Abstract
The actuarial senescence (i.e., the rate of increase in adult mortality with age) was related to body mass, development period, and age at sexual maturity across 124 taxonomic families of terrestrial vertebrates. Model selection based on Akaike's information criterion values adjusted for small size showed that the rate of aging decreases with increasing body mass, gestation period, age at maturity, and possession of flight. Among families of mammals, actuarial senescence was related to extrinsic mortality rate (standardized regression coefficient = 0.215), gestation period (−0.217), and age at maturity (−0.553). Although rate of aging in birds also was related to the embryo development period, birds grow several times more rapidly than mammals, and therefore, the connection between rate of early development and rate of aging is unclear. The strong vertebrate-wide relationship between rate of aging, or life span, and age at maturity can be explained by density-dependent feedback of adult survival rate on the recruitment of young individuals into the breeding population. Thus, age at maturity seems to reflect extrinsic mortality, which, in turn, influences selection on mechanisms that postpone physiological and actuarial senescence. Because rate of embryo development influences rate of aging independently of the age at maturity, in a statistical sense, the evolutionary diversification of development and aging seem to be connected in both birds and mammals; however, the linking mechanisms are not known.
Keywords: birds, embryo development, mammals, reptiles, sexual maturity
Most biologists accept that the rate of aging has a genetic basis and is under selection (1–3). However, evolutionary adaptations that influence the rate of aging and differentiate potential life span among species are poorly understood. Mechanisms related to the rate of aging that evolve under selection might, or might not, correspond to molecular and biochemical processes of interest to biologists who investigate the aging process in humans and model organisms. These processes include the production of reactive oxygen species (ROS) and control of oxidative damage (4, 5), telomere shortening, which influences cell replication (6–8), various signaling pathways that produce antagonisms between development and aging (9–11), and inflammation responses that produce antagonisms between disease prevention and tissue damage (12). However, other processes, particularly developmental mechanisms that influence the quality of the adult individual, might be brought into play by evolution.
Comparative analyses of the rate of aging, or some proxy such as maximum life span, have been used to support various ideas about aging (13–16). For example, the pervasive relationship between life span and body mass was viewed as support for a relationship between metabolism and life span—the so-called rate-of-living hypothesis (17, 18). However, comparative analyses also have been used to test falsifiable hypotheses. In the case of the rate of living hypothesis, for example, the observation that bats and birds live longer than cursorial mammals of similar size allowed biologists to reject a simple connection between metabolism and life span (19), although oxidative damage might nonetheless play an important role in aging (20, 21).
Comparative analyses also can be used to distinguish among competing hypotheses. For example, the idea that rate of aging can be modified independently of metabolic rate (or body mass) by selection on life span is supported by the strong correlation between rate of aging and extrinsic mortality rate, which limits the maximum potential life span independently of body mass (22). Clearly, broader comparisons have greater power to contrast the predictions of multiple hypotheses and uncover the most general relationships. In this sense, comparative analyses have been used primarily to identify patterns in the connections between life span and other aspects of the life history of organisms as a way of suggesting potential mechanisms of general importance.
As information about life histories of organisms has accumulated and with the advent of more powerful analytical techniques, including phylogenetically informed comparative analyses (23, 24), the search for pattern connected to aging has broadened. Earlier studies primarily concerned the relationship between life span and body size or metabolic rate (25). More recently, comparative analyses have extended to other life-history traits, particularly developmental schedules, leading to the concept of a slow–fast continuum in life histories (15, 26–28). Among mammals, maximum longevity has been related to age at maturity (29) and postnatal growth rate (30). In a broad analysis of bird and mammal data, de Magalhães et al. (31) concluded that age at sexual maturity, corrected for body mass, bears the most consistent relationship to maximum recorded life span, with the exclusion of metabolic rate and postnatal growth rate.
