Abstract
Trust plays an important role in the formation and maintenance of human social relationships. But trusting others is associated with a cost, given the prevalence of cheaters and deceivers in human society. Recent research has shown that the peptide hormone oxytocin increases trust in humans. However, oxytocin also makes individuals susceptible to betrayal, because under influence of oxytocin, subjects perseverate in giving trust to others they know are untrustworthy. Testosterone, a steroid hormone associated with competition and dominance, is often viewed as an inhibitor of sociality, and may have antagonistic properties with oxytocin. The following experiment tests this possibility in a placebo-controlled, within-subjects design involving the administration of testosterone to 24 female subjects. We show that compared with the placebo, testosterone significantly decreases interpersonal trust, and, as further analyses established, this effect is determined by those who give trust easily. We suggest that testosterone adaptively increases social vigilance in these trusting individuals to better prepare them for competition over status and valued resources. In conclusion, our data provide unique insights into the hormonal regulation of human sociality by showing that testosterone downregulates interpersonal trust in an adaptive manner.
Keywords: hormones, judgment, oxytocin, competition, vigilance
The hormonal regulation of human social relationships has recently been approached by several disciplines, including psychology, economics, and neuroscience (1–5). Of the many important findings, the discovery that oxytocin increases interpersonal trust (3), as well as the perseveration of trust toward the untrustworthy (2), has been of considerable interest given current debates about the evolution of prosocial behavior in humans and other animals (1). Here we investigate whether testosterone, a hormone associated with success in competition for resources and dominance (6), and an alleged inhibitor of sociality (4), may counteract the role of oxytocin in interpersonal trust. More specifically, we investigate whether, and in what way, testosterone administration in humans decreases interpersonal trust with unfamiliar others.
Humans are highly social and cooperative animals, and their interpersonal relationships importantly rely upon trust. Without trust, suspicion spreads through human social interaction, allowing fear to threaten relationships by instilling vigilance for treachery and betrayal. Compared with other animals, humans are much more likely to trust and cooperate with genetically unrelated and unfamiliar others, and these differences might constitute social adaptations that underlie their evolutionary success (3). Trust has, however, a downside: naïve, trusting humans run a much greater risk of being misguided and deceived by others. In the same way that we have evolved capacities to help others, we have also evolved capacities to deceive and cheat. Thus, those who are willing to believe what others say, or fail to probe the motivations underlying their actions, may fall prey to considerable economic and social costs.
Although humans are essentially social animals (7), competition for resources also underlies the evolution of our species. It is thus critical to understand both the evolutionary and moment-to-moment dynamic between competition and trust, as both have played a critical role in the construction and destruction of society (8).
Recent research in humans using an economic exchange task has shown that administration of oxytocin, a peptide hormone known for its role in attachment and bonding (9), increases interpersonal trust in an economic game, as evidenced by higher monetary allocations to unfamiliar others (3). Other studies have also shown, however, that oxytocin induces perseverative trust: following oxytocin administration, subjects continue to allocate substantial amounts of funds to untrustworthy others, despite being told that their opponents had repeatedly violated their trust (2). These findings highlight the Janus face of trust: high levels of interpersonal trust are beneficial in social interactions, but may place individuals at great personal risk (8).
Testosterone, a steroid hormone with potentially toxic consequences for human sociality (4), might counteract the maladaptive aspects of trust. Testosterone has been associated with social dominance and success in competition (6), and may restrain interpersonal trust to ensure social scrutiny for status and economic concerns. Indeed, testosterone levels in humans correlate positively with financial gain on the stock market and, as such, appear predictive of economic shrewdness (10). These findings, however, are only correlational and thus do not clarify testosterone's relation with interpersonal trust. To explore the possible causal role of testosterone in trusting behavior and, in particular, to test whether testosterone decreases interpersonal trust in humans, we investigated the effect of a single administration of testosterone to healthy volunteers in a trust experiment. In a double-blind, counterbalanced design, we sublingually administered either 0.5 mg of testosterone or a placebo to 24 adult females on two separate days (72 h interval between treatments). Only women participated, because the parameters (quantity and time course) for inducing neurophysiological effects after a single sublingual administration of 0.5 mg of testosterone have been established in women (11), but are unknown in men (for details, see Methods and Materials).
