Abstract
The force frequency relationship (FFR), first described by Bowditch 139 years ago as the observation that myocardial contractility increases proportionally with increasing heart rate, is an important mediator of enhanced cardiac output during exercise. Individuals with heart failure have defective positive FFR that impairs their cardiac function in response to stress, and the degree of positive FFR deficiency correlates with heart failure progression. We have identified a mechanism for FFR involving heart rate dependent phosphorylation of the major cardiac sarcoplasmic reticulum calcium release channel/ryanodine receptor (RyR2), at Ser2814, by calcium/calmodulin–dependent serine/threonine kinase–δ (CaMKIIδ). Mice engineered with an RyR2-S2814A mutation have RyR2 channels that cannot be phosphorylated by CaMKIIδ, and exhibit a blunted positive FFR. Ex vivo hearts from RyR2-S2814A mice also have blunted positive FFR, and cardiomyocytes isolated from the RyR2-S2814A mice exhibit impaired rate-dependent enhancement of cytosolic calcium levels and fractional shortening. The cardiac RyR2 macromolecular complexes isolated from murine and human failing hearts have reduced CaMKIIδ levels. These data indicate that CaMKIIδ phosphorylation of RyR2 plays an important role in mediating positive FFR in the heart, and that defective regulation of RyR2 by CaMKIIδ-mediated phosphorylation is associated with the loss of positive FFR in failing hearts.
Keywords: calcium channels, excitation–contraction coupling
When heart rate increases during exercise, contractility must increase concomitantly; otherwise cardiac output falls as a result of decreased diastolic filling time. Early studies on the mechanism underlying the positive force frequency relationship (FFR) (1) demonstrated that systolic Ca2+ transient amplitude is elevated at faster pacing frequencies (2). This observation has been linked to frequency dependent activation of calcium/calmodulin–dependent serine/threonine kinase–δ (CaMKIIδ) (3, 4). CaMKIIδ, a dodecameric holoenzyme found in cardiac muscle, is activated by Ca2+-calmodulin when Ca2+ levels are high and, when activated, autophosphorylates at Thr287, enabling the enzyme to remain active even when Ca2+ levels are low (5, 6). In the heart, it is hypothesized that CaMKIIδ cycles between a phosphorylated active state during systole (when the heart muscle is contracting) and a dephosphorylated resting state during diastole (when the heart is relaxing). At faster heart rates, CaMKIIδ does not have sufficient time to dephosphorylate and return to a resting state before the onset of the next Ca2+ transient. This leads to a progressive increase in the level of autophosphorylated and activated CaMKIIδ (3, 7, 8).
Activated CaMKIIδ phosphorylates multiple Ca2+-handling proteins including the L-type calcium channel (LTCC, or Cav1.2), phospholamban (PLN), and RyR2. CaMKIIδ phosphorylation of the β2a subunit of the LTCC at Thr498, and of RyR2 at Ser2814, increases the open probability of both channels (9, 10), while phosphorylation of PLN at Thr17 reduces the inhibitory effect of PLN on the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a), which is responsible for SR Ca2+ uptake (11, 12).
We previously reported that cardiac RyR2 is progressively phosphorylated by CaMKIIδ at Ser2814 in a rate-dependent manner in ex vivo rabbit hearts and that the correlation between faster heart rates and phosphorylation of RyR2-Ser2814 is not observed in failing rat hearts (10). In the present study we have generated RyR2-S2814A mice that have a targeted mutation that eliminates the CaMKIIδ phosphorylation site in the RyR2 channel to determine whether CaMKIIδ phosphorylation of RyR2 at Ser2814 is required for normal positive FFR, and whether impaired CaMKIIδ phosphorylation of RyR2 plays a role in the loss of positive FFR in failing hearts.
Results
We used site-directed mutagenesis and homologous recombination to substitute Ala for Ser2814 in the murine RyR2 channel (Fig. S1 A–E). RyR2-S2814A mice have a birth rate consistent with Mendelian inheritance and exhibit normal cardiac morphology and function at rest (Fig. S1F and Table S1).
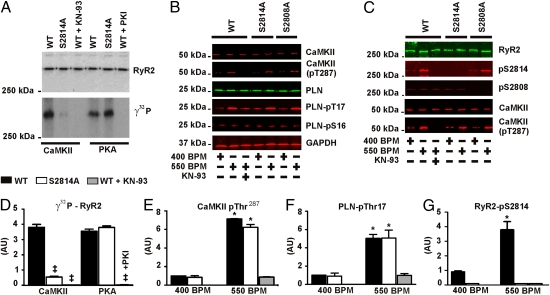
To determine whether Ser2814 is the major CaMKIIδ phosphorylation site on murine RyR2, we incubated RyR2 immunoprecipitated from WT or RyR2-S2814A mouse hearts with [γ32P]-ATP and exogenous CaMKIIδ. CaMKIIδ incorporated significantly less [γ32P]-ATP into RyR2-S2814A channels, confirming that Ser2814 is the major CaMKIIδ phosphorylation site in murine RyR2 (Fig. 1 A and D). Moreover, the stoichiometry of phosphorylation of rabbit RyR2 by CaMKII is 1.5 ± 0.07 mol phosphate per mol of RyR2 (10), consistent with the presence of one major CaMKIIδ site on each of the four monomers that comprise the homotetrameric channel. cAMP-dependent protein kinase (PKA) readily phosphorylated RyR2-S2814A channels (as determined by incorporation of [γ32P]-ATP into the channel in the presence of PKA; Fig. 1 A and D). Others have reported that both PKA and CaMKII phosphorylate a single site on RyR2, Ser2808 (13, 14). However, the present data, obtained by using RyR2-S2814A expressed in the hearts of genetically altered mice, combined with our previous studies using site directed mutagenesis of heterologously expressed RyR2 channels, and RyR2-S2808A mice (15), indicate that Ser2808 is the PKA phosphorylation site and Ser2814 is the CaMKII phosphorylation site on RyR2 (10).