Although most comparative studies have related life span to life-history attributes of individuals, such as metabolic rate and development periods, life span also varies in relation to environmental variables—a predictable outcome of evolutionary differentiation but also of direct phenotypic responses of aging to the environment. For example, the rate of aging, assessed by the increase in mortality rate as a function of age [actuarial senescence (AS)], increases with the extrinsic mortality rate experienced by young (presumably nonsenescent) adults (22). This relationship is consistent with a prominent evolutionary theory of aging (3, 22, 32–36), which states that selection to postpone the effects of senescence is strongest in species that enjoy low extrinsic risk of death. Mortality rates in nature depend on aspects of the environment as well as the adaptations of individuals that influence risk, such as the arboreal habit in mammals (37), which also should be correlated with the rate of aging. Wasser and Sherman (38) recently analyzed the relationship of maximum recorded life span in 40 avian families to several ecological, physiological, and behavioral traits, finding significant support for effects of body mass (P < 0.0001), diet (herbivore > carnivore = omnivore; P = 0.013), breeding (social > nonsocial; P = 0.028), and isolation (island > mainland; P = 0.054); breeding latitude, breeding habitat, nest-site location, and migratory behavior were not significant effects.
The present study differs from, and extends, previous comparative studies in that (i) rate of aging is based on actuarial data rather than maximum longevity, (ii) mammals, birds, reptiles, and amphibians are analyzed together to determine commonalities broadly across vertebrates, (iii) several life-history variables are added, particularly the gestation/incubation period, (iv) multivariate analyses are used to account for correlated variation among independent variables and to determine unique statistical contributions to the rate of aging, (v) adult body mass is treated as an independent variable rather than calculating residuals of the other variables with respect to mass (which presumes primary importance for body mass), and (vi) analyses are based mostly on mean values for taxonomic families rather than phylogenetically independent contrasts because of the considerable error in estimating the rate of aging (as well as maximum life span).
Results
The general result of the comparative analysis is that rate of aging, quantified as the parameter omega (ω) of the Weibull function (22), is negatively related to age at maturity, the possession of flight (scored as 0 or 1, essentially the distinction between birds and other vertebrates, except bats), and the duration of embryo growth (Fig. 1). Body mass makes no additional unique contribution to variation in ω. When flight is removed from the regression, incubation/gestation period becomes nonsignificant, because flight serves as a proxy for the difference between mammals and birds. The incubation or gestation periods differ substantially between birds and mammals (39), and therefore, without distinguishing these observations taxonomically, the influence of embryo growth rate on the rate of aging evaporates, although the statistical effect is strong within each class.
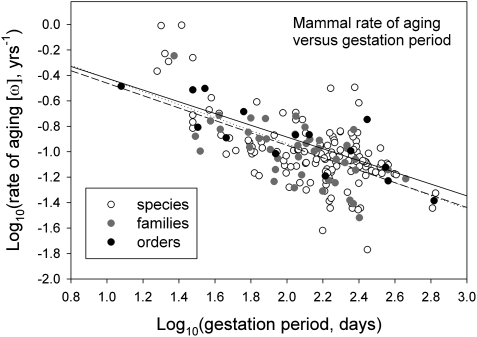
Fig. 1.
Relationship between the rate of aging (ω) and gestation period (GP) in mammals analyzed at the levels of species, family, and order. Regression equations at each level are: order (F1,13 = 29.5, P < 0.0001, r2 = 0.694), ω = 0.040 (± 0.176 SE) − 0.463 (0.085) GP; family (F1,50 = 48.0, P < 0.0001, r2 = 0.490), ω = 0.027 (0.147) – 0.489 (0.071) GP; species (F1,158 = 100.5, P < 0.0001, r2 = 0.389), ω = 0.088 (0.110) – 0.512 (0.051) GP.
Family-level analyses included 124 taxa (Amphibia = 9, Aves = 45, Mammalia = 53, Reptilia = 17). The standard deviations of the log10-transformed variables were: adult body mass, 1.290 (n = 118); ω, 0.237 (n = 124); m0, 0.354 (n = 124); neonate mass, 1.301 (n = 89); incubation or gestation period, 0.472 (n = 104); postnatal growth rate, 0.703 (n = 65); age at maturity, 0.387 (n = 90). Adult and neonate mass exhibited the broadest variation; gestation period and postnatal growth rates had high variability, compared with the remaining traits, because of the pronounced differences between birds and mammals.
The small sample sizes for neonate mass and postnatal growth rate limited the scope of comparative analyses. In a stepwise regression of logω with all of the independent variables (n = 58 families), neither neonate mass nor postnatal growth rate were significant effects. Thus, these variables were deleted from subsequent analyses, leaving age at maturity (n = 90 families) with the limiting sample size.
