Abstract
Feedback plays important roles in sensory processing. Mushroom bodies are believed to be involved in olfactory learning/memory and multisensory integration in insects. Previous cobalt-labeling studies have suggested the existence of feedback from the mushroom bodies to the antennal lobes in the honey bee. In this study, the existence of functional feedback from Drosophila mushroom bodies to the antennal lobes was investigated through ectopic expression of the ATP receptor P2X2 in the Kenyon cells of mushroom bodies. Activation of Kenyon cells induced depolarization in projection neurons and local interneurons in the antennal lobes in a nicotinic receptor-dependent manner. Activation of Kenyon cell axons in the βγ-lobes in the mushroom body induced more potent responses in the antennal lobe neurons than activation of Kenyon cell somata. Our results indicate that functional feedback from Kenyon cells to projection neurons and local interneurons is present in Drosophila and is likely mediated by the βγ-lobes. The presence of this functional feedback from the mushroom bodies to the antennal lobes suggests top-down modulation of olfactory information processing in Drosophila.
In the fruit fly, the olfactory system is important for identifying food sources, avoiding predators, and recognizing mating partners (1). The primary Drosophila olfactory neurons are the olfactory receptor neurons (ORNs) present in the two olfactory organs of the fruit fly, the antennae and the maxillary palps (2, 3). Chemical stimuli detected by the olfactory receptors in the ORNs are converted into electrical signals, which are transmitted to the secondary neurons, the projection neurons (PNs), in the antennal lobes. The antennal lobes are important olfactory coding centers that consist of PNs as well as inhibitory and excitatory local interneurons (LNs) (4 –6). The PNs receive signals from the primary ORNs and also receive lateral inhibitory/excitatory inputs from the LNs (7 –10). After processing in the antennal lobes, olfactory information is relayed by PNs to the mushroom bodies and the protocerebrum region of the fly brain (11 –13).
Feedback is important in sensory processing. For example, in the mammalian thalamocortical system, a large number of thalamus neurons are modulated by cortical feedback mechanisms (14). Such top-down cortical feedback regulation is critical for visual perception (15). Anatomical and functional studies of the mammalian olfactory system indicate that there is also functional feedback from the cortex to the olfactory bulb (16, 17). Several lines of evidence also suggest the existence of feedback in the insect olfactory pathway. Olsen and Wilson (8) showed that spontaneous excitatory postsynaptic activity in PNs is suppressed presynaptically via lateral inhibition by LNs in antennal lobes, suggesting modulation of the primary ORNs by the secondary LNs. In addition, Tanaka et al. (18) demonstrated that odor stimulation can induce spikes and subthreshold membrane potential oscillations in PNs that are phase-locked to odor-elicited local field-potential oscillations in mushroom bodies. These results indicate the existence of feedback within the antennal lobe of Drosophila. Finally, by injecting cobalt into the α-lobe of the mushroom bodies of the honey bee, Rybak and Menzel identified a projection from the α-lobe to the antennal lobe (19). A neuron (the antennal lobe feedback neurons, ALF-1) with similar projection pattern was reported by Kirschner et al. (20). The results of these studies suggest feedback from the mushroom body to the antennal lobe in the honey bee. Based on these observations, we investigated whether functional feedback from mushroom bodies to antennal lobes is present in Drosophila.
Drosophila mushroom bodies are predominately composed of Kenyon cells (KCs) (21). Because of the small size of KCs, it is difficult to stimulate these cells using conventional electrophysiological methods. Taking advantage of fly genetics, and the absence of the ionotropic ATP receptor P2X2 gene in the Drosophila genome (22), Zemelman et al. established a UAS-P2X2 system for precise activation of specific neurons in the fly brain using exogenous ATP (23). In this study, we have used the UAS-P2X2 system for activation of KCs in combination with the patch-clamp method for recording of PN and LN activity. Using these techniques, we showed in this study the existence of functional feedback from KCs in the mushroom bodies to PNs and LNs in the antennal lobes.
Results
Depolarization Responses in PNs and LNs Resulted from Activation of Mushroom Bodies.
