, and thus dopaminergic signaling in the reward pathway, appear to be critically involved in addiction, but the strength of this involvement may vary with stage of the addiction process. For example, drug initiation appears to be driven predominantly by the reward system, and people report that they began using drugs for their positive or euphoric effects. Studies in animals have revealed that drug initiation, or acquisition, can be blocked by dopamine antagonists or by inducing lesions in the reward pathway. However, accumulating evidence indicates that after chronic drug exposure, systems and signaling pathways outside the reward pathway (for example, dopaminergic and nondopaminergic signaling in cortical areas, other striatal areas, amygdala, hippocampus; for review see reference 100) become involved in driving drug-taking and -seeking behaviors. On the basis of the idea that this reward pathway is critical for drug reinforcement, much of the hunt for pharmacotherapeutic agents to combat drug addiction has focused on dopaminergic signaling. Although the rationale for using dopaminergic agents appears to be obvious, the plethora of research in this area has failed to produce an accepted effective pharmacotherapy. This failure is due, in part, to severe adverse effects, including nausea, headaches, hypertension, tachycardia, and psychosis-like symptoms, experienced after the administration of dopaminergic agents.
Retrospective reports from drug abusers reveal that drug use was maintained not only because of its rewarding properties but also because drug abusers feel compelled to use the drug to alleviate craving or drug-withdrawal symptoms.105 As a result, research has shifted toward modeling what may be fundamental dimensions of human drug addiction that are not captured in the traditional reinforcement paradigm. These features include vulnerability to drug abuse (that is, why some people become addicted and not others), the transition from controlled use to compulsive and uncontrolled drug use, and relapse to drug use after a period of abstinence. Although addiction involves drug reinforcement, it also involves drug craving and a loss of control over use.
Vulnerability to drug abuse.
Animal models of the initiation or acquisition phase have been developed, and they have been useful in identifying biological and behavioral factors in vulnerability to the reinforcing effects of drugs of abuse that may apply to prevention efforts in humans.15 Optimally, acquisition of drug self-administration is investigated in drug-naive and experimentally naive animals that are maintained under food-satiated conditions and tested under low-dose conditions. Under these conditions, individual differences are maximized, and some rats acquire self-administration whereas others do not. The question that is addressed is, “Which animals can detect the reinforcing effects of this low drug dose?”
A simple method of evaluating acquisition is to give an animal access to a drug during a daily experimental session, with deliveries available contingent upon an operant response (that is, lever press).16,24 Acquisition of drug self-administration then is measured as the number of sessions needed to reach a criterion level of intake, which can be standardized and adjusted for dose and drug availability. Often the ratio of active to inactive lever press responses is used in conjugation with the intake criteria. All of the study animals are included in the analysis, regardless of whether they acquire self-administration or not, and the focus is on how rapidly acquisition of self-administration takes place and the percentage of animals in each group that acquire drug self-administration.
As occurs in humans, environmental factors (such as feeding conditions, the presence of an alternative nondrug reinforce, and drug history) can greatly affect rates of acquisition of drug self-administration in animals. For example, in a study that examined the effects of feeding condition and palatability of the diet, rates of acquisition became slower with increasing levels of food satiation, particularly when the food options were enriched.17
In addition, these acquisition methods have revealed a number of physiologic and organismic factors that predict vulnerability to drug self-administration, including basal and stress-elicited corticosterone levels,78 dopamine release in brain regions associated with drug reward,32,40 genetic strain,93,104 innate saccharin preference,33,75 levels of impulsivity,77 age,91 and sex.52 For example, we previously showed that female rats acquire cocaine and heroin self-administration at a faster rate than do male rats and that a greater percentage of female rats acquire cocaine self-administration than do male rats.53
The transition from controlled use to compulsive and uncontrolled drug use.
Two of the fundamental features of drug addiction in humans—loss of control over use and the resulting excessive or compulsive use of the drug—have been modeled in animals using several different methods (for review, see reference 83). Early studies with monkeys and rats showed that, like those in humans, patterns of self-administration in laboratory animals that are given unlimited access conditions (that is, 24-h sessions wherein each response was reinforced under a fixed-ratio 1 schedule) were characterized by dysregulated and binge patterns of use. Under these conditions, toxicity can develop rapidly, particularly with unlimited access to psychostimulant drugs and opiates, thereby necessitating the use of procedures that limit access to these drugs in some way.
Recent studies have attempted to capture these features—dysregulated patterns of use and excessive consumption—without the toxicity. For example, dysregulated and excessive drug intake without serious toxicity has been observed to occur under 24-h access conditions with low-unit doses of drug18 and under continuous access conditions that limit the number of hours of access each day (that is, 6 to 12 h daily1) or each period of continuous access (that is, 72 h).101 Another method that has been used with limited toxicity is to give animals 24-h access to a drug in discrete trials throughout the light:dark cycle.27 This method has been used for cocaine self-administration, and the results have shown that the regularity of patterns of use break down and intake progressively increases as access conditions increase. Under short-access conditions (that is, 1 to 2 discrete trials per hour, 1.5 mg/kg per infusion), rats consume low levels of cocaine, and intake is relatively stable over time and confined to the dark phase of the light:dark cycle.82 However, under extended access conditions (that is, 4 discrete trials per hour, 1.5 mg/kg per infusion), rats self-administer high levels of cocaine in a pattern that is dysregulated from the diurnal cycle (that is, responding occurs at high levels during both dark and light phases).
Importantly, both increased motivation for cocaine66 and increased drug-seeking behavior46,59,85—additional key features of cocaine addiction—occur after excessive drug self-administration when examined after an abstinence period. For example, we reported that 10 d of access to cocaine under a discrete trial procedure (4 trials per hour) produced a sustained increase from baseline levels of progressive-ratio responding for cocaine when assessed after a 7-d abstinence period.66 Similar results have recently been reported after extended access to self-administered heroin and methamphetamine by using similar procedures.2,85,108 These parallel results between extended access drug self-administration paradigms in animals and drug-addicted humans validate their use as an animal model of addiction and suggest that these models of extended drug access may be useful in determining predictive factors during the transition from controlled to uncontrolled drug use.