Fig. 1.
CaMKIIδ phosphorylates RyR2 at Ser2814 in response to increased heart rate. (A) RyR2 immunoprecipitated from hearts from WT, RyR2-S2814A knock-in mice, and WT samples treated with either 1 μM KN-93 or PKI incubated with CaMKIIδ or PKA in the presence of [γ32P]-ATP. (B) Lysates of low- and high-frequency paced WT, RyR2-S2814A, RyR2-S2808A, and KN-93–treated WT hearts. Blots show total CaMKIIδ, activated CaMKIIδ (pThr287), and total PLN levels. The phosphorylation status of PLN at Ser16 and Thr17 are also shown. (C) RyR2 immunoprecipitated from baseline and high-frequency paced hearts. Blot shows phosphorylation status of RyR2-Ser2808 and RyR2-Ser2814, as well as the levels of CaMKIIδ and activated CaMKIIδ complexed with RyR2. (D–G) Quantitative summary of data (‡P < 0.05 vs. WT; *P < 0.05 vs. baseline). All experiments were performed in triplicate. AU, arbitrary unit.
We next examined the effects of heart rate on CaMKIIδ activity and RyR2 phosphorylation using ex vivo heart preparations. Hearts from WT, RyR2-S2814A, and RyR2-S2808A (15) mice were isolated and allowed to equilibrate under retrograde perfusion using a Langendorff ex vivo preparation (16). The baseline pacing frequency was set at 400 beats/min, slightly greater than the average intrinsic heart rate of the isolated hearts, and was increased to 550 beats/min during the experiment. One group of WT hearts was pretreated with 600 nM of the CaMKIIδ inhibitor KN-93 for 10 min.
All hearts paced at 550 beats/min, except those treated with KN-93, exhibited significantly increased activation of CaMKIIδ present in heart lysate (Fig. 1 B and E) and in complex with RyR2 (Fig. 1C) compared with baseline, as determined by measuring phosphorylation of CaMKIIδ at Thr287 (6). Phosphorylation of PLN at Thr17 was also significantly increased in the 550 beats/min paced samples compared with baseline, but not in the KN-93–treated samples (Fig. 1 B and F). Immunoblots confirmed that there were no differences in the total CaMKIIδ levels in heart lysate and in complex with RyR2 (Fig. 1 B and C). The increased CaMKIIδ activity corresponded with a significant increase in RyR2 phosphorylation at Ser2814 (Fig. 1 C and G), but not at Ser2808 (Fig. 1C).
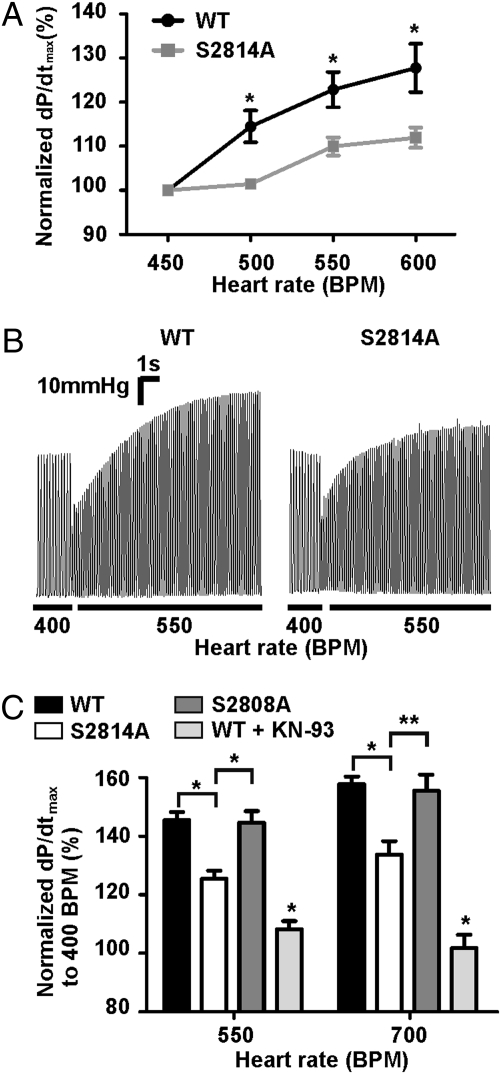
After determining that pacing activates CaMKIIδ and induces phosphorylation of murine RyR2 at Ser2814, we investigated the FFR of RyR2-S2814A mice in vivo. To minimize the effects of changes in sympathetic tone on contractility, the mice were treated with 1 mg/kg i.p. propranolol, a nonselective β-adrenergic receptor blocker, 5 min before the experiment. Using intracardiac pacing and pressure-volume catheters in intact animals, we found that WT mice exhibited a robust positive FFR with cardiac contractility (dP/dtmax), increasing by 28 ± 5% when the pacing rate was increased from 450 beats/min to 600 beats/min. In contrast, the RyR2-S2814A mice exhibited a blunted positive FFR with contractility increasing only 12 ± 2% at 600 beats/min (WT, n = 9; RyR2-S2814A, n = 8; P < 0.05 for WT vs. RyR2-S2814A percentage increase; Fig. 2A). Thus, CaMKII phosphorylation of RyR2 at Ser2814 contributes to the positive FFR.
