Abstract
An insertion polymorphism of the angiotensin-I converting enzyme gene (ACE) is common in humans and the higher expressing allele is associated with an increased risk of diabetic complications. The ACE polymorphism does not significantly affect blood pressure or angiotensin II levels, suggesting that the kallikrein-kinin system partly mediates the effects of the polymorphism. We have therefore explored the influence of lack of both bradykinin receptors (B1R and B2R) on diabetic nephropathy, neuropathy, and osteopathy in male mice heterozygous for the Akita diabetogenic mutation in the insulin 2 gene (Ins2). We find that all of the detrimental phenotypes observed in Akita diabetes are enhanced by lack of both B1R and B2R, including urinary albumin excretion, glomerulosclerosis, glomerular basement membrane thickening, mitochondrial DNA deletions, reduction of nerve conduction velocities and of heat sensation, and bone mineral loss. Absence of the bradykinin receptors also enhances the diabetes-associated increases in plasma thiobarbituric acid-reactive substances, mitochondrial DNA deletions, and renal expression of fibrogenic genes, including transforming growth factor beta1, connective tissue growth factor, and endothelin-1. Thus, lack of B1R and B2R exacerbates diabetic complications. The enhanced renal injury in diabetic mice caused by lack of B1R and B2R may be mediated by a combination of increases in oxidative stress, mitochondrial DNA damage and over expression of fibrogenic genes.
Keywords: diabetes mellitus complications, kinins
The angiotensin-I converting enzyme (ACE) is a dipeptidyl carboxypeptidase, named because it removes two amino acids from the carboxyl terminus of the inactive peptide angiotensin I and converts it into the active blood pressure-raising peptide, angiotensin II. However, ACE is also a kininase and converts the active vasodilatory kinins into inactive metabolites by removing two amino acids from their carboxyl termini (1). Prior experimental findings (2) and computer simulations (3) show that modest changes in ACE levels affect the levels of its substrates much more than its products, indicating that relatively small changes in the levels of ACE affect kinin levels more than angiotensin II levels.
The common insertion/deletion (I/D) polymorphism of the ACE gene in humans is due to the presence or absence of an Alu retrotransposon in the 16th intron of the gene. The polymorphism is associated with up to a twofold difference in relative plasma ACE levels (4), but the polymorphism does not significantly affect blood pressure or angiotensin II or aldosterone levels (5). Nevertheless, the I and D human ACE alleles are associated with different risks for developing diabetic complications including nephropathy (6), neuropathy (7), retinopathy (8), myocardial infarction (9), stroke (10), and osteoporosis (11). In all these diabetes-associated conditions, it is the D allele, associated with higher serum levels of ACE, that confers the increased risk. Acting in the reverse direction, ACE inhibitors (ACEIs) have well-documented beneficial effects on diabetic nephropathy (12), diabetic neuropathy (13), diabetic retinopathy (14), osteoporosis (15, 16), and coronary artery diseases (17) that exceed what can be attributed solely to changes in blood pressure. These findings suggest that the kallikrein-kinin system (KKS) may partly mediate the protective effects of low levels of ACE on diabetic complications and that reduced levels of the kinins mediate some of the harmful effects of the ACE D allele. In support of this postulate, a recent study has demonstrated that lack of tissue kallikrein gene increases microalbuminuria in a mouse model of type I diabetes (18). In addition, we have demonstrated that genetically diabetic mice that also lack one of the bradykinin receptors, B2R, develop a more severe kidney pathology by age 6 months than their diabetic littermates expressing B2R (19). By age 12 months, they develop senescence-associated phenotypes that are more severe than those in their diabetic littermates that have the B2R, including alopecia, skin atrophy, kyphosis, osteoporosis, testicular atrophy, lipofuscin accumulation in renal proximal tubule and testicular Leydig cells, and apoptosis in the testis and intestine (20).
The other bradykinin receptor, B1R, is expressed at much lower levels than B2R in the kidney of WT mice, but its expression is markedly enhanced in the kidney of Akita diabetic or B2R knocked out (B2R-null) mice (19). Whether this increase in B1R expression is beneficial or harmful is debatable (21, 22). Because the loci coding for B1R and B2R are only 11 kb apart, it is impractical to make mice lacking both B1R and B2R by simply crossbreeding B1R-null mice and B2R-null mice. Consequently, to unambiguously explore the role of KKS, we have generated mice lacking both receptors (BRKO) by deleting the genomic region that includes both genes (23). (There are no identified or predicted ORFs between the loci for B1R and B2R.) Another group has generated BRKO mice by disrupting B1R using embryonic stem (ES) cells obtained from B2R-null mice (24) and have shown that the response to kinins, as attested by contractility studies in smooth muscle cells, is lacking, indicating that signaling via B1R and B2R mediates most of the effects of the kinins.