The P2X2 protein was specifically expressed in KCs using 247-Gal4, an enhancer trap line that has been shown to be specific for KCs (24, 25). Whole-cell recording from KCs showed that brief focal applications of 10 mM ATP (0.3-s pulse) near the somata of KCs elicited large depolarizations in the KCs (up to 60 mV) for a prolonged period. More transient depolarizations of smaller amplitudes were induced by 1 mM ATP pulses (Fig. S1). These ATP-induced KC depolarization responses were predominantly mediated by expression of the P2X2 channel, as only a slight depolarization (∼2 mV) was induced by 10 mM ATP, and no detectable response was induced by 1 mM ATP in KCs of UAS-P2X2 flies (Fig. S1). Thus, the 247-Gal4:UAS-P2X2 system can be used for specific activation of KCs.
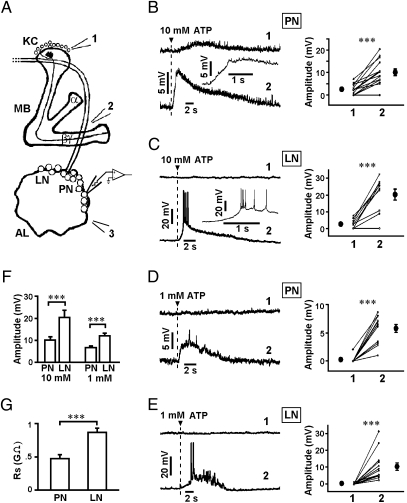
The calyces and the α/β-, α′/β′-, and γ-lobes of the mushroom bodies are formed by the dendrites and axonal projections of KCs, respectively. Through use of ATP application and whole-cell recording from antennal lobes, we found that application of 10 mM ATP at the KC somata (position 1, Fig. 1A) and βγ-lobes (position 2, Fig. 1A) of the mushroom bodies induced significant depolarization responses in 34 of 54 (59%) and 20 of 22 (91%) PNs recorded in the antennal lobes, respectively. Stimulation of the lobes generally resulted in larger depolarization responses than stimulation of the somata, as shown by results from paired recording (Fig. 1B). Application of 10 mM ATP at the KC somata and βγ-lobes of the mushroom bodies induced significant depolarization responses in 8 of 24 (33%) and 11 of 12 (92%) LNs recorded, respectively. Again, LN depolarization responses induced by ATP stimulation at the KC somata were significantly smaller than those induced by stimulating the lobes, and many of the latter responses were accompanied by action potentials (Fig. 1C). The average amplitude of the depolarization responses induced by lobe stimulation (excluding action potentials) was significantly larger for LNs than PNs (Fig. 1F). This phenomenon may be attributed to the higher input resistance (Rs) of LNs (Fig. 1G ). The PN and LN responses induced by 1-mM ATP pulses were generally smaller than those induced by 10 mM ATP (Fig.1 D–F). These results suggest the existence of functional feedback from KCs in mushroom bodies to antennal lobe neurons.
Fig. 1.
Depolarization responses in PNs and LNs resulted from activation of mushroom bodies. (A) KC cell bodies or βγ-lobes of mushroom bodies were activated by local puffing of ATP at positions marked 1 and 2, respectively. PN or LN responses were monitored by whole-cell recording. (B–E) Representative traces showed the responses in PNs and LNs resulted from activation of KC cell bodies (position 1) or βγ-lobes (position 2) by 10 mM ATP (B, C) or 1 mM ATP (D, E). Inset depicts the response evoked by ATP application at position 2 with higher time resolution. Graphs on the right show the peak amplitudes of depolarization responses evoked by paired ATP applications at positions 1 and 2 (Materials and Methods). Points with error bars depict the average ± SEM. Data sets marked *** are significantly different with P < 0.001 (paired t test). (F) Average amplitudes of depolarization responses in PNs and LNs evoked by local puffing of 10 mM ATP and 1 mM ATP at position 2. (G) The average input resistance of PNs (n = 10) and LNs (n = 6). Data sets marked *** are significantly different with P < 0.001 (t test).
PN and LN Responses Depend on Specific Expression of P2X2 in KCs.