Fig. 2.
Mice lacking the CaMKIIδ phosphorylation site on RyR2 have a blunted FFR. (A) In vivo contractility as a function of pacing frequency. Contractility represented as percentage change in dP/dtmax from baseline (WT, n = 9; S2814A, n = 8; *P < 0.05). (B) Representative left ventricular pressure traces of ex vivo contractility data from Langendorff perfused hearts paced from 400 beats/min to 550 beats/min. (C) Pooled results of ex vivo contractility study for hearts paced from baseline heart rate of 400 beats/min to 550 or 700 beats/min represented as percentage change in dP/dtmax from baseline heart rate (WT, n = 13; S2814A, n = 9; S2808A, n = 4; WT + KN-93, n = 5; asterisk over KN-93 bar indicates significant difference vs. WT; *P < 0.01; **P < 0.05).
To further explore the mechanism underlying the blunted FFR in the RyR2-S2814A mice we performed ex vivo experiments in which we measured heart rate–dependent contractility changes using Langendorff perfused hearts (16). Isolated murine hearts were paced from 400 beats/min to 550 beats/min or 700 beats/min. At 550 beats/min the contractility (dP/dtmax) of WT mouse hearts increased 45 ± 3% greater than baseline whereas the RyR2-S2814A mouse hearts increased by 25 ± 3% (Fig. 2 B and C; WT, n = 13; RyR2-S2814A, n = 9; P < 0.001 for WT vs. RyR2-S2814A percentage increase). At 700 beats/min the contractility of WT mouse hearts increased 58 ± 5% greater than baseline whereas the RyR2-S2814A mouse hearts increased by only 33 ± 5% (Fig. 2C; WT, n = 13; RyR2-S2814A, n = 9; P < 0.01 for WT vs. RyR2-S2814A percentage increase). The positive FFR in RyR2-S2808A mice was comparable to that observed in WT mice (Fig. 2C). WT hearts pretreated with 600 nM KN-93 for 10 min had no positive FFR (Fig. 2C). There were no differences in baseline dP/dtmax between WT and RyR2-S2814A mice (WT = 2,555 ± 96 mmHg/s; RyR2-S2814A = 2,445 ± 85 mmHg/s; WT, n = 13; S2814A, n = 9; P = not significant).
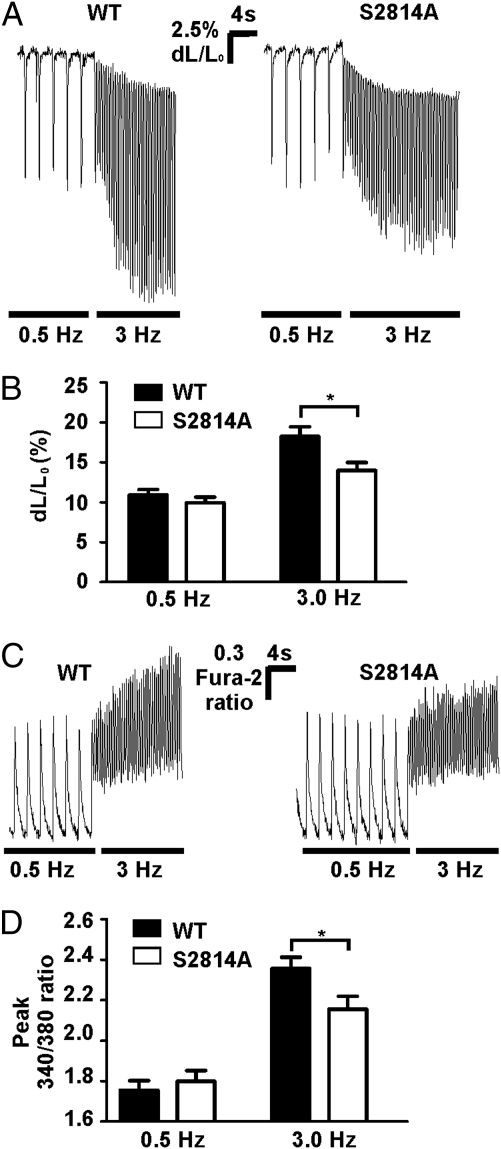
We next examined FFR in isolated cardiomyocytes to determine whether Ca2+ handling was altered in the RyR2-S2814A mice at the cellular level. Using video edge-detection, we found that WT cardiomyocytes had a fractional shortening of 10.8 ± 0.7% at 0.5 Hz and 18.0 ± 1.2% at 3.0 Hz, whereas RyR2-S2814A myocytes had a fractional shortening of 9.8 ± 0.7% at 0.5 Hz and 13.8 ± 1.0% at 3.0 Hz (WT, n = 37 cells; RyR2-S2814A, n = 35; n = 4 mice per group; P < 0.01 for WT vs. RyR2-S2814A fractional shortening at 3.0 Hz; Fig. 3 A and B).
