Abstract
Although multiple follicles are present in mammalian ovaries, most of them remain dormant for years or decades. During reproductive life, some follicles are activated for development. Genetically modified mouse models with oocyte-specific deletion of genes in the PTEN-PI3K-Akt-Foxo3 pathway exhibited premature activation of all dormant follicles. Using an inhibitor of the Phosphatase with TENsin homology deleted in chromosome 10 (PTEN) phosphatase and a PI3K activating peptide, we found that short-term treatment of neonatal mouse ovaries increased nuclear exclusion of Foxo3 in primordial oocytes. After transplantation under kidney capsules of ovariectomized hosts, treated follicles developed to the preovulatory stage with mature eggs displaying normal epigenetic changes of imprinted genes. After in vitro fertilization and embryo transfer, healthy progeny with proven fertility were delivered. Human ovarian cortical fragments from cancer patients were also treated with the PTEN inhibitor. After xeno-transplantation to immune-deficient mice for 6 months, primordial follicles developed to the preovulatory stage with oocytes capable of undergoing nuclear maturation. Major differences between male and female mammals are unlimited number of sperm and paucity of mature oocytes. Thus, short-term in vitro activation of dormant ovarian follicles after stimulation of the PI3K-Akt pathway allows the generation of a large supply of mature female germ cells for future treatment of infertile women with a diminishing ovarian reserve and for cancer patients with cryo-preserved ovaries. Generation of a large number of human oocytes also facilitates future derivation of embryonic stem cells for regenerative medicine.
Keywords: intracellular signaling, oocyte maturation, phosphatase with TENsin homology deleted in chromosome 10, infertility treatment, phosphatidylinositol-3-kinase
Human ovaries contain follicles as basic functional units. The total number of follicles is determined early in life, and follicle depletion leads to reproductive senescence. Human follicles begin development during the fourth month of fetal life, and each human ovary contains ≈400,000 follicles at birth. Unknown intraovarian mechanisms activate a small number of dormant primordial follicles at a rate of ≈1,000 per month to initiate growth, and follicle depletion occurs at menopause when <1,000 follicles remain (1). For follicles not activated, the default pathway is to remain dormant for years or decades. Once activated, primordial follicles develop through primary and secondary stages before acquiring an antral cavity (2). After gonadotropin stimulation, a few antral follicles reach the preovulatory stage and respond to the preovulatory gonadotropin surge to release mature eggs for fertilization.
Studies using mutant mice indicated that oocyte-specific deletion of the Phosphatase with TENsin homology deleted in chromosome 10 (PTEN) gene promotes the growth of all primordial follicles in neonatal and adult animals (3–5). The PTEN gene encodes a phosphatase enzyme that negatively regulates the PI3K-Akt signaling pathway. Deletion of PTEN in the oocyte increases protein kinase B (Akt) phosphorylation and nuclear export of downstream Foxo3 proteins (4). Indeed, Foxo3 gene deletion also activated all dormant primordial follicles in mice (6). Here, we treated neonatal mouse ovaries in vitro with a PTEN inhibitor and a PI3K activating peptide to activate dormant primordial follicles, followed by transplanting them into the kidney capsule of FSH-treated adult ovariectomized recipients to promote follicle growth. We generated mature eggs capable of developing into viable and fertile offspring. Using human cortical fragments containing primordial follicles, we also activated dormant human follicles to develop into large antral follicles containing mature oocytes after xeno-transplantation into immune-deficient mice.