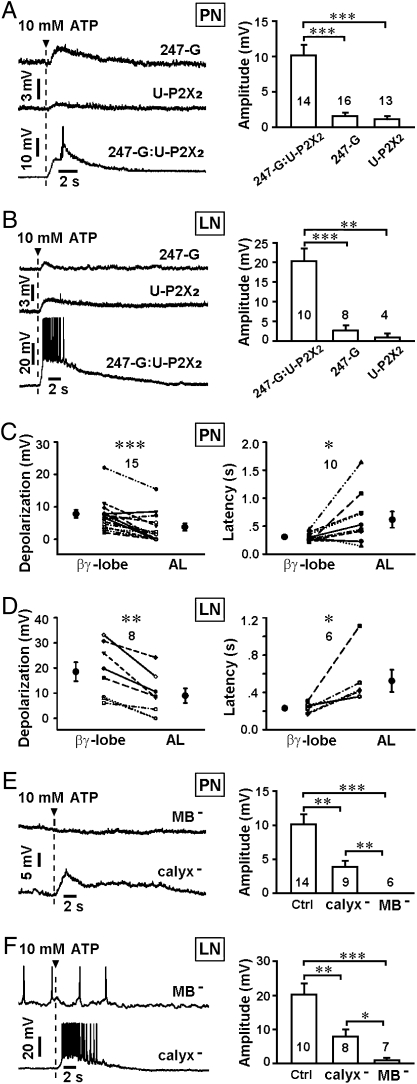
The feedback responses in PNs and LNs induced by ATP stimulation of mushroom bodies were not caused by mechanical artifacts of local puffing, because puffing extracellular solution (ECS) elicited no response (Fig. S2). Furthermore, the responses were not a result of nonspecific activation of other endogenous ATP receptors or channels in the fly brain, as focal application of 10 mM ATP to the βγ-lobes of 247-Gal4 flies and UAS-P2X2 flies induced very little response in PNs and LNs (Scale bar, 3 mV), differing significantly from the robust depolarization responses (Scale bar, 10 mV) evoked in 247-Gal4:UAS-P2X2 flies (Fig. 2 A and B). In addition, we also excluded the possibility of leaky expression of P2X2 in PNs and LNs via the following controls. First, when ATP was directly puffed on the antennal lobes of 247-Gal4:UAS-P2X2 flies (position 3, Fig. 1A), the induced depolarization responses were markedly smaller and significantly slower than those resulting from application of ATP to the βγ-lobes of the mushroom bodies (Fig. 2 C and D). Second, we found that ablation of the mushroom body calyx (including the KC somata) (Fig. S3) decreased the response in PNs and LNs elicited by ATP application at the βγ-lobes (position 2, Fig. 1A). Furthermore, removal of the entire mushroom bodies (including the lobes) (Fig. S3) completely abolished PN/LN depolarization in response to ATP application at the same position (Fig. 2 E and F). These results indicate that feedback responses in antennal lobes were a result of specific expression of P2X2 in KCs.
Fig. 2.
Mushroom body activation-induced PN/LN responses depend on specific expression of P2X2 in KCs. (A and B) (Left) Representative responses from PNs/LNs of 247-Gal4, UAS-P2X2 and 247-Gal4:UAS-P2X2 flies caused by local puffing of 10 mM ATP at βγ-lobes. (Right) Average peak amplitudes ± SEM (**, P < 0.01; ***, P < 0.001 by t test). (C and D) Average peak amplitudes (Left) and latencies (Right) of PNs/LNs responses evoked by puffing of ATP on the βγ-lobes of mushroom bodies or directly to antennal lobes (position 3 in Fig. 1A). Results are shown as the average ± SEM (*, P < 0.05; **, P < 0.01; and ***, P < 0.001 by paired t test). (E and F) (Left) Representative responses of PNs/LNs recorded from fly brains after removal of the calyx (calyx¯) or mushroom body (MB¯), with ATP puffing at position 2 shown in Fig. 1A. (Right) Average peak amplitudes ± SEM (*, P < 0.05; **, P < 0.01; and ***, P < 0.001 by t test). Number in line/bar graph indicates sample size.
