Abstract
The balance between anabolic and catabolic signaling pathways is critical in maintaining cartilage homeostasis and its disturbance contributes to joint diseases such as osteoarthritis (OA). A unique mechanism that modulates the activity of cell signaling pathways is controlled by extracellular heparan endosulfatases Sulf-1 and Sulf-2 (Sulfs) that are overexpressed in OA cartilage. This study addressed the role of Sulfs in cartilage homeostasis and in regulating bone morphogenetic protein (BMP)/Smad and fibroblast growth factor (FGF)/Erk signaling in articular cartilage. Spontaneous cartilage degeneration and surgically induced OA were significantly more severe in Sulf-1−/− and Sulf-2−/− mice compared with wild-type mice. MMP-13, ADAMTS-5, and the BMP antagonist noggin were elevated whereas col2a1 and aggrecan were reduced in cartilage and chondrocytes from Sulf−/− mice. Articular cartilage and cultured chondrocytes from Sulf−/− mice showed reduced Smad1 protein expression and Smad1/5 phosphorylation, whereas Erk1/2 phosphorylation was increased. In human chondrocytes, Sulfs siRNA reduced Smad phosphorylation but enhanced FGF-2-induced Erk1/2 signaling. These findings suggest that Sulfs simultaneously enhance BMP but inhibit FGF signaling in chondrocytes and maintain cartilage homeostasis. Approaches to correct abnormal Sulf expression have the potential to protect against cartilage degradation and promote cartilage repair in OA.
Keywords: osteoarthritis, heparan sulfate, Smad, Erk
Cartilage homeostasis is controlled by extracellular factors such as mechanical loading, cytokines, and growth factors that are translated by intracellular signaling pathways to changes in cell survival, activation, and differentiation. Abnormal activation of these signaling pathways during development results in skeletal dysplasias and, in mature joints, leads to osteoarthritis (OA) (1).
Bone morphogenetic proteins (BMPs) regulate various stages of cartilage and bone development (2–4), promote chondrogenesis from mesenchymal stem cells, and stimulate cartilage repair (5–7). BMPs bind and signal through serine/threonine kinase receptors (8–10). BMP-7 binding to the BMP receptor type IB leads to receptor dimerization so that the type I receptor phosphorylates Smad1/5/8 and stimulates expression of genes that promote cartilage repair (7, 11) and may protect against OA (6, 12). The BMP antagonist noggin is a secreted protein that interacts with heparan sulfate proteoglycans (HSPGs) at the cell membrane where it binds and prevents BMP-2, -4, -6, and -7 from activating their receptors (13).
In contrast, fibroblast growth factor-2 (FGF-2) stimulates catabolic responses in chondrocytes by activating Erk1/2 (14). Models have been proposed to describe the interaction of HSPGs with FGFs and their receptors, yet the precise molecular details remain to be determined (15). It has been established that FGF signaling requires HSPGs to form stable ligand/receptor complexes, apparently by protecting the FGF ligand from proteolytic degradation and by enhancing and stabilizing cell surface ligand/receptor interactions (16).
Extracellular heparan sulfate 6-O endosulfatases Sulf-1 and Sulf-2 (Sulfs) localize to the cell membrane and the extracellular matrix and regulate several major cell signaling pathways (17, 18). Sulfs release the BMP antagonist noggin (19) and interfere with FGF-HS-FGF receptor-1 ternary complex formation (20). Although BMP and FGF were known to antagonize one another during chondrogenesis (21, 22), their roles in cartilage homeostasis and corresponding signaling mechanisms were unknown. We recently reported increased expression of Sulfs in human OA cartilage (23). The present study demonstrates that Sulf-deficient mice develop more severe OA pathology and Sulfs regulate the balance between BMP and FGF signaling to maintain cartilage homeostasis.
Results
Spontaneous and Surgical OA in Sulf−/− Mice.