Fig. 3.
Cardiomyocytes lacking the CaMKIIδ phosphorylation site on RyR2 have a blunted FFR. (A) Representative fractional shortening traces from isolated cardiomyocytes paced at 0.5 Hz and then stepped directly to 3.0 Hz. (B) Pooled fractional shortening data (WT, n = 37 cells; RyR2-S2814A, n = 35 cells; n = 4 mice per group) presented as change in cell length normalized to baseline cell length. At 3.0 Hz the fractional shortening of WT cardiomyocytes was significantly higher than RyR2-S2814A cardiomyocytes (*P < 0.01). Both WT and RyR2-S2814A myocytes had significant increases in fractional shortening at 3.0 Hz compared with 0.5 Hz (P < 0.001 and P < 0.05, respectively). (C) Representative Ca2+ transients from cardiomyocytes loaded with Fura-2. Data presented as the 340/380 ratio of the dye. (D) Pooled Ca2+ fluorescence ratio data (WT peak, 2.35 ± 0.06, n = 30 cells; RyR2-S2814A peak, 2.15 ± 0.05, n = 32 cells; n = 4 mice per group; *P < 0.05).
To determine whether the blunted FFR observed in the RyR2-S2814A mice was associated with a reduction in the rate-dependent increase in the Ca2+ transient amplitude, we loaded WT and RyR2-S2814A cardiomyocytes with the Ca2+ indicator Fura-2 and examined Ca2+ transients at 0.5 Hz and 3.0 Hz pacing frequencies. We found that the peak Ca2+ transient amplitude increased by 35.0 ± 1.9% in the WT cells at 3.0 Hz compared with 0.5 Hz, whereas the magnitude of the increase was 20.3 ± 1.5% in the RyR2-S2814A myocytes (WT, n = 30 cells; RyR2-S2814A, n = 32 cells; n = 4 mice per group; P < 0.0001 for WT vs. RyR2-S2814A percentage increase in peak Ca2+ transient amplitude; Fig. 3 C and D). Frequency-dependent increase in baseline Ca2+ in WT myocytes has been reported (17). Similarly, we observed comparable frequency-dependent increases in baseline Ca2+ in both WT and RyR2-S2814A myocytes (Fig. 3C). We also observed comparable frequency-dependent increases in the baseline fractional shortening (Fig. 3A), which is likely a result of the increased baseline Ca2+. Thus, the blunted FFR observed in RyR2-S2814A mice in vivo and ex vivo correlates with a reduced rate-dependent increase in fractional shortening and peak Ca2+ transient amplitude in cardiomyocytes.
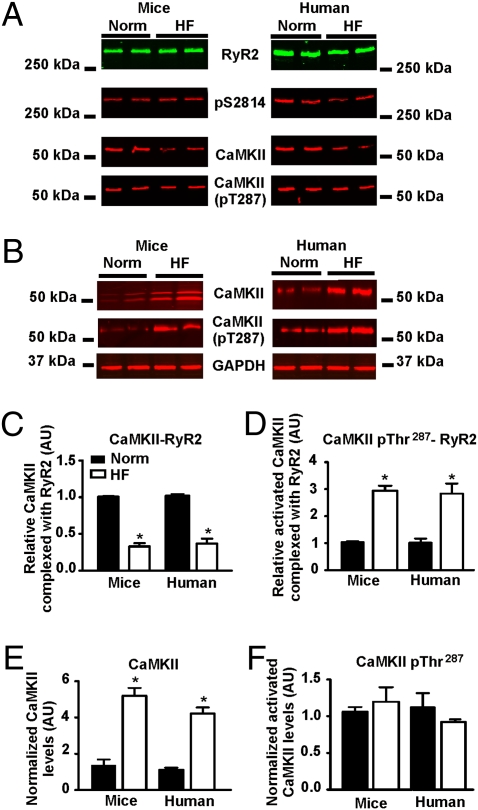
Having determined that CaMKIIδ phosphorylation of RyR2 plays a role in achieving positive FFR, we next sought to determine whether this mechanism is impaired in heart failure, in which positive FFR is blunted (18–21). We have previously demonstrated that the levels of calstabin2, protein phosphatases 1 and 2A (22), and phosphodiesterase 4D3 (23) are reduced in the RyR2 macromolecular complex in failing hearts. In addition, we have reported that the rate-dependent increase in CaMKIIδ-mediated phosphorylation of RyR2 at Ser2814 is not observed in a rat model of post–myocardial infarction (MI) heart failure (10). RyR2 was immunoprecipitated from mouse and human hearts with and without heart failure. CaMKIIδ levels were reduced in the cardiac RyR2 macromolecular complexes from both murine and human failing hearts (Fig. 4 A and C). In addition, the CaMKIIδ in the RyR2 complex was already activated, as determined by measuring the phosphorylation of CaMKIIδ at Thr287 (Fig. 4 A and D). Total CaMKIIδ levels were elevated in cardiac lysates from failing murine and human hearts (Fig. 4 B and E), consistent with previous reports in failing rabbit hearts (24). However, there was no change in the ratio of activated CaMKIIδ in heart lysate (Fig. 4 B and F). Thus, despite an increase in total cellular CaMKIIδ, there is a significant reduction in the amount of activatable CaMKIIδ in the RyR2 macromolecular complex in failing hearts.