We used facial trustworthiness evaluations as a measure of interpersonal trust to control for the inherent rewarding properties of economic exchange tasks. The association of testosterone with reward and risk-taking is very strong, and could potentially interfere with the measure for trust in an economic exchange task (3, 4). Importantly, trustworthiness judgments of nonfamiliar faces is not only a highly validated procedure (12, 13) unconfounded by reward, but these judgments are also highly correlated with investments in an economic-trust task (14). A recent study showed higher trustworthiness ratings to unfamiliar others after oxytocin administration compared with placebo, demonstrating the validity of using a comparable paradigm for measuring trustworthiness (15). For these reasons, the trustworthiness task is our method of choice for measuring the effect of testosterone administration on subjects’ interpersonal trust levels.
Results
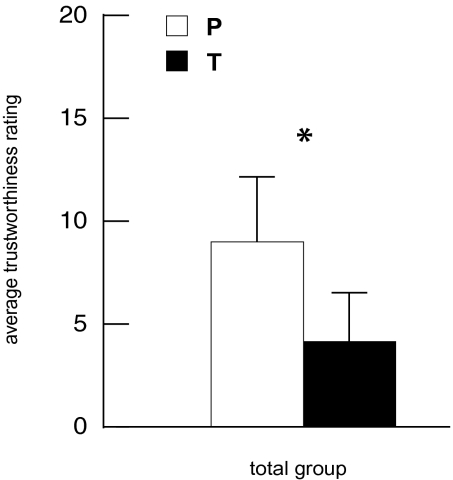
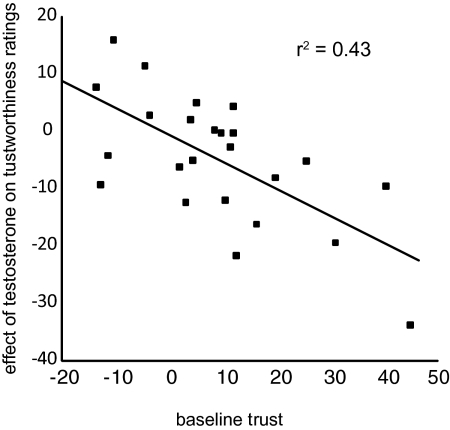
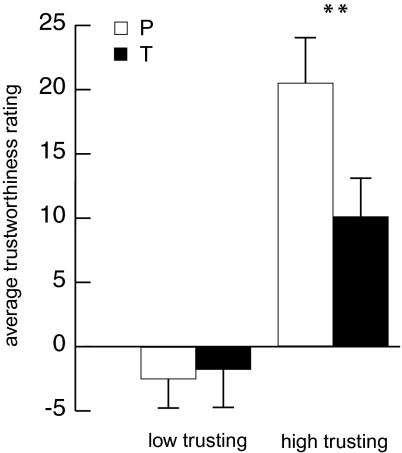
In agreement with our hypothesis, we show a significant overall reduction in trustworthiness ratings after testosterone compared with placebo [F(1, 23) = 4.56, P = 0.044; Fig. 1]. This significant reduction has an effect size of Cohen's d = 0.36. To address the possibility of individual differences, we applied a linear regression to examine whether subjects’ individual basic trust levels (observed from their ratings in the placebo condition) predicted testosterone-induced changes in trustworthiness. This analysis yielded a statistically significant correlation (r = −0.66, P = 0.001; Fig. 2), with individuals’ baseline trust levels explaining 43% of the variance in the effect of testosterone on interpersonal trust. To better qualify this effect, we applied a median split on the 24 basic-trust levels to create groups of 12 high- and 12 low-trusting subjects. Analyses (Fig. 3) showed no effects in low-trusting subjects [F(1, 11) = 0.79, NS], but high-trusting subjects presented a substantial reduction in interpersonal trust following testosterone administration [F(1, 11) = 10.89, P = 0.007]. The effect sizes of testosterone's effect in the low- and high-trust group are, respectively, d = 0.08 and d = 0.92. Note that the absence of an effect in the low-trust group is not caused by a floor effect. That is, the dependent measure could range from −100 to 100, which did not restrict the ratings of the faces in the low-trust group, because the average scores ranged from −13.5 to 8.5 and were normally distributed. In sum, testosterone administration reduced interpersonal trust, but only in subjects who were generally trusting, and therefore more at risk for deceit.
Fig. 1.