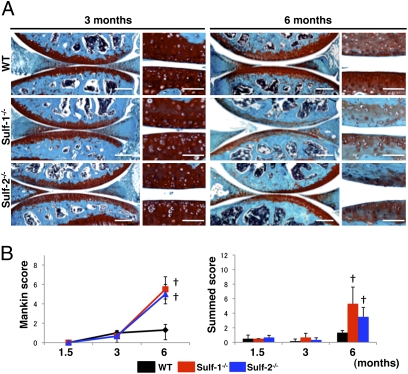
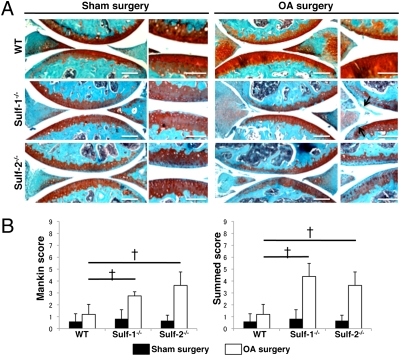
Similar to our previous observations on OA-affected human articular cartilage (23) Sulf-1 and Sulf-2 are overexpressed in mouse joints that are subjected to experimental OA (Fig. S1). In 2-month-old mice Sulfs were increased 4 weeks and more strongly 8 weeks after OA surgery. In 6-month-old mice Sulfs were already increased 2 weeks after surgery and this earlier up-regulation was related to an earlier development of cartilage degeneration in the older mice (Fig. S2). To determine the role of Sulfs in cartilage homeostasis we analyzed Sulf−/− mice. Knee joint sections from wild-type (WT) and Sulf−/− mice were stained with safranin O. At 1.5 and 3 months of age, all strains had an intact articular cartilage surface and homogeneous glycosaminoglycan (GAG) staining (Fig. 1A). At 6 months, Sulf-1−/− and Sulf-2−/− mice showed lower cell density and GAG loss in the tibial and femoral articular cartilage and in the menisci (Fig. 1A). Both Mankin and summed scores that reflect pathological changes in histology were significantly increased in Sulf−/− mice compared with WT, indicating spontaneous cartilage degeneration in Sulf−/− mice (Fig. 1B). The three strains of mice were then subjected to surgically induced OA and the severity of cartilage degeneration was analyzed 4 weeks after surgery. Following OA surgery, knee joints from all mouse strains showed degenerative changes in menisci, predominantly at the anterior sides (Fig. 2A). WT knee joints showed only mild cartilage degeneration as previously reported (24). Loss of safranin O staining and roughening of the articular surface were accelerated in Sulf−/− knee joints, indicating more severe cartilage degeneration (Fig. 2A). Both modified Mankin scores and summed OA scores were not significantly different in the sham surgery groups, whereas OA scores in the mice subjected to surgery were significantly increased in Sulf−/− compared with WT mice (Fig. 2B). These results indicate that deficiency in either Sulf-1 or Sulf-2 leads to more severe spontaneous and surgically induced OA joint pathology.
Fig. 1.
Spontaneous cartilage degeneration in Sulf−/− mice. (A) Knee joints were harvested from WT and Sulf−/− mice at 1.5, 3, and 6 months of age to determine spontaneous development of changes in joint tissues. Sections were stained with safranin O and cartilage degeneration was quantified by using a modified Mankin score and a summed score (n = 6–8 animals per time point). (B) At 6 months, Sulf−/− mouse knee joints showed significantly more severe cartilage degeneration than WT. †, P < 0.01. (Scale bars: low magnification, 200 μm; high magnification, 50 μm.)
Fig. 2.
Surgically induced OA is more severe in Sulf−/− mice. (A) Surgical OA was induced in WT and Sulf−/− mice at the age of 8 weeks (n = 8–10 mice per group) by resecting the medial meniscotibial ligament and harvesting the knee joints 4 weeks after surgery for safranin O staining. Arrow indicates roughening of the articular surface. (B) Sulf−/− mice showed significantly increased Mankin and summed scores compared with WT after surgery. †, P < 0.01. (Scale bars: low magnification, 200 μm; high magnification, 50 μm.)
