Abstract
Interferon regulatory factor (IRF) 5 is a key transcription factor for the activation of innate immune responses downstream of Toll-like receptor signaling. Based on recent genetic analyses, IRF5 is a focus for its potential involvement in systemic lupus erythematosus (SLE), although how IRF5 contributes to SLE is uncertain. In this study, we demonstrate a requirement for IRF5 in the development of murine SLE via its role in B lymphocytes. We show that antinuclear autoantibodies and Ig glomerular deposits, hallmarks of SLE, are absent in Irf5−/− mice challenged to develop SLE by pristane injection. In particular, production of autoantibodies of the IgG2a subtype, the most prominent isotype in inducing autoimmunity, requires IRF5. Finally, we provide evidence for the critical role of this transcription factor in the secretion of pathogenic antibodies through its direct control of class switch recombination of the γ2a locus. By demonstrating a B-cell-intrinsic role, this study places IRF5 in a context that may have implications for understanding the pathogenesis of human SLE.
Keywords: autoimmunity, B lymphocytes, TLR signaling
B lymphocytes are central to the development of systemic lupus erythematosus (SLE). The loss of B-cell tolerance is well-documented to increase the incidence of SLE disease in mice and humans, and the effectiveness of anti-B-cell therapies in the treatment of SLE further demonstrates their crucial involvement (1–3). B cells can contribute to SLE by their ability to present antigens to autoreactive T cells, activate inflammatory responses by secretion of cytokines and chemokines, and, especially, secrete autoantibodies. Indeed, a cardinal feature of SLE is the presence of autoantibodies with specificities against DNA and nuclear ribonucleoprotein (nRNP) antigens, typically IgG1 and IgG3 (IgG2a and IgG2b in the mouse), in the circulation and in end organs as immune complex (IC) deposits (4–6). ICs may be particularly important to the propagation of SLE by virtue of their dual ability to induce tissue damage and serve as “autoadjuvants” (1, 7).
Activated B cells can switch the constant region of its immunoglobulin (Ig) heavy chain through the process of class switch recombination (CSR), a DNA break and nonhomologous end-joining event within the Ig heavy-chain locus in which IgM/IgD-expressing B cells are made to express IgG, IgA, or IgE isotypes. CSR is necessary for the production of Ig with original antigen specificity but enhanced immune effector functions. The IgG2a isotype in particular plays a critical role in protective and pathological immune conditions in mice; IgG2a is the predominant isotype elicited during virus infection, most effective in directing antibody-dependent cellular cytotoxicity and most potent in inducing autoimmune anemia (8–11). This is due to its relatively higher and lower affinity for activating and inhibitory Fcγ receptors, respectively (12). IgG2a CSR can be induced in B cells by a number of stimuli including a combination of Toll-like receptor (TLR) 4 plus interferon γ receptor (IFNGR) or by ligands to TLR9 (13). Initiation of Ig CSR requires the germ-line transcription (GLT) of so-called sterile mRNA transcripts at the germ-line promoters of the to-be-rearranged α, ε, or γ constant region gene. Although T-bet and Stat1 transcription factors are shown to regulate GLT of IgG2a, a detailed mechanism still remains unknown (14, 15). Interestingly, both of these transcription factors are involved in the production of SLE-related autoantibodies (14, 16).
IRF5 is a member of the interferon regulatory factor (IRF) gene family that is known for its activation of TLR-mediated innate immune responses, particularly the induction of type I IFN and proinflammatory cytokine genes (17, 18). A potential role for IRF5 in SLE pathogenesis in humans is indicated by the observation that several polymorphisms that increase IRF5 mRNA stability and protein abundance raise the susceptibility to develop SLE (19, 20). In view of IRF5’s ability to regulate the induction of type I IFNs and the involvement of type I IFNs in the pathogenesis of SLE, the current conventional wisdom is that IRF5 may drive SLE development by causing the aberrant production of type I IFN through TLR9 and/or TLR7 signaling activated by ICs (21, 22). This is supported by the ample genetic evidence that TLR7 and TLR9 are involved in the development of lupus-like disease in mice. TLR7 promotes autoimmunity and the development of autoantibodies with specificities against nRNP antigens, whereas TLR9 drives the development of anti-double-stranded (ds) DNA antibodies (23). However, the complexity of TLR9’s contribution to autoimmunity is provided by the findings that autoimmune-prone TLR9-deficient mice have exacerbated symptoms of disease (24, 25).
Recently, IRF5 was demonstrated to be required for murine lupus in autoimmune-prone FcγRIIb−/−Yaa mice, although no mechanism was described (26). Whereas type I IFN production induced in murine dendritic cells (DCs) by stimulation with human IC is IRF5-dependent, albeit partially, it is unclear whether this is a mechanism by which IRF5 contributes to SLE disease development (27). Moreover, a study found that IRF5 is involved in the differentiation of splenic B220+CD138+ B cells in older mice through its regulation of Blimp-1 expression (28), suggesting that IRF5 can regulate humoral immune responses. In the present study, we demonstrate that IRF5’s contribution to murine SLE-like disease induced by pristane injection is likely due in part to its role in B cells. In particular, we reveal that IRF5 is required for secretion of IgG2a- and IgG2b-isotype-switched antibodies in vivo and in vitro, and provide molecular evidence that IRF5 regulates GLT of the IgG2a locus. Finally, we discuss our findings of IRF5’s role in B cells and their implications for human SLE.