Testosterone induced a significant decrease in interpersonal trust in the total group (n = 24). A repeated-measures ANOVA (testosterone placebo) showed [F(1, 23) = 4.56, *P = 0.044]. White bars represent placebo (P), black bars represent testosterone (T), and error bars represent SEM.
Fig. 2.
Plot of the baseline trust ratings, correlated against the effect of testosterone on trust judgments. The points on the left side of the graph, representing subjects who displayed low interpersonal trust in baseline measures, are clustered around zero for an effect of testosterone, indicating that their behavior was not affected by hormone treatment. In contrast, in the subjects displaying high interpersonal baseline trust, represented by the points on the right side of the graph, testosterone significantly decreased interpersonal trust.
Fig. 3.
Separate repeated-measures ANOVAs for the low- and high-trusting subject groups showed that low-trusting participants were completely unaffected by testosterone administration [F(1, 11) = 0.79, NS], whereas high-trusting participants showed a sizeable reduction in the evaluation of facial trustworthiness [F(1, 11) = 10.89, **P = 0.007]. White bars represent placebo (P), black bars represent testosterone (T), and error bars represent SEM.
To control for potential secondary mood-generated effects of testosterone on interpersonal trust, we administered the shortened version of the profile-of-mood-states (POMS) (16) before the trustworthiness task for both the placebo and testosterone conditions; the POMS includes the subscales tension–anxiety, depression, anger, fatigue, and vigor. Paired t tests for the subscales showed nonsignificant effects (all P’s > 0.24, two-tailed). Furthermore, subjects were asked after the experiment to indicate or guess the day they received testosterone. Subjects’ scores were at chance (binomial = 0.84, two-tailed), and there was no statistically significant relationship between the subjective guess of the day of testosterone administration and trustworthiness ratings; an ANOVA that used testosterone-induced change in trust as a between-subject factor and correct-versus-incorrect guesses as a between-subject factor was not significant [F(1, 22) = 0.18, NS].
In sum, the effects of testosterone on interpersonal trust are not mediated by either mood or subjective preconceptions (17); they are pure effects of the hormone on behavior.
Furthermore, as can be seen in Materials and Methods, testosterone levels that were measured from saliva before the experiment did not predict the trustworthiness scores, and the individual variance in these baseline testosterone levels between conditions did also not explain the effect of testosterone administration on interpersonal trust.
Discussion
Our findings license the conclusion that testosterone decreases interpersonal trust, and in an apparently adaptive manner. The hormone acted selectively on our high-trusting subjects, defensibly to down-regulate their trust to a level more advantageous in the competition for resources. Our data coincide with correlational evidence showing that higher testosterone levels predict financial gain on the stock market (10), but seem somewhat at odds with recent findings of more fair bargaining behavior on the ultimatum game after testosterone administration (17). However, the down-regulation of trust after testosterone administration at present was restricted to the high trusting, thus most socially naïve half of our subject group, and may for that reason be adaptive in the competition for status and resources. The ultimatum game, however, is an economic paradigm that measures fairness and not trust (3); in the ultimatum game, fair offers are logically more often accepted. With fair offers, the proposer takes control over the game and both players make money. In sum, more fair offers by the proposer in the ultimatum game after testosterone administration are also adaptive for achieving status and resources (17). Hence, the context (i.e., trusting behaviors against fairness behaviors) in the above cases obviously defined the—at first sight—differential effects of the hormone, which ultimately have common ground. In many mammalian species, testosterone's role in social behaviors is simply confined to motivating aggression in competition for status and resources. In humans, however, the hormone seems to motivate for rational decision-making, social scrutiny, and cleverness (17), the apparent tools for success in a modern society (4, 6, 18; but see ref. 19). Viewed from this perspective, testosterone's relation to risk-taking behaviors in humans (20) might also be reevaluated, as success on the stock market cannot be established by unrestrained risk-taking, but requires a fine-tuned grasp of the balance between financial threat and reward (10).
