Abstract
Although recent studies suggest that climate change may substantially accelerate the rate of species loss in the biosphere, only a few studies have focused on the potential consequences of a spatial reorganization of biodiversity with global warming. Here, we show a pronounced latitudinal increase in phytoplanktonic and zooplanktonic biodiversity in the extratropical North Atlantic Ocean in recent decades. We also show that this rise in biodiversity paralleled a decrease in the mean size of zooplanktonic copepods and that the reorganization of the planktonic ecosystem toward dominance by smaller organisms may influence the networks in which carbon flows, with negative effects on the downward biological carbon pump and demersal Atlantic cod (Gadus morhua). Our study suggests that, contrary to the usual interpretation of increasing biodiversity being a positive emergent property promoting the stability/resilience of ecosystems, the parallel decrease in sizes of planktonic organisms could be viewed in the North Atlantic as reducing some of the services provided by marine ecosystems to humans.
Keywords: biodiversity, climate change, ecosystem services, carbon cycles, fisheries
Warming of the climate system and its potential consequences for the biosphere are increasingly documented (1). The rise in global temperature has altered all Earth's subsystems (e.g., cryosphere) including the oceans (2). Consistent with the fact that oceans have absorbed 84% of the heat added to the climate system over the last 40 years (3), and temperature influences almost all biological processes and ecosystems from individual cells to the whole biosphere (4), there were reports of many climate-induced alterations of marine ecosystems ranging from biogeographical and phenological changes to abrupt ecosystem shifts (5, 6). The increasing temperature is also expected to accelerate the global rate of species extinction (7) and reduce the efficiency of the biological carbon pump (8–10).
The aims of our study were to assess the effects of climate change on biodiversity of marine plankton in the last decades and predict the potential consequences of ongoing climate change on the functioning and some services of planktonic ecosystems. Our approach was to (i) search for basin-scale and long-term latitudinal changes in biodiversity patterns of key marine planktonic groups, (ii) identify hydro-climatological variables that covaried with planktonic biodiversity, (iii) use observed covariations to predict the potential impact of current climate change, (iv) investigate spatiotemporal links between biodiversity and size of plankton, and (v) analyze the potential effects of climate-change-related modifications of current planktonic biodiversity on some of the services provided by marine ecosystems to humans (i.e., the biological carbon pump and fisheries in the North Atlantic).
Results and Discussion
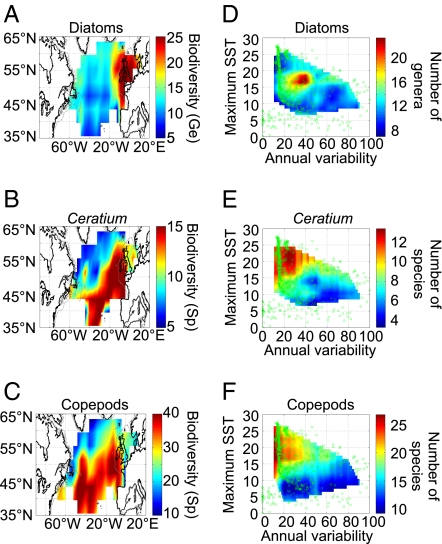
We first examined the geographical distributions and multidecadal latitudinal changes in biodiversity of three key planktonic groups by using data from the Continuous Plankton Recorder (CPR) survey in the North Atlantic (SI Text). The three planktonic groups were as follows: for phytoplankton, diatoms at the genus level; for photosynthethic protists, the dinoflagellate genus Ceratium at the species level; and for mesozooplankton, calanoid copepods at the species level (called copepods hereinafter). Biodiversity was estimated by applying a first-order jackknife procedure on the number of species (Ceratium and copepods) or genera (diatoms) (SI Text). Whereas Ceratium and copepods exhibited similar spatial patterns in biodiversity, it was not so for diatoms (Fig. 1 A–C). Clear ecological partitioning was observed between diatoms, which are characteristic of mixed waters (11) and were most diverse in continental-shelf ecosystems, and Ceratium, which is an indicator of stratified waters (11) and was most diverse in stable and warmer oceanic ecosystems.
Fig. 1.