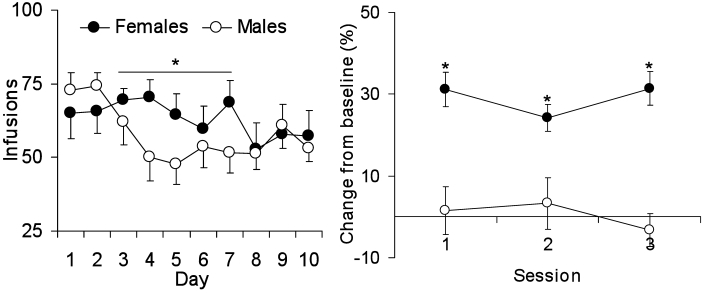
Although few studies have examined individual differences by using extended access drug self-administration procedure, one factor that is known to predict vulnerability is sex. For example, results from studies from our laboratory have revealed that female rats take more cocaine under extended-access conditions and appear to require less drug exposure than do male rats to display increased motivation for cocaine (Figure 3).54 Other factors include sweet preference and level of reactivity to novelty, both of which appear to influence the appearance of drug escalation or dysregulation as well as motivational changes after extended-access self-administration.58,76 Notably, the underlying neurobiology associated with extended-access drug self-administration appears to be different than that associated with short-access drug self-administration.9,13,26,31,99
Figure 3.
An example of using the extended-access drug self-administration procedure to predict vulnerability, in this case sex differences in levels of cocaine intake. (Left) Male and female rats were compared in their responses for cocaine under extended access conditions by using a discrete trial procedure (4 trials/h, 1.5 mg/kg/infusion). Results show that female rats take more cocaine over a 10-d access period as compared with male rats. (Right) When responding is assessed 10 d after extended-access cocaine self-administration, female but not male rats show enhanced levels of progressive-ratio responding as compared with female and male rats tested after short-access cocaine self-administration (for example, maximum of 20 infusions/d for 5 d). n = 7 to 12. *Significant (P < 0.05) difference between male and female rats.
Relapse to drug use.
Various types of stimuli can precipitate drug craving and relapse to drug use. These factors include environmental stressors, internal cues such as reexposure to small ‘priming’ doses of the drug, and external cues such as specific places and people that were associated with drug use. Animal models of relapse have been developed and have provided important information on the neurobiologic mechanisms underlying vulnerability to relapse to drug abuse.48,92
One model that has been used to investigate mechanisms underlying relapse is the reinstatement paradigm.44 With this procedure, animals are trained to self-administer a drug and once stable responding is achieved, it is extinguished by discontinuing drug delivery. After responding reaches some criterion of unresponsiveness, the ability of various stimuli to reinstate drug-seeking is determined under conditions of nonreinforcement (that is, responses are no longer reinforced by drug). A stimulus is said to reinstate responding if it causes an increase in responding that formerly was reinforced by the drug. The results from preclinical studies have revealed that the conditions that reinstate drug seeking in laboratory animals are similar to those that trigger relapse in humans and include exposure to stressors, small doses of the drug itself, and cues associated with the drug, thereby demonstrating the predictive validity of this model.44 As such, the reinstatement paradigm can be useful for studying factors influencing relapse to drug use. Results from reinstatement studies have revealed a number of factors that predict vulnerability during this phase, including responsiveness to the acute and chronic locomotor-activating effects of psychostimulants,25 locomotor responses to novelty,98 pattern of drug intake prior to reinstatement testing,98 and sex.52
Conditioned place preference.
In addition to self-administration, conditioned place preference experiments provide additional information on the rewarding effects of drugs of abuse. In this model, animals receive a dose of a drug or vehicle and are placed into 1 of 2 sides of an experimental chamber, which have different visual (that is, vertical versus horizontal lines on the walls) and tactile (that is, grid versus bars for the floor) cues. The drug is always paired with the same set of visual and tactile stimuli, as is the vehicle with the other, producing an association with the respective stimuli. Drug administrations and pairings are repeated over the course of several days, and the amount of time spent in each chamber is recorded. After the pairings, a test day is conducted on which no drug is given and the animal is allowed to freely roam the 2 chambers. The preference for one environment over the other confers information regarding the motivational state created by the drug. If the drug induces a positive state or ‘rewarding’ effect, the subject is expected to spend more time in the drug-paired environment. For example, in one study, vehicle or cocaine (1, 3, 5, 7.5, 10, or 20 mg/kg IP) was administered to different groups of male and female rats.114 After cocaine administration, rats were placed in what was designated to be the drug-associated side of the chamber for 30 min. After vehicle injections, subjects were placed in the alternate side of the chamber for 30 min, thereby pairing drug effects or vehicle with the specific set of environmental cues. After repeated pairings, rats were allowed access to both sides of the chamber, and time spent in each side was recorded. Adult male rats spent more time in the drug-associated side after conditioning with 10 and 20 mg/kg cocaine but not with lower doses, indicating the development of conditioned place preference to the higher doses. This same experiment also revealed that conditioned place preference developed at lower doses of cocaine in female than male rats, showing that this model is also useful for investigating individual differences in sensitivity to the rewarding effects of drugs of abuse.
In the converse to conditioned place preference, drugs with negative effects result in the animal spending less time in the drug-associated side. This phenomenon is known as conditioned place aversion. For example, morphine-dependent rats treated with the opioid antagonist naloxone during training spend significantly less time in the drug-associated side, indicating conditioned place aversion.68 Therefore the place conditioning procedure can 1) provide information on the potential rewarding effects of novel drug through the development of place preference;7,67,102 2) be used to assess the development of physical dependence by providing a measure of withdrawal effects indicated by the development of place aversion,68 and 3) be used to screen potentially therapeutic compounds, that is drugs with the ability to block conditioned place preference.56,62
Preclinical Abuse Liability Assessment
Drug discrimination.
An experimental model frequently used as a component of the overall assessment of the abuse liability of a novel drug is the drug-discrimination paradigm. Drug-discrimination studies are considered to provide an animal model of the subjective effects of drugs in humans.4,39 Drugs that cross-substitute for each other in animal discrimination procedures match very well with drugs that humans report having similar subjective effects.41,80,88 Although the parameters of individual studies may vary, such as in type of operant response and type of reinforcement, all drug discrimination procedures rely on state-dependent learning and the repeated pairing of a drug effect with an operant behavioral response, which results in presentation of a reinforcer of that behavior.39 For example, an animal is placed into an operant chamber equipped with 2 response levers. On some days, the animal is pretreated with a known drug of abuse (the drug to be ‘trained’) and is reinforced with a food pellet for pressing the left lever. On other days, the animal receives an injection of vehicle before being placed in the chamber and is reinforced with a food pellet for pressing the right lever. Over time and continued pairing of one lever with drug and the other with vehicle, the animal learns to press the left lever whenever it receives an injection of the training drug and to press the right lever when it is injected with vehicle. Once a subject has acquired discrimination between drug and vehicle conditions, novel compounds can be evaluated for substitution for the training drug. If a novel drug produces responding on the lever associated with the training drug, it is considered to have discriminative stimulus effects like those of the training drug and would be predicted to produce subjective effects like those of the training drug in humans. If the novel drug engenders responding mostly on the vehicle-associated lever, it does not share discriminative stimulus effects with the training drug. The discriminative stimulus effects of a drug have repeatedly been demonstrated to be centrally mediated and pharmacologically specific;4,39,87,103 that is, drugs that bind to the same receptor and produce similar pharmacologic effects tend to substitute and cross-substitute for each other in drug discrimination procedures. For example, methadone and heroin will substitute in animals trained to discriminate morphine, but cocaine will not substitute.