When log10ω was regressed as a function of the logarithms of body mass (m0), incubation or gestation period, and age at maturity, including taxonomic class as an effect (n = 87 families), class was a significant effect (F3,79 = 10.4; P < 0.0001, explaining 13.2% of the total variance), with birds exhibiting significantly lower log10ω than the other classes by factors of 1.56–1.90. Taxonomic class, however, is redundant on flight, which, in the absence of class as a variable, explained 10.0% of the total variance (F1,81 = 22.1; P < 0.0001); with flight and class in the same model, neither was uniquely significant (both P > 0.05). In subsequent analyses, I retained flight as the biologically more interpretable variable.
Akaike's information criterion (AIC) adjusted for small sample size (AICc) comparisons of models with different combinations of the independent variables indicated low weights for initial mortality rate (0.37) and adult body mass (0.68), with the remaining variables having weights of 0.97–1.00. With m0 deleted from the model (n = 87), weighted estimates of variable coefficients were: mass, −0.038; gestation period, −0.156; age at maturity, −0.322; flight, −0.192. Thus, age at maturity was a strong predictor of the rate of aging (and maximum recorded life span) across the vertebrate classes. Rate of aging decreases with increasing body mass (standardized regression coefficient = −0.208), gestation period (−0.289), and age at maturity (−0.524) and with possession of flight (Appendix S1).
The analysis was repeated among families of mammals (n = 50) with complete data (flight deleted and length of the weaning period added). Adult mass, neonate mass, and weaning period had variable weights less than 0.6 and were subsequently excluded. In the final model (n = 51), the weighted regression coefficients were: initial mortality rate, 0.143; gestation period, −0.161; age at maturity, −0.389. The standardized regression coefficients were 0.215, −0.217, and −0.553, respectively ((Appendix S2).
One of the striking contrasts in the life histories of birds and mammals is the disparity in rate of development, both during the embryo period and after birth or hatching (Fig. 2). In both birds and mammals, the rate of aging is statistically related to the length of the embryo development period (r = −0.43, n = 141 species; r = −0.62, n = 160) and the rate of postnatal growth (r = 0.28, n = 91; r = 0.73, n = 72). However, the relationship is displaced between the classes (Fig. 3); only gestation period remains a marginally significant effect, and this is only for mammals in multiple regressions that include age at maturity.
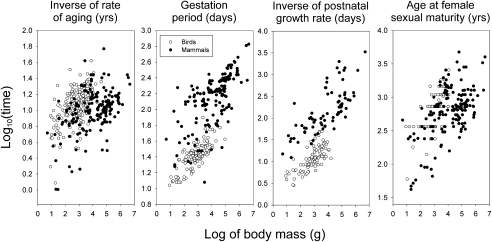
Fig. 2.
Species-level relationships between potential life span (1/ω, in years) and lengths of the embryo, postnatal growth, and prereproductive periods as a function of body mass in birds (open symbols) and mammals (solid symbols). The relationship between birds and mammals with respect to the rate of aging most closely matches that with respect to age at sexual maturity.
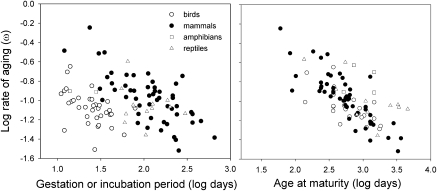
Fig. 3.
Family-level relationships between the rate of aging (ω) and the embryo development period (Left) and age at maturity (Right). The relationships for each of the classes match well with respect to age at maturity; crocodilians and tortoises are outliers among the reptiles, because they have relatively high rates of aging for their ages at maturity.
Discussion
The most striking result of this analysis is the strong relationship between rate of aging and age at sexual maturity across terrestrial vertebrates. This relationship exists virtually to the exclusion of statistical contributions of body and neonate mass, lengths of the embryo development and weaning periods, and rate of postnatal development. The result parallels the results of de Magalhães et al. (31), which are based on maximum reported life span rather than an actuarial rate of aging, as in this analysis. Ricklefs (39) found displaced relationships between the rate of aging and the rate of embryo growth in birds and mammals, but the numbers of species were small and age at maturity was not included in the multiple-regression analyses. The result also parallels the observation of Charnov (15, 40, 41) that age at maturity directly parallels the mean adult life span, such that the dimensionless ratio of these numbers is invariant.