Results
IRF5 Regulates Autoantibody Production in a Murine Model System for SLE.
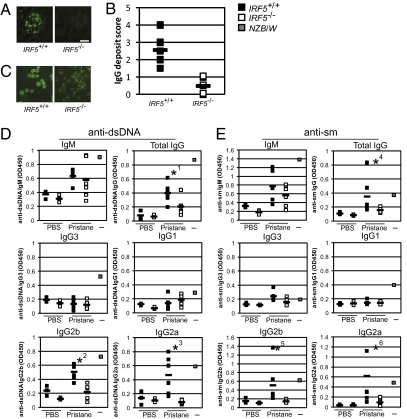
Intraperitoneal injection of pristane oil into C57BL/6 mice induces a condition of chronic apoptosis and inflammation within the peritoneal cavity (29). These mice progressively develop key features of human SLE disease such as elevated expression of type I IFN and IFN-inducible genes, secretion of autoantibodies against ds- and single-stranded (ss) DNA, nRNP, Sm and Su self-antigens, and development of IC-mediated kidney disease. To examine IRF5’s contribution to the development of SLE-like disease in mice, IRF5 homozygous null (Irf5−/−) and wild-type (Irf5+/+) mice were subjected to a single injection of pristane oil, and then 10 months postinjection serum and kidney from these mice were analyzed for the presence of autoantibodies and IgG glomerular deposits, respectively. We observed that IgG deposits assayed by immunofluorescence are far more obvious in Irf5+/+ glomeruli (Fig. 1A). When the prevalence and intensity of IgG-infiltrated glomeruli were scored by an observer blinded to the genotype of each section, a significant absence of IgG deposits in Irf5−/− mice kidney sections is noted (Fig. 1B). These data demonstrate that IRF5 is required for nephritis-related IgG deposits in glomeruli induced to develop by pristane treatment and are consistent with those by Richez et al. (26).
Fig. 1.
IRF5 is required for pristane-induced IgG glomerular deposits and anti-nuclear autoantibodies. (A) Glomeruli evaluated for IgG deposits by immunostaining from one of six Irf5−/− and one of six Irf5+/+ mice injected with pristane. (Scale bar, 50 μm.) (B) Immunostained sections described in A were scored 0–4 on the prevalence and intensity of IgG-infiltrated glomeruli. Averages of two scores for each section (squares) and each genotype (filled rectangles) are shown. (C) Antinuclear antibodies in sera obtained from A assayed by fluorescent immunostaining of Hep2 cells. A representative section from each genotype is shown. All sera tested were positive for cytoplasmic staining. (D and E) ELISA was performed for (D) anti-dsDNA and (E) anti-sm autoantibodies in the sera of mice in A and three each PBS-injected Irf5+/+ and Irf5−/− mice. Serum from a 6-month-old NZB/W female mouse (intermediate) was used as a positive control. OD450 values from within the linear range for total IgG, IgM, IgG3, IgG1, IgG2b, and IgG2a isotypes are shown. Individual samples and averages are represented by open squares and filled rectangles, respectively. *1P = 0.030; *2P = 0.001; *3P = 0.005; *4P = 0.108; *5P = 0.071; *6P = 0.076.
The presence of antinuclear antibodies (ANAs) in the sera of these mice was evaluated by immunostaining of Hep2 cells. All Irf5+/+ mouse sera were positive for either homogeneous or speckled nuclear staining, whereas all Irf5−/− sera were negative for both (Fig. 1C). The absence of homogeneous or speckled nuclear staining in Irf5−/− sera indicates that IRF5 is necessary for the development of ANAs specific for dsDNA and nRNP antigens, respectively (30, 31). To test this, we measured anti-dsDNA autoantibody levels by ELISA and found that Irf5−/− sera contain significantly reduced amounts of anti-dsDNA total IgG (Fig. 1D). The levels of anti-dsDNA IgG2a and IgG2b in particular are drastically reduced or absent in sera from Irf5−/− mice as compared with the sera from Irf5+/+ mice, whereas IgM autoantibodies are unaffected. In fact, in this SLE model system, anti-dsDNA IgG antibodies are almost exclusive of the IgG2a and IgG2b isotypes, indicated by the comparison of pristane-injected Irf5+/+ sera with control saline-injected Irf5+/+ sera. This is similar to the findings of Ehlers et al., who found IgG2a and IgG2b dominated the autoantibody repertoire in FcγRIIb−/− mice (32). We also measured anti-sm Ig, an anti-nRNP autoantibody, in the sera of Irf5+/+ and Irf5−/− mice. Although anti-sm IgMs are comparable between the two groups, there is a substantial reduction in anti-sm total IgG and complete absence of anti-sm IgG2a and IgG2b in Irf5−/− sera (Fig. 1E). Thus, these data demonstrate that IRF5 is required for the production of IgG2a and IgG2b ANAs in pristane-treated mice.
IRF5 Is Required by B Cells for IgG2a Secretion.