In addition, we also investigated whether ATP activation of other types of neurons or other brain regions could induce responses in PNs/LNs. P2X2 was expressed in the gustatory neurons [Gr66a-Gal4 (26)], octopaminergic neurons [TDC2-Gal4 (27)], the fan-shaped bodies of the central complex [C205-Gal4 (28)], and the giant fiber system [A307-Gal4 (29)] of the fly brain. As shown in Fig. S4, ATP-mediated activation of these neurons or brain regions did not induce any significant responses in PNs/LNs, These results further confirm the specificity of the functional feedback from the mushroom bodies to the antennal lobes.
Feedback Is Mediated by Cholinergic Transmission.
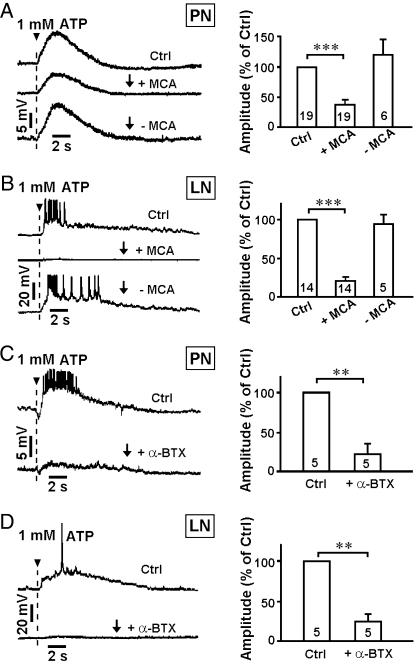
We next examined whether the functional feedback from the mushroom bodies to the antennal lobes was dependent upon synaptic transmission and whether specific neurotransmitters mediate the feedback (Materials and Methods). Because ACh is the major excitatory neurotransmitter in the fly brain, we first tested whether inhibitors of AChRs were capable of blocking the feedback response. Mecamylamine hydrochloride (MCA), a reversible AChR antagonist (30), effectively blocked feedback from mushroom bodies to PNs and LNs (Fig. 3 A and B). We then conducted wash-out experiments using the MCA antagonist. Inhibition caused by MCA was effectively removed through the wash-out procedure (Fig. 3 A and B). To further confirm that ACh is required for feedback, the irreversible AChR blocker α-bungarotoxin (α-BTX) (31) was applied in the assay. α-BTX also significantly inhibited the responses in PNs and LNs elicited by activation of mushroom bodies (Fig. 3 C and D). These results indicate that the functional feedback from mushroom bodies to antennal lobes is dependent upon ACh. In addition, we also explored whether the feedback response could be inhibited by a glutamate receptor (GluR) blocker [D(-)-2-amino-5-phosphor-nopentanoic acid (APV) plus 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)] (31), a GABAAR inhibitor (picrotoxin, PTX) (18, 32), or an octopamine receptor (OCTR) and D1/D2R antagonist (fluphenazine dihydrochloride, Flu) (33). PN and LN responses were unaffected by inhibition of glutamate, GABA, octopamine, or dopamine receptors (Fig. S5). Together, these data indicate that cholinergic transmission specifically mediates functional feedback from the mushroom bodies to the antennal lobes.
Fig. 3.
Functional feedback is mediated by cholinergic transmission. (A–D) (Left) Responses from PNs/LNs evoked by ATP puffing at position 2, before and after application of MCA (A and B) or α-BTX (C and D), and after MCA washout (A and B). (Right) Average peak amplitudes ± SEM (**, P < 0.01; ***, P < 0.001 by paired t test). Number in column indicates sample size.
Candidate Neurons Underlying Functional Feedback.