Spatial distributions and long-term latitudinal changes in biodiversity of diatoms (genus level), Ceratium dinoflagelates (species level), and calanoid copepods (species level) in the extratropical North Atlantic Ocean, 1960–2007, in relation to maximum and annual variability in SST. Mean spatial distribution (1960–2007) of biodiversity of (A) diatoms, (B) Ceratium, and (C) copepods is shown. Plankton biodiversity as a function of two variables, i.e., maximum SST and an index of annual variability in SST, is shown for (D) diatoms, (E) Ceratium, and (F) copepods.
We tested whether the biodiversity of the three planktonic groups was related to maximum sea surface temperature (SST) and an index of annual variability in SST calculated as the coefficient of variation of monthly SST. Temperature is often invoked when investigating processes and explanations of latitudinal gradients in biodiversity (12) (SI Text). Other factors, either atmospheric (e.g., wind speed and intensity) or chemical (e.g., nutrients, salinity, and oxygen), seem to be of less significance to planktonic biodiversity (12, 13). Among the indicators of temperature, maximum SST is one of the best predictors of the spatial distribution of some marine organisms in the North Atlantic (14, 15). The annual variability in SST is also known to be important for the biodiversity of our three planktonic groups (11, 16). In the present study, biodiversity of the three planktonic groups was significantly related to maximum SST and the index of annual variability in SST (Fig. 1 D–F). In multiregression linear models (SI Text), the two variables explained 38% (coefficient of multiple correlation, R = 0.62; probability, P < 0.001; degrees of freedom, n = 377), 36% (R = 0.60, P < 0.001, n = 292) and 9% (R = 0.31, P < 0.001, n = 352) of the total variance of the biodiversity of copepods, Ceratium, and diatoms, respectively. Partial correlation coefficients (SI Text) showed that for copepods and the genus Ceratium, the positive relation of biodiversity with maximum temperature was stronger than its negative relation with annual variability in SST, whereas for diatoms, the positive relation of biodiversity with annual variability in SST was stronger than its positive relation with maximum temperature (Table S1). Annual SST and the index of annual variability in SST were significantly negatively correlated (r = −0.70, P < 0.001, n = 29,523) during the period 1960–2005 on the basis of data from a grid of 1° longitude × 1° latitude for the world's oceans. Projections from the Intergovernmental Panel on Climate Change (2007) indicate that thermal stratification of the water column will intensify throughout the world's oceans in the next decades (17). Our results suggest that increasing maximum temperature may favor the biodiversity of both copepods and Ceratium and that decreasing annual variability in SST may contribute to reduce biodiversity and abundance of diatoms, which are major contributors to carbon export (8, 9, 18).
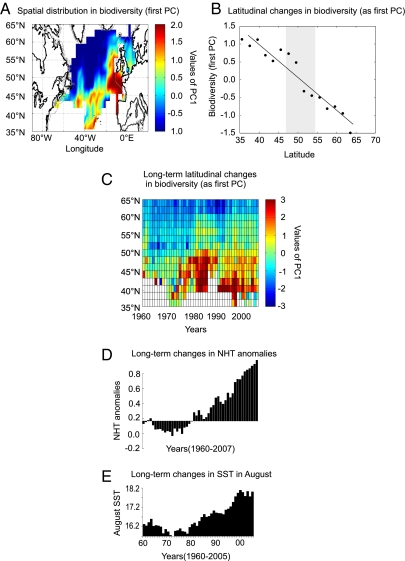
We investigated spatial and latitudinal changes in the combined biodiversity of the three planktonic groups in relation to global and regional changes in SST (SI Text). A standardized principal component analysis (PCA) was performed on the table of geographical cells × biodiversity of the three planktonic groups and showed a clear contrast between lower biodiversity in regions south of the Oceanic Polar Front and higher biodiversity observed over the European shelf edges (19) (Fig. 2A). When the data were meridionally averaged, the first principal component (Fig. 2B) mirrored the latitudinal gradient in biodiversity between 35°N and 65°N (Fig. 2A) (12, 20). A second PCA was performed on the multidecadal latitudinal changes in biodiversity of the three planktonic groups (Fig. 2C) and summarized the latitudinal northward shifts in biodiversity observed for the three groups (Fig. 1 A–C). A southward retreat occurred after a pronounced increase in biodiversity between 40°N and 50°N at the end of the 1980s, but the northward shift continued after the mid-1990s above 60°N. The stepwise changes in latitudinal distribution of biodiversity coincided with pronounced increases in both global atmospheric and regional sea surface temperatures (Fig. 2 D and E). Such latitudinal increases at the ecoregional scale have also been recently reported for fish in the North Sea (21).