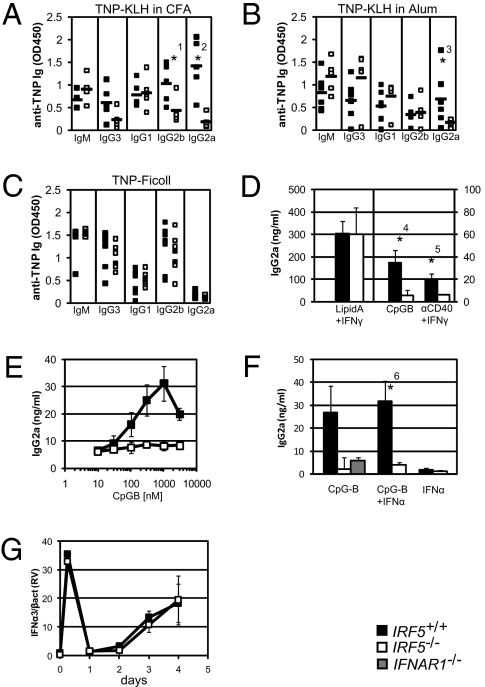
To address IRF5’s role more generally in B-cell–mediated humoral immune respon-ses, groups of Irf5−/− and Irf5+/+ mice were immunized with either 2,4,6-trinitrophenol(TNP)-keyhole limpet hemocyanin (KLH) in complete Freund’s adjuvant (CFA), TNP-KLH in Alum, or TNP-Ficoll. Fourteen days later, sera were collected and ELISA against anti-TNP antibodies performed for IgM and all four IgG subclasses. A selective defect in the production of IgG2a antibodies is observed in the sera of Irf5−/− mice immunized with TNP-KLH in CFA (Fig. 2A) and TNP-KLH in Alum (Fig. 2B). In addition, anti-TNP IgG2b antibodies were also reduced in Irf5−/− mice immunized with TNP-KLH in CFA as compared with control mice (Fig. 2A). As expected, anti-TNP IgG2a antibodies are not detected in the sera of Irf5+/+ or Irf5−/− mice immunized with TNP-Ficoll (Fig. 2C). These results reveal that IRF5 is required for the in vivo secretion of IgG2a elicited during a routine immune system challenge with a T-cell–dependent antigen mixed with either a TLR-dependent (CFA) or TLR-independent (Alum) adjuvant (33).
Fig. 2.
Defective Ig antibody responses in Irf5−/− mice and B cells. Sera from Irf5+/+ (filled squares) and Irf5−/− (empty squares) mice immunized for 14 days with either (A) TNP-KLH in CFA, (B) TNP-KLH in Alum, or (C) TNP-Ficoll were assayed by ELISA for relative amounts of anti-TNP IgM, IgG3, IgG1, IgG2b, and IgG2a as indicated. Averages are represented by filled rectangles. Linear range OD450 values are shown (n = 5–7 mice per group). (D) Supernatant from Irf5+/+ and Irf5−/− purified B cells stimulated in vitro with either lipid A plus IFNγ (n = 4), CpG-B (n = 10), or anti-CD40 plus IFNγ (n = 3) for 4 days were analyzed for IgG2a secretion by ELISA. Note that the vertical axis scale for lipid A plus IFNγ differs from CD40 plus IFNγ and CpG-B. Mean and standard deviation (SD) are shown. (E) As in D, IgG2a ELISA was performed on supernatant following a dose curve of CpG-B (10, 30, 100, 300, 1,000, and 3,000 nM) (n = 3). (F) IgG2a ELISA as in D on supernatants of Irf5+/+, Irf5−/−, and Ifnar1−/− (intermediate) B cells following CpG-B alone (n = 4), CpG-B plus IFNα (n = 4), or IFNα alone (n = 2) treatment for 4 days. (G) Real-time RT-PCR for IFNα3 mRNA in Irf5+/+ and Irf5−/− B cells stimulated with CpG-B for the indicated number of days. Mean and SD relative values (RV) normalized to β-actin expression from three independent experiments are shown. *1P = 0.032; *2P = 0.002; *3P = 0.097; *4P = 0.000; *5P = 0.073; *6P = 0.002.
In determining whether B cells are intrinsically defective in class switching to IgG2a, we next purified splenic B cells by indirect magnetic separation, stimulated the cells with cytokines or mitogens, and then assayed for IgG2a antibody secretion by ELISA. We observed that Irf5−/− B cells secrete IgG2a antibodies normally upon lipid A (a TLR4 ligand) plus IFNγ treatment (Fig. 2D). Thus, Irf5−/− B cells do not possess a general defect in Ig secretion. However, Irf5−/− B cells are completely defective in IgG2a secretion upon CpG-B (ODN1668, a TLR9 ligand) or anti-CD40 plus IFNγ stimulation (Fig. 2D). Moreover, the requirement for IRF5 is irrespective of the concentration of CpG-B (Fig. 2E). Steady-state levels of TLR9 and Myd88 mRNA transcripts are equivalent in Irf5+/+ and Irf5−/− B cells before and following CpG-B stimulation, indicating that Irf5−/− B cells are competent for TLR9 stimulation (Fig. S1 A and B). Indeed, transcripts of IRF4, a transcription factor essential for class switch recombination and antibody secretion (34), are induced normally in Irf5−/− B cells following CpG-B stimulation (Fig. S1C). These in vitro data clearly show a B-cell-intrinsic defect in Irf5−/− mice and a critical and signaling-specific role for IRF5 in IgG2a production.
Given that IgG2a CSR is induced by IFNγ and inhibited by interleukin (IL) 4, we tested whether the in vivo defect observed (Fig. 2 A–C) may also be due to an intrinsic defect in T-cell polarization. Untreated, nonpolarized, or Th1- or Th2-polarized Irf5+/+ and Irf5−/− CD4+ T cells were examined for their IFNγ and IL4 steady-state mRNA levels by real-time RT-PCR. No differences were observed in the expression of transcripts for either cytokine under these conditions (Fig. S2 A and B). Thus, it is unlikely that the defect in IgG2a secretion observed in vivo upon the immunization and pristane treatment protocols described above is due to a T-cell-intrinsic defect. However, it remains possible that IRF5-dependent IgG2a responses still involve a non-B-cell component. For instance, because IRF5 is required for IL12 cytokine production by DCs and macrophages, Th1 responses may be reduced in these mice (18).