Fig. 4.
CaMKIIδ is depleted from RyR2 in heart failure. (A) RyR2 immunoprecipitated from human and mouse normal and heart failure hearts probed for phosphorylation at RyR2-Ser2814, CaMKIIδ, and CaMKIIδ-Thr287. (B) Lysates from human and mouse normal and heart failure hearts probed for total CaMKIIδ and CaMKIIδ-pT287. (C–F) Quantitative summary of immunoblot data. Graphs represent the relative amount of CaMKIIδ associated with RyR2 (C), relative amount of activated CaMKIIδ associated with RyR2 normalized to the amount of CaMKIIδ present in the channel complex (D), total CaMKIIδ in heart lysate (E), and relative amount of activated CaMKIIδ normalized to the total amount of CaMKIIδ in mouse and human cardiac lysates (F). All experiments were performed in triplicate. AU, arbitrary unit. *P < 0.05.
Although the existence of a diastolic SR Ca2+ leak via defective RyR2 channels, and its role in causing heart failure progression (25), is gaining acceptance (26), there has been disagreement as to the mechanism that causes the leak (27). We have previously reported that in failing hearts PKA hyperphosphorylation of RyR2 at Ser2808 causes depletion of calstabin2 from the channel, resulting in a diastolic SR Ca2+ leak that promotes heart failure progression and arrhythmias (15, 23, 25, 28). Others have proposed that chronic CaMKIIδ-mediated hyperphosphorylation of RyR2 causes the diastolic SR Ca2+ leak that leads to heart failure progression (29). Indeed, others have reported that deletion or genetic inhibition of CaMKIIδ is cardioprotective (30, 31). In the present study, there were no differences in RyR2-Ser2814 phosphorylation between control and heart failure (Fig. 4A), in agreement with our previous observations (15). To test the possibility that preventing CaMKIIδ-mediated phosphorylation of RyR2 could be protective, we examined heart failure progression in post-MI WT and RyR2-S2814A mice. Based on echocardiographic assessments of heart function, RyR2-S2814A mice were not protected against heart failure progression (Fig. 5). Thus, CaMKIIδ-mediated phosphorylation of RyR2 is not required for heart failure progression in mice, and the protective effects of CaMKIIδ inhibition in heart failure are likely not explained by reduction of CaMKIIδ-dependent phosphorylation of RyR2.
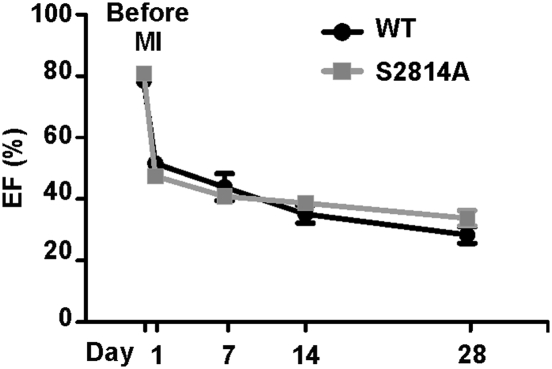
Fig. 5.
Preventing CaMKIIδ phosphorylation of RyR2 does not impart cardioprotection in heart failure. Ejection fraction of WT and RyR2-S2814A mice before and after MI followed by echocardiography for 4 weeks (WT, n = 10; S2814A, n = 11).
Discussion
Cardiac output is the product of stroke volume and heart rate. However, faster heart rates alone reduce diastolic filling time, which would reduce stroke volume. By augmenting contractility, positive FFR can prevent this reduction in stroke volume at faster heart rates and ensure an enhanced cardiac output (32). In the present study we show that CaMKIIδ-dependent phosphorylation of RyR2 at Ser2814 plays a role in the rate-dependent increase in the peak systolic Ca2+ transient amplitude and enhanced myocardial contractility at faster heart rates.
The role of elevated intracellular [Ca2+] and recruitment of additional actin/myosin pairs in the FFR was suggested in 1955 by Moulin and Wilbrandt (33, 34). Direct evidence for this hypothesis was provided 20 years later by Allen and Blinks (2), who, by using the fluorescent Ca2+ sensor aequorin, found that intracellular [Ca2+] was elevated at higher stimulation frequencies. The elevation in total intracellular [Ca2+] at higher frequencies has been attributed to increased Ca2+ influx through LTCC (ICa,L) and SERCA2a activity, which increases the fraction of cytosolic Ca2+ pumped into the SR (35–38). Indeed, Zhao et al. have shown that CaMKIIδ phosphorylation of PLN and increased activity of SERCA2a are required for positive FFR in mice (38).