Fig. 2.
Spatial distribution and mean and long-term latitudinal changes in plankton biodiversity (diatoms, Ceratium, and copepods) in the extratropical North Atlantic Ocean, 1960–2007. The index of biodiversity here is the first principal component (PC) resulting from standardized PCA. (A) Spatial distribution of plankton biodiversity (first PC, 58% of total variance). The PCA was performed on a table containing spatial information on biodiversity of the three plankton groups (Fig. 1 A–C). (B) Mean latitudinal change of biodiversity after averaging the values of the first PC in Fig. 1A by latitudinal bands of 2 °C. Long-term latitudinal changes of plankton biodiversity are shown (first PC, 62% of total variance). The PCA was performed on a table containing long-term latitudinal information on biodiversity of the three plankton groups (SI Text). (D) Long-term changes in Northern Hemisphere Temperature (NHT) anomalies, 1960–2007. (E) Long-term changes in mean average temperature in August in the North Atlantic, 1960–2005.
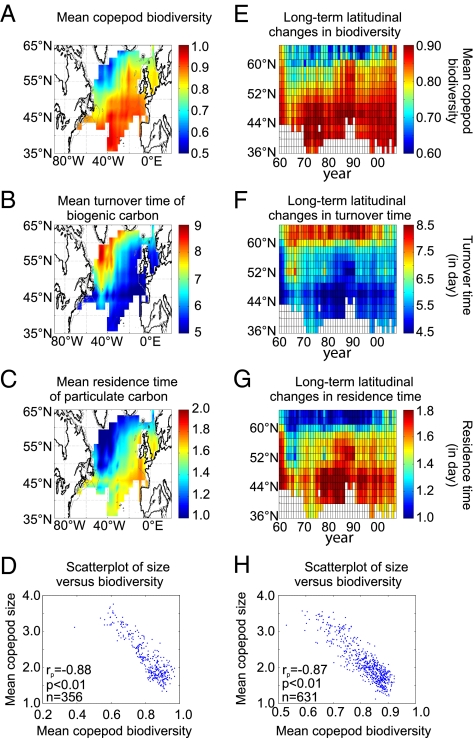
Community body size largely determines the types and strengths of flows of energy and materials in ecosystems, affecting both ecological networks and the way ecosystems are structured and function (22, 23). Biomass, production, predator/prey interactions, cannibalism, and carbon export are among the quantities or processes that respond to the size structure of an ecosystem (22–24). In CPR, zooplankton organisms are sampled more quantitatively than phytoplankton, so we focused our analysis of possible relationships between biodiversity and size structure of planktonic assemblages on copepods. We assessed biodiversity on the basis of copepod species abundances by applying a first-order jackknife procedure to an index of biodiversity based on abundance called the Gini coefficient (SI Text). Although the spatial pattern of copepod biodiversity was similar to that based on species richness (Fig. 3A vs. Fig. 1C), the multidecadal northward shift in biodiversity was more clearly evidenced when using biodiversity based on species abundances, with two major stepwise shifts identified at the beginning of the 1980s and in the mid-1990s. There was a highly significant inverse relationship between biodiversity and the mean community body size of copepods in both space (extratropical North Atlantic, Fig. 3D, r = −0.88, P < 0.01) and time (multidecadal scale, Fig. 3H, r = −0.87, P < 0.01). Such a general decrease in size of copepods co-occurred with temperature warming in the North Atlantic and the Baltic and North Seas (15, 22). Because the CPR tends to collect small individuals less efficiently than plankton nets (25), the observed decreases in mean community size probably underestimate the real trends.
Fig. 3.
Relationships between the spatial distributions and long-term latitudinal changes in biodiversity and two size-derived functional characteristics of copepods in the extratropical North Atlantic. Biodiversity was measured by first-order jackknife performed on the Gini coefficient. Mean spatial distributions (1960–2007) are shown of (A) copepod biodiversity, (B) minimum turnover time of carbon incorporated in copepods (in days), and (C) mean residence time above 50 m of sinking copepod particles (in days). (D) Relationship between the mean size of copepods and biodiversity based on mean spatial distributions (1960–2007). Long-term latitudinal changes are shown in (E) copepod biodiversity, (F) minimum turnover time of carbon incorporated in copepods, and (G) mean residence time above 50 m of sinking copepod particles. (H) Relationship between long-term latitudinal changes in size and biodiversity. Linear correlation (rp), probability of H0 (p), and degrees of freedom (n) are indicated in D and H.