In view of the reasonable hypothesis that IRF5 is responsible for the development of SLE-related autoantibodies by its aberrant production of type I IFN, we examined the role of type I IFN in inducing IgG2a secretion of Irf5+/+ and Irf5−/− B cells. First, exogenous type I IFN stimulation does not rescue the defect in CpG-B-induced IgG2a secretion by Irf5−/− B cells in vitro (Fig. 2F), although these cells respond to type I IFN, as indicated by the phosphorylation of Stat1 (Fig. S3). Moreover, no differences are found in type I IFN mRNA levels measured in CpG-B-stimulated Irf5+/+ and Irf5−/− B cells (Fig. 2G). These data collectively rule out the possibility that IRF5 regulates IgG2a secretion through its ability to regulate type I IFN gene expression, which is demonstrated by the absence of IgG2a secretion by type I IFN receptor-deficient B cells upon CpG-B stimulation (Fig. 2F) (35). We thus conclude that the defect in IgG2a autoantibody secretion in the absence of IRF5 is independent of any secondary effects IRF5 may have on type I IFN expression and suggest, rather, that IRF5 intrinsically regulates IgG2a secretion in B cells. These data do not, however, exclude the involvement of IRF5-induced type I IFN signaling contributing to autoimmunity through other mechanisms (discussed below).
IRF5 Regulates B-Cell Activation Following TLR9 Stimulation.
Although a major focus on B cells in the context of SLE has been on their secretion of autoantibodies, B cells are also known to drive SLE development through antibody-independent mechanisms (36). Pathogenic B cells unable to secrete Ig could still support spontaneous T-cell activation, T-cell infiltration, and early mortality in MRL/Mplpr/lpr mice by a mechanism(s) postulated to be mediated by B-cell costimulation and presentation of antigen to autoreactive T cells and/or secretion of mediators of local inflammation (36). Beyond its induction of IgG2a CSR, we have data to suggest that IRF5 regulates the activation of CpG-B-stimulated B cells more generally. For one, we previously demonstrated that IRF5 is required for IL6 secretion by B cells (18). Interestingly, IL6 is required for the production of anti-dsDNA, but not anti-sm, autoantibodies in pristane-injected mice (37). However, because exogenous IL6 does not rescue IgG2a secretion by B cells (Fig. S4A), we contend that the IgG2a defect is independent of IRF5-dependent IL6 production. In addition, ccl3 and ccl4 mRNA expression are also absent in TLR9-stimulated Irf5−/− B cells (Fig. S4 B and C), which implicates IRF5 in orchestrating inflammatory responses. Somewhat unexpectedly, CpG-B-treated Irf5−/− B cells have higher surface expression of costimulatory proteins CD80 and CD86 but normal CD40 expression (Fig. S4 D–F); these data would seem to argue against a role for autoimmune-prone alleles of IRF5 in the costimulation and activation of autoreactive T cells. Finally, Irf5−/− B cells undergo less proliferation than Irf5+/+ B cells following TLR9 stimulation in vitro, suggesting that autoimmune-prone IRF5 alleles could promote the enhanced clonal expansion of autoreactive B cells (Fig. S4G).
Regulation of IgG2a GLT by IRF5.
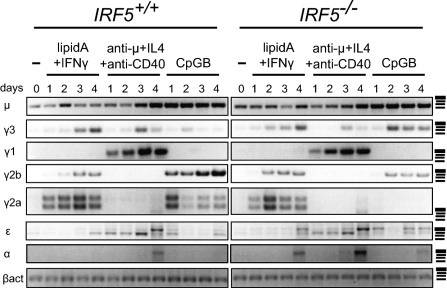
To determine whether IRF5 affects IgG2a secretion at the level of GLT, we next measured germ-line transcripts of all heavy-chain genes by semiquantitative RT-PCR for Irf5+/+ and Irf5−/− B cells untreated or treated with lipid A plus IFNγ, anti-μ plus IL4 plus anti-CD40, or CpG-B. Markedly, γ2a transcripts were exclusively absent in Irf5−/− B cells treated with CpG-B (Fig. 3), indicating that the IgG2a secretion defect is before or at the stage of GLT. γ2b transcripts were also reduced in CpG-B-treated Irf5−/− cells, further suggesting that IRF5 regulates switching to IgG2b as well. γ2a GLT is normal in Irf5−/− B cells upon stimulation with lipid A plus IFNγ (Fig. 3), an observation consistent with their normal IgG2a production by these stimuli (Fig. 2D). Finally, that Irf5−/− B cells express γ3 transcripts indicates that TLR9-stimulated Irf5−/− B cells do not possess a general defect in CSR or Ig secretion.
Fig. 3.