Here, we show that the lack of both B1R and B2R enhances the nephropathy, neuropathy, and osteopathy observed in male mice with the Akita diabetogenic mutation in the Insulin 2 gene (Ins2). These results demonstrate that the KKS plays a protective role in these diabetic complications and suggest that vasopeptidase inhibitors and KKS agonists, like ACE inhibitors, could be beneficial for treatment or prevention of diabetic complications.
Results
Baseline Effects of Lack of Bradykinin B1 and B2 Receptors on Akita Diabetic Mice.
Table 1 shows baseline data at 12 months age of WT male mice, mice with the gene coding for B2R knocked out (B2R-null), mice with the genes coding for both B1R and B2R knocked out (BRKO), mice heterozygous for the diabetogenic Akita mutation in the Ins2 gene (Akita), Akita mice with the gene coding for B2R knocked out (B2R-null-Akita), and Akita mice with the genes coding for both bradykinin receptors knocked out (BRKO-Akita). Table S1 lists the P values, assessed by two-way ANOVA, for the effects on the various parameters of bradykinin receptors genotype and the Akita mutation and for the presence of any interaction between them. The data show that the bradykinin receptor mutations by themselves affect only two of the baseline parameters: plasma insulin levels (increased to approximately 1.6× normal in B2R-null and approximately 2.5× normal in BRKO), and plasma thiobarbituric acid-reactive substances (TBARS) (increased to approximately 2.6× normal in B2R-null and approximately 3.0× normal in BRKO). Absence of the receptors does not change systolic blood pressure. The Akita mutation by itself has many of the expected effects of untreated type I diabetes: decreased body weight (BW) and heart weight (HW), increased kidney to body weight ratio (KW/BW), and increased plasma glucose, plasma triglycerides, and plasma TBARSs. It has no significant effect on systolic blood pressure. When the bradykinin receptor mutations and the Ins2 mutation are combined in the B2R-null-Akita or BRKO-Akita mice, there is a marked additive increase in plasma TBARSs (to approximately 4.6× normal in B2R-null and approximately 5.8× normal in BRKO). Not surprisingly, the high plasma insulin levels caused by the BRKO mutation are abolished by the profound decrease in insulin production that is a consequence of the Akita mutation. The BRKO mutation does not modify the effects of the Akita diabetogenic mutation on BW, HW, KW/BW, plasma glucose, and plasma triglyceride. Thus, the most notable effect on the diabetic profile of absence of the two bradykinin receptors is that the already high level of plasma TBARSs caused by the diabetes is additively increased to approximately five times normal, indicating a large increase in oxidative stress.
Table 1.
Baseline characteristics of the 6 genotypes of male animals at age 12 months
| WT | B2R-null | BRKO | Akita | B2R-null-Akita | BRKO-Akita | |
| Number of mice | 5 | 5 | 5 | 5 | 5 | 10 |
| Body weight, g | 32.0 ± 1.6 | 31.1 ± 1.2 | 37.4 ± 1.6 | 22.5 ± 1.3* | 21.2 ± 1.5* | 23.2 ± 1.4* |
| Kidney weight, mg | 245 ± 19 | 237 ± 35 | 248 ± 17 | 239 ± 14 | 252 ± 10 | 255 ± 15 |
| KW/BW, ‰ | 7.80 ± 0.71 | 7.48 ± 0.89 | 6.81 ± 0.63 | 10.67 ± 0.50* | 12.21 ± 0.96† | 10.91 ± 0.54* |
| Heart weight, mg | 238 ± 22 | 205 ± 21 | 184 ± 22 | 112 ± 4* | 110 ± 8* | 151 ± 18 |
| HW/BW, ‰ | 7.50 ± 0.76 | 6.60 ± 0.61 | 5.00 ± 0.76 | 5.75 ± 0.60 | 5.22 ± 0.17 | 6.42 ± 0.65 |
| Glucose, mg/dL | 142 ± 14 | 145 ± 9 | 154 ± 32 | 798 ± 58* | 783 ± 44* | 750 ± 32* |
| Insulin, μmol/L | 0.59 ± 0.10 | 0.95 ± 0.10¶ | 1.61 ± 0.10‡,§ | 0.11 ± 0.08* | 0.09 ± 0.05* | 0.09 ± 0.08* |
| Urea nitrogen, mg/dL | 20.3 ± 1.4 | 22.1 ± 2.8 | 22.8 ± 3.0 | 32.3 ± 4.2 | 35.6 ± 2.8† | 25.8 ± 2.8 |
| Creatitine, mg/dL | 0.10 ± 0.03 | 0.16 ± 0.04 | 0.12 ± 0.03 | 0.14 ± 0.03 | 0.18 ± 0.03 | 0.13 ± 0.03 |
| Total cholesterol, mg/dL | 54.9 ± 9.0 | 105.9 ± 10.9 | 85.8 ± 9.0 | 77.7 ± 7.1 | 62.9 ± 13.1 | 67.3 ± 7.6 |
| Triglyceride, mg/dL | 55.1 ± 25.1 | 76.4 ± 14.5 | 48.1 ± 25.1 | 105.8 ± 19.8 | 93.0 ± 27.6 | 133.8 ± 21.2† |
| TBARSs, mmol/L | 6.5 ± 1.0 | 17.2 ± 1.6‡ | 19.0 ± 2.6‡ | 22.5 ± 1.2* | 29.7 ± 2.0¶,* | 37.9 ± 2.0‡,§,* |
| Systolic BP, mmHg | 108 ± 3 | 113 ± 1 | 114 ± 5 | 115 ± 2 | 115 ± 2 | 115 ± 3 |
Data are shown as mean ± SEs. WT, wild type; B2R-null, mice lacking B2R; BRKO, mice lacking both B1R and B2R; Akita, mice with heterozygous Akita mutation in Ins2 gene; B2R-null-Akita, B2R-null mice with heterozygous Akita mutation in Ins2 gene; BRKO-Akita, BRKO mice with heterozygous Akita mutation in Ins2 gene; TBARSs, thiobarbituric acid-reactive substances; BP, blood pressure.