Given that global warming may shift mean community body size, we investigated potential consequences of such a shift for ecosystem functioning and carbon cycles. Information on size structure of copepods was converted into the minimum turnover time of carbon incorporated in these organisms and the mean residence time above 50 m of sinking copepod particles (i.e., fecal pellets) by using allometric equations (24) (SI Text). It has been shown that (a) the minimum turnover time of carbon incorporated in organisms is directly related to the size of organisms and (b) the mean residence time of sinking particles produced by organisms in surface waters is inversely proportional to size and the downward export of sinking particles is inversely related to their mean residence time at the surface; hence downward export is directly related to the size of organisms that produce the sinking particles (24). Because of the strong inverse relationships between biodiversity and size of copepods (Fig. 3 D and H), there were highly significant correlations between copepod biodiversity and both the minimum turnover time of carbon in copepods (negative) and the mean residence time of sinking copepod particles (positive) (Fig. 3 A–C). The correlation between biodiversity and the minimum turnover time of carbon in copepods (r = −0.88, P < 0.01) indicates that the northward increase in biodiversity was accompanied by a reduction in the minimum turnover time of carbon in these organisms, i.e., a quicker circulation of the carbon incorporated in smaller organisms that had shorter life cycles. This quicker circulation of carbon would be one component of the increase in ecosystem metabolism that is likely to accompany the rise in temperature in the Atlantic Ocean (15). The correlation between biodiversity and the mean residence time of sinking copepod particles (r = 0.86, P < 0.01) indicates that the increase in biodiversity was also accompanied by an increase in the mean residence time above 50 m of sinking copepod fecal pellets. Although it is debated whether copepod fecal pellets contribute significantly to the downward biological carbon pump (26), if the increase in the mean residence time of biogenic carbon in surface waters affected the whole planktonic ecosystem, we can reasonably speculate that food-web-controlled downward carbon export could be weaker in a warmer ocean and thus contribute to a positive feedback to climate change.
In addition to the above size-related changes in minimum turnover time of carbon in copepods and mean residence time above 50 m of sinking fecal pellets, the mean total biomass of copepods started to decrease from the end of the 1990s above 50°N (SI Text and Fig. S1). This decrease is an additional factor that could have contributed to decrease the copepod-related downward carbon export.
The extratropical North Atlantic Ocean is an important region for carbon export (9), and it is thought that the biological pump will be less efficient in a warmer world because of changes in phytoplanktonic types (floristic shifts) and reduced upward mixing of nutrients due to increased stratification of the oceans (8, 9). Deepening of the nutricline, as a result of increased stratification, would shift the phytoplanktonic community from diatoms (major exporters of carbon to depth) to coccolithophorids (27). Our results indicate that the biological carbon pump could be reduced not only because of lower nutrient inputs into the euphotic zone (18), but also because organic carbon would reside longer in surface waters where it would be processed through smaller-sized zooplankton and thus dissipated through more complex food webs (6) and additionally because the total biomass of copepods may decrease. Decreases in community size in relation to more stable and warmer environments have also been found for phytoplankton in the Atlantic (28) and fish in the North Sea (21). It therefore appears that increasing temperature has led to smaller-sized community assemblages across multiple pelagic trophic levels.