Results
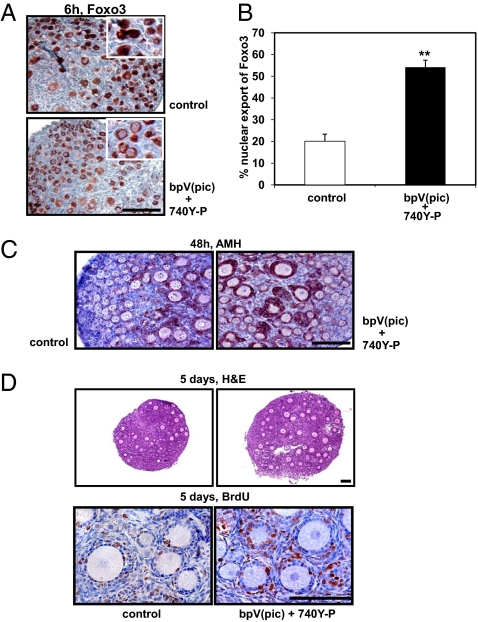
We hypothesized that transient incubation of neonatal mouse ovaries in vitro with bpV(pic), a PTEN inhibitor (7–9), allows the activation of dormant follicles. Ovaries containing mainly primordial follicles were obtained from neonatal mice at day 3 of age. Pairs of ovaries were incubated with bpV(pic) (100 μM) or culture media (control group) for 24 h. In addition to bpV(pic), some ovaries were treated for 48 h with a cell-permeable phospho-peptide (740Y-P) capable of binding to the SH2 domain of the p85 regulatory subunit of PI3K to stimulate enzyme activity (10). Activated PI3K converts phosphatidylinositol (4, 5)-bisphosphate (PIP2) to phosphatidylinositol (3-5)-trisphosphate (PIP3), whereas the PTEN inhibitor prevents the conversion of PIP3 back to PIP2. Accumulated PIP3, in turn, could stimulate the phosphorylation of Akt and increase the nuclear exclusion of the transcriptional factor Foxo3. As shown in Fig. 1A, treatment of neonatal ovaries with bpV(pic) and the PI3K activating peptide (740Y-P) increased the nuclear export of Foxo3 in oocytes of primordial follicles at 6 h after incubation. Quantitative analyses indicated that 54% of oocytes in primordial follicles exhibited Foxo3 export (Fig. 1B). At 48 h after treatment, granulosa cells of growing follicles in the treatment group showed increased staining of anti-Mullerian hormone (AMH), a marker for secondary/preantral follicles (11) (Fig. 1C).
Fig. 1.
Activation of the PI3K-Akt-Foxo3 pathway in oocytes and increased follicle development after treatment with bpV(pic) and 740Y-P. (A) Nuclear exclusion of Foxo3 in oocytes of primordial follicles at 6 h after treatment with bpV(pic) and 740Y-P (Insets, higher magnification). (Scale bar: 100 μm.) (B) Increases in the fraction of oocytes showing nuclear export of Foxo3. (C) AMH staining in granulosa cells of primary and secondary follicles at 2 days after in vitro culture. (D) BrdU incorporation into granulosa cells of activated follicles at 5 days after transplantation (Upper, hematoxylin and eosin staining; Lower, BrdU staining). (Scale bars: 100 μm.)
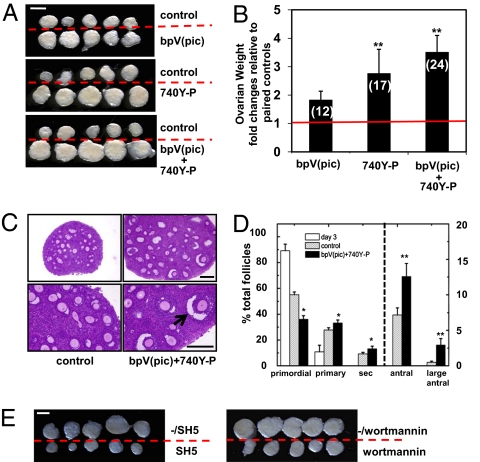
Paired ovaries (treated and untreated) from the same donor were then transplanted under separate sides of the kidney capsule in the same ovariectomized adult (8-week-old) recipient. One day after transplantation, hosts received daily i.p. injection of FSH (2 IU/day) to promote follicle development. At 5 days after transplantation, bromodeoxyuridine (BrdU) labeling analyses showed increased proliferation of granulosa cells in growing follicles of treated ovaries as compared with controls (Fig. 1D). At 14 days after transplantation, increases in ovarian sizes of treated groups were evident as compared with paired controls (Fig. 2A); cotreatment of bpV(pic) plus 740Y-P showed most pronounced increases based on ovarian weight determination (Fig. 2B), reaching 3.4-fold of the control level. Histological analyses further demonstrated the presence of large antral follicles (>250 μm in diameter) in ovaries treated with bpV(pic) plus 740Y-P (Fig. 2C, arrow) as compared with controls. Follicle counting of serial ovarian sections indicated 1.8- and 6-fold increases between control and treatment groups in the number of antral and large antral follicles, respectively (Fig. 2D). Also, in vitro cotreatment with bpV(pic) plus 740Y-P with or without inhibitors to Akt (SH-5) or PI3K (Wortmannin), followed by transplantation, demonstrated the mediatory roles of these enzymes in follicle activation (Fig. 2E).