*P < 0.01 vs. the nondiabetic group of the same bradykinin receptor genotype;
†P < 0.05 vs. the nondiabetic group of the same bradykinin receptor genotype;
‡P < 0.01 vs. WT (in nondiabetic groups) or Akita (in diabetic groups);
§P < 0.05 vs. B2R-null (in nondiabetic groups) or B2R-null-Akita (in diabetic groups);
¶P < 0.05 vs. WT (in nondiabetic groups) or Akita (in diabetic groups).
Lack of Bradykinin Receptors and Diabetic Nephropathy.
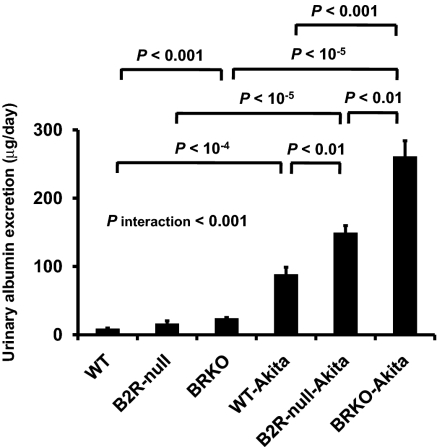
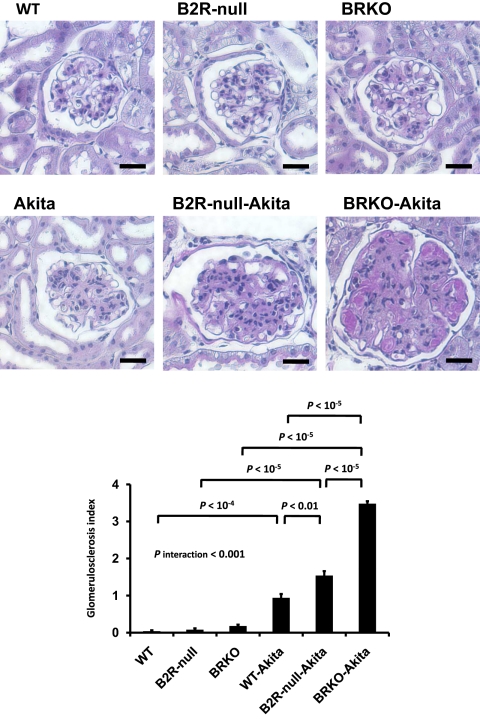
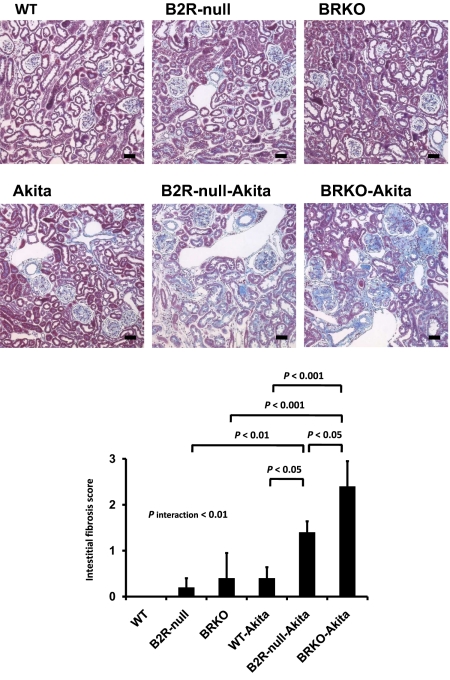
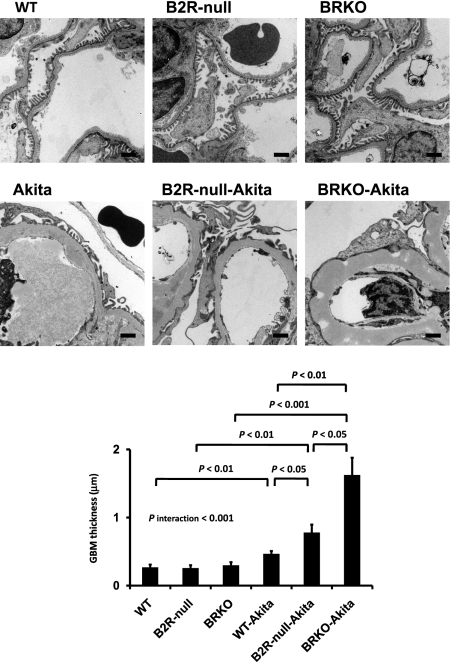
Urinary albumin excretion, already approximately 9.8× normal in the Akita diabetic mice and approximately 1.8× and approximately 2.7× normal in the B2R-null and BRKO mice, is increased to approximately 16.6× and approximately 29.0× normal in the B2R-null and BRKO-Akita mice and there is a strong positive interaction between the two mutations (P of interaction < 0.001; Fig. 1). In light microscopy, mesangial hypercellularity, accumulation of periodic acid-Schiff (PAS)-positive extracellular matrix, intra-arteriolar hyalinosis (insudation) in the renal glomerulus (Fig. 2), and tubulointerstitial fibrosis (Fig. 3) are observed in the Akita diabetic mice. The glomerular sclerosis and interstitial fibrosis caused by Akita diabetes are markedly enhanced by lack of B2R and by lack of both receptors at the age of 12 months, even though histological changes in the nondiabetic B2R-null or BRKO mice are not remarkable at this age (Figs. 2 and 3). Electron microscopy shows the thickening of the glomerular basement membrane and foot process effacement of podocytes caused by Akita diabetes. The thickening of basement membrane in the Akita diabetic mice is markedly enhanced by lack of B2R (approximately 2.9× normal) and by lack of both B1R and B2R (approximately 6.1× normal), although lack of the receptors in the absence of diabetes has little or no effect (Fig. 4). The effects on glomerular basement membrane thickening and podocyte effacement of combining the two mutations are more than additive, as are the albuminuria, glomerulosclerosis and interstitial fibrosis. Thus, the diabetic nephropathy in Akita mice is superadditively enhanced by lack of bradykinin receptors.