Reduced Cellularity and Abnormal ECM, MMP-13, ADAMTS-5, and noggin Expression Patterns in Sulf−/− Mice.
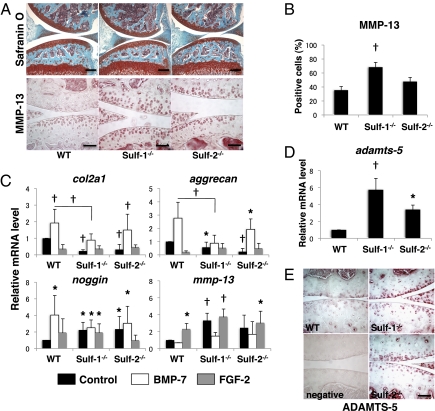
In knee joints from Sulf−/− mice, the number of chondrocytes in articular cartilage was reduced from the age of 4 weeks (Fig. S3). The expression of MMP-13 was increased, especially in Sulf-1−/− compared with WT (Fig. 3 A and B). To determine changes in gene expression patterns in Sulf−/− chondrocytes, col2a1, aggrecan, noggin, and mmp-13 mRNAs were measured by Taqman PCR. Sulf−/− chondrocytes showed reduced basal mRNA levels of col2a1 and aggrecan and increased levels of mmp-13 and noggin compared with WT (Fig. 3C, black bars). Although BMP-7 stimulation increased col2a1, aggrecan, and noggin expression in Sulf−/− cells compared with the WT control, these levels were still lower than in WT chondrocytes stimulated with BMP-7 (Fig. 3C, white bars). This result supports a role of Sulfs in mediating BMP responses. FGF-2 increased mmp-13 in both WT and Sulfs−/− cells, and especially Sulf-1−/− had the highest basal mmp-13 (Fig. 3C, gray bars). Thus, Sulf deficiency is associated with increased mmp-13 and noggin expression whereas col2a1 and aggrecan are reduced (Fig. 3C, black bars). Furthermore ADAMTS-5, a major aggrecan-degrading enzyme in cartilage, was overexpressed in Sulfs−/− compared with WT chondrocytes (Fig. 3 D and E).
Fig. 3.
Abnormal ECM, MMP-13, ADAMTS-5, and noggin expression patterns in Sulf−/− mice. (A) Knee joint sections from mice at the age of 4 weeks were stained with safranin O and for MMP-13 (n = 6). (Scale bars: low magnification for safranin O, 200 μm; high magnification for MMP-13, 50 μm.) (B) Articular cartilage from Sulf−/− mice contained more MMP-13-positive cells especially in Sulf-1−/− compared with WT mice. †, P < 0.01. (C) mRNA expression in articular chondrocytes from WT and Sulf−/− mice. col2a1, mmp-13, and noggin expression was analyzed by Taqman PCR in chondrocytes from WT, Sulf-1−/−, and Sulf-2−/− mice (n = 6–8). (D) mRNA expression of adamts-5 was examined in chondrocytes from WT, Sulf-1−/−, and Sulf-2−/− mice (n = 6). Results are shown as mRNA levels of the indicated genes relative to gapdh (*, P ≤ 0.05; †, P < 0.01). (E) ADAMTS-5 expression was examined in 3-month-old WT, Sulf-1−/−, and Sulf-2−/− mice by immunohistochemistry. (Scale bars: 50 μm.)
Smad and Erk Expression and Activation in Sulf−/− Mice.