At present, there is little understanding of the neurobiological mechanisms by which testosterone acts on interpersonal trust. Nonetheless, animal data have shown that the amygdala is an important target of this hormone in the brain (21). Human neuroimaging studies support this finding by demonstrating the involvement of the human amygdala in the detection of facial threat (22), in social evaluations of faces (23), and specifically, in evaluations of trustworthiness from faces (24). Furthermore, social evaluations of faces are impaired in patients with bilateral lesions to the amygdala, and these patients appear more trusting in their interactions with strangers (13). However, the amygdala does not stand alone in the social evaluation of faces; in particular, the orbitofrontal cortex (OFC), which shows strong connectivity to the amygdala, also plays an important role in these social processes. Moreover, the amygdala and OFC are thought to act in concert in the regulation of many social behaviors (25, 26), and the communication of these structures is affected by testosterone. In humans, administration of testosterone induces rapid reductions in the functional connectivity between amygdala and OFC in response to facial threat (27) and, conversely, seems to activate the amygdala–brainstem defense circuit (5). Interestingly, animal research shows that testosterone may induce amygdala–brainstem functional connectivity by acting on the social peptide vasopressin (21, 28). Vasopressin, whose expression is regulated by testosterone (29), increases outputs of the amygdala to the brainstem by acting on distinct neuronal populations within the amygdala (28).
Oxytocin, the hormone that increases interpersonal trust (3), acts in a manner opposite to vasopressin, decreasing the outputs to the brainstem (28, 30), but also increasing the involvement of frontal cortical regions, such as the OFC (31). Thus, testosterone and oxytocin seem to act as hormonal antagonists at the level of the amygdala, providing an adaptive balance in behavioral responses to social cues. In sum, we suggest that testosterone in the present study may have induced a prefrontal limbic shift in social-emotional processing by regulating peptide expression in the amygdala (21, 28). This shift toward evolutionary older brain regions puts the brain in a defensive or vigilant mode (28, 31–33), and consequently may have down-regulated interpersonal trust. A socially vigilant stance is vital for gaining and maintaining dominance or leadership, and for success in competition for resources (10, 17, 18).
In conclusion, we show that testosterone plays a causal role in reducing interpersonal trust among unfamiliar individuals. The way in which testosterone decreases trust is consistent with its role in economic decision-making and competitive interactions. The attribution of trust toward unfamiliar others was especially decreased in subjects who run the greatest risk of being misled by others, that is, those who grant trust easily. Consequently, testosterone increased social vigilance in trusting humans, presumably to better prepare them for the hard-edged competition over status and valued resources. These findings provide insight into the hormonal regulation of human sociality by showing that the hormone testosterone down-regulates interpersonal trust in an adaptive manner.
Materials and Methods
Subjects.
The Ethics Committee of the University Medical Centre Utrecht approved the protocol of our experiment wherein 24 healthy young women (mean age 20.2) participated. All women received testosterone and placebo, in randomized order, with a 72-h latency between sessions. Subjects had no (history of) psychiatric disorders or neurological or endocrine abnormalities. They did not smoke, and used no medication other than contraceptives. We controlled for influences of hormonal change due to menstrual cycle by only including women who used single-phase contraceptives, and testing them during the 3-week period they were on these contraceptives and not during menstruation (see also ref. 34). In this 3-week contraceptive period, menstrual-cycle influences are virtually absent. Moreover, any effects of the contraceptives would be equal during the placebo or testosterone condition.
Substance Administration.
The drug samples consisted of 0.5 mg of testosterone, 5 mg of (the carrier) cyclodextrine, 5 mg of ethanol, and 5 mL of water. Testosterone was omitted from the placebo samples, and both testosterone and placebo were administered sublingually. Previous experimental research established the time course of changes in blood levels of testosterone and physiological responsiveness in typical young women after a single sublingual administration of 0.5 mg of testosterone (11). A 10-fold increase in total testosterone was observed 15 min after intake with testosterone levels returning to baseline within 1.5 h (11). It was also shown that this single administration of testosterone significantly elevated vaginal pulse amplitude in healthy young women, which peaks around 4 h. Thus, physiological effects after single sublingual administrations of 0.5 mg testosterone peak 2.5 h after the testosterone level in the blood has returned to baseline. Note, that vaginal pulse amplitude, a centrally driven response evoked by erotic material, is the only physiological measure known to possess a nonhabitual nature, thus allowing multiple measures throughout the day (11, 35). There is no method available to assess the time course of effects of testosterone in human males, whereas in females, the present time-course method may have unique applicability in the treatment of sexual dysfunction (35, 36). Crucially, the reliability and generalizability of behavioral effects after a 4-h delay has been successfully established in more than 20 studies, addressing sexual, social, and emotional behaviors in young typical women (e.g., refs. 5, 17, 35, and 37–41). Therefore, in the present protocol, a 4-h delay between testosterone administration and measurement of mood and the trustworthiness ratings was again used.