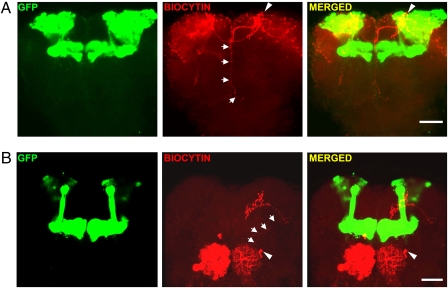
Cobalt injection into the α-lobe of honey bee mushroom bodies previously identified a projection from the mushroom body to the antennal lobe (19), although the precise location of the somata of the labeled projection is unknown. Analogous labeling experiments are difficult to perform in Drosophila because of the small size of the fly brain. However, through the use of whole-cell loading of biocytin and post hoc staining of neurons near mushroom bodies and antennal lobes, we have identified neuronal candidates that may contribute to this functional feedback. For example, a mushroom body extrinsic neuron was found to extend projections toward the antennal lobes (Fig. 4A and MovieS1). In addition, we also observed a neuron from the antennal lobes that possessed extensive processes covering most regions of the antennal lobes, as well as projections to the α- and β-lobes of the mushroom bodies (Fig. 4B and MovieS2). These two types of unique projections may mediate feedback regulation from mushroom body cells to antennal lobe neurons.
Fig. 4.
Identification of candidate neurons underlying functional feedback from mushroom bodies to antennal lobes. Neurons recorded using the whole-cell method were loaded with biocytin, and the morphology of the neuron was examined by post hoc staining combined with confocal microscopy. (A) Example of a neuron with the cell body located near the mushroom body calyx in a 247-Gal4:UAS-GFP fly. Biocytin-stained projections from the neuron are shown in red, GFP-labeled mushroom bodies in green. The soma (arrowhead) is located in or next to the mushroom body calyx, the dendrites are primarily distributed in the calyx, and the axon projects (arrows) to the ipsi-lateral antennal lobe, proto-cerebrum, and the contralateral mushroom body (For additional data, see Movie S1). (Scale bar, 50 μm.) (B) An example of a neuron found in the antennal lobes of the 247-Gal4:UAS-GFP fly. The soma of the neuron (arrowhead) is located in the dorso-lateral antennal lobe, and the processes (red, with white arrows to show the shaft of the process) project to the mushroom body lobes (green) and spread over the majority of the antennal lobes (For additional data, see Movie S2). (Scale bar, 50 μm.)
Discussion
Using the UAS-P2X2:ATP system, we explored whether functional feedback exists from the mushroom body to the antennal lobe. Our results showed that ATP is sufficient to effectively activate P2X2-expressing KCs in the mushroom body. We also observed depolarization responses in PNs and LNs after activation of P2X2-expressing KCs by ATP. Furthermore, activation of the βγ-lobes of mushroom body is more effective in inducing depolarization responses in PNs and LNs than activation of the somata of KCs. Pharmacological experiments showed that the induced responses were dependent upon AChR, but not GluR, GABAAR, OCTR, or D1/D2R. These results suggest that there is functional feedback from the mushroom body to the antennal lobe in Drosophila.
In Drosophila, odors characteristically evoked vigorous responses in PNs of antennal lobes (34). In vivo recordings revealed that some KCs fire sparse action potential to odor stimuli (35). However, several KCs strongly respond to certain odors (e.g., isoamyl acetate), producing prolonged burst-spiking in the presence of the odor (for at least 1 s) (36). In addition, the mushroom bodies are involved in the integration of several sensory modalities, and KCs receive other types of information in addition to olfactory inputs. For example, Wang et al. (37) showed that in olfactory learning, both odor and unconditioned stimuli can induce prolonged calcium elevation in KCs, and the odor-evoked KC responses were markedly enhanced by unconditioned stimuli. Thus, our ATP activation of KCs simulates the conditions when KCs are highly activated during odor stimulation or after conditioning, and the feedback activation of PNs may serve useful functions in odor information processing.
In the Drosophila olfactory system, local circuits in the antennal lobe play important roles in coding and processing odor information from primary ORNs, and the processed information is transmitted by PNs to mushroom bodies and lateral horns (11 –13). Functional feedback from the mushroom body to the antennal lobe may modulate the spontaneous activity and the odor-evoked firing pattern of PNs, thus contributing to the gain control and filtering/sharpening of odor signals during memory retrieval. Yu et al. (38) found that forward conditioning with different odors enhanced the responses in antennal lobes and recruited different PNs synapses for a short-term cellular memory trace. These authors hypothesized that unconditioned stimuli may modulate PN activities via higher-order neurons. Our finding of the functional feedback from the mushroom body to the antennal lobe provides the evidence in support of this hypothesis.