Self-administration procedures.
Although drug discrimination provides a preclinical model of the subjective effects, it does not provide a measure of drug ‘liking’ or drug reward. As discussed earlier, there is generally good correlation between those drugs that are self-administered by laboratory animals and those that are recreationally abused by humans.3,35,42 This correlation provides the principal rationale for using self-administration to predict abuse potential. The majority of abuse liability studies use a substitution procedure. Subjects are trained to self-administer (intravenously or orally) a known drug of abuse from a similar pharmacologic class or therapeutic indication as the novel medication to be assessed under a fixed-ratio schedule of reinforcement. Unlike many models of addiction, substitution procedures typically use limited access to the training drug. When the subject reliably responds for the positive control (training drug) and extinguishes when all that is available is a negative control solution (saline or vehicle), substitution testing with various doses of the novel drug is begun. If the novel compound maintains responding above the negative control, the novel compound is considered a positive reinforcer of behavior. Additional information about relative strength of reinforcement can be obtained by utilizing a progressive-ratio schedule of reinforcement, in which each subsequent infusion requires the subject to elicit more and more responses. The work requirement is raised until responding ceases. This maximum work level, the ‘break point,’ can be compared between drugs to assess relative reinforcing efficacy.
Physical dependence.
Of particular concern for any compound intended for chronic administration, such as in the treatment of chronic pain, is the effect of cessation of that drug. Impressive evidence shows that physical dependence can play an important role in compulsive drug use. When the withdrawal syndrome is unpleasant, physical dependence can be a key determinant regarding continued use of a drug. Methods for evaluating novel drugs or drug formulations for physical dependence production include substitution procedures and primary dependence procedures. Substitution procedures require creating physical dependence to a known drug of abuse through repeated or continuous administration of that drug. Once dependence has been established, either administration of the novel drug is substituted for administration of the dependence-inducing drug (scenario 1) or, if an appropriate antagonist is available, withdrawal is precipitated and the novel medication is administered to determine whether it can reverse the withdrawal signs (scenario 2). If spontaneous withdrawal occurs (scenario 1) or the drug is unable to reverse precipitated withdrawal (scenario 2), the substituted drug is not similar to the dependence-inducing drug; that is, it does not produce the same kind of physical dependence as the known drug. Conversely, if withdrawal does not occur or precipitated withdrawal is reversed, the novel drug is predicted to have dependence-producing potential similar to that of the known drug. Substitution studies rely upon the phenomenon of cross-dependence between pharmacologically similar compounds. For example, morphine, codeine, and heroin all show substitution for one another in these dependence procedures, thereby reflecting similar sites of cellular action and the production of similar neuroadaptations during repeated exposure.8,14 Similarly, CNS depressants that produce their effects through GABAA receptor ion channels (such as ethanol, benzodiazepines, and barbiturates) also demonstrate substitution indicative of cross-dependence.14,90 However, ethanol and morphine dependence results in different withdrawal syndromes, and they do not cross substitute. The purpose of a primary dependence study is to determine whether repeated or continuous administration of the novel drug itself will produce neuroadaptive changes that result in physical dependence as indicated by the presence of a withdrawal syndrome upon cessation of the drug or after administration of an antagonist. After animals undergo repeated or continuous exposure to the novel drug, administration is stopped and the animals are observed for signs of physical withdrawal. If withdrawal occurs, the test drug is likely to produce physical dependence if self-administered (whether for medical or nonmedical purposes) by humans over time. This approach may be more relevant when the mechanism of action of the novel medication does not share mechanisms of action with known drugs of abuse or when the mechanism of action is unclear.
Veterinary, Husbandry, and IACUC Issues
Animal models of substance abuse and addiction present a host of challenges to laboratory animal professionals. The research models are often chronic and complex and introduce specific issues related to husbandry, chronic instrumentation, special housing needs, food and water restriction, and pain and distress. However, with a single exception, namely withdrawal, these issues are not entirely unique to abuse-related preclinical research. The IACUC and animal care staff need to also recognize that this research involves complex behavioral responses that can be disrupted by a variety of environmental factors, administration of drugs whose effect(s) may or may not be known, and a requirement for diligent monitoring of physiologic and behavioral status often for many months.
IACUCs should understand that most of the drugs used or tested are reinforcing, and humans find their use highly rewarding and will go to great lengths to administer these drugs. Generally it is not difficult to train animals in the necessary behaviors because they, like humans, find the result rewarding.6,82 The animal use protocol may contain terms that are unfamiliar to the IACUC, such as fixed-ratio schedules, progressive-ratio schedules, conditioned place preference, reversal learning task, drug reinforcement, reinstatement, and extinction (Figure 1). The principal investigator should clearly describe the experimental paradigms and various conditions to which the animals will be subjected. This description should include items such as the procedure for training self-administration, how withdrawal response will be monitored and measured, what environmental stimulus is used to signal drug availability, the schedule of drug administration and response requirements, and the use of positive or negative reinforcement to maintain behavior in drug discrimination procedures. Paramount to all these studies are the reinforcing effects of drugs of abuse, and it is these effects that allow researchers to study behaviors such as drug craving, relapse, motivational status, and genetic differences in drug preference.49,60 Because access to the study drug is carefully controlled, the risk of overdose or death is minimal. Once animals are trained in the protocol of a drug abuse study, they become very valuable and investigators will go to great lengths to ensure their health and longevity.
The use of nonhuman primates may raise IACUC concern. In substance abuse research, the use of nonhuman primates are only utilized when it affects the validity of the model and their use is very often based on the drug(s) class of interest. The intravenous self-administration paradigm has been extensively validated in nonhuman primates for use in abuse liability assessment.3-5,64 This model has been shown to provide excellent correlation between drugs which monkeys self-administer and those abused by humans. Results with rodent models have been less reliable in their relevance to assessing human abuse potential across all drug classes. For example, N-methyl D-aspartate antagonists share reinforcing effects in humans and nonhuman primates,12,70,113 but these drugs have been very difficult to show as reinforcers in rodent species, for which specific and sometimes limited testing parameters may be required.30,84 This consideration becomes particularly important when assessing novel classes of drugs for which predictability in rodents is unknown.