IRF5 is required for γ2a germ-line transcription upon TLR9 stimulation. Semiquantitative RT-PCR was performed for germ-line mRNA transcripts induced in Irf5+/+ (left panels) and Irf5−/− (right panels) purified B cells either untreated (−) or stimulated with lipid A plus IFNγ, anti-μ plus IL4 plus anti-CD40, or CpG-B for the indicated number of days. Representative gels from μ (n = 3), γ3 (n = 3), γ1 (n = 3), γ2b (n = 3), γ2a (n = 6), ε (n = 2), and α (n = 2) are shown. β-Actin (βact; n = 6) is a loading control. All signals were confirmed to be in the linear range of amplification. DNA molecular weight markers indicated in the right column from top to bottom are ≈500 (wide), 400, 300, and 200 bp.
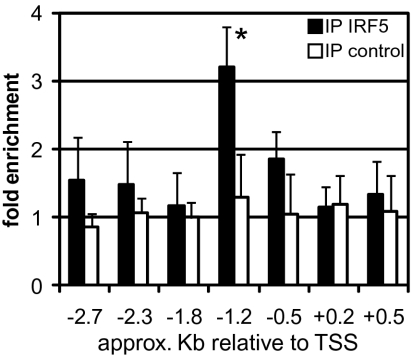
To further consolidate the above results, we next sought to determine whether IRF5 directly binds to the γ2a germ-line promoter. Chromatin immunoprecipitation (ChIP) was performed for IRF5 on putative consensus IRF binding sites [IFN-stimulated response elements (ISRE), AANNGAAA (38)] within the promoter region using chromatin prepared from wild-type B cells stimulated with CpG-B for 24 h. On the germ-line promoter of the IgG2a heavy chain (Igh-1a), we observed an enrichment of a consensus ISRE, GAATAGAAAC, located ~1.2 kb upstream of the transcription start site as compared with the signal from ChIP of an irrelevant antibody, but little or no enrichment for six other ISREs (Fig. 4). These data therefore further lend support to the notion that IRF5 directly acts on the ISRE within the γ2a germ-line promoter, although they do not exclude the possibility that additional proximal and distal ISREs are also involved in IgG2a GLT as well. The transcription factor T-bet is required for IgG2a GLT and CSR following TLR4 (14) and TLR9 (39) stimulation. T-bet mRNA transcripts are not induced in Irf5−/− B cells following CpG-B stimulation (Fig. S1D). Hence, we envisage cooperation between IRF5 and T-bet following TLR9 signaling for the induction for GLT and subsequent CSR, although the detailed mechanism of such cooperation remains to be clarified. Notwithstanding, these data, which go to the central point of this study, strongly suggest that IRF5 binds to the Igh-1a gene promoter, where it directs γ 2a GLT.
Fig. 4.
IRF5 binds to the γ2a germ-line promoter. Real-time PCR for chromatin immunoprecipitation samples was performed with an antibody against IRF5 or an irrelevant protein (control) for putative IRF5 binding sites within the Igh-1a locus. Mean and SD values are from seven independent experiments. Approximate positions of each binding site relative to the transcription start site (TSS, +1) are given (52). *P = 0.007.
IRF5 Is Dispensable for Collagen-Induced Arthritis and Anti-Collagen Autoantibodies.
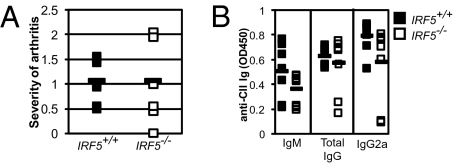
Because IRF5 is required for the production of pathogenic (IgG2a) autoantibodies in the context of murine SLE and the concomitant development of SLE as well as for IgG2a in immunized mice, we hypothesized that IRF5 may be required for the development of other forms of autoimmunity, such as rheumatoid arthritis. To test this, we challenged groups of Irf5+/+ and Irf5−/− mice to develop collagen-induced arthritis (CIA) by immunization with chicken collagen II (CII) (40). Surprisingly, the severity of paw swelling (Fig. 5A) as well as IgM, total IgG, and IgG2a sera levels of anti-CII autoantibodies (Fig. 5B) were found to be equivalent between the experimental and control groups. Thus, at least under these experimental conditions, IRF5 is not required for clinical manifestation of arthri-tis or the development of collagen-specific autoantibodies, including those of the IgG2a subtype. As several reports have implicated the role of TLR4 signaling in this CIA model (41), these data together with the in vitro experiment shown in Fig. 2D suggest that IRF5 is dispensable for the development of autoimmunity in which TLR4 signaling is involved. These results in toto underscore the selective contribution of IRF5 to IgG2a CSR in response to certain stimuli, particularly those affecting SLE development.
Fig. 5.
IRF5 is dispensable for collagen-induced arthritis and anti-CII autoantibody production. (A) Hind paws of CII-challenged Irf5+/+ (filled, n = 7) and Irf5−/− (empty, n = 8) mice were scored for severity of arthritis. Squares represent the average values from each mouse 5 weeks after the start of the experiment. Averages of each group are given by filled rectangles. (B) ELISA for anti-CII autoantibodies in the sera of mice described in A. Linear range OD450 values for IgM, total IgG, and IgG2a isotypes are shown.