Physiological Levels and Potential Neuroendocrine Mechanisms.
The 10-fold increase in testosterone levels that our method induces (11) seems rather high in the light of increases seen in treatment studies. However, it is important to note that there are important differences between the chronic treatments, which do not consider a time course of effects, and our single-administration approach. Our single sublingual administration of 0.5 mg testosterone produces an increase in absolute levels of testosterone in most cases higher than that seen with chronic treatment, but within and during a very short period. Crucially, it is conjectured by van der Made et al. (35) that this increase will not produce a proportional increase in the free fraction of testosterone; the amount of testosterone reaching the brain will be much less. A sex hormone binding globulin (SHBG) saturation threshold mechanism has been postulated: The increase of testosterone into the body will first bind to SHBG (and to albumin, to a smaller extend) before being able to produce an increase in the free fraction (35). The increase of testosterone produced by the sublingual 0.5 mg administration method does not compare with the 10-fold increase in total testosterone in the blood, but would be large enough to pass this putative SHBG threshold, resulting in a short increase in the free testosterone fraction. This short increase, however, is responsible for cognitive, affective, and behavioral effects observed a few hours later, which have been reported in numerous studies in human females, as noted previously.
Generalizability of Effects to Males.
The parameters (quantity and time course) for inducing neurophysiological effects after a single sublingual administration of 0.5 mg of testosterone are thus known in women, but not in men. Nonetheless, based on findings from our correlational research on testosterone and human social behavior in which we used males and females, we expect the effects of testosterone administration to be similar for males and females (41, 42). Moreover, we have repeatedly shown that testosterone administration in females results in more male-typical social behavior (37, 43). Finally, others have shown that testosterone administration in females (17) seems to increase status-seeking behavior, and this finding agrees with correlations between endogenous testosterone levels and status-related behaviors shown in men (for a review, see ref. 18) and women (44–47). This research adds to the growing evidence that testosterone plays an important role in female social behavior (48–51). In sum, the relation between testosterone and social behavior apparently has much communality in human males and females.
Behavioral Experiment.
The stimuli in the trustworthiness task consisted of 150 grayscale frontal pictures of unfamiliar faces with neutral emotional expressions, of which 100 were adapted from Adolphs et al. (13) and 50 were taken from the Psychological Image Collection at Stirling (PICS; http://pics.psych.stir.ac.uk/). For our within-subjects design, we created two sets of 75 stimuli that were matched based on trustworthiness ratings in a previous study with 36 healthy adult subjects (52).
On each test day all stimuli of one set were presented once, in random order, both sets being counterbalanced with administration order. Pictures were presented in the middle of a 17-inch LCD display subtending a visual angle of ≈8 ° on a gray background. Directly below the stimulus a visual analog scale was presented ranging from (left to right) “very untrustworthy” to “neutral” to “very trustworthy.” For each stimulus, subjects were presented with the question “How trustworthy do you think this person is?” and asked to answer by clicking on the scale with a mouse cursor. After the response to each trial, a button appeared with the word next; the subject's response to the scale could be adjusted until this button was clicked, and then it disappeared. For each presentation trial, the scale was reset to the neutral position. The stimuli were presented using software written in E-Prime (Psychology Software Tools, Inc.). Subjects performed trustworthiness ratings once on each set, counterbalanced with order of administration.
For data analysis, the scale positions were coded from −100 (very untrustworthy) to 0 (neutral) to +100 (very trustworthy) in steps of 1. These scores were averaged for each subject and both test sessions to obtain individual measures of trustfulness in testosterone and placebo conditions.
Testosterone Saliva Measurement.
Salivary sampling was chosen to obtain baseline testosterone levels. Salivary testosterone has proven to be a reliable noninvasive biomarker in the social (42, 44, 46, 49, 51) and clinical sciences (36, 53), and has also been successfully applied in economic research (10, 54). Salivary sampling avoids possible confounding influences induced by (anticipation of) blood sampling procedures, which in humans are known to induce substantial stress, and increases in stress hormones such as cortisol (55). Our sampling method was based upon Granger et al. (56), which has been successfully applied in several previous studies (5, 10).