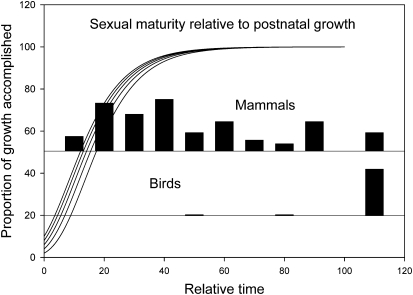
The relationship between age at maturity and potential life span is consistent with the distribution of species along a slow–fast continuum, but the continuum concept (28) breaks down when embryonic and postnatal growth and development are considered. Indeed, birds and mammals contrast strikingly with respect to the onset of maturity relative to postnatal growth (Fig. 4). Most mammals become sexually mature before they have completed postnatal growth, whereas virtually all birds mature sexually long after they are fully grown. Thus, no single pattern of life, including development, maturity, and aging, varies among vertebrate species only by expansion or contraction of a common time scale. These aspects of the life history apparently evolve, to a large extent, independently. Indeed, in comparisons across the vertebrate classes, rate of development predicts longevity relatively poorly; even within each vertebrate class, the statistical association of longevity with development is weak, if it exists at all, when age at maturity also is included in the equation.
Fig. 4.
Distribution of the ages at sexual maturity relative to postnatal growth rate. Black bars represent the relative numbers of species that become sexually mature at different times relative to their postnatal growth. Growth curves in the background are Gompertz functions that describe mass at time t as W(t) = Aexp[−bexp(−kt)], where A is the asymptote of the growth curve, k is the growth-rate constant (1/time), and b is ln[A/W(0)]. The curves have asymptotes of 100 units and initial masses [W(0)] of 2, 4, 6, 8, and 10 units, and they are plotted as a function of relative time (kt). When W(0) = 2, for example, 50% of the asymptote is reached at kt = 1.7, 90% at kt = 3.6, and 95% at kt = 4.3. The relative time at sexual maturity for each species is the product of the species’ growth-rate constant (k; [1/days]) and age at sexual maturity (days), resulting in a dimensionless number. Because birds grow very rapidly compared with mammals, most species of bird mature well after reaching full size, whereas many species of mammal mature well below their eventual adult mass.
Why is age at maturity the important variable? One might argue a direct cause–effect relationship [maturity → aging; i.e., that individuals do not begin to age until they become reproductively mature, at which time endocrine mechanisms change (42) and individuals must allocate resources between self-maintenance and the production of offspring (43, 44)]. If this were the case, however, it is not clear why the rate at which mortality increases with age is lower in species that mature later, as found in this study, where 1/ω (directly related to life span) is approximately proportional to the square root of the age at maturity (Fig. 3). Although rate of aging and development seemingly must be generally linked, the acquisition of sexual maturity in birds at widely different ages (in many cases, a decade or more after growth is completed) does not seem to reflect a developmental process proceeding at different rates so much as differential postponement of sexual development.
It is also not clear why males and females of most species have similar life expectancies, given their different reproductive roles (10, 45). In captive populations of birds and mammals, the number of offspring produced up to a certain age does not predict the longevity of an individual beyond that age, suggesting that reproduction per se does not interfere with self-maintenance processes that influence life span (46). Moreover, mice and dogs ovariectomized before maturation do not live longer than controls (47, 48), suggesting that coming into reproductive condition has little effect on the somatic physiology of aging, although implantation of young ovaries into older, ovariectomized mice seems to extend life (47).
Another possibility is that adult mortality applies selection on longevity in accordance with evolutionary models of the rate of aging and also directly affects the age at maturity through density-dependent feedbacks on young individuals (age at maturity ← extrinsic adult mortality → rate of aging). Accordingly, age at maturity and rate of aging would be fortuitously related by their independent links to extrinsic mortality. A balanced population requires that annual recruitment equals adult mortality. Annual recruitment is a function of the number of offspring produced and their survival to maturity. If, in comparisons across species, adult mortality decreases faster than annual reproductive success, then the age at maturity must be extended to balance the population equation, assuming that older individuals are dominant over younger individuals and can prevent them from reproducing successfully.