Discussion
Using a pristane-induced murine SLE model, we show that IRF5 is required for the development of ANAs and IC glomerular deposits, two cardinal features of SLE. Similar findings were independently made by another group (26) and were anticipated from studies identifying autoimmune-prone alleles of human IRF5 (19, 20). In the present study, we go beyond the previous by examining IRF5’s molecular mechanism in promoting SLE-like autoimmune disease. We demonstrate that the absence of pathogenic ANAs in pristane-treated Irf5−/− mice is likely due to IRF5’s direct regulation of class switching to IgG2a in B cells. IgG2a has been demonstrated to be the most potent isotype in mediating pathogenic (and protective) immune processes because of its enhanced induction of FcR-mediated effector functions (12). In particular, we observe that anti-dsDNA and anti-sm IgG2a (and to a lesser extent IgG2b) autoantibodies are reduced or absent in pristane-injected Irf5−/− mice, whereas IgM autoantibodies remain unaffected. As the development of autoantibodies against dsDNA and nRNP has been shown to be promoted by TLR9 and TLR7 signaling, respectively (23), our results indicate that IRF5 functions downstream of these receptors. These findings are reminiscent of the absence of antinuclear autoantibodies noted in Myd88−/−MRLlpr/lpr and Myd88−/−FcγRIIB−/− mice (32, 42); MyD88 is an essential adapter protein for TLR9 and TLR7 signaling upstream of IRF5 (18).
At first glance, it is confounding that IRF5 is required for the in vivo secretion of IgG2a more generally, as demonstrated by the absence of anti-TNP IgG2a antibodies in Irf5−/− immunized mice. This appears to be at odds with normal IgG2a GLT, CSR, and secretion upon stimulation of Irf5−/− B cells by lipid A plus IFNγ, although IRF5 can be activated by TLR4 signaling (18). We postulate that IRF5 is perhaps dispensable for IgG2a CSR induced by this combination of stimuli because of a redundancy with the transcription factor T-bet. Furthermore, the broad in vivo defect in CSR in Irf5−/− mice may be consistent with IRF5 functioning downstream of TLR9 and TLR7 which, although not required, are crucial for augmenting adjuvant-induced humoral responses including and especially the production of IgG2a-switched antibodies (43, 44). This notion is also supported by our findings that IRF5 is dispensable for the production of collagen-specific autoantibodies, including IgG2a, in the context of arthritis development. We hypothesize that this is likely due to IRF5 not being required for the activation of B cells in the context of TLR2 and/or TLR4 stimulation, pathways that are implicated in the progression of CII-induced arthritis (41). Thus, we speculate that IRF5’s role in autoimmunity may be restricted to the development of autoantibodies that only require TLR7 and TLR9 signaling, that is, nRNP and dsDNA autoantigens.
The requirement for IRF5 in the in vivo production of IgG2a may also be explained by the activation of IRF5 independent of TLR signaling, which is demonstrated in the immunization of Irf5−/− mice with Alum, a TLR-independent adjuvant, as well as upon stimulation of Irf5−/− B cells with anti-CD40 plus IFNγ in vitro. Whereas IRF5 is well-known to be activated downstream of TLR9 and TLR7, this is not so for IFNGR or CD40 signaling. We have shown previously that IRF5 nuclear translocation is induced upon TLR9 stimulation by MyD88-mediated recruitment of the E3 ubiquitin ligase TRAF6 (18). Because TRAF6 is also activated downstream of CD40 (45), we speculate that this may be the mechanism by which IRF5 is required for IgG2a secretion upon CD40 plus IFNGR stimulation.
The in vivo IgG2a secretion defect, and protection from SLE more generally, is perhaps also due to IRF5’s role in non-B cells, such as in the regulation of type I IFN by DCs. IC from SLE patients mixed with apoptotic cells or purified autoantigens induces type I IFN from murine DCs by a mechanism that involves FcγRIIA internalization and delivery of autoantigens to intracellular TLR7 and TLR9 (23, 46). Type I IFN, in turn, promotes the activation of autoreactive B cells by enhancing B-cell survival, inducing TLR7 expression, and triggering secretion of IgG2a (21, 47). As IRF5 is required for IC-mediated type I IFN expression by DCs and for IgG2a secretion by B cells, our system cannot experimentally determine which of the two is more critical for SLE disease pathogenesis. However, because exogenous type I IFN does not rescue the IgG2a secretion defect in Irf5−/− B cells in vitro and because prior generation of pathogenic autoantibodies is necessary for IC formation and uptake by DCs leading to type I IFN secretion, we hypothesize that “IRF5 in B cells” precedes “IRF5 in DCs” as the earlier and more crucial trigger for SLE disease development. Indeed, this is supported by the demonstration that autoreactive B cells are distinctly capable of internalizing, via BCR endocytosis, and responding to autoantigens in the absence of prior autoantibody production (42). Consistent with our data, more convincing data are provided by Richez et al., who found that type I IFN receptor-deficient mice are not protected from SLE on an autoimmune-prone background (26).
In humans, the murine IgG2a and IgG2b isotypes are thought to correspond to IgG1 and IgG3, respectively. Analogous to their murine counterparts, IgG1 and IgG3 are the most predominant subclasses of autoantibodies in SLE patients (4–6). IgG3 titers, in particular, positively correlate with renal pathogenicity and are reduced in remissive SLE patients as compared with patients with active disease (48, 49). As in murine B cells, activation of TLR9 in human B cells (in combination with other stimuli) potently induces proliferation, differentiation to antibody-secreting plasma cells, and germ-line transcription of IgG1 and IgG3 (50). Additional studies are required to clarify to what extent IRF5 and its autoimmune-prone alleles are involved in the activation and/or induction of CSR of autoreactive human B cells. Nevertheless, our present study demonstrates that IRF5 is required for manifestations of SLE-like disease in mice due to, at least in part, an intrinsic defect in B-cell switching to IgG2a. These findings may offer insight into IRF5’s role in SLE pathogenesis, one that is B-cell-intrinsic and independent of type I IFN production, and reaffirms the association between IRF5 polymorphisms and increased risk to SLE disease in humans.