In the present study we engineered RyR2-S2814A mice to directly test whether CaMKIIδ phosphorylation of RyR2 contributes to positive FFR by enhancing SR Ca2+ release. Our data show that CaMKIIδ-dependent phosphorylation of RyR2 is required for normal rate-dependent increases in the Ca2+ transient and fractional shortening of cardiomocytes and cardiac contractility. The observation that positive FFR was almost completely abolished in hearts treated with the CaMKII inhibitor KN-93, but only partially reduced in RyR2-S2814A mice, hearts, and cardiomyocytes, agrees with the results from Zhao et al. and others that additional targets of CaMKIIδ are also required for normal positive FFR (i.e., PLN and Cav1.2) (35–38). Moreover, our data also agree with studies showing that mice have positive FFR (38, 39); however, other studies have not reported positive FFR in mice (40, 41), possibly because of differences in experimental approaches.
We previously reported that CaMKII phosphorylation of RyR2 at Ser2814 activates the channel by sensitizing it to Ca2+-dependent activation (10). Bers and colleagues have proposed that CaMKIIδ phosphorylation of RyR2 results in increased excitation–contraction coupling gain that could contribute to positive FFR (42). In their experiments, by using prepulse conditioning to set SR load and voltage clamp to control ICa,L, Bers and colleagues found that inhibiting CaMKII with KN-93 reduced Ca2+ transients and cell shortening amplitudes (42). These studies, however, were limited by the possible nonspecific effects of KN-93 and the contributions of other targets of CaMKII. In the present study we have engineered mice with RyR2 specifically lacking the CaMKII phosphorylation site to test the role of CaMKII phosphorylation of RyR2 in rate-dependent increases of cardiac contractility. Our data demonstrate that phosphorylation of RyR2 at Ser2814 increases the amplitude of the systolic Ca2+ transient and contributes to enhancing cardiac contractility at faster heart rates.
Loss of positive FFR in heart failure has been primarily attributed to reduction in SR Ca2+ load, secondary to reduced SERCA2a activity or expression (43). We have previously reported that rats with heart failure lack rate-dependent increased phosphorylation of RyR2 by CaMKIIδ (10). In the present study we have demonstrated that, in heart failure, despite increased cellular levels of CaMKIIδ, there is depletion of CaMKIIδ from the RyR2 complex and that the enzyme complexed with the channel is already activated at baseline heart rates (Fig. 4). Thus, in failing hearts the levels of total and activated CaMKII in the RyR2 complex are not reflected in the cellular levels of this enzyme. This reduction in the RyR2-associated CaMKIIδ in failing hearts likely explains why CaMKIIδ phosphorylation of the RyR2 channel does not increase at higher heart rates in heart failure (10), and suggests that impaired rate-dependent CaMKIIδ phosphorylation of the RyR2 channel contributes to the blunted positive FFR observed in heart failure (21). Although it has been suggested that CaMKIIδ phosphorylation of RyR2 is enhanced in heart failure and that this may contribute to worsening cardiac function, the RyR2-S2814A mice were not cardioprotected in ischemic heart failure (Fig. 5).
Taken together, our data show that the rate-dependent increase in CaMKIIδ activity and subsequent phosphorylation of RyR2 at Ser2814 plays an important role in positive FFR. Moreover, in heart failure, there is less CaMKIIδ in the RyR2 macromolecular complex, and what remains is already activated at baseline heart rates. The presence of a smaller amount of CaMKIIδ in the RyR2, which is already activated, may result in the relative deficiency in the rate-dependent activation of RyR2 by CaMKIIδ and the blunted positive FFR observed in failing hearts.
Materials and Methods
Generation of RyR2-S2814A Mouse.
The targeting vector containing exon 57 of Ryr2 was subcloned from a positively identified B6 BAC clone. The TCT > GCA mutations (S > A) within exon 57 were generated using four primers in a three-step PCR mutagenesis. Primers 1 through 4 (5′-3′: ACTTGGTACCATGTAAATGAGCTAAATACC, TGCCACCTGGGTGATTGACAAAAAG, TTTGTCAATCACCCAGGTGGCAATAGATGCTGCACATGGATACAGC, ACTTGGTACCCGTACGACTTGTTAGGACTTATGTCATCAAC) were designed to amplify a fragment with the size of 450 bp that incorporates the mutations at the desired position. The point mutations were engineered into primers 2 and 3. The PCR fragment carrying the point mutations was then used to replace the WT sequence using conventional subcloning methods (Fig. S1 A–D). The targeting vector was electroporated into ES cells derived from hybrid C57BL/6N × 129SvEv mice (Taconic), which, after selection, were implanted into blastocysts in C57BL/6N mice. Chimeras were crossed with C57BL/6N to generate the F1. After confirmation of germ line transmission and cross with EIIa cre mice to excise the neomycin cassette, mice were backcrossed for more than six generations into the C57BL/6J strain. ES cell preparation and implantation were performed at inGenious Targeting Laboratory.