Fig. 2.
Activation of dormant primordial follicles after in vitro treatment with bpV(pic) and 740Y-P, followed by transplantation into the kidney capsule of ovariectomized hosts. (A) Ovaries at 14 days after transplantation. Isolated ovaries for control vs. bpV(pic), control vs. 740Y-P, and control vs. bpV(pic) plus 740Y-P groups. (Scale bars: 1 mm.) (B) Ovarian weight increases as fold changes relative to paired nontreated controls. Numbers of paired ovaries used for analyses are shown in parentheses. (C) Ovarian histology showing follicle development to the large antral stage (arrow) at 14 days after treatment with bpV(pic) and 740Y-P followed by transplantation. (Scale bars: 200 μm.) (D) Distribution of follicles in ovaries without and with exposure to bpV(pic) and 740Y-P. Follicle dynamics of day 3 ovaries before transplantation is included for comparison. s, secondary follicle. (E) Ovarian morphology at 14 days after transplantation of grafts treated with bpV(pic) and 740Y-P with or without inhibitors to Akt (SH5) or PI3K (Wortmannin). The symbol -/ indicates groups without the inhibitors. (Scale bar: 1 mm.)
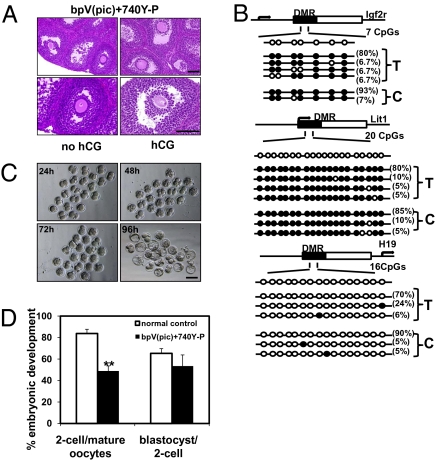
To test the maturity of oocytes from activated follicles, neonatal ovaries treated with bpV(pic) plus 740Y-P were transplanted for 18 days with FSH treatment. At 8 h before killing, hosts received saline or an ovulatory dose of hCG to evaluate nuclear maturation of oocytes. As shown in Fig. 3A Upper Left, large antral follicles were present in saline-treated (no hCG) animals. Oocytes in these follicles showed intact germinal vesicles and compact cumulus cells (Fig. 3A Lower Left). In contrast, ovaries from hCG-treated group contained oocytes showing germinal vesicle breakdown (Fig. 3A Upper Right) in preovulatory follicles. These oocytes had no nuclear membrane but showed meiotic spindles (Fig. 3A Lower Right) and were surrounded by loosely associated cumulus cells, representing cumulus expansion. Immunofluorescence analyses of β-tubulin further indicated normal meiotic spindle formation in mature oocytes (Fig. S1A). For ovarian grafts with or without pretreatment with bpV(pic) and 740Y-P followed by FSH and hCG exposure in vivo (Fig. S1B), histological analyses indicated that 32% and 5% of antral follicles contained mature oocytes showing signs of germinal vesicle breakdown, respectively (Fig. S1C).
Fig. 3.