Materials and Methods
Mice.
Irf5−/− mice were described previously (18). Ifnar1-deficient mice were purchased from B&K Universal Group and backcrossed onto the C57BL/6 genetic background for more than eight generations. Irf5−/− and Irf5+/+ littermate control mice were housed in specific pathogen free conditions under University of Tokyo guidelines. Male and female mice between 6 and 12 weeks old were used for all experiments. Ten months following a single i.p. injection of 0.5 mL pristane, sera and kidneys were harvested from mice. For immunizations, 50 μg of TNP-KLH emulsified in 1:1 (vol/vol) CFA or Alum, or 50 μg of TNP-Ficoll in PBS, were injected i.p. Ten milligrams of chicken collagen II (CII; Chemicon International) dissolved in 50 mM acetic acid and emulsified in 1:1 (vol/vol) CFA was injected subdurally. A second injection was performed 21 days later. Arthritis in hind limbs was scored by an experienced individual in a blinded manner every other day (51). Sera were collected 5 weeks following initial injections.
Cells.
Splenic B cells were purified by Magnetic-activated cell sorting column separation (Miltenyi Biotec). Unless indicated otherwise, B cells were plated at a final concentration of 1.5 × 106 cells/mL and stimulated with combinations of 10 μg/mL lipid A, 150 ng/mL IFNγ, 10 μg/mL anti-μ, 10 ng/mL IL4, 10 μg/mL anti-CD40 mAB, anti-IFNγ mAB, 10 μg/mL IFNα1, and 1,000 nM CpG-B.
ELISA and Immunofluorescence.
For IgG2a-capture ELISA, goat anti-mouse IgG2c, anti-IgG2c horseradish peroxidase (HRP), and purified IgG2c antibodies were from Bethyl Laboratories. Anti-TNP ELISA was performed using standard procedures. Anti-double-stranded DNA and anti-sm ELISA were performed as follows. Either 50 μg/mL sm antigen from calf thymus (IVAX Diagnostics) in PBS bound to a 96-well Maxisorp Immuno plate (Nunc) for 16 h or S1 nuclease-treated calf thymus DNA at 25 ng/mL covalently conjugated to a NucleoLink 96-well plate according to the manufacturer's instructions was prepared. Plates were blocked with 3% BSA in PBS, incubated with dilutions of sera (1:25–1:400), washed three times, exposed to HRP-conjugated secondary detection antibodies, washed six times, and then developed. For anti-CII ELISA, wells were coated with 4 μg/mL CII, washed, incubated with sera (1:200–1:5,000 diluted), and processed as described above. Hep2 cells were cytospun on glass slides, briefly dried, fixed with cold acetone for 90 s, again briefly dried, and then blocked with 3% BSA and 1% fetal calf serum in PBS. Sera from Irf5−/− and Irf5+/+ mice following 10 months of PBS or pristane injection were diluted 1:100 and incubated 16 h. Slides were washed, incubated with anti-IgG FITC-conjugated antibody, briefly air dried, fixed, and then mounted. For Ig-deposit visualization, kidneys were frozen in OCT media and then sectioned to 0.6 μm. Immunofluorescence was visualized using an iXON DU897_BV camera (Andor Technology) with 500-ms exposure. Photographs were captured using NIS Elements AR2 software (Nikon).
Chromatin Immunoprecipitation and Real-Time RT-PCR.
ChIP protocol was performed according to the manufacturer's instructions (Millipore). Briefly, chromatin prepared from 7 × 106 B cells stimulated with CpG-B plus anti-IFNγ for 24 h was incubated with equivalent amounts of either anti-IRF5 antibody (Cell Signaling), anti-PLCγ antibody (Santa Cruz Biotechnology), or rabbit sera for 16 h at 4 °C. Four percent of the material was subjected to analysis by quantitative PCR using SYBR Premix Ex Taq (Takara). Putative IRF binding sites (ISRE) within the promoter and first exon of Igh-1a were manually located by searching for the consensus sequence AANNGAAA (38). Primer sequences used to amplify target sequences are available upon request. Real-time RT-PCR for IFNα3 mRNA was performed using PrimeScript (Takara) and SYBR Premix Ex Taq according to the manufacturer's instructions. Samples were normalized to β-actin.
Statistical Analysis.
An unpaired, two-tailed Student's t test was performed between indicated samples for all statistical analyses.
Supplementary Material
Acknowledgments
We thank Jeffrey V. Ravetch for his invaluable advice, Makato Nakasato for his help with the CIA experiment, Rie Takeda and Masashi Shishido for their technical assistance, Allen Cheung for his help as a blind observer, and Erna Magnúsdóttir for her suggestions for ChIP. This work was supported in part by grants for Advanced Research on Cancer Grant-in-Aid for Scientific Research on Priority Areas (17012005 and 19041021) or for Scientific Research (A) (19209016) and (B) (19390109 and 21390089), and by the Global Center of Excellence Program “Integrative Life Science Based on the Study of Biosignaling Mechanisms” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. D.A.S. is a former postdoctoral fellow of the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005599107/-/DCSupplemental.
References
- 1.Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20:517–527. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- 2.Anolik JH. B cell biology and dysfunction in SLE. Bull NYU Hosp Jt Dis. 2007;65:182–186. [PubMed] [Google Scholar]
- 3.Bhat P, Radhakrishnan J. B lymphocytes and lupus nephritis: New insights into pathogenesis and targeted therapies. Kidney Int. 2008;73:261–268. doi: 10.1038/sj.ki.5002663. [DOI] [PubMed] [Google Scholar]
- 4.Blanco F, et al. IgG subclasses in systemic lupus erythematosus and other autoimmune rheumatic diseases. Lupus. 1992;1:391–399. doi: 10.1177/096120339200100609. [DOI] [PubMed] [Google Scholar]
- 5.Manolova I, Dancheva M, Halacheva K. Predominance of IgG1 and IgG3 subclasses of autoantibodies to neutrophil cytoplasmic antigens in patients with systemic lupus erythematosus. Rheumatol Int. 2002;21:227–233. doi: 10.1007/s00296-002-0174-2. [DOI] [PubMed] [Google Scholar]
- 6.Tiikkainen U, Wangel A, Appleton SL, Arthur D. Subclasses of IgG anticardiolipin antibodies in patients with systemic lupus erythematosus. Scand J Immunol. 1991;34:265–271. doi: 10.1111/j.1365-3083.1991.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 7.Yung S, Chan TM. Anti-DNA antibodies in the pathogenesis of lupus nephritis—The emerging mechanisms. Autoimmun Rev. 2008;7:317–321. doi: 10.1016/j.autrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipps TJ, Parham P, Punt J, Herzenberg LA. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985;161:1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 11.Fossati-Jimack L, et al. Markedly different pathogenicity of four immunoglobulin G isotype-switch variants of an antierythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity Fcγ receptor III. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. Fcγ receptors: Old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 14.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci USA. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Zhang JJ. Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation. J Immunol. 2005;175:7419–7424. doi: 10.4049/jimmunol.175.11.7419. [DOI] [PubMed] [Google Scholar]
- 16.Thibault DL, et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118:1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon α genes. J Biol Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- 18.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 19.Sigurdsson S, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham RR, et al. Argentine and Spanish Collaborative Groups. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38:550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 21.Kyogoku C, Tsuchiya N. A compass that points to lupus: Genetic studies on type I interferon pathway. Genes Immun. 2007;8:445–455. doi: 10.1038/sj.gene.6364409. [DOI] [PubMed] [Google Scholar]
- 22.Kozyrev SV, Alarcon-Riquelme ME. The genetics and biology of Irf5-mediated signaling in lupus. Autoimmunity. 2007;40:591–601. doi: 10.1080/08916930701510905. [DOI] [PubMed] [Google Scholar]
- 23.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Ehlers M, Ravetch JV. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007;28:74–79. doi: 10.1016/j.it.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Richez C, et al. IFN regulatory factor 5 is required for disease development in the FcγRIIB−/−Yaa and FcγRIIB−/− mouse models of systemic lupus erythematosus. J Immunol. 2010;184:796–806. doi: 10.4049/jimmunol.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuda K, et al. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 28.Lien C, et al. Critical role of IRF-5 in regulation of B-cell differentiation. Proc Natl Acad Sci USA. 2010;107:4664–4668. doi: 10.1073/pnas.0911193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen SR, et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 32.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Gregorio E, D'Oro U, Wack A. Immunology of TLR-independent vaccine adjuvants. Curr Opin Immunol. 2009;21:339–345. doi: 10.1016/j.coi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Sciammas R, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Oganesyan G, et al. IRF3-dependent type I interferon response in B cells regulates CpG-mediated antibody production. J Biol Chem. 2008;283:802–808. doi: 10.1074/jbc.M704755200. [DOI] [PubMed] [Google Scholar]
- 36.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards HB, et al. Interleukin 6 dependence of anti-DNA antibody production: Evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii Y, et al. Crystal structure of an IRF-DNA complex reveals novel DNA recognition and cooperative binding to a tandem repeat of core sequences. EMBO J. 1999;18:5028–5041. doi: 10.1093/emboj/18.18.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng SL, Li J, Lin L, Gerth A. The role of T-bet in B cells. Nat Immunol. 2003;4:1041. doi: 10.1038/ni1103-1041a. author reply 1041. [DOI] [PubMed] [Google Scholar]
- 40.Inglis JJ, et al. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res Ther. 2007;9:R113. doi: 10.1186/ar2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y, et al. Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat Immunol. 2004;5:380–387. doi: 10.1038/ni1054. [DOI] [PubMed] [Google Scholar]
- 42.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 45.Pullen SS, Dang TT, Crute JJ, Kehry MR. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J Biol Chem. 1999;274:14246–14254. doi: 10.1074/jbc.274.20.14246. [DOI] [PubMed] [Google Scholar]
- 46.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19:11–23. doi: 10.1016/j.smim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 48.Amoura Z, et al. Presence of antinucleosome autoantibodies in a restricted set of connective tissue diseases: Antinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosus. Arthritis Rheum. 2000;43:76–84. doi: 10.1002/1529-0131(200001)43:1<76::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 49.Lin GG, Li JM. IgG subclass serum levels in systemic lupus erythematosus patients. Clin Rheumatol. 2009;28:1315–1318. doi: 10.1007/s10067-009-1224-x. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 51.Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1:2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- 52.Collins JT, Dunnick WA. Germline transcripts of the murine immunoglobulin γ 2a gene: Structure and induction by IFN-γ. Int Immunol. 1993;5:885–891. doi: 10.1093/intimm/5.8.885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.