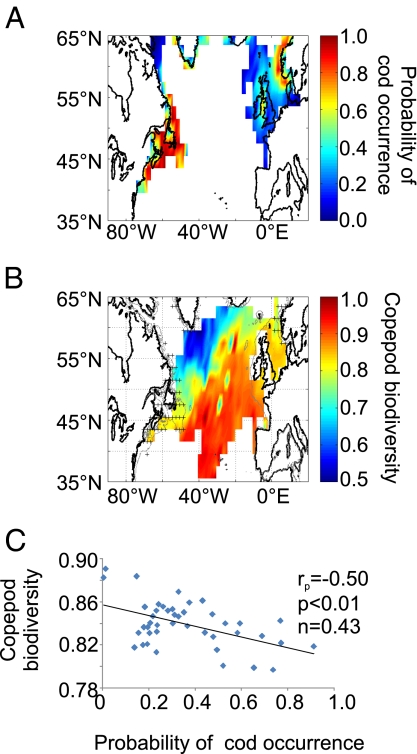
To assess the potential consequences of increasing biodiversity and diminishing size of organisms for ecosystem services, in addition to the biological carbon pump, we use here the example of Atlantic cod (Gadus morhua L.). This subarctic species has been heavily exploited in the North Atlantic for decades and, consequently, overfishing had major impacts on cod in the whole basin (29). In addition to overfishing, climate-driven changes in planktonic composition and thus biodiversity have negatively influenced some stocks of Atlantic cod at the edges of their distributional ranges or environmental (thermal) niches (e.g., North Sea), whereas climate-driven effects were much less important at the centers of niches (e.g., Iceland) (30). In the present study, we found a negative correlation between the probability of cod occurrence (SI Text) and copepod biodiversity (Fig. 4). However, we caution that this correlation does not imply a direct negative link between the two variables, but suggests instead that cod are sensitive to the partitioning of energy and biomass among planktonic components. For example, a recent study showed that although the stomach contents of cod larvae can include large amounts of small copepods (mainly Pseudocalanus spp.) (31), cod had high probability of occurrence only in areas where the percentage of Pseudocalanus abundance (small copepods) in the joint abundance of Pseudocalanus and Calanus finmarchicus (large energetic copepods) was <50% (32). This result stresses the significance of the negative relationship between copepod biodiversity and mean community size (Fig. 3 D and H) for North Atlantic cod fisheries.
Fig. 4.
Spatial relationships between the biodiversity of copepods and the probability of cod occurrence in the extratropical North Atlantic. (A) Probability of cod occurrence. (B) Mean spatial distribution of copepod biodiversity (as measured by first-order jackknife performed on the Gini coefficient). Crosses indicate a probability of cod occurrence >0.5. (C) Relationship between copepod biodiversity and probability of cod occurrence. Linear correlation (rp), probability of H0 (p), and degrees of freedom (n) are indicated.
Ecologists often see the biodiversity of an ecosystem as beneficial in terms of resilience, stability, and ecosystem services (33), but in economic terms, increasing planktonic biodiversity may be detrimental to marine bioresources particularly in higher-latitude fisheries. It is known that simpler food webs and lower biodiversity ecosystems, such as those found in cold-temperate ecoregions (e.g., the North Sea and Grand Banks), have often been characterized by large populations of exploitable fish species. As temperature rises and pelagic biodiversity increases, traditional cold-temperate fisheries that were built on the exploitation of large gadoids will have to adapt to exploiting stocks of smaller-sized fish, and it is thought that the shift in the size structure of exploited fish will devalue the fisheries (21). However, the relationship between temperature, planktonic biodiversity, and fish is far more complex than described here because of other synergistic pressures such as overfishing, which exerts a significant control on the biomass of the spawning stocks of exploited and other species (33).
Our ocean-basin scale study showed a pronounced and rapid reorganization in biodiversity of phytoplankton and zooplankton in the extratropical North Atlantic that paralleled a decrease in the mean size of copepods. We suggested that this large-scale reorganization impacted the structure and functioning of pelagic ecosystems, which could have affected the services of these ecosystems to human societies, i.e., the biological carbon pump and the Atlantic cod. Although the most conspicuous factors that seem to explain the observed changes are the increase in maximum SST and decrease in annual variability in SST, these changes are likely driven by both climate change and the complex interplay between climate and ocean variability.
Methods
Long-term monthly SST data for the period 1960–2005 were obtained from the Comprehensive Ocean-Atmosphere Data Set 1-degree enhanced dataset provided by the comprehensive National Oceanic and Atmospheric Administration–Cooperative Institute for Environmental Research in Environmental Sciences Climate Diagnostics Center Database (Boulder, Colorado) (34). From these data, we created an index of annual variability in SST by calculating the coefficient of variation of SST (35).
Northern Hemisphere Temperature anomalies from 1958 to 2007 were provided by the Hadley Centre for Climate Prediction and Research, Meteorological Office, London, UK.