The relationship between recruitment and adult survival can be described simply (i.e., without age dependence in adult survival and reproductive success) as PaB (recruitment) = M, where B is the number of independent offspring produced per reproducing adult each year, Pa is the probability that offspring survive to maturity (age = a), and M is the annual adult mortality (1 − S). Suppose that Pa is simply the adult survival rate raised to the power of the age (years) at maturity. Now, we have SaB = M, which can be rearranged to give an expression for the age at maturity as a function of S and B (Eq. 1),
 |
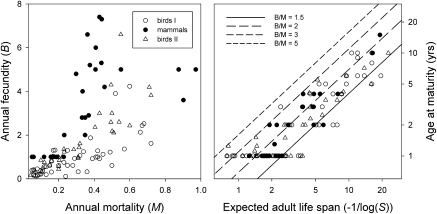
In birds and mammals, B is directly proportional to M (15, 49, 50), and therefore, the numerator of Eq. 1 is roughly constant across species. In addition, annual prereproductive survival is proportional to, but somewhat lower than, adult S. If first-year prereproductive survival were proportion c (< 1) of adult survival, then −log(c) would be added to the numerator in Eq. 1. Under strong density dependence, with older individuals socially dominant to younger individuals, the age at maturity will depend closely on the value of S (Fig. 5), specifically on −1/log(S) with a slope equal to log(B/M). Notice that −log(S) is equal to the instantaneous annual adult mortality rate [m; i.e., S = exp(−m)] and that 1/m is the expected adult life expectancy assuming age-independent mortality.
Fig. 5.
(Left) Annual fecundity (B) increases linearly in relation to annual mortality (1 − S) in samples of avian and mammalian life histories. Data for birds are from (birds I) Saether and Bakke (60) (50 species; female offspring per female) and (birds II) Ricklefs (49) (34 species; offspring per pair), and data for mammals are from Millar and Zammuto (61) (29 species; litter size). The slopes of the relationships passed through the origin were 3.34 (± 0.25 SE), 5.68 (± 0.37 SE; M < 0.8), and 11.34 (± 0.80 SE), respectively. (Right) The relationship between age at maturity (a) and −1/ln(S). The straight lines show the expected relationship between age at maturity and expected adult life span for different ratios of annual fecundity to annual adult mortality (B/M) in a stable population (Eq. 1). The slopes of the linear relationships [log(B/M)] were 0.519 (± 0.028 SE), 0.477 (± 0.024 SE), and 0.674 (± 0.028 SE), respectively. (Appendix S3 has further details.
The strong dependence of the age at maturity on annual survival of adults suggests that the close relationship between the rate of aging, or maximum potential longevity, and the age at maturity is a fortuitous consequence of the dependence of both life-history variables on the extrinsic mortality rate. These relationships provide insights into the evolution of both the age at sexual maturity and the rate of aging. In the first case, although fecundity decreases with decreasing adult mortality, the number of offspring produced each year as potential recruits to the adult population, nonetheless, exceeds the number of adult deaths by approximately the same ratio, regardless of the annual adult mortality. Thus, assuming that population size is regulated by density-dependent factors, young individuals must wait longer to enter the breeding population in species with lower annual adult mortality. Density dependence evidently is exerted most strongly on the ability of young individuals to gain breeding places in the population, indicative of strong social feedback from adults (51, 52).
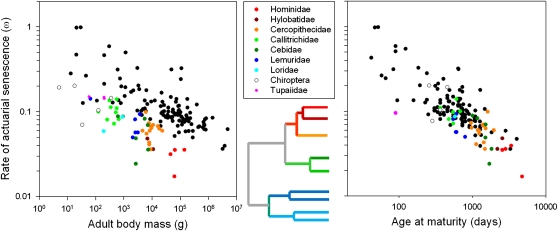
The relationship between age at maturity and rate of aging is consistent with evolutionary theories relating the rate of aging primarily to extrinsic mortality. Although humans have exceptionally long potential life spans, our longevity apparently results from the safety of our lives as primates rather than other features of our anatomy, physiology, or behavior. Longevity is a feature of primates of all types. Relative to adult body mass, the primates share exceptional life spans and slow rates of actuarial senescence with bats and a few other mammals (Fig. 6). When plotted as a function of the age at maturity, however, rate of aging in both primates and bats falls into line with other mammals and other vertebrates.
Fig. 6.