Testosterone in saliva was measured after diethylether extraction using a competitive radioimmunoassay employing a polyclonal antitestosterone antibody (AZG 3290; a gift from J. J. Pratt, Groningen, The Netherlands). [1,2,6,7-3H]-Testosterone (TRK402; Amersham Nederland BV) was used as a tracer following chromatographic verification of its purity. The lower limit of detection was 10 pmol/L, and interassay variation was 16.1, 11.5, and 5.1% at 21, 100, and 230 pmol/L, respectively (n = 4, 5, 5). Samples of two subjects were contaminated, showing out-of-normal range levels, and therefore not included in further analysis.
Our analyses showed that testosterone levels measured from saliva before administration did not differ between the testosterone and placebo administration condition in the complete group [F(1, 21) = 2.19, NS] or in the high-trusting subject group, which was accountable for our effects [F(1, 11) = 1.27, NS]. Furthermore, differences in baseline testosterone levels between subjects' placebo and testosterone condition (entered as a covariate in the original analyses) did not explain any variance in the effects of testosterone administration on trust in the complete group [F(1, 20) = 0.42, NS] or in the high-trusting group [F(1, 10) = 1.24, NS]. Finally, low-trusting subjects compared with high-trusting subjects did not show higher baseline testosterone levels in their placebo [F(1, 20) = 0.72, NS] or testosterone condition [F(1, 20) = 0.87, NS] in the experiment. Thus, our findings on testosterone administration cannot be attributed to variation in baseline testosterone levels in subjects between conditions, or to differences in baseline testosterone between conditions in general. Finally, testosterone baseline levels can also not account for our exclusive effect in the high-trust group.
Acknowledgments
We thank Ralph Adolphs for the use of his stimulus material and Marc Hauser for constructive feedback on the manuscript. We also thank the reviewers for their comments, which helped us greatly to improve the manuscript. Funding for this study was provided by a Utrecht University High Potential Grant and the Hope for Depression Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Delgado MR. Fool me once, shame on you; fool me twice, shame on oxytocin. Neuron. 2008;58:470–471. doi: 10.1016/j.neuron.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 4.van Honk J. In: Methods in Social Neuroscience. Harmon–Jones E, Beer JS, editors. New York: Guilford; 2009. pp. 45–69. [Google Scholar]
- 5.Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol Psychiatry. 2008;63:263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Adolphs R. The social brain: Neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond JM. Guns, Germs and Steel: The Fates of Human Societies. New York: Norton; 1997. [Google Scholar]
- 9.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 10.Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci USA. 2008;105:6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuiten A, et al. Time course of effects of testosterone administration on sexual arousal in women. Arch Gen Psychiatry. 2000;57:149–153. doi: 10.1001/archpsyc.57.2.149. discussion 155-146. [DOI] [PubMed] [Google Scholar]
- 12.Todorov A, Duchaine B. Reading trustworthiness in faces without recognizing faces. Cogn Neuropsychol. 2008;25:395–410. doi: 10.1080/02643290802044996. [DOI] [PubMed] [Google Scholar]
- 13.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 14.van ’t Wout M, Sanfey AG. Friend or foe: The effect of implicit trustworthiness judgments in social decision-making. Cognition. 2008;108:796–803. doi: 10.1016/j.cognition.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Shacham S, Reinhardt LC, Raubertas RF, Cleeland CS. Emotional states and pain: Intraindividual and interindividual measures of association. J Behav Med. 1983;6:405–419. doi: 10.1007/BF00846327. [DOI] [PubMed] [Google Scholar]
- 17.Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E. Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature. 2010;463:356–359. doi: 10.1038/nature08711. [DOI] [PubMed] [Google Scholar]
- 18.Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21:353–363, discussion 363–397. [PubMed] [Google Scholar]
- 19.Zak PJ, et al. Testosterone administration decreases generosity in the ultimatum game. PLoS One. 2009;4:e8330. doi: 10.1371/journal.pone.0008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Honk J, et al. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29:937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Koolhaas JM, Van den Brink THC, Roozendaal B, Boorsma F. Medial amygdala and aggressive behavior: Interaction between testosterone and vasopressin. Aggr Behav. 1990;16:223–229. [Google Scholar]