The CPR survey is an upper-layer planktonic monitoring program that has regularly collected samples in the North Atlantic and adjacent seas at monthly intervals since 1946 (25, 36, 37). Among the monitored taxa, we chose to investigate the biodiversity of three major planktonic groups: diatoms at the genus level (35 genera), the dinoflagellate genus Ceratium at the species level (47 species), and calanoid copepods at the species level (109 species). We selected these three taxa because they represent key components of pelagic ecosystems, are good integrators of climate, and are well identified in the CPR survey. Diatoms were selected because of their importance as primary producers and carbon exporters. Among dinoflagellates, the genus Ceratium was investigated because this diverse taxon is an important component of marine photosynthetic protists. Copepods contribute to transfer energy and materials between smaller and larger components of the food web (38).
Data on probability of cod occurrence used in Fig. 4 (62,160 data points) were downloaded from Fishbase (http://www.fishbase.org). These data originated from a relative habitat suitability (RES) model developed to predict global distributions of marine mammals (39) and subsequently adapted to map the probability of occurrence of other marine organisms, especially fish. The model for a given fish species is constructed from estimates of the environmental tolerance of that species (i.e., its ecological niche) with respect to depth, salinity, temperature, primary productivity, sea-ice concentration, and distance to land. The RES approach incorporates expert knowledge into the ecological niche model. Computationally, RES (called procedure “aquamap” in Fishbase) is a trapezoidal model that calculates the probability of cod occurrence (which varies from 0 when absence is highly probable to 1 when presence is highly probable) from the environmental variables listed in the previous sentence. Hence, the probabilities of cod occurrence derived from the RES model are not influenced by overexploitation, contrary to actual fish abundance or biomass data, because they are based on environmental data only. The computed values largely reflect the probability of occurrence of cod ≥1 year.
Description of the numerical procedures applied to the data are described in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. John Alistair Lindley and Prof. Peter Burkill for useful comments on the research. We are grateful to past and present members and supporters of the Sir Alister Hardy Foundation for Ocean Science whose continuous efforts have allowed the long-term establishment and maintenance of the unique CPR data set; the survey depends on the owners, masters, and crews of the ships that tow the CPRs. This work was supported by the Centre National de la Recherche Scientifique, France, and the European Network of Excellence for Ocean Ecosystems Analysis and Marine Biodiversity and Ecosystem Functioning.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913855107/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change WGI . Climate Change 2007: Impacts, Adaptation and Vulnerability. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 2.Barnett TP, et al. Penetration of human-induced warming into the world's oceans. Science. 2005;309:284–287. doi: 10.1126/science.1112418. [DOI] [PubMed] [Google Scholar]
- 3.Levitus S, Antonov J, Boyer T. Warming of the world ocean, 1955–2003. Geophys Res Lett. 2005;32:L02604. [Google Scholar]
- 4.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 5.Beaugrand G, Luczak C, Edwards M. Rapid biogeographical plankton shifts in the North Atlantic Ocean. Glob Change Biol. 2009;15:1790–1803. [Google Scholar]
- 6.Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 7.Thomas H, Bozec Y, Elkalay K, de Baar HJW. Enhanced open ocean storage of CO2 from shelf sea pumping. Science. 2004;304:1005–1008. doi: 10.1126/science.1095491. [DOI] [PubMed] [Google Scholar]
- 8.Bopp L, Aumont O, Cadule P, Alvain S, Gehlen G. Response of diatoms distribution to global warming and potential implications: A global model study. Geophys Res Lett. 2005;32:L19606. [Google Scholar]
- 9.Sarmiento JL, et al. Response of ocean ecosystems to climate warming. Glob Biogeochem Cycles. 2004;18 [Google Scholar]
- 10.Ready J, et al. Predicting the distributions of marine organisms at the global scale. Ecol Modell. 2010;221:467–478. [Google Scholar]