The anatomy and physiology of the brain, and of specific receptor systems in particular, can provide justification for the species selection. In some instances, rodents lack the primary drug target or have known differences in the drug target from primates, such as different receptor numbers, distribution, or receptor subtypes expressed. For example, opioid receptors in particular are more similar in these characteristics between humans and nonhuman primates than between humans and other mammalian species, such as rodents.61,65,94,95
In addition, the pharmacokinetic profile of the drug may play a key role in the results of testing, thus providing another compelling reason for the use of primate versus rodent subjects. This factor is especially important in abuse liability self-administration experiments and physical dependence studies. Previous studies have demonstrated that differences in biodisposition can affect self-administration behavior between drugs with similar pharmacologic effects.63,110 Because physical dependence relies on continued occupancy of drug receptor sites, primary dependence studies may be extremely difficult to extrapolate from rodents to humans if the half-life of the drug is very different. This consideration is even more essential when evaluating sustained release or drug combination formulations. Comprehensive pharmacokinetic studies in humans, monkeys, rats, and dogs have shown that monkeys provide the most qualitatively and quantitatively accurate predictions of human pharmacokinetic parameters and the least-biased predictions compared with other species.43,106,107
Regardless of the species used, the protocol also should describe anticipated negative effects related to the drugs used. The effects that are relevant are those affecting pain and distress and food and water consumption, adverse reactions to novel compounds, and effects related to drug withdrawal, especially when novel compounds are introduced. When considering withdrawal, the adverse effects depend on the type of drug and the health status of the animals.89 It is important to note that not all drugs of abuse produce physical dependence. Extensive exposure to psychostimulants is associated with psychologic dependence, and this feature is a key component of many of the addiction models discussed earlier. However, cessation of these drugs does not result in physiologic responses reflecting alterations in the body's homeostatic set points. In addition, many drug abuse models specifically design the dosing regimen to avoid the development of tolerance and dependence because these are not the questions being asked and may actually modify the results obtained. For studies designed to evaluate the production of physical dependence, withdrawal syndromes are not alike across all pharmacologic classes. Opioid withdrawal in rodents is characterized by diarrhea, rhinorrhea, teeth chattering, ‘wet dog shakes,’ and decreased food consumption (anorexia) leading to weight loss. Although unpleasant, opiod withdrawal in rodents is not typically life-threatening.38 Behaviors associated with nicotine withdrawal include writhes, gasps, shakes, tremors, teeth chattering, chewing, ptosis, and scratching.57 A heightened startle response is an indication of increased irritability after termination of nicotine infusion. Withdrawal from CNS depressants such as ethanol, benzodiazepines, and barbiturates conversely cause anxiety, elevated blood pressure, and potentially life-threatening seizures.51,79 In fact, one of the tests for assessing ethanol withdrawal severity in rats is susceptibility to audiogenic seizures.45 Protocols including this test may be categorized as Pain Category E (that is, unrelieved pain or distress), because administration of any sedative or analgesic drug would compromise seizure behavior. Although the seizures generally are short-lived (less than 60 s), careful monitoring is required, as is a contingency plan to treat any complications. In contrast, some models of nicotine dependence produce subtle signs of withdrawal and may actually necessitate the use of highly sensitive measures such as responding for food reinforcement to measure withdrawal.57
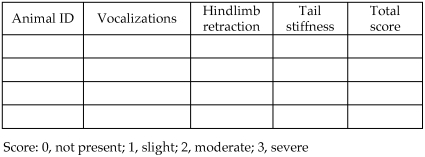
For those studies designed to produce physical dependence as evaluated by withdrawal, the IACUC should consider the constellation of withdrawal signs associated with the pharmacologic class of target drugs and the dose and dosing frequency to be used before automatically categorizing the pain category as E. Although higher and more frequent dosing enhance the severity of dependence and withdrawal, care also should be taken to ensure that frequency of administration is adequate to maintain blood levels sufficiently high at all times to prevent periodic inadvertent spontaneous withdrawal. During assessment of withdrawal, a plan should be in place for the response to withdrawal signs, including contingency plans for potentially life-threatening signs (such as seizures) and when and how withdrawal will be terminated. Typically cessation of withdrawal is accomplished by administration of the dependence-producing drug at a point based on scoring of the degree of severity of withdrawal or following a set time point when the withdrawal syndrome is mild. Checklists or numeric scoring systems for monitoring withdrawal, such as the scoring system used for monitoring alcohol withdrawal (Figure 4), can be useful. Also important is the observation schedule during spontaneous withdrawal assessment—for drugs with long half-lives, onset of spontaneous withdrawal can be protracted, and the committee should be sure monitoring is sufficient to observe animals when withdrawal ensues. The final consideration is disposition of the subjects. Whether subjects are to be euthanized at the end of the study or proceed to other projects, as is the case with many nonhuman primate subjects, they should not be abruptly removed from any drug on which they are physically dependent, but rather should be gradually weaned from the drug or euthanized while still receiving the drug to avoid inadvertent withdrawal.
Figure 4.
Alcohol dependence withdrawal scoring. This scoring sheet is useful for determining the time course of the alcohol withdrawal syndrome. Animals are scored based on presence and severity of above clinical manifestations. A total score ≥ 3 indicates signs of withdrawal. Higher scores signify peak expression of withdrawal. A low score is indicative of an animal at the beginning or end stage of withdrawal or lack of dependence induction.
A potential concern in all these studies is the repeated administration of drug solutions. Drugs should be obtained at a high level of purity, to avoid potential contamination and adverse events related to unknown contaminants. Drugs that cannot be obtained in sterile condition should be filter-sterilized prior to injection. The potential for adverse reactions to novel drugs requires careful monitoring of clinical condition. Animals should be weighed on a regular schedule (depending on type of study), and food and water consumption monitored at least qualitatively. Signs of drug toxicity or distress warrant permanent or temporary removal from the study or at least decreasing of the drug dose and close monitoring of the animal's health status.
The clinical and husbandry issues in drug abuse research vary in intensity depending on the type of study. An abuse liability self-administration study may present very different clinical issues than does a physical dependence or addiction study. One consideration involves the potential for pain or development of drug withdrawal signs, as discussed previously. In these studies, choosing an appropriate analgesic that will not interfere with any components of the particular study is always a concern. This challenge is complicated further when surgery or other invasive procedures are involved. However these challenges are not unique to substance abuse research and must be managed successfully in all research protocols. Careful planning and coordination of veterinary and animal care with the investigative staff is imperative, especially in the early stages of protocol development. For example, use of a nonsteroidal antiinflammatory drug such as carprofen or a long-acting local anesthetic to provide analgesia after vascular catheterization or other required surgical procedure allows the investigator to avoid administering an opioid.
Another challenge is related to the length of time animals remain on study. Investigators have invested substantially in training the animals for many of these models and will continue to maintain these valuable resources as long as they remain healthy. Old animals are common, and eventually many of the geriatric and chronic diseases that are rarely seen in other research studies become a consideration in these animals. All species should be monitored for conditions such as cancer, amyloidosis (from chronic antigenic stimulation by catheters, repeated injection, and implants), and organ failure (either from aging or chronic drug exposure).74 For rodents, diseases such as Mycoplasma pulmonis and pinworms may become clinically apparent with age.29 The presence of any rodent viral disease in the facility has potential to affect drug study animals.