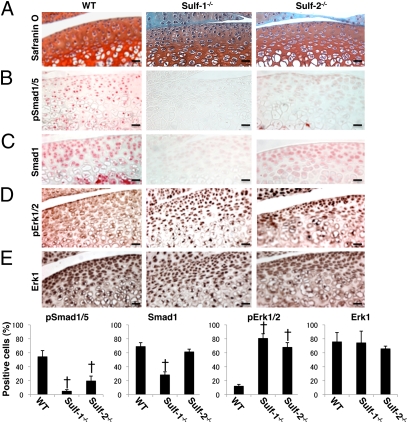
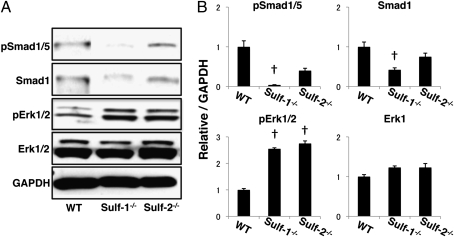
To analyze the role of Sulfs in BMP-7 and FGF-2 signaling, knee joint sections from WT, Sulf-1−/−, and Sulf-2−/− mice were stained with antibodies for total Smad1 and Erk1/2 protein expression and with antibodies specific for phosphorylated forms of Smad1/5 and Erk1/2. Articular cartilage structure, cell density, and organization did not appear different in the Sulf−/− mice compared with WT at the age of 2 weeks (Fig. 4A). Cells positive for Smad1/5 phosphorylation were present in all layers of cartilage in WT mice, whereas their numbers were significantly lower in the Sulf−/− mice, especially in the Sulf-1−/− (Fig. 4B). Smad1 protein expression was also detected throughout all zones of articular cartilage in WT mice, but was profoundly reduced in Sulf-1−/− mice (Fig. 4C). In contrast, Erk1/2 phosphorylation in Sulf−/− mice was markedly increased compared with that in WT (Fig. 4D). Erk1/2 protein expression was similar in all three strains of mice (Fig. 4E). Chondrocytes were isolated from hip and knee articular cartilage of WT, Sulf-1−/−, and Sulf-2−/− mice. Western blotting showed reduced basal levels of Smad1 protein expression and phosphorylation in Sulf-1−/− compared with WT. In contrast, basal Erk1/2 phosphorylation in chondrocytes from Sulf−/− mice was significantly higher than in WT (Fig. 5 A and B). These observations indicate increased Erk1/2 activation and reduced Smad1/5 activation in Sulf−/− mice.
Fig. 4.
Smad and Erk expression and phosphorylation in murine knee joints. (A–E) Knee joint sections from 2-week-old mice were stained with safranin O and immunostained for total and phosphorylated forms of Smad1 and Erk1/2. For quantification total cell numbers and positive cells were counted in cartilage from WT and Sulf−/− mice (n = 6; †, P < 0.01). Results are expressed as percentage of positive cells. (Scale bars: 20 μm.)
Fig. 5.
Smad1/5 and Erk1/2 expression and phosphorylation in chondrocytes from WT and Sulf−/− mice. (A) Western blot analysis of pSmad1/5, Smad1, pErk1/2, and Erk1/2. Protein expression and phosphorylation of Smad1/5 were reduced, but Erk1/2 was increased in Sulf−/− mice. (B) Densitometry of the autoradiographs was performed with NIH Image J software (n = 4; †, P < 0.01).
Sulfs Simultaneously Regulate BMP-7 and FGF-2 Cell Signaling Pathways.