Fig. 1.
Urinary albumin excretion in 12-month-old male WT mice (n = 5), mice with a null mutation in the gene coding for B2R (B2R-null; n = 5), mice with a null mutation in the genes coding for B1R and B2R (BRKO; n = 5), mice heterozygous for the Akita mutation in the Ins2 gene (Akita; n = 5), B2R-null mice heterozygous for Akita mutation (B2R-null-Akita; n = 5), and BRKO mice heterozygous for Akita mutation (BRKO-Akita; n = 10). Absence of B2R or of both B1R and B2R increases urinary albumin excretion in Akita diabetic mice. Urinary albumin excretion is significantly higher in Akita mice lacking both B1R and B2R than in Akita mice lacking B2R. Data are presented as means ± SEs. P interaction (Methods) is the P value for interaction of the effects of bradykinin receptor genotypes and Akita mutation.
Fig. 2.
Histopathology of kidneys in 12-month-old male WT, B2R-null, BRKO, Akita, B2R-null-Akita, and BRKO-Akita mice. Absence of bradykinin receptors enhances renal histological changes in Akita diabetic mice. PAS staining of renal glomeruli and glomerulosclerosis index. Lack of bradykinin receptors increases diabetic glomerulosclerosis. (Scale bar, 100 μm.)
Fig. 3.
Gomori's trichrome staining of renal cortex and interstitial fibrosis score. Absence of bradykinin receptors increases the interstitial fibrosis in Akita diabetic mice. (Scale bar, 100 μm.)
Fig. 4.
Electron micrographs of glomeruli. Lack of bradykinin receptors enhances the increase in the thickness of basement membrane occurring in diabetic mice. (Scale bar, 1 μm.)
Effect on Mitochondrial Mutation of Lack of Bradykinin Receptors and Akita Diabetes.
The D-17 deletion in mitochondrial DNA is an index of DNA damage and has been shown to be increased by aging in mice (25). Fig. S1 shows that its incidence is also increased by Akita diabetes (approximately 5× normal) and by lack of B1R and B2R (approximately 2× normal). The increase in mitochondrial DNA deletion caused by Akita diabetes is enhanced by lack of the bradykinin receptors in a superadditive manner (approximately 12× normal; P of interaction < 0.001).