Food or caloric restriction is necessary for some of the animal models described. These animals are sometimes maintained below their free-feed weight in an effort to encourage response to food and drug reinforcements. Studies have shown that ad libitum food access does not necessarily provide the optimum feeding conditions, and longer healthier life spans are associated with limited caloric intake across multiple species.20,34,81 Nonetheless, regular monitoring of weight gain and loss is essential when caloric intake is limited. The IACUC should ensure that the protocol clearly defines to what extent food restriction is permitted, for how long, the amount of weight loss permitted, the frequency of weighing, and the action taken if weight loss exceeds that limit (for example, increase feed or euthanasia).
Often, because the drugs being tested have behavioral effects or because they need to be administered by means of a chronic indwelling device, animals need to be housed singly to safeguard the device or to protect the animals from aggressive behavior that may be associated with a drug effect. This need presents challenges for environmental enrichment. Although some compensation for the lack of cage conspecifics is desirable, enrichment itself can be a variable affecting experimental results and therefore may need to be minimized or avoided if scientifically justified.55
Many animals used in these studies require chronic indwelling devices such as jugular vein catheters. Improper catheter placement, inability to protect the catheter from injury and dislodgement, loss of patency due to thrombosis or occlusion, development of chylothorax due to thoracic duct damage, and infection are common problems. These issues can be addressed with appropriate training, use of appropriate catheter materials, and careful attention to aseptic surgical technique. To extend the use of an animal (both rodents and primates), investigators may catheterize a number of large veins other than the jugular, including the femoral (rodents and primates), brachial (primates), subclavian (primates), and even collateral veins that surface (primates). Catheter occlusion can be addressed by using dilute heparin solution infused continuously, heparin-impregnated catheters, and alternative catheter materials.28 Commercial serine proteases, such as alteplase and urokinase, may be effective in unblocking an occluded catheter.
Another consideration is the pharmacologic makeup of novel drugs being tested and their ability to be administered effectively. When working with these compounds, it is wise to assume nothing and verify everything. For example, some of these drugs are formulated to be active at an extreme pH. With most drugs, blood is primarily responsible for pH buffering; however, care should be taken whenever using a drug with pH at either extreme of the range.10,86 Depending on the volume to be administered, a pH of 5 to 9 is recommended for intravenous administration, pH 6 to 8 for intramuscular or intraperitoneal administration, and approximately pH 7 for subcutaneous administration to avoid pain and necrosis at the injection site. Whenever possible, investigators should identify the class of drug or its anticipated receptor target so that when unknown reactions occur, the veterinary staff can better anticipate the organ systems that might be impaired and how to treat the clinical effects.
Controlling the administration of inhalant drugs can be much less predictable than for other dosing routes, especially when working with smoking chambers. Critical in these studies is verifying and maintaining adequate oxygen flow.50,72 An animal lying quietly in its chamber may be manifesting behavior expected from the pharmacologic effect of the administered drug or could be experiencing hypoxia due to equipment malfunction and inadequate available oxygen.
Specialized equipment used in drug abuse and addiction research studies include rotating swivel arms or special jackets to protect catheters or other devices from tangling or becoming damaged, testing chambers used for behavioral assessments, and inhalation chambers. Sanitation of these chambers can be difficult, and both researchers and veterinary staff must be aware of trafficking of animals if animals being tested in the same chamber or room originate from different barrier facilities. If the chamber or behavior platform is simple, then sanitation and the control of disease transmission are equally so. However, an operant chamber typically contains sensitive electronic parts and intricate mechanical components, thereby complicating sanitation. Inhalation chambers may be elaborate as well, with many components requiring disassembly for adequate sanitation. Manually wiping all exposed surfaces with a disinfectant such as a quaternary ammonium compound is one option; doing so at the end of a work day or at the end of a work week allows odors to dissipate prior to beginning the next test session. Other possibilities include periodically placing the entire operant chamber in an ethylene oxide or hydrogen peroxide sterilization chamber. Another important consideration is preparation for power failures. If emergency power is not available to the testing equipment, all continuous-infusion pumps should be equipped with automatic shut-off mechanisms.
Finally, animal care and veterinary staff must recognize the importance of minimizing environmental changes, which can dramatically alter the behavior of animals on (and therefore research results from) drug studies. When staff changes occur, so do the odors, sounds, and work routines that surround the animals. Anecdotal accounts suggest that certain perfumes affect behavioral responding. Construction noises and bedding changes can create frustration for an investigator if advance notice is not provided.109 Rodents tend to be nocturnal, and strong evidence suggests that studies run during a reverse light cycle can produce more stable responding.82 The presence or absence of enrichment can affect behavior, and many of the drugs used to treat illness have the potential to interfere with the drugs being tested, which in turn can affect behavioral response. If changing environmental conditions is unavoidable, investigators must be informed in a timely manner so that they can plan accordingly to minimize effects on research outcomes.
Conclusion
Substance abuse is perceived by many as a human, self-inflicted disease. Other than in the area of pain research, some people may not readily see the societal benefits derived from these studies. The public questions why animals are made to ‘suffer’ for a problem that people inflict on themselves. Animal rights extremists may exploit this sentiment as they attempt to generate opposition to this area of research. However, addictive diseases and their comorbid clinical conditions (such as HIV, hepatitis C, cirrhosis) are biomedical diseases with massive personal and societal costs, and brain structures and responses often are chronically affected in long-term addicts.
With regard to the validity of animal models for furthering our understanding of substance abuse and addictive drugs, traditional self-administration procedures have firmly established that drugs of abuse function as reinforcers in animals. Although the reinforcing effects of drugs are certainly important in the acquisition and maintenance of the addiction process, it is becoming increasingly apparent that other factors are involved. The shift to focusing on vulnerability factors for addiction and the use of models that more closely mimic characteristics of addiction in humans is likely to advance our ability to understand the key factors involved in addiction, and ultimately, identify potential pharmacologic and environmental treatments. Furthermore, the search for pain-relieving drugs with less abuse liability is a key area of research with potential to benefit vast numbers of persons suffering from chronic pain. Preclinical models of substance abuse provide an excellent screening process for evaluation of new medications. Although data obtained during clinical trials provide helpful information, the use of animal models to evaluate these compounds under controlled conditions is essential. In addition, some of the most appropriate reference drugs for comparison may be prohibitive for testing in humans (for example, abused inhalants and class I compounds such as LSD), requiring that testing be done in preclinical models. Both the IACUC and veterinarian play crucial roles in overseeing and assisting this area of research and tackling sometimes difficult ethical issues. The models often require long-term studies in either rodents or nonhuman primates, surgical procedures including chronic instrumentation, food or caloric restriction, specialized housing and testing equipment, substantial use of controlled substances with its associated issues of drug reinforcing behavior and withdrawal symptoms, and administration of novel drugs with potentially unknown clinical effects. Investigators will benefit from the guidance of informed animal care and veterinary staff, and as with all areas of animal research, consideration of alternatives (replacement, reduction, and refinement) is important to continually improve these animal models.