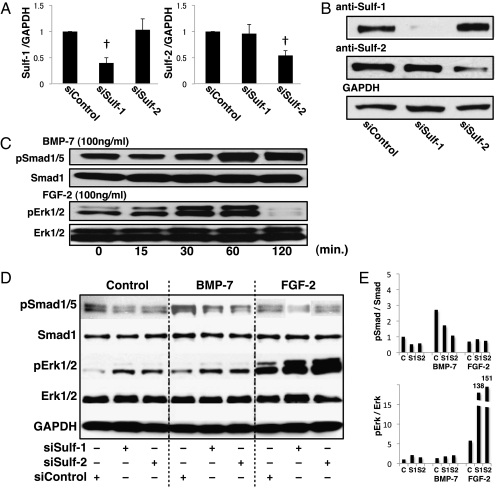
To further analyze the function of Sulfs in BMP-7 and FGF-2 signaling pathways, human chondrocytes were treated with Sulf siRNA and this treatment reduced protein and mRNA expression of Sulf-1 by 70% and Sulf-2 by 50% (Fig. 6 A and B). Human chondrocytes were then stimulated with BMP-7 or FGF-2 and cell lysates were prepared after 0–120 min to examine Smad1/5 and Erk1/2 phosphorylation by Western blotting. As expected, in control cells Smad1/5 and Erk1/2 phosphorylation increased in a time-dependent manner until 60 min after stimulation with BMP-7 or FGF-2, respectively (Fig. 6C). Basal (no BMP-7 or FGF-2) Smad1/5 phosphorylation was reduced by 30%, whereas Erk1/2 phosphorylation was increased 1.5- to 2-fold in Sulf-1 and Sulf-2 siRNA-treated human chondrocytes. BMP-7-induced Smad phosphorylation was inhibited by 40–60% in Sulf-1 and Sulf-2 siRNA-treated cells. In contrast, Sulf-1 and Sulf-2 siRNA greatly enhanced FGF-2-induced Erk1/2 phosphorylation by >100-fold compared with control siRNA (Fig. 6 D and E). Thus, Sulfs promote BMP-induced Smad activation while inhibiting FGF-induced Erk activation in normal human chondrocytes.
Fig. 6.
Sulfs regulate BMP-7-induced Smad1/5 and FGF-2-induced Erk1/2 phosphorylation in human chondrocytes. (A and B) Human articular chondrocytes were transfected with siRNA for Sulf-1 or Sulf-2. RNA was isolated for qPCR and proteins were isolated for Western blotting after 48 h. Sulfs were knocked down specifically by 50–70% (n = 5; †, P < 0.01). (C) Western blotting shows the expression of Smad1/5 and Erk1/2 phosphorylation in response to BMP-7 and FGF-2 stimulation of human chondrocytes from 0 to 120 min. (D) Maximal Smad1/5 and Erk1/2 phosphorylation is observed at 60 min. Chondrocytes were transfected with Sulf siRNA to determine changes in Smad1/5 and Erk1/2 phosphorylation in response to BMP-7 and FGF-2 (each 100 ng/mL) after 60 min by Western blotting. Sulf-1 and Sulf-2 siRNA reduced Smad1/5, but increased Erk1/2 phosphorylation, and densitometry of the autoradiographs was performed with NIH Image J software (E). These representative data are from chondrocytes obtained from a 61-year-old male donor with normal articular cartilage.
Discussion
A unique extracellular mechanism that controls several signaling pathways involves the endosulfatases Sulf-1 and Sulf-2, which are localized at the cell surface and in the ECM (18, 19, 25). The Sulfs remove the 6-O sulfate from heparan sulfate proteoglycans such as syndecan, perlecan, agrin, or glypican that are expressed in articular cartilage (26). Previously we reported overexpression of Sulfs in human OA cartilage (23). To determine the role of Sulfs in articular cartilage, the present study used mice with deletions in Sulf genes. Sulf-1−/− and Sulf-2−/− mice appeared normal at birth and had no detectable skeletal malformations. However, by the age of 6 months, the knee joints of Sulf-1−/− and Sulf-2−/− mice showed early OA-like changes with reduced glycosaminoglycan content and lower cartilage cellularity. This phenotype of the Sulf−/− mice suggests that Sulfs exert a protective or homeostatic function in cartilage and menisci. In the surgical OA model, disease severity was also significantly increased in the Sulf-1−/− and Sulf-2−/− mice with degenerative changes not only in articular cartilage but also in the menisci. Furthermore, when surgical OA was performed in 6-month-old mice, Sulfs expression was increased at earlier time points compared with that in 2-month-old mice. This finding is consistent with earlier and more severe OA in the older mice and suggests that increased Sulfs expression precedes OA-like joint pathology (Fig. S2).