Effect of Lack of Bradykinin Receptors and Akita Diabetes on Renal Expression of Fibrogenic Genes.
Expression of transforming growth factor β1 (TGFβ1), connective tissue growth factor (CTGF) and endothelin-1 (EDN1) is known to be increased in diabetic nephropathy (26–28). Furthermore, in vivo overexpression of TGFβ1 (29), CTGF (30), or EDN1 (31) is associated with the development of nephrosclerosis (26–28), suggesting that overexpression of these genes contributes to the pathogenesis of diabetic nephropathy. Fig. S2 shows that the levels of mRNAs for these genes are greater in the kidney in BRKO Akita mice than in Akita mice at age 12 months and that this effect is also superadditive (Fig. S2). The increased incidence of mitochondrial mutations and in the expression of these fibrogenic genes is relevant to the enhanced diabetic nephropathy of the BRKO-Akita mice.
Lack of Bradykinin Receptors and Diabetes-Induced Osteoporosis.
Bone mineral loss is another serious complication of type I diabetes (32). Fig. 5 shows bone mineral density determined with dual emission x-ray absorptiometry (DEXA) at age 12 months. Both the Akita diabetic mice and the BRKO mice have densities very significantly less than WT mice. The BRKO-Akita mice have still lower bone mineral densities, although the effects of the two mutations are less than additive (P of negative interaction < 0.005).
Fig. 5.
Bone mineral density. Densities were assessed with dual emission x-ray absorptiometry in 12-month-old male WT (n = 5), Akita (n = 5), BRKO (n = 5), and BRKO Akita (n = 10) mice. Absence of bradykinin receptors decreases bone mineral density in Akita diabetic mice.
Lack of Bradykinin Receptors and Diabetic Neuropathy.
We have previously reported that Akita diabetic mice on a C57BL/6 genetic background are resistant to diabetic neuropathy, as judged by the minimal effects of Akita diabetes on latencies of heat sensation and nerve conduction velocity at age 24 weeks (33). Nevertheless, at the same age and on the same genetic background, the response times to a thermal stimulus (tail flick and hind paw withdrawal) are prolonged in the BRKO-Akita mice relative to the Akita, BRKO, or WT mice (Fig. 6). Likewise, nerve conductance velocities of the BRKO-Akita mice are less than in the other three genotypes (Fig. 6). Thus, concomitant absence of bradykinin receptors induces a neuropathy not otherwise observed in diabetic Akita mice.
Fig. 6.
Neurological status measurements made in 6-month-old male WT (n = 5), Akita (n = 8), BRKO (n = 8), and BRKO Akita (n = 10) mice. Hind paw, reaction time for hind paw withdrawal; Tail flick, reaction time for tail flick; sciatic motor NCV, sciatic motor nerve conduction velocity; sural NCV, sural (sensory) nerve conduction velocity. Absence of bradykinin receptors enhances neuropathy in Akita diabetic mice.
Discussion
Kinins and their derivatives generated from kininogens by kallikreins and other serine proteases compose the entire KKS. To date, two receptors for kinins have been identified in mammals: B1R and B2R. Whereas B2R is constitutively expressed, B1R is expressed at only low levels in normal tissues, but it is induced following tissue injury or after treatment with endotoxins or cytokines (34). Both B1R and B2R are overexpressed in ischemia and diabetes mellitus (19, 35). B1R is also markedly induced in the absence of B2R (19, 36), suggesting some functional redundancy between the two receptors.
Both the receptors are coupled with Gq-proteins (34), and their stimulation activates phosphatidylinositol-specific phospholipases, which elevates intracellular [Ca2+] and leads to activation of endothelial nitric oxide synthase (eNOS) (37, 38). The kinins, acting through B2R also increase the expression of inducible NOS (iNOS) (39). Our previous finding that preprandial levels of plasma nitrite/nitrate are lower in BRKO mice than in WT (23), and another report showing that urinary nitrite/nitrate excretion in B2R-null mice is lower than in WT (40) suggest that the KKS is also important in basal NO production.
NO reversibly suppresses mitochondrial oxidative metabolism (41), at least in part by inhibiting cytochrome c oxidase, which is a key enzyme in the electron transport chain (42). Kinins also facilitate the synthesis of prostanoids, including prostaglandin (PG) E2 and I2, which elevate intracellular cAMP levels (43). Recent studies have shown that cAMP decreases mitochondrial respiration by activating the NADH-ubiquinone oxidoreductase activity of complex I and by inhibiting cytochrome c oxidase (44, 45). Thus, it is likely that the KKS contributes to reducing oxidative stress via NO and PGs. In support of this possibility, we have shown that bradykinin reduces mitochondrial superoxide generation in human EA.hy926 vascular endothelial cells, an effect that is partly reversed by an NOS inhibitor (20). When bradykinin is administered to rats made hyperglycemic with streptozotocin (STZ), it also reduces their oxidative stress phenotype, as judged by hydrogen peroxide and malondialdehyde levels (46).