- 11.Margalef R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta. 1978;1:493–509. [Google Scholar]
- 12.Rombouts I, et al. Global latitudinal variations in marine copepod diversity and environmental factors. Proc R Soc B. 2009;276:3053–3062. doi: 10.1098/rspb.2009.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaugrand G, Edwards M, Brander K, Luczak C, Ibañez F. Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecol Lett. 2008;11:1157–1168. doi: 10.1111/j.1461-0248.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 14.McMahon CR, Hays GC. Thermal niche, large-scale movements and implications of climate change for a critically endangered marine vertebrate. Glob Change Biol. 2006;12:1330–1338. [Google Scholar]
- 15.Beaugrand G. Decadal changes in climate and ecosystems in the North Atlantic Ocean and adjacent seas. Deep Sea Res II. 2009;56:656–673. [Google Scholar]
- 16.Begon M, Townsend CR, Harper JL. Ecology. From Individuals to Ecosystems. Oxford: Blackwell; 2006. [Google Scholar]
- 17.Intergovernmental Panel on Climate Change WGI . Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 18.Hashioka T, Yamanaka Y. Ecosystem change in the western North Pacific associated with global warming using 3D-NEMURO. Ecol Modell. 2007;202:95–104. [Google Scholar]
- 19.Beaugrand G, Ibañez F, Lindley JA. Geographical distribution and seasonal and diel changes of the diversity of calanoid copepods in the North Atlantic and North Sea. Mar Ecol Prog Ser. 2001;219:205–219. [Google Scholar]
- 20.Hillebrand H. Strength, slope and variability of marine latitudinal gradients. Mar Ecol Prog Ser. 2004;273:251–267. [Google Scholar]
- 21.Hiddink JG, ter Hofstede R. Climate induced increases in species richness of marine fishes. Glob Change Biol. 2008;14:453–460. [Google Scholar]
- 22.Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci USA. 2009;106:12788–12793. doi: 10.1073/pnas.0902080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward G, et al. Body size in ecological networks. Trends Ecol Evol. 2005;20:402–409. doi: 10.1016/j.tree.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Legendre L, Michaud J. Flux of biogenic carbon in oceans: Size-dependent regulation by pelagic food webs. Mar Ecol Prog Ser. 1998;164:1–11. [Google Scholar]
- 25.Batten SD, et al. CPR sampling: The technical background, materials, and methods, consistency and comparability. Prog Oceanogr. 2003;58:193–215. [Google Scholar]
- 26.Turner JT. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol. 2002;27:57–102. [Google Scholar]
- 27.Cermeño P, et al. The role of nutricline depth in regulating the ocean carbon cycle. Proc Natl Acad Sci USA. 2008;105:20344–20349. doi: 10.1073/pnas.0811302106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen MR, Ingram WJ. Constraints on future changes in climate and the hydrologic cycle. Nature. 2002;419:224–232. doi: 10.1038/nature01092. [DOI] [PubMed] [Google Scholar]
- 29.Brander KM. Global fish production and climate change. Proc Natl Acad Sci USA. 2007;104:19709–19714. doi: 10.1073/pnas.0702059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaugrand G, Kirby RR. Spatial changes in the sensitivity of Atlantic cod to climate-driven effects in the plankton. Clim Res. 2010;41:15–19. [Google Scholar]
- 31.Heath MR, Lough RG. A synthesis of large-scale patterns in planktonic prey of larval and juvenile cod (Gadus morhua) Fish Oceanogr. 2007;16:169–185. [Google Scholar]
- 32.Beaugrand G, Kirby RR. Climate, plankton and cod. Glob Change Biol. 2010;16:1268–1280. [Google Scholar]
- 33.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 34.Woodruff S, Slutz R, Jenne R, Steurer P. A comprehensive ocean-atmosphere dataset. Bull Am Meteorol Soc. 1987;68:1239–1250. [Google Scholar]
- 35.Legendre P, Legendre L. Numerical Ecology. Amsterdam: Elsevier Science; 1998. [Google Scholar]
- 36.Warner AJ, Hays GC. Sampling by the Continuous Plankton Recorder survey. Prog Oceanogr. 1994;34:237–256. [Google Scholar]
- 37.Reid PC, et al. The Continuous Plankton Recorder: Concepts and history, from plankton indicator to undulating recorders. Prog Oceanogr. 2003;58:117–173. [Google Scholar]
- 38.Mauchline J. The Biology of Calanoid Copepods. San Diego: Academic Press; 1998. [Google Scholar]
- 39.Kaschner K, Watson R, Trites AW, Pauly D. Mapping world-wide distributions of marine mammal species using a relative environmental suitability (RES) model. Mar Ecol Prog Ser. 2006;316:285–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.