Histochemical staining and electrophysiological studies in mammalian olfactory systems have shown that the olfactory bulb (and accessory olfactory bulb) receives feedback from the cortex and the CA1 area of the hippocampus (39). Rybak and Menzel have injected cobalt into the α-lobe of the mushroom body of the honey bee and identified a fiber projecting from the mushroom body to the antennal lobe (19). Although the identity of the cell body of the labeled fiber is unclear, their results suggest the existence of a feedback connection from the mushroom body to the antennal lobe in honey bees (19, 40). Interestingly, two-thirds of GH146-Gal4-labeled PNs send projections to the mushroom body calyx (5), and two GH146-Gal4-labeled GABAergic neurons in the lateral antennal lobe also send projections to the mushroom body lobes (41), suggesting that axo-axonal synaptic activation of PNs and LNs by KCs could contribute to the functional feedback from the mushroom bodies. In addition, Menzel and colleagues proposed the model in which KCs and PNs are linked by specific GABAergic neurons (PCT neuron) in the honey bee (42, 43). Drosophila analogs of PCT neurons also exist in the mushroom body calyx (44). Our results suggest that in addition to such inhibitory modulation at the PN's axon terminals, excitatory feedback responses to PNs could also be induced by KCs in the mushroom bodies via other excitatory connections. For example, we found two neurons that send projections to both the antennal lobes and KC axons in the βγ-lobes of the mushroom bodies. One of the neurons (Fig. 4B) is similar to the honey bee ALF-1 neuron identified in the honey bees (20). Kirschner et al. suggest that the honey bee ALF-1 neuron might provide a feedback circuit from the mushroom body neuropil to the antennal lobe, as its soma lies close to the vertical lobe of the mushroom body and dense processes with blebby endings spread across the entire antennal lobe. The other neuron (Fig. 4A) appears to be an extrinsic mushroom body neuron, with the soma located in or next to the mushroom body calyx and the axon projecting to the ipsi-lateral antennal lobe. This neuron is similar to the cobalt-labeled feedback neuron identified in the honey bee (19). These findings suggest that candidates for mediating feedback regulation from mushroom body cells to antennal lobe neurons indeed exist in Drosophila.
Further studies are needed to test whether these candidate neurons indeed act as olfactory feedback neurons. For example, it is necessary to examine whether activation of the mushroom body can induce excitatory responses in these candidate neurons, and furthermore, whether activation of these candidate neurons can cause depolarization in PNs or LNs. If some of these candidate neurons could be identified with specific GAL4 lines, then the activities of those neurons could be manipulated and its potential role in the feedback circuit, as well as in olfactory coding and learning and memory, could be thoroughly investigated. The results of these studies would not only identify bona fide feedback neurons, but also shed light on our understanding of the circuitry mechanism and physiological role of the functional feedback.
Materials and Methods
Drosophila Stocks.
Flies were reared on standard cornmeal agar medium at 25 °C and 60% relative humidity. All experiments were performed on adult female flies 1 to 2 days after eclosion.
Electrophysiology.