We have previously reported that absence of the predominant bradykinin receptor, B2R, exacerbates many of the pathologic consequences of diabetes (19) and induces premature aging (20). The interpretation of these findings with respect to the KKS is complicated by a marked increase in the expression of the other bradykinin receptor, B1R, which is normally expressed at low levels but is induced by absence of B2R, by diabetes, and by their combination.
In our present study, we observe that the pathological changes characteristic of Akita diabetes, including albuminuria, renal histopathology, bone mineral loss, and neuropathy, are all enhanced in the BRKO-Akita mice that lack both bradykinin receptors. The enhancement of albuminuria and renal histopathology in Akita mice caused by absence of both receptors is greater than that in Akita mice lacking only B2R, thus establishing that expression of B1R has beneficial effects in the B2R-Akita mice. The effects on any particular phenotype of combining the BRKO and Akita mutations may be simply additive, more than additive, or less than additive. But, even when the two mutations combine less than additively, the pathology observed in the BRKO-Akita mice is invariably worse than that in either the Akita or the BRKO animals. The renal diabetic phenotype (including albuminuria, interstitial fibrosis, and glomerular basement membrane thickening) is most sensitive to the BRKO-Akita combination, even though the BRKO mice exhibit minimal renal pathology.
The expression of TGFβ1, CTGF, and EDN1 is increased in diabetic nephropathy (26–28), and glomerulonephritis has been reported in albumin promoter-driven TGFβ1 transgenic mice, which have more than 10-fold TGFβ1 levels of controls (29). Transgenic expression in mice of EDN1 driven by its own promoter is associated with age-dependent development of glomerulosclerosis (31). Furthermore, in streptozotocin-induced diabetes, podocyte-specific CTGF overexpression causes enhanced proteinuria and glomerular mesangial expansion (30). Here we find that the renal expressions of TGFβ1, CTGF, and EDN1 are all increased in the mice made diabetic with the Akita mutation. The BRKO mutation also increases the expression of these genes when alone or when combined with the Akita mutation, and the BRKO-Akita combination of the two mutations increases the expression of TGFβ1 superadditively and that of CTGF and EDN1 additively. The increased expression of these fibrogenic genes probably contributes to the enhanced diabetic nephropathy we find in the BRKO-Akita mice.
Thus far, the effect of KKS on the neuropathy induced by long-lasting diabetes has been focused on the suppressive effect of B1R on diabetic hyperalgesia (47). However, another important aspect of diabetic neuropathy is the hypoalgesia caused by polyneuropathy in peripheral nerves, which can lead to lower limb amputations that have a major impact on the quality of life and disability of diabetic patients (48). In the present study, we find that response times to thermal stimuli are increased in the BRKO-Akita mice relative to either the simply diabetic Akita mice or the BRKO mice. Nerve conduction velocities of the BRKO-Akita mice are also decreased by the combination of diabetes and absence of the bradykinin receptors, in this case more than additively, thus demonstrating the importance of the KKS in minimizing the neurological effects of diabetes.
Osteoporosis is a well established manifestation of aging. It is also one of complications in type I diabetes (32), although the mechanisms leading to this osteoporosis are not clear. We find that lack of both bradykinin receptors results in a severe reduction in bone mineral density both in Akita diabetic and nondiabetic mice, demonstrating the importance of the KKS in bone mineralization. However, although osteoblasts express both B1R and B2R, their stimulation with bradykinin increases expression of the receptor activator for NF-κB ligand, which is known to be involved in osteoclastogenesis (49). This in vitro observation consequently suggests that acute stimulation of KKS can cause bone resorption. In contrast to these results with cultured cells, we find that lack of either B2R (20) or of both B1R and B2R decreases bone mineral density in living mice. The reason for the discrepancy between the results of the in vitro experiments and our current in vivo experiments is unclear, although the concentration of bradykinin used in the tissue culture system (3 μM) is much greater than that encountered in vivo (<1 pM) (50).
Several of our findings are relevant to the mechanism underlying the effects of an impaired KKS on the complications induced by diabetes. The first finding is that BRKO mice are insulin resistant, which is in agreement with a previous report that B2R-null mice are insulin-resistant (51). Thus the KKS may play an important role in insulin sensitivity in mice. When the Akita mutation is added to the BRKO mutation, the development of high insulin levels is prevented, consequently the two mutations interact negatively on insulin levels in the BRKO-Akita mice. In contrast, the increase in plasma TBARSs caused by the BRKO mutation is additively increased by the Akita mutation. Finding increased oxidative stress, detected as the increase in plasma TBARSs, suggests that oxidative stress is a second factor leading to the pathological changes that are exhibited by these mice.