Immunoprecipitation and Immunoblot Analysis.
Cardiac homogenates were prepared by homogenizing whole hearts in 3 to 5 mL/g of the following (in mM): 10 Tris-maleate, 1 EDTA, 20 NaF, 2 Na3VO4, pH 7.4, plus protease inhibitors. Mouse heart failure samples were taken from mice 4 weeks after MI. All human studies were performed according to protocols approved by the Institutional Review Board of the New York Presbyterian Hospital and the heart samples were acquired as previously described (44). For heart lysate CaMKIIδ and PLN immunoblots, cardiac homogenates (50 μg) were heated to 95 °C (CaMKIIδ only) and size-fractionated by SDS/PAGE (10% and 15%, respectively). For RyR2 and complexed CaMKIIδ blots, RyR2 was immunoprecipitated using anti-RyR2 (5029) (45) from 500 μg of cardiac homogenate and size fractionated by PAGE (6% and 10%, respectively). Proteins were transferred to nitrocellulose membranes, which were then blocked with Licor Odyssey blocking buffer mixed 1:1 with PBS solution. Primary antibodies used were as follows: total RyR2 1:2,000 (MA3-925; Thermo Scientific), phospho-specific RyR2-Ser2808 and RyR2-Ser2814 1:5,000 (10), total CaMKII 1:500 (Santa Cruz Biotechnology), CaMKII-Thr286 1:1,000 (06–881; Millipore), total PLN 1:1,000 (MA3-922; Thermo Scientific), phospho-specific PLN-Ser16 1:1,000 (20R-P121A; Fitzgerald), and PLN-Thr17 1:2,000 (sc-17024-R; Santa Cruz Biotechnology). The membranes were washed three times with 0.1% Tween-20 and incubated with infrared-labeled secondary antibodies at 1:10,000 (Licor Biosystems).
In Vitro Phosphorylation Assay.
RyR2 was immunoprecipitated using anti-RyR2 (5029) (45) from 500 μg of cardiac homogenate. The immunoprecipitations were washed three times in modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 0.9% NaCl, 5.0 mM NaF, 1.0 mM Na3VO4, 1% Triton X-100, and protease inhibitors) and then washed with CaMKII Reaction Buffer (NEB). The sample was then split, with one half being used for total RyR2 immunoblot and the other for in vitro phosphorylation. Briefly, 250 U CaMKIIδ was preactivated as per manufacturer's instructions (NEB). The immunoprecipitated RyR2 was supplemented with the CaMKII reaction buffer, 100 μM ATP, and [γ32P]-ATP for a final specific activity of 250 μCi/μmol and incubated at 30 °C for 15 min. One group of WT samples were supplemented with 1 μM KN-93 (Calbiochem). PKA phosphorylation was performed as previously described (46). Reactions were stopped by addition of SDS-sample buffer. Quantification of blots was performed using a PhosphorImager.
In Vivo Animal Studies.
All experiments were conducted by blinded observers and in accordance with protocols approved by the Institutional Animal Care and Use Committees of Columbia University. Three-month-old male C57BL/6J mice (WT mice were obtained from Jackson Labs) were intubated and anesthetized with 1.5% isoflurane. Pressure-volume (PVR-1045) and EP catheters (EPR-800; Millar Instruments) were inserted into the left ventricle and right ventricle through incisions in the common carotid artery and jugular vein, respectively. The PV catheter was connected to a PC running IOX software (EMKA), which automatically calculated dP/dtmax, and pacing was achieved using an Multichannel System STG3008 pacer controlled by a PC running MC Stimulus II software (Multichannel Systems). Mice received 1 mg/kg propranolol i.p. 5 min before every experiment. MI and echocardiography assessment of cardiac function were performed as previously described (15).
Ex Vivo FFR.
Mice were anesthetized using 1.5% isoflurane and injected with 200 IU heparin. Hearts were surgically removed and cannulated on the tip of a modified 22-gauge needle and perfused retrogradely at 3 mL/min (37 °C) with a modified Krebs solution (in mM): 118.5 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 11 glucose, 1.8 Ca2+. A balloon catheter was inserted into the left ventricle and connected to an APT-300 pressure transducer (ADInstruments), which was connected to a Powerlab digitizer (ADInstruments). The ECG was monitored using voltage electrodes placed on the heart. All data were recorded on a PC running LabChart 6 software (ADInstruments). The hearts were allowed to equilibrate for 10 to 15 min before each experiment and were paced using a small needle positioned on the right atrium that was controlled by an STG2004 pacer connected to a PC running MC Stimulus II software (Multichannel Systems). Hearts used for biochemistry were rapidly removed from the system and flash frozen in liquid nitrogen.
Fractional Shortening and Calcium Fluorescence in Isolated Cardiomyocytes.