Whole-cell recordings of PNs, LNs, and KCs of the fruit flies were carried out in vitro. The recordings were performed following the protocol described by Gu and O'Dowd (31), with slight modifications. Briefly, the entire brain was dissected, and the peri-neural sheath was gently removed in ECS containing 103 mM NaCl, 3 mM KCl, 5 mM TES, 10 mM trehalose, 10 mM glucose, 7 mM sucrose, 26 mM NaHCO3, 1 mM NaH2PO4, 1.5 mM CaCl2, and 4 mM MgCl2 (adjusted to 280 mOsm, pH 7.3). The dissected fly brains were transferred to a glass-bottom recording chamber containing ECS and were continuously perfused with ECS bubbled with 95% O2 and 5% CO2 (2 mL/min) throughout the experiments. Dissected fly brains were immobilized using a platinum frame during recording as described in Fig. 1 from Gu and O'Dowd (31). The PNs/LNs and KCs were identified by their relative position and morphology using a 60× water objective and DIC optics. Current-clamp and voltage-clamp recordings were performed using patch-clamp electrodes (9-10 MΩ for PNs/LNs and 13–15 MΩ for KCs) filled with internal solution (160 mM Potassium D-gluconate, 10 mM hepes, 4 mM MgATP, 0.5 mM Na3GTP, 1 mM EGTA, adjusted to 265 mOsm, pH 7.3). Cells were used for recording if the Rm value was greater than 500 MΩ and the MP value was lower than −50 mV. A small constant hyperpolarizing current was injected during recording, immediately after break-in, to bring the membrane potential of neurons to approximately −60 mV. All electrophysiological recordings were carried out using a Nikon E600FN upright microscope equipped with a 100 W mercury lamp (OSRAM) and a GFP filter (BP 450–480). Signals were acquired with an Axon-700B multiclamp amplifier, and were digitized at 10 kHz and filtered at 2 kHz using a 1322A D-A converter. Data were analyzed using Clampex 9.0 software (Molecular Devices). For neurons with action potentials, the membrane potential at the threshold of the firing action potential was taken as the peak amplitude.
ATP was puffed by an electrically gated valve (Picospritzer III, Parker Hannifin Corp.) under the control of pulse generator (Master-8 stimulator, AMPI). The tip of the pipette was positioned as close as possible to the stimulated brain region (KCs or βγ-lobes of the mushroom bodies). Perfusion was applied to the recording chamber with a flow direction that minimized the potential effects of diffusion of ATP to PNs. To provide further characterization of the puffing method, a visual profile of ejected solution containing blue dye (Trypan blue) near the site of application under the same puffing conditions (0.3 s, 4 psi, pipette tip opening of 1 μm) is shown in MovieS3. Solution ejected toward the mushroom bodies was very restricted to the desired target region, with no visible diffusion to the antennal lobes. The following drugs were bath applied in experiments as noted: 5 μM α-BTX, 20 μM CNQX, 100 μM APV, 50 μM Flu, 150 μM MCA, and 100 μM PTX.
Every PN/LN were initially identified by their electrophysiological properties, and further confirmed by their morphology after post hoc biocytin staining. One-percent biocytin was added to the internal pipette solution. After electrophysiological recording, the brain was fixed in phosphate-buffered 4% formaldehyde at 4 °C for 1 h and subjected to biocytin staining.
Biocytin Staining and Confocal Imaging.
Morphology and identity of recorded single neurons were confirmed by post hoc staining of biocytin. Briefly, after fixation for 1 h on ice, the brains were washed in PBS with 0.1% Triton X-100 several times and blocked in blocking buffer (0.1M Tris-HCl, 0.1% Triton X-100, 10% goat serum) for 3 h on ice. Brains were incubated in mouse nc82 antibody (1:50 dilution, gift from E. Buchner, University of Würzburg, Germany) with or without rabbit anti-GFP antibody (1:1,000 dilution, Molecular Probes) overnight at 4 °C. Following incubation in the primary antibodies, brains were washed three times at 20-min intervals in PBS. Brains were then incubated in goat anti-rabbit:Alexa Fluor 488 (1:200 dilution, Molecular Probes), rhodamine-avidin (1:500 dilution, Vector Laboratories), and goat anti-mouse:Alexa Fluor 633 (1:200 dilution, Molecular Probes) for 2 h at room temperature. An LSM 510 Pascal confocal microscope (Zeiss) with a 20× objective was used to acquire optical slices through the antennal lobes. The position of the soma was determined by both the position of electrode tip and the intense biocytin staining. The photographs in Fig. S3 were taken by LSM confocal microscopy, using laser and DIC channels.
Supplementary Material
Acknowledgments
We thank G. Miesenböck (University of Oxford) for providing UAS-P2X2 flies, E. Buchner (University of Würzburg) for providing nc82 antibody, and Dr. M.M. Poo for critical reading of and comments on the manuscript. This work was supported by the 973-2006CB806604, NSF30625020, NSF30621062, and Chinese Academy of Sciences Bai Ren Project and KSCX1-YW-R-31 (to Z.W.) and China Postdoctoral Foundation-20080430704 and Shanghai Postdoctoral Fund-07R214155 (to A.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914912107/-/DCSupplemental.