Retrieval of mature mouse oocytes for epigenetic analyses, in vitro fertilization, and early embryonic development. (A) Histology of ovaries with or without hCG treatment at 18 days after transplantation after pretreatment with bpV(pic) and 740Y-P. (A Left) An ovary before hCG treatment showing preovulatory follicles with an oocyte at the GV stage. (A Right) An ovary after hCG treatment showing preovulatory follicles with an oocyte exhibiting germinal vesicle breakdown. (Scale bars: 100 μm.) (B) Methylation status of the differentially methylated regions (DMRs) of two maternal imprinted genes, Igf2r and Lit1, and one paternal imprinted gene H19 in mature oocytes retrieved from transplanted ovaries after in vitro activation (T) and those from superovulated control ovaries (C). Each row represents a unique methylation profile within the pool of clones sequenced with the frequency of that methylation state indicated on the right side of each row. Each circle within the row represents a single CpG site (open circles, nonmethylated cytosines; filled circles, methylated cytosines). n = 15–20 sequenced clones. (C) Early embryonic development of retrieved mature oocytes after in vitro fertilization. Representative figures for embryos reaching two-cell (24 h), four-cell (48 h), morula (72 h), and blastocyst (96 h) stages. (D) Efficiency of early embryonic development. Percentage of mature oocytes retrieved from transplanted grafts capable of developing into two-cell embryos and blastocysts as compared with mature oocytes obtained from ovaries from superovulated controls without transplantation.
We isolated mature oocytes from activated follicles at 18 days after transplantation to evaluate epigenetic changes and developmental potential. Maternal-specific methylation of imprinted genes is established during ovarian follicle development (12). In mature oocytes (Fig. 3B), methylation patterns in the differential methylated regions (DMR) of two maternal imprinted genes, Igfr2 and Lit1, were comparable between those obtained after transplantation (T) or from superovulated control mice (C). For both groups, a paternal imprinted gene H19 showed minimal methylation. After in vitro fertilization using donor sperm, mature oocytes from transplanted ovaries developed into two-cell embryos (24h), four-cell embryos (48h), morula (72h), and blastocysts (96h) during culture (Fig. 3C). Although the fraction of mature oocytes developing into two-cell embryos was lower for oocytes from transplanted ovaries than those from superovulated ovaries without transplantation (normal control), a similar percentage of 2-cell embryos progressed to blastocysts for both groups (Fig. 3D). A total of 118 two-cell embryos were transferred into 10 pseudopregnant females, leading to the delivery of 20 healthy pups capable of developing into adults. These progeny (Fig. S1D) showed normal fertility with the derivation of healthy offspring.
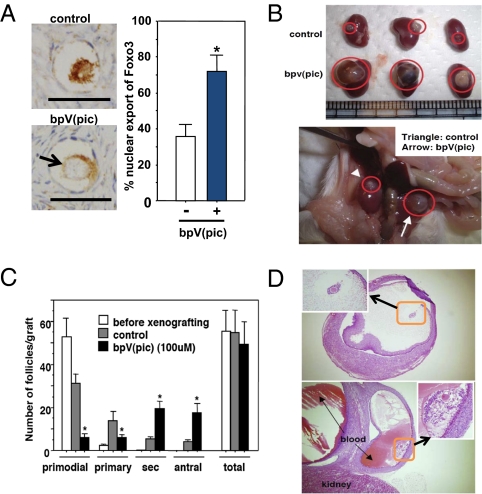
To activate human dormant follicles, ovarian cortical fragments containing primordial follicles were obtained from patients with benign ovarian tumor. After cutting into 1 mm3 cubes, cortical tissues were treated with bpV(pic). As shown in Fig. 4A Left, nuclear exclusion of Foxo3 was evident in the treated group as compared with controls at 1 h after culture, showing a 2-fold increase (Fig. 4A Right). Pairs of cortical cubes were treated with bpV(pic) or saline for 24 h before xeno-transplantation into each side of the kidney capsule of ovariectomized, severe combined immunodeficiency (SCID) mice. Three days after transplantation, animals were treated i.p. with FSH (1 IU per animal) every 48 h for 24 weeks. At 36 h before sample retrieval, hCG (20 IU per animal) was injected s.c.. As shown in Fig. 4B, major increases in graft sizes were apparent in the bpV(pic)-treated group as compared with controls (Fig. 4B Upper, paired grafts with and without bpV(pic) treatment;Fig. 4B Lower, in situ kidney picture of one host). Serial sectioning was performed for four pairs of ovarian grafts together with ovarian cubes before xeno-transplantation. Follicle counting indicated each graft contained ≈55 follicles (Fig. 4C). Before xeno-grafting, 96% of follicles were at the primordial stage with <4% at the primary stage and none at more advanced stages. After transplantation, 40% and 89% of follicles left the dormant primordial pool and developed into larger follicles in control and PTEN inhibitor-treated groups, respectively. In the bpV(pic)-treated group, ≈4-fold increases in the number of secondary and antral follicles were found. Oocyte maturation status of large follicles was also evaluated. In the control group, 8 antral follicles (>1 mm diameter) were present in four ovarian grafts with 7, 0, and 1 oocytes at germinal vesicle (GV), metaphase I (MI), and metaphase II (MII) stages, respectively. In four paired grafts treated with bpV(pic), 32 antral follicles were found with 5, 15, and 12 oocytes at GV, MI, and MII stages, respectively. Thus, 4- and 27-fold increases in the number of antral follicles and mature (MI and MII) oocytes were found after bpV(pic) treatment. As shown in Fig. 4D, two representative preovulatory follicles in the bpV(pic)-treated group contained mature MII oocytes (Fig. 4D Inset). These mature human oocytes were not fertilized because of ethical concerns.
Fig. 4.
Activation of human primordial follicles from patients with benign ovarian tumor. (A Left) Increased nuclear export of Foxo3 in primordial follicles after 1 h of treatment with 100 μM bpV(pic). Arrow, positive staining in diminished cytoplasmic space due to fixation-induced shrinkage. (Scale bars: 50 μm.) (A Right) Percentage of primordial oocytes showing Foxo3 nuclear export in control and bpV(pic)-treated groups. (B) Ovarian morphology at 6 months after xeno-transplantation into SCID mice (Upper, kidneys with ovarian grafts; Lower, in situ kidney picture of one host). (C) Distribution of follicles at different stages in grafts with or without bpV(pic) treatment. Follicle distribution in cortical cubes before xeno-grafting is provided for comparison. s, secondary follicles. (D) Representative sections showing the development of two large antral follicles in bpV(pic)-treated group with mature oocytes exhibiting germinal vesicle breakdown after hCG treatment (insets).
Discussion
We performed short-term and ovary-specific treatment of rodent and human ovaries with a PTEN inhibitor and/or a PI3K activator to increase Foxo3 nuclear extrusion in primordial oocytes, leading to the activation of dormant primordial follicles. Subsequent allo- or xeno-transplantation into kidney capsules of FSH-treated hosts allowed optimal follicle development. Once activated, follicles in grafts continue to grow to the antral stage with oocytes capable of undergoing nuclear maturation. For activated murine follicles, mature oocytes could be retrieved for in vitro fertilization and embryo transfer, followed by the delivery of healthy pups with proven fertility. Although attempts were not made to fertilize mature human oocytes obtained after xeno-transplantation because of ethical concerns, morphological evaluation indicated germinal vesicle breakdown of these oocytes and future studies using nonhuman primates are needed to ensure fertilization capacity and embryonic development potential. Because epigenetic modification of DNA methylation in the differential methylated regions of key imprinted genes took place in oocytes during folliculogenesis (13) and increased frequencies of imprinting disorders (e.g., Angelman and Beckwith-Wiedemann Syndromes) are associated with assisted reproductive technology for human infertility treatment (14, 15), we examined the methylation of two maternally imprinted (Igfr2 and Lit1) and one paternally imprinted (H19) genes in mature oocytes. We found similar patterns for oocytes from activated and superovulated control ovaries.