References
- 1.Ahmed SH, Koob GF. 1998. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SH, Walker JR, Koob GF. 2000. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421 [DOI] [PubMed] [Google Scholar]
- 3.Ator NA, Griffiths RR. 1987. Self-administration of barbiturates and benzodiazepines: a review. Pharmacol Biochem Behav 27:391–398 [DOI] [PubMed] [Google Scholar]
- 4.Balster RL. 1991. Drug abuse potential evaluation in animals. Br J Addict 86:1549–1558 [DOI] [PubMed] [Google Scholar]
- 5.Balster RL, Bigelow GE. 2003. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend 70:S13–S40 [DOI] [PubMed] [Google Scholar]
- 6.Banks ML, Gould RW, Czoty PW, Nader MA. 2008. Relationship between response rates and measures of reinforcing strength using a choice procedure in monkeys. Behav Pharmacol 19:365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardo MT, Rowlett JK, Harris MJ. 1995. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19:39–51 [DOI] [PubMed] [Google Scholar]
- 8.Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. 2004. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12:163–172 [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shahar O, Moscarello JM, Ettenberg A. 2006. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res 1095:148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin E, Oropello JM, Abalos AM, Hannon EM, Wang JK, Fischer E, Iberti TJ. 1994. Effects of acid–base correction on hemodynamics, oxygen dynamics, and resuscitability in severe canine hemorrhagic shock. Crit Care Med 22:1616–1623 [PubMed] [Google Scholar]
- 11.Bozarth M. 1990. Drug addiction as a psychobiological process, p 112–134 : Warburton DM. Addiction controversies. London (UK): Harwood Academic Publishers [Google Scholar]
- 12.Brady KT, Woolverton WL, Balster RL. 1982. Discriminative stimulus and reinforcing properties of etoxadrol and dexoxadrol in monkeys. J Pharmacol Exp Ther 220:56–62 [PubMed] [Google Scholar]
- 13.Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. 2008. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 33:2969–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunton LL, Lazo JS, Parker KL. 2006. Goodman and Gilman's the pharmacological basis of therapeutics, 11th ed New York (NY): McGraw–Hill [Google Scholar]
- 15.Campbell UC, Carroll ME. 2000. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol 8:312–325 [DOI] [PubMed] [Google Scholar]
- 16.Carroll ME, Lac ST. 1993. Autoshaping IV cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology (Berl) 110:5–12 [DOI] [PubMed] [Google Scholar]
- 17.Carroll ME, Lac ST. 1998. Dietary additives and the acquisition of cocaine self-administration in rats. Psychopharmacology (Berl) 137:81–89 [DOI] [PubMed] [Google Scholar]
- 18.Carroll ME, Lac ST, Nygaard SL. 1989. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl) 97:23–29 [DOI] [PubMed] [Google Scholar]
- 19.Carroll ME, Mattox AJ. 1997. Drug reinforcement in animals, p 3–38 : Johnson BA, Roache JD. Drug addiction and its treatment: nexus of neuroscience and behavior. New York (NY): Lippencott–Raven Press [Google Scholar]
- 20.Carroll ME, Meisch RA. 1979. Effects of food deprivation on etonitazene consumption in rats. Pharmacol Biochem Behav 10:155–159 [DOI] [PubMed] [Google Scholar]
- 21.Cartwright WS. 1999. Costs of drug abuse to society. J Ment Health Policy Econ 2:133–134 [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention, National Center for Health Statistics 2006. Health, United States, 2006, with chartbook on trends in the health of Americans with special feature on pain. Washington (DC): US Department of Health and Human Services [Google Scholar]
- 23.Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. 1984. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 82:6–13 [DOI] [PubMed] [Google Scholar]
- 24.Davis BA, Clinton SM, Akil H, Becker JB. 2008. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred high-responder and low-responder rats. Pharmacol Biochem Behav 90:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fattore L, Piras G, Corda MG, Giorgi O. 2009. The Roman high- and low-avoidance rat lines differ in the acquisition, maintenance, extinction, and reinstatement of intravenous cocaine self-administration. Neuropsychopharmacology 34:1091–1101 [DOI] [PubMed] [Google Scholar]
- 26.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. 2005. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759 [DOI] [PubMed] [Google Scholar]
- 27.Fitch TE, Roberts DC. 1993. The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend 33:119–128 [DOI] [PubMed] [Google Scholar]
- 28.Foley PL, Barthel CH, Brausa HR. 2002. Effect of covalently bound heparin coating on patency and biocompatibility of long-term indwelling catheters in the rat jugular vein. Comp Med 52:243–248 [PubMed] [Google Scholar]
- 29.Fox JG. 2007. The mouse in biomedical research. Amsterdam (the Netherlands): Academic Press [Google Scholar]
- 30.French ED, Lopez M, Peper S, Kamenka JM, Roberts DC. 1995. A comparison of the reinforcing efficacy of PCP, the PCP derivatives TCP and BTCP, and cocaine using a progressive ratio schedule in the rat. Behav Pharmacol 6:223–228 [PubMed] [Google Scholar]
- 31.George O, Mandyam CD, Wee S, Koob GF. 2008. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33:2474–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glick SD, Raucci J, Wang S, Keller RWJ, Carlson JN. 1994. Neurochemical predisposition to self-administer cocaine in rats: individual differences in dopamine and its metabolites. Brain Res 653:148–154 [DOI] [PubMed] [Google Scholar]
- 33.Gosnell BA, Krahn DD, Yracheta JM, Harasha BJ. 1998. The relationship between intravenous cocaine self-administration and avidity for saccharin. Pharmacol Biochem Behav 60:229–236 [DOI] [PubMed] [Google Scholar]
- 34.Greenberg JA, Boozer CN. 2000. Metabolic mass, metabolic rate, caloric restriction, and aging in male Fischer 344 rats. Mech Ageing Dev 113:37–48 [DOI] [PubMed] [Google Scholar]
- 35.Griffiths RR, Balster RL. 1979. Opioids: similarity between evaluations of subjective effects and animal self-administration results. Clin Pharmacol Ther 25:611–617 [DOI] [PubMed] [Google Scholar]