The rate of actuarial senescence (ω) in mammals as a function of body mass (Left) and age at maturity (Right). Whereas bats (Chiroptera) and primates age slowly for their size, their rate of aging falls into line with other mammals relative to the age at maturity. Note: If you cannot distinguish the colors in this figure and would like more information, please contact the author.
The apparent primacy of extrinsic mortality in determining rates of aging in vertebrates suggests shared underlying mechanisms that regulate the rate of physiological deterioration of the body, or at least, the rate of increase in susceptibility to terminal diseases. That the rate of aging responds to extrinsic selective pressures in the same way in mammals, birds, and reptiles suggests that the costs of mechanisms that prevent or repair damage to the body are similar among different groups of vertebrates, despite differences in metabolic rates, body-temperature control, rates of development, and other physiological parameters. In addition, the observation that the proportion of adult mortality resulting from aging-dependent causes increases with the potential longevity of species (22, 53) indicates that the costs of mechanisms to prolong life increase with greater potential longevity or that the availability of such mechanisms is exhausted in long-lived organisms. Accordingly, we should not expect to see substantial improvements in human life span through ordinary biological mechanisms. Evidently, these have been exploited fully over the millions of years of vertebrate evolution.
Materials and Methods
Rates of actuarial senescence for mammals (168 species), birds (207), reptiles (39), and amphibians (12) were estimated from the parameters of Weibull functions fitted to the relationship between survival and age in captive and wild populations (Appendix S4). The Weibull function is (Eq. 2)
where mx is the mortality rate at age x, m0 is the initial, or extrinsic, mortality rate experienced by young adults, a is a scaling parameter equal to the age-related component of mortality at age 1, and b is the power of the relationship between the age-related component of mortality and age. The relationship between survival to age x (lx) and age (Eq. 3),
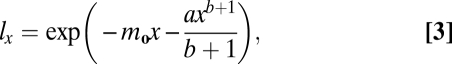 |
was used to fit the survival data by nonlinear regression. A derived rate of aging (ω), defined as a1/(b + 1), is proportional to the overall rate of aging and has units of 1/time (22). Further properties of Weibull aging parameters and comparison with Gompertz parameters, also commonly used to fit age-related mortality and survival data (54, 55), are discussed by Ricklefs and Scheuerlein (56). Because values of ω are similar for populations of the same or related species in the wild and captivity (53, 57), values of ω estimated from both sources can be combined in comparative studies, although extrinsic mortality (m0) is generally lower in captive and domesticated animals (53). ω is strongly correlated with maximum recorded life span both for the datasets used to estimate ω and also for independent datasets ((Appendix S5).
Life-history variables included in this analysis, in addition to extrinsic mortality estimated from survival curves, were body mass, neonate mass, gestation or incubation period, weaning period (for mammals), postnatal growth rate (mammals and birds), and female age at maturity. Life-history variables were largely obtained from the AnAge online database (58) (http://genomics.senescence.info/species/) maintained by J. P. de Magalhães. The life-history data and literature sources are detailed in (Appendix S6. Body mass for several species of reptiles and amphibians was estimated from a mass–length relationship ((Appendix S7); extrinsic mortality for captive populations of only mammals was estimated from a regression equation for m0(wild) as a function of m0(captive) based on a limited number of species for which both values were available ((Appendix S8).
Life-history variables were log10 transformed to make variances closer to equal, linearize relationships between the variables, and make variation scale-independent (i.e., related to proportional rather than absolute differences between observations).
Because most of the variation in rate of actuarial senescence at the level of species is caused by stochastic variation and measurement error, analyses were performed on taxonomic family means (Fig. 1). Although relationships between variables generally have higher coefficients of determination (r2) at higher taxonomic level, sample sizes increase and standard errors of regression coefficients often decrease at lower taxonomic levels. The decision to analyze most of the data using family means reflects a compromise between these trends. Phylogenetically informed analyses, including taxonomically nested analyses of variance and covariance as well as contrasts analysis, of the relationship between rate of aging and body mass in mammals show that only variation at the deeper nodes in the tree is significant ((Appendices S9 and ((S10). Thus, the increase in sample size from using species-level data conveys little additional information concerning variation in the rate of aging.