An additional factor that impinges on the pathology of the BRKO-Akita mice, particularly the renal pathology, is a marked increase in renal mitochondrial damage. The combined enhancing effects of the BRKO mutation on the increased renal mitochondrial damage and increased renal expression of fibrogenic genes already caused by diabetes are likely explanations for our finding that the kidney is particularly sensitive to the BRKO mutation.
In conclusion, absence of both bradykinin receptors enhances the nephropathy, neuropathy, and bone mineral loss caused by insulin-dependent diabetes in mice, together with increased oxidative stress, mitochondrial mutations, and expression of fibrogenic genes. These results demonstrate that B1R and B2R moderate the development of complications in diabetic mice and suggest that activation of KKS could be beneficial in reducing the severity of complications in diabetic patients, particularly those carrying the D allele of the ACE gene.
Methods
Animals.
Mice lacking B2R gene or having the diabetogenic Akita mutation (C96Y) in the insulin 2 gene of the C57BL/6 strain were purchased from Jackson Laboratory. Mice lacking both B1R and B2R of a pure C57BL/6 background were generated as previously described (23). All experiments were approved by the University of North Carolina Institutional Animal Care and Use Committee. Bone mineral densities in femurs were measured with DEXA (LUNAR PIXImus2; GE Healthcare) in the Body Composition Core of the Clinical Nutrition Research Center at the University of North Carolina. Systolic blood pressures and pulse rates were measured with the tail-cuff method (52).
Measurement of Biochemical Parameters.
Mice were anesthetized with 2.5% isoflurane, and blood was collected from retro-orbital sinuses with heparinized glass pipettes (Fisher Scientific). The samples were centrifuged (7,000 g for 5 min) to separate plasma. Plasma samples were frozen and stored at –80 °C before analysis for biochemical parameters. Plasma glucose levels were determined with the glucose oxidase method (Wako Chemical USA, Inc.). Plasma insulin levels were determined with ELISA (Crystal Chem Inc.). Plasma urea nitrogen and creatinine concentrations were determined with the Vitros 250 Chemistry system (Ortho-Clinical Diagnostics). Plasma total cholesterol (Wako) and triglyceride (Stanbio Laboratory) were measured with enzymatic colorimetric methods. Plasma levels of TBARSs were determined as described (53). Urinary albumin was determined with ELISA (Albuwell M; Exocell).
Sensory Testing, Tail Flick, and Hind Paw Withdrawal.
Thermal sensitivities were determined with an analgesia apparatus (Model 336TG; Life Sciences) as previously described (33). Tail flick responses were elicited with an adjustable red light emitter (range 60–170 °C), and the time for the animal to respond was recorded electronically. For hind paw withdrawal times, the mice were placed in compartments on a warm (32 °C) glass plate and allowed to habituate for 10 min. The light source was maneuvered under the hind paw, and the time to paw withdrawal was recorded. The light source was set at 25 °C and the temperature increased to 70 °C over the course of 10 s. A threshold of 10 s was applied to prevent injury to the mice.
Measurement of Nerve Conduction Velocity.
Sciatic motor nerve conduction velocity (SMNCV) and sural nerve conduction velocity (Sural NCV) were assessed as previously described (54). Briefly, animals were anesthetized with 30/2.5 mg/kg i.p. ketamine:xylazine. Hind limb skin temperature was monitored using a thermistor and was maintained at approximately 34 °C with a warming lamp. SMNCV was recorded by stimulating proximally at the sciatic notch and distally at the ankle. Sural NCV was determined by stimulating the sural nerve distally at the ankle and recording at the fourth and fifth digit. Conduction velocity was calculated using the onset latency and distance.
Histological Evaluation.
The left kidney and heart were fixed with 4% (wt/vol) paraformaldehyde. Tissues were embedded in paraffin, stained with PAS reagent and hematoxylin or with Gomori's Trichrome reagent, and examined under an optical microscope. Renal tissue was also examined under an electron microscope after fixation with 15% (wt/vol) glutalaldehyde. The glomerulosclerosis index and interstitial fibrosis score were evaluated in a blind fashion as previously described (55, 56).
Quantitative RT-PCR.
Total RNA was extracted from the kidney and heart. mRNAs for TGFβ1, CTGF, and EDN1 were assayed by quantitative reverse transcription-PCR as previously described (20).
Quantification of Mitochondrial DNA Deletion Mutants.
Large deletions between two homologous sequences in mtDNA have been reported in mice (25). We assayed the most common deletion mutation, D-17, in renal mtDNA samples using quantitative PCR with primers flanking the D-17 deletion and with the template mtDNAs cut at an Mlu I site present in the region deleted in D-17 as previously described (20).