- 36.Griffiths RR, Bigelow GE, Henningfield JE. 1980. Similarities in animal and human drug-taking behavior, p 1–90 : Mello NK. Advances in substance abuse. Stamford (CT): JAI Press [Google Scholar]
- 37.Griffiths RR, Brady JV, Bradford LD. 1979. Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens., p. 163–208 : Thompson T, Dews P. Advances in behavioral pharmacology, vol 2 New York (NY): Academic Press [Google Scholar]
- 38.Higgins GA, Sellers EM. 1994. Antagonist-precipitated opioid withdrawal in rats: evidence for dissociations between physical and motivational signs. Pharmacol Biochem Behav 48:1–8 [DOI] [PubMed] [Google Scholar]
- 39.Holtzman SG. 1990. Discriminative stimulus effects of drugs: relationship to potential for abuse, p 193–210 : Adler MW Cowan A Testing and evaluation of drugs of abuse. Modern methods in pharmacology, vol 6 New York (NY): Wiley–Liss [Google Scholar]
- 40.Homberg JR, van der Akker M, Raaso HS, Wardeh G, Binnekade R, Schoffelmeer AN, de Vries TJ. 2002. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci 15:1542–1550 [DOI] [PubMed] [Google Scholar]
- 41.Johanson CE. 1990. The evaluation of the abuse liability of drugs. Drug Saf 5 Suppl 1:46–57 [DOI] [PubMed] [Google Scholar]
- 42.Johanson CE, Balster RL. 1978. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull Narc 30:43–54 [PubMed] [Google Scholar]
- 43.Jolivette LJ, Ward KW. 2005. Extrapolation of human pharmacokinetic parameters from rat, dog, and monkey data: molecular properties associated with extrapolative success or failure. J Pharm Sci 94:1467–1483 [DOI] [PubMed] [Google Scholar]
- 44.Katz JL, Higgins ST. 2003. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 168:21–30 [DOI] [PubMed] [Google Scholar]
- 45.Keane B, Leonard BE. 1989. Rodent models of alcoholism: a review. Alcohol Alcohol 24:299–309 [DOI] [PubMed] [Google Scholar]
- 46.Kippin TE, Fuchs RA, See RH. 2006. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 187:60–67 [DOI] [PubMed] [Google Scholar]
- 47.Koob GF. 2003. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol 13:442–452 [DOI] [PubMed] [Google Scholar]
- 48.Le A, Shaham Y. 2002. Neurobiology of relapse to alcohol in rats. Pharmacol Ther 94:137–156 [DOI] [PubMed] [Google Scholar]
- 49.Li TK. 2000. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J Stud Alcohol 61:5–12 [DOI] [PubMed] [Google Scholar]
- 50.Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR. 2001. The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend 63:107–116 [DOI] [PubMed] [Google Scholar]
- 51.Little HJ. 1991. The benzodiazepines: anxiolytic and withdrawal effects. Neuropeptides 19 Suppl:11–14 [DOI] [PubMed] [Google Scholar]
- 52.Lynch WJ. 2006. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41 [DOI] [PubMed] [Google Scholar]
- 53.Lynch WJ, Carroll ME. 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroine in rats. Psychopharmacology (Berl) 144:77–82 [DOI] [PubMed] [Google Scholar]
- 54.Lynch WJ, Taylor JR. 2004. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29:943–951 [DOI] [PubMed] [Google Scholar]
- 55.Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. 2007. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. Eur J Neurosci 25:761–771 [DOI] [PubMed] [Google Scholar]
- 56.Maldonado C, Rodriquez-Arias M, Castillo A, Aguilar MA, Minarro J. 2007. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaine-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry 31:932–939 [DOI] [PubMed] [Google Scholar]
- 57.Malin DH, Goyarzu P. 2009. Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol 192:401–434 [DOI] [PubMed] [Google Scholar]
- 58.Mantsch JR, Ho A, Schlussman SD, Kreek MJ. 2001. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 157:31–39 [DOI] [PubMed] [Google Scholar]
- 59.Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. 2004. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement, and brain mRNA levels in rats. Psychopharmacology (Berl) 175:26–36 [DOI] [PubMed] [Google Scholar]
- 60.Markou A, Weiss F, Gold LH, Barak Caine S, Schulteis G, Koob GF. 1993. Animal models of drug craving. Psychopharmacology (Berl) 112:163–182 [DOI] [PubMed] [Google Scholar]
- 61.Mathieu-Kia AM, Fan LQ, Kreek MJ, Simon EJ, Hiller JM. 2001. Mu-, delta-, and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer's disease patients. Brain Res 893:121–134 [DOI] [PubMed] [Google Scholar]
- 62.McCallum SE, Glick SD. 2009. Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett 458:57–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meisch RA. 1987. Factors controlling drug reinforced behavior. Pharmacol Biochem Behav 27:367–371 [DOI] [PubMed] [Google Scholar]
- 64.Mello NK, Negus SS. 1996. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424 [DOI] [PubMed] [Google Scholar]
- 65.Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O'Donnell D. 2003. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol 465:349–360 [DOI] [PubMed] [Google Scholar]
- 66.Morgan D, Brebner K, Lynch WJ, Roberts DC. 2002. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol 13:389–396 [DOI] [PubMed] [Google Scholar]
- 67.Mucha RF, Van der Kooy D, O'Shaughnessy M, Bucenieks P. 1982. Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105 [DOI] [PubMed] [Google Scholar]
- 68.Myers KM, Carlezon WA., Jr 2010. D-Cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol Psychiatry 67:85–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Center on Addiction and Substance Abuse 2009. Shoveling up II: the impact of substance abuse on federal, state, and local budgets. New York (NY): Columbia University [Google Scholar]
- 70.Nicholson KL, Mansbach RS, Menniti FS, Balster RL. 2007. The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-D-aspartate antagonist CP101 606 in rats and rhesus monkeys. Behav Pharmacol 18:731–743 [DOI] [PubMed] [Google Scholar]
- 71.National Institute on Drug Abuse. [Internet] 2007. Drugs, brains, and behavior: the science of addiction. [Cited 05 Feb 2010.] Available at http://www.drugabuse.gov/scienceofaddiction/sciofaddiction.pdf.