Data were analyzed in a multiple-regression framework using AICc to weight models, calculate importance values for the independent variables, and estimate regression parameters (59). Briefly, models were weighted by ΔAICc values (i.e., the difference compared with the model with the lowest or best AICc score). Variable weights were calculated as the sum of weights of the models in which they are included. The regression parameter for each variable was calculated from the parameter in each of the models, including that variable, multiplied by the model weight. AIC criteria do not test the statistical validity of independent variables in the model but rather, generate an overall importance value for each variable. Analyses were run in SAS version 9.2 using the STEPWISE, REG, and GLM procedures.
Supplementary Material
Acknowledgments
Joao Pedro de Magalhães and Steve Austad provided helpful comments on the manuscript. I am grateful to Dr. Nate Flesness and the Board of Directors of the International Species Inventory System (ISIS) for providing demographic data. Dr. Alex Scheuerlein produced most of the estimates of Weibull function parameters. Dr. Olaf Bininda-Emonds kindly provided a phylogeny for the mammal species in this analysis. My work on aging was supported by Grants AG16895-01 and AG20263-01 from the National Institutes of Health Institute on Aging. I would also like to acknowledge the generous support of the Board of Curators of the University of Missouri and the Alexander von Humboldt Foundation.
Footnotes
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005862107/-/DCSupplemental.
References
- 1.Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- 2.Partridge L, Barton NH. Evolution of aging: Testing the theory using Drosophila. Genetica. 1993;91:89–98. doi: 10.1007/BF01435990. [DOI] [PubMed] [Google Scholar]
- 3.Rose MR. Evolutionary Biology of Aging. New York: Oxford University Press; 1991. [Google Scholar]
- 4.Barja G. Aging in vertebrates, and the effect of caloric restriction: A mitochondrial free radical production-DNA damage mechanism? Biol Rev Camb Philos Soc. 2004;79:235–251. doi: 10.1017/s1464793103006213. [DOI] [PubMed] [Google Scholar]
- 5.Kujoth GC, Bradshaw PC, Haroon S, Prolla TA. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, Vleck CM. Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp Gerontol. 2007;42:610–618. doi: 10.1016/j.exger.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 8.Monaghan P, Haussmann MF. Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- 10.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 11.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- 12.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 13.Calder WA., III . Size, Function, and Life History. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- 14.Promislow DEL. Senescence in natural populations of mammals: A comparative study. Evolution. 1991;45:1869–1887. doi: 10.1111/j.1558-5646.1991.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 15.Charnov EL. Life History Invariants. Some Explorations of Symmetry in Evolutionary Biology. New York: Oxford University Press; 1993. [Google Scholar]
- 16.Promislow DE. On size and survival: Progress and pitfalls in the allometry of life span. J Gerontol. 1993;48:B115–B123. doi: 10.1093/geronj/48.4.b115. [DOI] [PubMed] [Google Scholar]
- 17.Kleiber M. The Fire of Life. New York: Wiley; 1961. [Google Scholar]
- 18.Pearl R. The Rate of Living. New York: Alfred Knopf; 1928. [Google Scholar]
- 19.Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: Evidence from the bats and marsupials. J Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- 20.Austad SN. Diverse aging rates in metazoans: Targets for functional genomics. Mech Ageing Dev. 2005;126:43–49. doi: 10.1016/j.mad.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Ricklefs RE. Evolutionary theories of aging: Confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am Nat. 1998;152:24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- 23.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 24.Harvey PH, Pagel MS. The Comparative Method in Evolutionary Biology. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- 25.Calder WAI., 3rd The comparative biology of longevity and lifetime energetics. Exp Gerontol. 1985;20:161–170. doi: 10.1016/0531-5565(85)90033-6. [DOI] [PubMed] [Google Scholar]
- 26.Stearns SC. The Evolution of Life Histories. New York: Oxford University Press; 1992. [Google Scholar]
- 27.Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol Evol. 2002;17:462–468. [Google Scholar]
- 28.Harvey PH, Purvis A. In: Advanced Ecological Theory: Principles and Applications. McGlade J, editor. Oxford: Blackwell Scientific; 1999. pp. 232–248. [Google Scholar]