References
- 1.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 2.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994;275(1):3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 3.Stocker RF, Singh RN, Schorderet M, Siddiqi O. Projection patterns of different types of antennal sensilla in the antennal glomeruli of Drosophila melanogaster . Cell Tissue Res. 1983;232:237–248. doi: 10.1007/BF00213783. [DOI] [PubMed] [Google Scholar]
- 4.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 5.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 7.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54(1):89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson RI. Neural and behavioral mechanisms of olfactory perception. Curr Opin Neurobiol. 2008;18:408–412. doi: 10.1016/j.conb.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 12.Jefferis GS, et al. Comprehensive maps of Drosophila higher olfactory centers: Spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128(6):1205–1217. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol. 2001;430(1):27–55. [PubMed] [Google Scholar]
- 15.Tiesinga P, Fellous JM, Sejnowski TJ. Regulation of spike timing in visual cortical circuits. Nat Rev Neurosci. 2008;9(2):97–107. doi: 10.1038/nrn2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci. 2007;27:5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Rosa-Prieto C, et al. Subicular and CA1 hippocampal projections to the accessory olfactory bulb. Hippocampus. 2009;19(2):124–129. doi: 10.1002/hipo.20495. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka NK, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci. 2009;29:8595–8603. doi: 10.1523/JNEUROSCI.1455-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybak J, Menzel R. Anatomy of the mushroom bodies in the honey bee brain: The neuronal connections of the alpha-lobe. J Comp Neurol. 1993;334:444–465. doi: 10.1002/cne.903340309. [DOI] [PubMed] [Google Scholar]
- 20.Kirschner S, et al. Dual olfactory pathway in the honeybee, Apis mellifera . J Comp Neurol. 2006;499:933–952. doi: 10.1002/cne.21158. [DOI] [PubMed] [Google Scholar]
- 21.Heisenberg M. Mutants of brain structure and function: What is the significance of the mushroom bodies for behavior? Basic Life Sci. 1980;16:373–390. doi: 10.1007/978-1-4684-7968-3_27. [DOI] [PubMed] [Google Scholar]
- 22.Littleton JT, Ganetzky B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron. 2000;26(1):35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 23.Zemelman BV, Nesnas N, Lee GA, Miesenbock G. Photochemical gating of heterologous ion channels: Remote control over genetically designated populations of neurons. Proc Natl Acad Sci USA. 2003;100:1352–1357. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 25.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila . Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 26.Scott K, et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila . Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 29.Allen MJ, Drummond JA, Moffat KG. Development of the giant fiber neuron of Drosophila melanogaster . J Comp Neurol. 1998;397:519–531. doi: 10.1002/(sici)1096-9861(19980810)397:4<519::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Wegener C, Hamasaka Y, Nässel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila . J Neurophysiol. 2004;91:912–923. doi: 10.1152/jn.00678.2003. [DOI] [PubMed] [Google Scholar]
- 31.Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci. 2006;26:265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila . J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 36.Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 39.Kiselycznyk CL, Zhang S, Linster C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn Mem. 2006;13:575–579. doi: 10.1101/lm.285706. [DOI] [PubMed] [Google Scholar]
- 40.Rybak J, Menzel R. Integrative properties of the Pe1 neuron, a unique mushroom body output neuron. Learn Mem. 1998;5(1–2):133–145. [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12(1):53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganeshina O, Menzel R. GABA-immunoreactive neurons in the mushroom bodies of the honeybee: An electron microscopic study. J Comp Neurol. 2001;437:335–349. doi: 10.1002/cne.1287. [DOI] [PubMed] [Google Scholar]
- 43.Szyszka P, Ditzen M, Galkin A, Galizia CG, Menzel R. Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J Neurophysiol. 2005;94:3303–3313. doi: 10.1152/jn.00397.2005. [DOI] [PubMed] [Google Scholar]
- 44.Yasuyama K, Meinertzhagen IA, Schürmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster . J Comp Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.