Using in vitro cultures, mutant animals, specific inhibitors, and passive immuno-neutralization tests, several ovarian paracrine factors have been found to be important for the activation of cultured murine primordial follicles (5), including kit ligand (16), PDGF (17), neurotrophins (18), leukemia inhibitory factor (19), vascular endothelial growth factor (20), bone morphogenetic proteins (21), and FGF proteins (22, 23). Although the exact factors involved in the activation of few primordial follicles to initiate growth under physiological states are still unknown, it is possible that key tyrosine kinase receptors respond to their ligands in oocytes by direct binding and activation of downstream PI3K and Akt enzymes. In addition, some factors could inhibit PTEN activity, also leading to increases in Akt phosphorylation. Indeed, treatment with the kit ligand activated Akt phosphorylation and suppressed Foxo3 activity in murine oocytes (24). Binding of tyrosine auto-phosphorylation sites on activated receptors to the SH2 domains of p85, the regulatory subunit of PI3K, releases an autoinhibitory constraint to stimulate the catalytic subunit of PI3K. To stimulate PI3K-Akt-Foxo3 signaling in primordial oocytes, we treated ovaries with a pan-specific PI3K activator. The synthetic 740Y-P peptide has a phosphorylated tyrosine residue with flanking sequences identical to the interaction site of activated PDGF receptor, together with a protein transduction domain (16 residues) of the fly Antennapedia protein (25) to facilitate plasma membrane penetration. Peptide 740Y-P is a potent stimulator of PI3K activity and mitogenic responses in myoblast cells (10) and promotes primordial germ cell migration by mimicking the action of the receptor tyrosine kinase c-kit (26). This PI3K-activating peptide likely increases intracellular PIP3 levels, mimicking the effects of ovarian ligands for tyrosine kinase receptors such as kit ligand, PDGF, leukemia inhibitor factor, and neurotrophins (17–19, 27). Coupled with the prevention of PIP3 conversion to PIP2 by the PTEN inhibitor, the PI3K activator stimulated nuclear exclusion of Foxo3 and follicle activation. With subsequent optimal follicle growth in kidney capsules, the present approach allowed efficient activation of dormant follicles and subsequent development to derive mature oocytes.
Most primordial follicles are dormant for months or decades in the ovary, likely due to local inhibitory signals. In contrast to the continuous activation of the PI3K-Akt-Foxo3 signaling pathway in PTEN null mice (3–5), our findings indicated that murine and human follicles only require short-term treatment to initiate growth. Once activated, follicles continue to grow; they appear to be nonresponsive to the inhibitory signals to remain dormant or are producing local stimulating factors to sustain growth. Although the development of preantral follicles are not gonadotropin-dependent, FSH treatment promotes early follicle development (28). Early studies indicated that 2 and 6 months are required for primordial follicles to reach the preovulatory stage in mouse and human, respectively (2). After treating hosts with FSH for only 14 days, murine primordial follicles in grafts progressed to the preovulatory stage. For xeno-transplanted human follicles, it is interesting to investigate whether a shorter duration is sufficient for antral follicle development. No malignancy was found in mouse or human ovarian grafts or hosts during prolonged transplantation, indicating the present short-term exposure to the PTEN inhibitor had minimal adverse effects despite the known function of PTEN as a tumor suppressor gene (29). These findings are consistent with a lack of tumorigenesis in mutant mice with PTEN deletion in oocyte or granulosa cells (3, 30).
Earlier studies demonstrated the delivery of only few live offspring by IVF of oocytes derived from cryopreserved primordial mouse follicles after in vivo transplantation (31), likely due to inefficient activation of dormant follicles. Likewise, only few human antral follicles developed from primordial follicles after xeno-transplantation into immune-deficient mice (32). One case of successful ovarian transplantation between monozygotic twins discordant for premature ovarian failure has been reported, and the patient delivered a healthy baby (33). In addition, ovarian auto-transplantation to the forearm has been successfully performed in women undergoing sterilizing cancer therapy, but less than a dozen pregnancies have been reported (34). Although primordial follicles are more resistant to cryo-damage as compared with larger follicles, ovarian cortical tissue cryopreservation for fertility preservation is still inefficient (34). The present in vitro activation (IVA) protocol could substantially increase the derivation of mature eggs from patients after auto-transplantation of fresh or frozen ovarian tissues. Although with low efficiency, advances have been made to develop murine primordial in vitro through oocyte maturation and fertilization to allow the birth of live pups (35, 36). Coupled with the present follicle activation approach, future improvement of follicle culture methods could bypass the need for in vivo transplantation to generate mature oocytes.