- 29.Prothero J. Adult life span as a function of age at maturity. Exp Gerontol. 1993;28:529–536. doi: 10.1016/0531-5565(93)90041-b. [DOI] [PubMed] [Google Scholar]
- 30.Ricklefs RE, Scheuerlein A. Comparison of aging-related mortality among birds and mammals. Exp Gerontol. 2001;36:845–857. doi: 10.1016/s0531-5565(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 31.de Magalhães JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol. 2007;62A:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlesworth B. Evolutionary mechanisms of senescence. Genetica. 1993;91:11–19. doi: 10.1007/BF01435984. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 34.Kirkwood TBL. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 35.Kirkwood TBL. Evolution of ageing. Mech Ageing Dev. 2002;123:737–745. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 36.Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 37.Shattuck MR, Williams SA. Arboreality has allowed for the evolution of increased longevity in mammals. Proc Natl Acad Sci USA. 2010;107:4635–4639. doi: 10.1073/pnas.0911439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasser DE, Sherman PW. Avian longevities and their interpretation under evolutionary theories of senescence. J Zool. 2010;280:103–155. [Google Scholar]
- 39.Ricklefs RE. Embryo growth rates in birds and mammals. Funct Ecol. 2010;24:588–596. [Google Scholar]
- 40.Charnov EL. On evolution of age of maturity and the adult lifespan. J Evol Biol. 1990;3:139–144. [Google Scholar]
- 41.Charnov EL. A note on dimensionless life histories for birds versus mammals. Evol Ecol. 1995;9:288–291. [Google Scholar]
- 42.Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- 43.Kirkwood TBL. In: Physiological Ecology: An Evolutionary Approach to Resource Use. Townsend CR, Calow P, editors. Oxford: Blackwell Scientific; 1981. pp. 165–189. [Google Scholar]
- 44.Kirkwood TBL, Holliday R. The evolution of ageing and longevity. Proc Biol Sci. 1979;205:531–546. doi: 10.1098/rspb.1979.0083. [DOI] [PubMed] [Google Scholar]
- 45.Toivonen JM, Partridge L. Endocrine regulation of aging and reproduction in Drosophila. Mol Cell Endocrinol. 2009;299:39–50. doi: 10.1016/j.mce.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Ricklefs RE, Cadena CD. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecol Lett. 2007;10:867–872. doi: 10.1111/j.1461-0248.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 47.Cargill SL, Carey JR, Müller HG, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2:185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waters DJ, et al. Exploring mechanisms of sex differences in longevity: Lifetime ovary exposure and exceptional longevity in dogs. Aging Cell. 2009;8:752–755. doi: 10.1111/j.1474-9726.2009.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricklefs RE. Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor. 2000;102:9–22. [Google Scholar]
- 50.Ricklefs RE. On the evolution of reproductive strategies in birds: Reproductive effort. Am Nat. 1977;111:453–478. [Google Scholar]
- 51.Ferrière R, Clobert J. Evolutionarily stable age at first reproduction in a density-dependent model. J Theor Biol. 1992;157:253–267. doi: 10.1016/s0022-5193(05)80624-1. [DOI] [PubMed] [Google Scholar]
- 52.Ergon T, MacKinnon JL, Stenseth NC, Boonstra R, Lambin X. Mechanisms for delayed density-dependent reproductive traits in field voles, Microtus agrestis: The importance of inherited environmental effect. Oikos. 2001;95:185–197. [Google Scholar]
- 53.Ricklefs RE. Insights from comparative analyses of aging in birds and mammals. Aging Cell. 2010;9:273–284. doi: 10.1111/j.1474-9726.2009.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavrilov LA, Gavrilova NS. The Biology of Life Span: A Quantitative Approach. New York: Harwood; 1991. [Google Scholar]
- 55.Wilson DL. The analysis of survival (mortality) data: Fitting Gompertz, Weibull, and logistic functions. Mech Ageing Dev. 1994;74:15–33. doi: 10.1016/0047-6374(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 56.Ricklefs RE, Scheuerlein A. Biological implications of the Weibull and Gompertz models of aging. J Gerontol A Biol Sci Med Sci. 2002;57:B69–B76. doi: 10.1093/gerona/57.2.b69. [DOI] [PubMed] [Google Scholar]
- 57.Ricklefs RE. Intrinsic aging-related mortality in birds. J Avian Biol. 2000;31:103–111. [Google Scholar]
- 58.de Magalhães JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol. 2009;22:1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 59.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 60.Saether BE, Bakke O. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology. 2000;81:642–653. [Google Scholar]
- 61.Millar JS, Zammuto RM. Life histories of mammals: An analysis of life tables. Ecology. 1983;64:631–635. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.