To address potential mediators of the enhanced cartilage degradation in Sulf−/− mice, we examined cartilage cellularity and levels of MMP-13, a major enzyme responsible for degrading type II collagen (27), and ADAMTS-5, a major aggrecan-degrading enzyme (28). The number of chondrocytes in Sulf−/−cartilage was significantly reduced compared with that in WT. Furthermore, both MMP-13 and ADAMTS-5 were significantly increased in cartilage from Sulf−/− mice and cultured chondrocytes from Sulf−/− mice. In addition, the levels of the major cartilage ECM proteins type II collagen and aggrecan were significantly lower in Sulf−/− chondrocytes. These results suggest that Sulfs regulate the overall balance of cartilage matrix synthesis and degradation.
To investigate signaling mechanisms that are regulated by Sulfs in cartilage, we focused on FGF-2/Erk and BMP-7/Smad because these are major pathways in chondrocytes (29) and targets of Sulfs (19, 20). BMP signaling is antagonized by secreted factors such as noggin (30, 31). The binding of noggin to the cell surface and its BMP antagonist activity are dependent on HS 6-O sulfate (13), and this interaction is modulated by Sulfs (19). We show here induction of noggin and reduced Smad phosphorylation and protein expression in Sulf−/−chondrocytes, indicating that Sulfs regulate BMP signaling not only via changes in HS sulfation, but also by controlling noggin expression that can be increased via the FGF-2/Erk pathway (32). Consequences of abnormal noggin and BMP-7/Smad signaling are changes in type II collagen expression as shown here in articular cartilage or during endochondral bone formation (33).
Analysis of knee cartilage from Sulf−/− mice showed not only reduced Smad1/5 phosphorylation but also increased Erk1/2 phosphorylation. Erk can be activated by several extracellular stimuli in cartilage (34), including FGF-2, which also induces MMP-13 (32, 35). When Sulf expression was inhibited by siRNA in human articular chondrocytes, FGF/Erk signaling was increased and BMP/Smad signaling was decreased, and this is consistent with the findings in the Sulf-deficient mice. Sulfated syndecan-3 promotes FGF signaling by stabilizing ligand receptor interactions (36). It is of interest that lack or inhibition of syndecan-4 protects against OA and this is also associated with changes in Erk activation and expression of MMPs and ADAMTS-5 (37). Furthermore, mice with deletion of the Sulfatase-Modifying Factor 1, which catalyzes a posttranslational formylglycination that is required for activation of all sulfatases (38), show a phenotype that is consistent with a positive effect of sulfatases in chondrogenesis that is related to limiting FGF signaling (39).
Sulf-1- and Sulf-2-deficient mice showed a similar phenotype, suggesting these Sulfs might have redundant function. Sulfs double-deficient mice are difficult to propagate due to fertility and postnatal survival problems (40–42). We were able to evaluate double Sulf-deficient mice up to 2 months of age and these mice showed reduced safranin O staining already at 1 month and more profound reduction at 2 months of age, further suggesting that Sulfs are critical for cartilage homeostasis (Fig. S4).
In conclusion, results from the present study suggest that Sulfs simultaneously regulate BMP-7 Smad1/5 and FGF-2 Erk1/2 pathways. BMP/Smad is significantly suppressed, whereas FGF/Erk is accelerated, when Sulfs are reduced. The overexpressed noggin suppresses BMP/Smad signaling. Subsequently, gene expression in Sulf-deficient mice is reduced for anabolic factors such as col2a1 and aggrecan and increased for the catabolic factors mmp-13 and adamts-5. In Sulf−/− mice, this leads to early and more severe OA-like changes.