- 72.Niyuhire F, Varvel SA, Martin BR, Lichtman AH. 2007. Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther 322:1067–1075 [DOI] [PubMed] [Google Scholar]
- 73.Office of National Drug Control Policy 2001. The economic costs of drug abuse in the United States, 1992–1998 Washington (DC): Executive Office of the President Office of National Drug Control Policy [Google Scholar]
- 74.Percy DH, Barthold SW. 2001. Pathology of laboratory rodents and rabbits. Ames (IA): Iowa State University Press [Google Scholar]
- 75.Perry JL, Anderson MM, Nelson SE, Carroll ME. 2007. Acquisition of intravenous cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav 91:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. 2006. Escalation of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 186:235–245 [DOI] [PubMed] [Google Scholar]
- 77.Perry JL, Nelson SE, Carroll ME. 2008. Impulsive choice as a predictor of acquisition of intravenous cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol 16:165–177 [DOI] [PubMed] [Google Scholar]
- 78.Piazza PV, Maccari S, Deminier JM, Le Moal M, Mormede P, Simon H. 1991. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA 88:2088–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinel JP. 1980. Alcohol withdrawal seizures: implications of kindling. Pharmacol Biochem Behav 13 Suppl 1:225–231 [DOI] [PubMed] [Google Scholar]
- 80.Preston KL, Bigelow GE. 1991. Subjective and discriminative effects of drugs. Behav Pharmacol 2:293–313 [PubMed] [Google Scholar]
- 81.Pugh TD, Klopp RG, Weindruch R. 1999. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging 20:157–165 [DOI] [PubMed] [Google Scholar]
- 82.Roberts DC, Brebner K, Vincler M, Lynch WJ. 2002. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend 67:291–299 [DOI] [PubMed] [Google Scholar]
- 83.Roberts DC, Morgan D, Liu Y. 2007. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry 31:1614–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rocha BA, Ward AS, Egilmez Y, Lytle DA, Emmett-Oglesby MW. 1996. Tolerance to the discriminative stimulus and reinforcing effects of ketamine. Behav Pharmacol 7:160–168 [PubMed] [Google Scholar]
- 85.Rogers JL, DeSantis S, See RE. 2008. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 199:615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothe KF, Schimek F. 1984. New aspects of acid–base balance influences of NH4Cl on intra- and extracellular acid–base equilibrium studies in the rat. J Med 15:135–148 [PubMed] [Google Scholar]
- 87.Schechter MD, Schechter JB, Calcagnetti DJ. 1992. Direct microinjection of cathinone into the rat brain produces discriminative stimuli. Pharmacol Biochem Behav 42:619–623 [DOI] [PubMed] [Google Scholar]
- 88.Schuster CR, Johanson CE. 1988. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser 4:161–175 [DOI] [PubMed] [Google Scholar]
- 89.Scorza FA, Arida RM, Cysneiros RM, Priel MR, de Albuquerque M, Cavalheiro EA. 2003. The effects of alcohol intake and withdrawal on the seizures frequency and hippocampal morphology in rats with epilepsy. Neurosci Res 47:323–328 [DOI] [PubMed] [Google Scholar]
- 90.Senay EC. 1989. Addictive behaviors and benzodiazepines. 1. Abuse liability and physical dependence. Adv Alcohol Subst Abuse 8:107–124 [DOI] [PubMed] [Google Scholar]
- 91.Shahbazi M, Moffet AM, Williams BF, Frantz KJ. 2008. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 196:71–81 [DOI] [PubMed] [Google Scholar]
- 92.Shalev U, Grimm JW, Shaham Y. 2002. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42 [DOI] [PubMed] [Google Scholar]
- 93.Shoaib M, Schindler CW, Goldberg SR. 1997. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 129:35–43 [DOI] [PubMed] [Google Scholar]
- 94.Sim-Selley LJ, Childers SR. 2002. Neuroanatomical localization of receptor-activated G proteins in brain. Methods Enzymol 344:42–58 [DOI] [PubMed] [Google Scholar]
- 95.Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. 1999. Mu and kappa1 opioid-stimulated [35S]guanylyl-5′-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience 94:651–662 [DOI] [PubMed] [Google Scholar]
- 96.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. 2003. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 290:2443–2454 [DOI] [PubMed] [Google Scholar]
- 97.Substance Abuse and Mental Health Services Administration 2008. Results from the 2007 national survey on drug use and health: national findings. Rockville (MD): Office of Applied Studies [Google Scholar]
- 98.Sutton MA, Karanian DA, Self DW. 2000. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology 22:626–641 [DOI] [PubMed] [Google Scholar]
- 99.Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. 2004. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem 89:1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas MJ, Kalivas PW, Shaham Y. 2008. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154:327–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tornatzky W, Miczek KA. 2000. Cocaine self-administration ‘binges’: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology (Berl) 148:289–298 [DOI] [PubMed] [Google Scholar]
- 102.Tzschentke TM, Magalas Z, De Vry J. 2006. Effects of venlafaxine and desipramine on heroin-induced conditioned place preference in the rat. Addict Biol 11:64–71 [DOI] [PubMed] [Google Scholar]
- 103.Valentino RJ, Herling S, Woods JH, Medzihradsky F, Merz H. 1981. Quaternary naltrexone: evidence for the central mediation of discriminative stimulus effects of narcotic agonists and antagonists. J Pharmacol Exp Ther 217:652–659 [PubMed] [Google Scholar]
- 104.Vargas-Irwin C, van den Oord EJ, Beardsley PM, Robles JR. 2006. A method for analyzing strain differences in acquisition of IV cocaine self-administration in mice. Behav Genet 36:525–535 [DOI] [PubMed] [Google Scholar]
- 105.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. 1997. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833 [DOI] [PubMed] [Google Scholar]
- 106.Ward KW, Smith BR. 2004. A comprehensive quantitative and qualitative evaluation of extrapolation of intravenous pharmacokinetic parameters from rat, dog, and monkey to humans. I. Clearance. Drug Metab Dispos 32:603–611 [DOI] [PubMed] [Google Scholar]
- 107.Ward KW, Smith BR. 2004. A comprehensive quantitative and qualitative evaluation of extrapolation of intravenous pharmacokinetic parameters from rat, dog, and monkey to humans. II. Volume of distribution and mean residence time. Drug Metab Dispos 32:612–619 [DOI] [PubMed] [Google Scholar]
- 108.Ward SJ, Lack C, Morgan D, Roberts DC. 2006. Discrete-trials heroin self-administration produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl) 185:150–159 [DOI] [PubMed] [Google Scholar]
- 109.Wersinger SR, Martin LB. 2009. Optimization of laboratory conditions for the study of social behavior. ILAR J 50:64–80 [DOI] [PubMed] [Google Scholar]
- 110.Winger G, Stitzer ML, Woods JH. 1975. Barbiturate-reinforced responding in rhesus monkeys: comparisons of drugs with different durations of action. J Pharmacol Exp Ther 195:505–514 [PubMed] [Google Scholar]
- 111.Wise RA. 1998. Drug-activation of brain reward pathways. Drug Alcohol Depend 51:13–22 [DOI] [PubMed] [Google Scholar]
- 112.World Health Organization. [Internet] 2009. Management of substance abuse. [Cited 03 Feb 2010]. Available at http://www.who.int/substance_abuse/facts/en/index.html.
- 113.Young AM, Woods JH. 1981. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther 218:720–727 [PubMed] [Google Scholar]
- 114.Zakharova E, Wade D, Izenwasser S. 2009. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav 92:131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]