Female mammalian gonads, unlike their male counterparts, have a limited number of mature germ cells, and the present approach allows the generation of a large number of mature oocytes. Peri-menopausal women and patients suffering from primary ovarian insufficiency/failure have no antral follicles, but a limited number of primordial follicles are still present (34, 37–39). The present follicle activation approach could benefit infertile patients with a diminishing pool of dormant follicles by providing a large supply of mature female germ cells. Generation of a large number of human mature oocytes also facilitates future derivation of embryonic stem cells for regenerative medicine. The same approach could also allow the generation of mature oocytes from primordial follicles of other mammals, including endangered species and economically important animals.
Materials and Methods
Short-Term in Vitro Incubations.
Paired ovaries of 3-day-old B6D2F1 mice were excised and washed three times in L-15 medium containing 3 mg/mL BSA before transferred individually to culture plate inserts (Millipore). From a given donor, one ovary served as the control and the contralateral one was treated with 100 μM bpV(pic) (Calbiochem) and/or 500 μg/mL 740Y-P (Tocris) for 24 h. After media change, the experimental group was further treated with medium containing only 740Y-P for another 24 h before transplantation. Ovaries were cultured in Waymouth medium MB752/1 supplemented with 0.23 mM pyruvic acid, 50 mg/L streptomycin sulfate, 75 mg/L penicillin G, 3 mg/mL BSA, 10% FBS, and 0.03 IU/mL FSH (NV Organon). Media (400 μL) were placed below the membrane insert to cover ovaries with a thin layer of medium. In some experiments, one ovary from the pair was cultured with bpV(pic) plus 740Y-P, whereas the contralateral one was treated with bpV(pic) plus 740Y-P together with the Akt inhibitor SH-5 (50 μM, Calbiochem) or the PI3K inhibitor Wortmannin (25 μM; Calbiochem), added 1 h earlier.
Ovarian Transplantation.
Host animals were anesthetized and kidneys externalized through a dorso-horizontal incision. Paired ovaries (control and treatment groups) from the same donor were randomly inserted under the kidney capsule of the same host that was ovariectomized to increase endogenous gonadotropin levels. One day after transplantation, hosts were treated daily with 2 IU FSH and killed at 14 or 18 days later.
Human Follicle Activation.
Ovarian fragments were obtained from patients with benign ovarian tumor but exhibiting normal menstrual cycles. Informed consent from the patient and approval from the Human Subject Committee of Akita University were obtained. Ovarian cortex was isolated and cut into small cubes (≈1 mm3). After treatment of cortical cubes for 1 h with bpV(pic), some samples were used for Foxo3 staining. Pairs of cortical cubes were treated with or without bpV(pic) (100 mM) for 24 h in MEM-α medium containing 10% human serum albumin (Mitsubishi Tanabe Pharma), 1% antibiotic/antimycotic solution (Invitrogen), and 0.3 IU/mL FSH before xeno-transplantation into each side of the kidney capsule of ovariectomized, immune-deficient SCID mice. An i.p. injection of antibiotic (4 μg/g body weight; gentamicin) was given while animals were anesthetized. Three days after transplantation, animals were treated i.p. with FSH (1 IU per animal) every 48 h for 24 weeks. At 36 h before sample retrieval, hCG (20 IU per animal) was injected s.c.. Ovarian grafts were recovered for histological analyses and follicle counting.
Experimental animals, follicle counting, immunohistochemistry, immunofluorescence, DNA methylation, and IVF and embryo transfer procedures are in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R21 HD060864 (to A.J.W.H.), Grant-In-Aid for Young Scientists B Grant 21791539 (to K.K.), the Yamaguchi Endocrine Research Association (to K.K.), Lalor Foundation postdoctoral fellowship (to Y.C.), and National Basic Research Program of China Grant 2007CB947401 (to E.K.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001198107/-/DCSupplemental.