Cardiomyocytes were isolated according to a modified version of Alliance for Cellular Signaling protocol PP00000125. Briefly, after the cells were isolated and the Ca2+ concentration brought to 1.8 mM, the cells were resuspended in a modified Tyrode solution (in mM): 137 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, 10 Hepes. The pH was adjusted to 7.4 with NaOH. Fractional shortening was measured using a Nikon Diaphot microscope connected to a video edge detection system (Crescent Electronics) driven by a PC running FeliX32 (Photon Technology International). For Ca2+ fluorescence measurements, cells were loaded for 20 min with 3 μM Fura 2-AM (Molecular Probes) and allowed to rest for at least 10 min before the experiment. Excitation light was produced using a DeltaramV high-speed random access monochromator (Photon Technology International). Fluorescence was recorded using an 814 Photomultiplier Detection System (Photon Technology International) connected to a PC running FeliX32 (Photon Technology International). Cells were paced at 0.5 Hz for at least 1 min before the frequency was increased to 3.0 Hz.
Statistics.
Data are expressed as mean ± SEM. A two-tailed Student t test was used for comparison between groups. P < 0.05 was accepted as significant.
Supplementary Material
Acknowledgments
We acknowledge Bi Xing Chen for her assistance with genotyping the knock-in mice. This work was supported by National Heart, Lung and Blood Institute Grants HL061503 and HL056180 (to A.R.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005843107/-/DCSupplemental.
References
- 1.Bowditch HP. Über die eigentümlichkeiten der reizbarkeit welche die muskelfasern des herzens zeigen (On the peculiarities of excitability which the fibers of cardiac muscle show) Ber Verh Saechs Akad Wiss. 1871;23:652–689. [Google Scholar]
- 2.Allen DG, Blinks JR. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978;273:509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- 3.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 4.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: A Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 6.Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- 7.Putney JW., Jr. Calcium signaling: Up, down, up, down…what's the point? Science. 1998;279:191–192. doi: 10.1126/science.279.5348.191. [DOI] [PubMed] [Google Scholar]
- 8.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 9.Grueter CE, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 11.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 12.Hagemann D, et al. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 14.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 15.Wehrens XH, et al. Ryanodine receptor/calcium release channel PKA phosphorylation: A critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: characteristics and cautions. Clin Exp Pharmacol Physiol. 2003;30:867–878. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- 17.Antoons G, Mubagwa K, Nevelsteen I, Sipido KR. Mechanisms underlying the frequency dependence of contraction and [Ca(2+)](i) transients in mouse ventricular myocytes. J Physiol. 2002;543:889–898. doi: 10.1113/jphysiol.2002.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bombardini T. Myocardial contractility in the echo lab: Molecular, cellular and pathophysiological basis. Cardiovasc Ultrasound. 2005;3:27. doi: 10.1186/1476-7120-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman MD, et al. Depression of systolic and diastolic myocardial reserve during atrial pacing tachycardia in patients with dilated cardiomyopathy. J Clin Invest. 1988;82:1661–1669. doi: 10.1172/JCI113778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, et al. Contractile reserve and intracellular calcium regulation in mouse myocytes from normal and hypertrophied failing hearts. Circ Res. 2000;87:588–595. doi: 10.1161/01.res.87.7.588. [DOI] [PubMed] [Google Scholar]
- 22.Reiken S, et al. beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 23.Lehnart SE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 25.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 26.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 27.Eisner DA, Trafford AW. Heart failure and the ryanodine receptor: Does Occam's razor rule? Circ Res. 2002;91:979–981. doi: 10.1161/01.res.0000045654.34731.ff. [DOI] [PubMed] [Google Scholar]
- 28.Lehnart SE, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier LS, et al. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 31.Ling H, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higginbotham MB, et al. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 33.Moulin M, Wilbrandt W. The effect of potassium and calcium on the staircase phenomenon in frog heart. Experientia. 1955;11:72–73. doi: 10.1007/BF02179035. [DOI] [PubMed] [Google Scholar]
- 34.Koch-Weser J, Blinks JR. The influence of the interval between beats on myocardial contractility. Pharmacol Rev. 1963;15:601–652. [PubMed] [Google Scholar]
- 35.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 36.Hudmon A, et al. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picht E, et al. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007;42:196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao W, et al. Threonine-17 phosphorylation of phospholamban: A key determinant of frequency-dependent increase of cardiac contractility. J Mol Cell Cardiol. 2004;37:607–612. doi: 10.1016/j.yjmcc.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Stull LB, Leppo MK, Marbán E, Janssen PM. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J Mol Cell Cardiol. 2002;34:1367–1376. doi: 10.1006/jmcc.2002.2065. [DOI] [PubMed] [Google Scholar]
- 40.Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redel A, Baumgartner W, Golenhofen K, Drenckhahn D, Golenhofen N. Mechanical activity and force-frequency relationship of isolated mouse papillary muscle: Effects of extracellular calcium concentration, temperature and contraction type. Pflugers Arch. 2002;445:297–304. doi: 10.1007/s00424-002-0931-9. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca(2+)-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: Physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Reiken S, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 45.Jayaraman T, et al. FK506 binding protein associated with the calcium release channel (ryanodine receptor) J Biol Chem. 1992;267:9474–9477. [PubMed] [Google Scholar]
- 46.Wehrens XH, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.