- 22.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 23.Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- 24.Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- 25.Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Emery NJ, Amaral DG. In: Cognitive Neuroscience of Emotion. Lane RD, Lynn N, editors. New York: Oxford Univ Press; 2000. pp. 156–191. [Google Scholar]
- 27.van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernández G. Testosterone reduces amygdala-orbitofrontal cortex coupling. Psychoneuroendocrinology. 2010;35:105–113. doi: 10.1016/j.psyneuen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 29.de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- 30.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 32.Mobbs D, et al. When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. New York: Plenum; 1990. [DOI] [PubMed] [Google Scholar]
- 34.Aarts H, van Honk J. Testosterone and unconscious positive priming increase human motivation separately. Neuroreport. 2009;20:1300–1303. doi: 10.1097/WNR.0b013e3283308cdd. [DOI] [PubMed] [Google Scholar]
- 35.van der Made F, et al. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med. 2009;6:777–790. doi: 10.1111/j.1743-6109.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 36.van der Made F, et al. Childhood sexual abuse, selective attention for sexual cues and the effects of testosterone with or without vardenafil on physiological sexual arousal in women with sexual dysfunction: A pilot study. J Sex Med. 2009;6:429–439. doi: 10.1111/j.1743-6109.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- 37.van Honk J, Schutter DJ. Testosterone reduces conscious detection of signals serving social correction: Implications for antisocial behavior. Psychol Sci. 2007;18:663–667. doi: 10.1111/j.1467-9280.2007.01955.x. [DOI] [PubMed] [Google Scholar]
- 38.Hermans EJ, Putman P, van Honk J. Testosterone administration reduces empathetic behavior: A facial mimicry study. Psychoneuroendocrinology. 2006;31:859–866. doi: 10.1016/j.psyneuen.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35:114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 40.van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.van Honk J, et al. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behav Neurosci. 2001;115:238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- 42.van Honk J, et al. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm Behav. 1999;36:17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- 43.Hermans EJ, et al. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Josephs RA, Newman ML, Brown RP, Beer JM. Status, testosterone, and human intellectual performance: Stereotype threat as status concern. Psychol Sci. 2003;14:158–163. doi: 10.1111/1467-9280.t01-1-01435. [DOI] [PubMed] [Google Scholar]
- 45.Cashdan E. Hormones, sex, and status in women. Horm Behav. 1995;29:354–366. doi: 10.1006/hbeh.1995.1025. [DOI] [PubMed] [Google Scholar]
- 46.Dabbs JM, Jr, Hargrove MF. Age, testosterone, and behavior among female prison inmates. Psychosom Med. 1997;59:477–480. doi: 10.1097/00006842-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Josephs RA, Sellers JG, Newman ML, Mehta PH. The mismatch effect: When testosterone and status are at odds. J Pers Soc Psychol. 2006;90:999–1013. doi: 10.1037/0022-3514.90.6.999. [DOI] [PubMed] [Google Scholar]
- 48.Mehta PH, Wuehrmann EV, Josephs RA. When are low testosterone levels advantageous? The moderating role of individual versus intergroup competition. Horm Behav. 2009;56:158–162. doi: 10.1016/j.yhbeh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. J Pers Soc Psychol. 2008;94:1078–1093. doi: 10.1037/0022-3514.94.6.1078. [DOI] [PubMed] [Google Scholar]
- 50.Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiol Behav. 2007;90:496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Newman ML, Sellers JG, Josephs RA. Testosterone, cognition, and social status. Horm Behav. 2005;47:205–211. doi: 10.1016/j.yhbeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Baas D, et al. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. Neuroimage. 2008;40:719–727. doi: 10.1016/j.neuroimage.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 53.Arregger AL, Contreras LN, Tumilasci OR, Aquilano DR, Cardoso EM. Salivary testosterone: A reliable approach to the diagnosis of male hypogonadism. Clin Endocrinol (Oxf) 2007;67:656–662. doi: 10.1111/j.1365-2265.2007.02937.x. [DOI] [PubMed] [Google Scholar]
- 54.Sapienza P, Zingales L, Maestripieri D. Gender differences in financial risk aversion and career choices are affected by testosterone. Proc Natl Acad Sci USA. 2009;106:15268–15273. doi: 10.1073/pnas.0907352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubbard JR, Kalimi M, Liberti JP. In: Handbook of Stress Medicine: An Organ System Approach. Hubbard JR, Workman EA, editors. Boca Raton, FL: CRC; 1997. pp. 309–322. [Google Scholar]
- 56.Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]