Materials and Methods
Mice.
Sulf-1−/− and Sulf-2−/− mice on C57BL/6 background were described earlier (41). All animal experiments were performed according to protocols approved by The Scripps Research Institute Institutional Animal Care and Use Committee. Knee joints were harvested from wild-type (WT) and Sulf−/− mice and stained with safranin O to determine spontaneous cartilage degeneration. Experimental OA was induced in WT and Sulf−/− mice at the age of 8 weeks by resecting the medial meniscotibial ligament in the right knee joint (24). Left knees were subjected to sham surgery. Pathological changes in all quadrants of the joint (medial tibial plateau, medial femoral condyle, lateral tibial plateau, and lateral femoral condyle) were evaluated after 4 weeks on safranin O-stained sections by modified Mankin score (43) and a modification of a semiquantitative scoring system (44). Murine articular chondrocytes were harvested from knee and femoral head cartilage of C57BL/6J and Sulf−/− mice at the age of 2–6 weeks and cultured as described (45). Chondrocytes were stimulated with BMP-7 or FGF-2 (both at 100 ng/mL; PeproTech) for 24 h and col2a1, aggrecan, noggin, and mmp-13 gene expression was determined by quantitative PCR.
Histology.
Mouse knee joint sections were stained with safranin O/fast green. Immunohistochemistry for Smad1 and Smad1/5 phosphorylation, Erk1/2 and Erk1/2 phosphorylation, MMP-13, and ADAMTS-5 was performed with specific antibodies (Erk1/2, Santa Cruz Biotechnology; MMP-13, Chemicon; ADAMTS-5, Abcam; others, Cell Signaling Technology). Negative controls were normal rabbit and goat IgG. Antibodies were applied and incubated as described (23). Immunohistochemistry signals were quantified as described previously (46).
Quantitative PCR (qPCR).
Gene expression was analyzed using TaqMan Gene Expression Assay probes for Sulf-1 (Hs00290918_m1), Sulf-2 (Hs00378697_m1), col2a1 (Mm01309565_m1), aggrecan (Mm00545807_m1), mmp-13 (Mm01168713_m1), noggin (Mm01297833_s1), adamts-5 (Mm01344182_m1), and gapdh (Hs99999905_m1, Mm99999915_g1) according to the manufacturer's protocol (Applied Biosystems). Gene expression levels were assessed relative to GAPDH.
Chondrocyte Isolation and siRNA Transfection.
Cartilage from human femoral condyles and tibial plateaus was obtained at autopsy from normal tissue donors and chondrocytes were isolated as described (47). Experiments were performed with high-density cultured chondrocytes in passages 1 and 2. Sulf expression was knocked down by siRNA (Ambion). Chondrocytes (0.35 × 106) were seeded in six-well plates, transfected with Sulf or control siRNA by using 5 μL Lipofectamine 2000 (Invitrogen), and cultured for 48 h.
Western Blotting and Densitometry.
Human chondrocytes were stimulated with BMP-7 or FGF-2 (both at 100 ng/mL; PeproTech) and cell lysates were prepared at the time points indicated. Sulf-1 and Sulf-2 proteins were determined by Western blotting using antibodies from Abcam as described previously (23). The Western blots were analyzed by densitometry using NIH image J software.
Statistical Analysis.
Statistically significant differences were determined with a two-tailed Student's t test between two groups and a one-way ANOVA between more than two. The results are reported as mean ± SEM. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Xingbin Ai (Boston University) for Sulf-deficient mice. Lilo Creighton, Diana C. Brinson, Eunnie Kim, and Jean Valbracht provided excellent technical support. This work was supported by National Institutes of Health Grants AI1072155 (to C.-H.W.), AG007996 and AG033409 (to M.K.L.), and AR050631 (to H.A.), the Sam and Rose Stein Endowment Fund, the Arthritis National Research Foundation (S.M.), and a postdoctoral fellowship from the Arthritis Foundation (to S.O.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913897107/-/DCSupplemental.