References
- 1.Macklon NS, Fauser BC. Aspects of ovarian follicle development throughout life. Horm Res. 1999;52:161–170. doi: 10.1159/000023456. [DOI] [PubMed] [Google Scholar]
- 2.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 3.Reddy P, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 4.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 6.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 7.Posner BI, et al. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- 8.Bevan AP, et al. In vivo insulin mimetic effects of pV compounds: Role for tissue targeting in determining potency. Am J Physiol. 1995;268:E60–E66. doi: 10.1152/ajpendo.1995.268.1.E60. [DOI] [PubMed] [Google Scholar]
- 9.Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 10.Derossi D, Williams EJ, Green PJ, Dunican DJ, Doherty P. Stimulation of mitogenesis by a cell-permeable PI 3-kinase binding peptide. Biochem Biophys Res Commun. 1998;251:148–152. doi: 10.1006/bbrc.1998.9444. [DOI] [PubMed] [Google Scholar]
- 11.Ueno S, et al. Mullerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology. 1989;125:1060–1066. doi: 10.1210/endo-125-2-1060. [DOI] [PubMed] [Google Scholar]
- 12.Obata Y, Kono T. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J Biol Chem. 2002;277:5285–5289. doi: 10.1074/jbc.M108586200. [DOI] [PubMed] [Google Scholar]
- 13.Lucifero D, Mann MR, Bartolomei MS, Trasler JM. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum Mol Genet. 2004;13:839–849. doi: 10.1093/hmg/ddh104. [DOI] [PubMed] [Google Scholar]
- 14.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox GF, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75:421–433. doi: 10.1095/biolreprod.106.051516. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131:1007–1015. doi: 10.1530/rep.1.00978. [DOI] [PubMed] [Google Scholar]
- 18.Ojeda SR, Romero C, Tapia V, Dissen GA. Neurotrophic and cell-cell dependent control of early follicular development. Mol Cell Endocrinol. 2000;163:67–71. doi: 10.1016/s0303-7207(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65–73. doi: 10.1016/s0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AE, et al. Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biol Reprod. 2007;76:218–223. doi: 10.1095/biolreprod.106.050880. [DOI] [PubMed] [Google Scholar]
- 21.Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. doi: 10.1016/s0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 23.Kezele P, Nilsson EE, Skinner MK. Keratinocyte growth factor acts as a mesenchymal factor that promotes ovarian primordial to primary follicle transition. Biol Reprod. 2005;73:967–973. doi: 10.1095/biolreprod.105.043117. [DOI] [PubMed] [Google Scholar]
- 24.Reddy P, et al. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Perez F, et al. Antennapedia homeobox as a signal for the cellular internalization and nuclear addressing of a small exogenous peptide. J Cell Sci. 1992;102:717–722. doi: 10.1242/jcs.102.4.717. [DOI] [PubMed] [Google Scholar]
- 26.Farini D, La Sala G, Tedesco M, De Felici M. Chemoattractant action and molecular signaling pathways of Kit ligand on mouse primordial germ cells. Dev Biol. 2007;306:572–583. doi: 10.1016/j.ydbio.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 28.McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod. 1997;57:990–998. doi: 10.1095/biolreprod57.5.990. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez F, Sellers WR. The PTEN tumor suppressor protein: An antagonist of phosphoinositide 3-kinase signaling. Biochim Biophys Acta. 2000;1470:M21–M35. doi: 10.1016/s0304-419x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 30.Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–178. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- 32.Gook DA, et al. Oocyte maturation, follicle rupture and luteinization in human cryopreserved ovarian tissue following xenografting. Hum Reprod. 2003;18:1772–1781. doi: 10.1093/humrep/deg365. [DOI] [PubMed] [Google Scholar]
- 33.Silber SJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 34.Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reproduction. 2008;136:681–689. doi: 10.1530/REP-08-0097. [DOI] [PubMed] [Google Scholar]
- 35.Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 37.Pal L, Santoro N. Premature ovarian failure (POF): Discordance between somatic and reproductive aging. Ageing Res Rev. 2002;1:413–423. doi: 10.1016/s1568-1637(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 38.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: Mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.