References
- 1.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- 3.Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- 4.Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 5.Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16:1121–1130. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Muneta T, Ju YJ, Mochizuki T, Sekiya I. Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther. 2008;10:R118. doi: 10.1186/ar2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig BB, et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ten Dijke P, et al. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 10.Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 11.Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC. Effects induced by BMPS in cultures of human articular chondrocytes: Comparative studies. Growth Factors. 2008;26:275–283. doi: 10.1080/08977190802291733. [DOI] [PubMed] [Google Scholar]
- 12.Badlani N, Inoue A, Healey R, Coutts R, Amiel D. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Osteoarthritis Cartilage. 2008;16:600–606. doi: 10.1016/j.joca.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Paine-Saunders S, Viviano BL, Economides AN, Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: A potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277:2089–2096. doi: 10.1074/jbc.M109151200. [DOI] [PubMed] [Google Scholar]
- 14.Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhoot GK, et al. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- 19.Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem. 2004;279:5604–5611. doi: 10.1074/jbc.M310691200. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, et al. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc Natl Acad Sci USA. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 22.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki S, et al. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10:R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Ai X, et al. Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J Biol Chem. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- 26.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell PG, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 29.Reddi AH. Cartilage morphogenetic proteins: Role in joint development, homoeostasis, and regeneration. Ann Rheum Dis. 2003;62(Suppl 2):ii73–ii78. doi: 10.1136/ard.62.suppl_2.ii73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 31.Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- 32.Li X, et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008;10:R48. doi: 10.1186/ar2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng Y, Valbracht J, Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest. 1996;98:2425–2430. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uria JA, et al. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol. 1998;153:91–101. doi: 10.1016/S0002-9440(10)65549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirsch T, Koyama E, Liu M, Golub EE, Pacifici M. Syndecan-3 is a selective regulator of chondrocyte proliferation. J Biol Chem. 2002;277:42171–42177. doi: 10.1074/jbc.M207209200. [DOI] [PubMed] [Google Scholar]
- 37.Echtermeyer F, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 38.Diez-Roux G, Ballabio A. Sulfatases and human disease. Annu Rev Genomics Hum Genet. 2005;6:355–379. doi: 10.1146/annurev.genom.6.080604.162334. [DOI] [PubMed] [Google Scholar]
- 39.Settembre C, et al. Proteoglycan desulfation determines the efficiency of chondrocyte autophagy and the extent of FGF signaling during endochondral ossification. Genes Dev. 2008;22:2645–2650. doi: 10.1101/gad.1711308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holst CR, et al. Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS One. 2007;2:e575. doi: 10.1371/journal.pone.0000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ai X, et al. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development. 2007;134:3327–3338. doi: 10.1242/dev.007674. [DOI] [PubMed] [Google Scholar]
- 42.Ratzka A, et al. Redundant function of the heparan sulfate 6-O-endosulfatases Sulf1 and Sulf2 during skeletal development. Dev Dyn. 2008;237:339–353. doi: 10.1002/dvdy.21423. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48:2509–2518. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- 44.Chambers MG, et al. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum. 2001;44:1455–1465. doi: 10.1002/1529-0131(200106)44:6<1455::AID-ART241>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 45.Salvat C, Pigenet A, Humbert L, Berenbaum F, Thirion S. Immature murine articular chondrocytes in primary culture: A new tool for investigating cartilage. Osteoarthritis Cartilage. 2005;13:243–249. doi: 10.1016/j.joca.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Zemmyo M, Meharra EJ, Kuhn K, Creighton-Achermann L, Lotz M. Accelerated, aging-dependent development of osteoarthritis in alpha1 integrin-deficient mice. Arthritis Rheum. 2003;48:2873–2880. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]
- 47.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146:75–85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.