Statistical Analysis.
Data are expressed as means ± SEs. To compare groups, we used the two-way ANOVA by least-square fit to determine the significance of the effects of and interactions between the two categorical parameters: bradykinin receptor genotype (WT, B2R-null, or BRKO) and the Akita heterozygous mutation in the Ins2 gene (Figs. 1–6, Figs. S1 and S2, and Table S1). Posthoc pairwise comparisons were by the Student's t test (JMP 6.0.0; SAS Institute Inc.).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK76160 to E.L.F., DK76131 and HL49277 to O.S., DK56350 to University of North Carolina Nutrition Obesity Research Center, and DK34987 to University of North Carolina Center for Gastrointestinal Biology and Diseases and by Career Development Award 2006-2-106 from Juvenile Diabetes Research Foundation International to M.K.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005144107/-/DCSupplemental.
References
- 1.Skidgel RA, Erdös EG. The broad substrate specificity of human angiotensin I converting enzyme. Clin Exp Hypertens A. 1987;9:243–259. doi: 10.3109/10641968709164184. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- 3.Smithies O, Kim HS, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 4.Rigat B, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachurié ML, Azizi M, Guyene TT, Alhenc-Gelas F, Ménard J. Angiotensin-converting enzyme gene polymorphism has no influence on the circulating renin-angiotensin-aldosterone system or blood pressure in normotensive subjects. Circulation. 1995;91:2933–2942. doi: 10.1161/01.cir.91.12.2933. [DOI] [PubMed] [Google Scholar]
- 6.Marre M, et al. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43:384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 7.Stephens JW, Dhamrait SS, Acharya J, Humphries SE, Hurel SJ. A common variant in the ACE gene is associated with peripheral neuropathy in women with type 2 diabetes mellitus. J Diabetes Complications. 2006;20:317–321. doi: 10.1016/j.jdiacomp.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Rabensteiner D, et al. ACE gene polymorphism and proliferative retinopathy in type 1 diabetes: Results of a case-control study. Diabetes Care. 1999;22:1530–1535. doi: 10.2337/diacare.22.9.1530. [DOI] [PubMed] [Google Scholar]
- 9.Cambien F, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 10.Doi Y, et al. Polymorphism of the angiotensin-converting enzyme (ACE) gene in patients with thrombotic brain infarction. Atherosclerosis. 1997;132:145–150. doi: 10.1016/s0021-9150(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 11.Sanada M, et al. Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis. 2004;176:387–392. doi: 10.1016/j.atherosclerosis.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD The Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 13.Malik RA, et al. Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: Randomised double-blind controlled trial. Lancet. 1998;352:1978–1981. doi: 10.1016/S0140-6736(98)02478-7. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Z, Chen H, Xu X, Li C, Gu Q. Effects of angiotensin-converting enzyme inhibitors and beta-adrenergic blockers on retinal vascular endothelial growth factor expression in rat diabetic retinopathy. Exp Eye Res. 2007;84:745–752. doi: 10.1016/j.exer.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Castrillón JL, et al. Effect of quinapril, quinapril-hydrochlorothiazide, and enalapril on the bone mass of hypertensive subjects: Relationship with angiotensin converting enzyme polymorphisms. Am J Hypertens. 2003;16:453–459. doi: 10.1016/s0895-7061(03)00845-8. [DOI] [PubMed] [Google Scholar]
- 16.Lynn H, Kwok T, Wong SY, Woo J, Leung PC. Angiotensin converting enzyme inhibitor use is associated with higher bone mineral density in elderly Chinese. Bone. 2006;38:584–588. doi: 10.1016/j.bone.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Mancini GB, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 18.Bodin S, et al. Kallikrein protects against microalbuminuria in experimental type I diabetes. Kidney Int. 2009;76:395–403. doi: 10.1038/ki.2009.208. [DOI] [PubMed] [Google Scholar]
- 19.Kakoki M, Takahashi N, Jennette JC, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci USA. 2004;101:13302–13305. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakoki M, et al. Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest. 2006;116:1302–1309. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagneux C, Bader M, Pesquero JB, Demenge P, Ribuot C. Detrimental implication of B1 receptors in myocardial ischemia: Evidence from pharmacological blockade and gene knockout mice. Int Immunopharmacol. 2002;2:815–822. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, et al. Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension. 2005;45:747–753. doi: 10.1161/01.HYP.0000153322.04859.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakoki M, McGarrah RW, Kim HS, Smithies O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2007;104:7576–7581. doi: 10.1073/pnas.0701617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cayla C, et al. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21:1689–1698. doi: 10.1096/fj.06-7175com. [DOI] [PubMed] [Google Scholar]
- 25.Tanhauser SM, Laipis PJ. Multiple deletions are detectable in mitochondrial DNA of aging mice. J Biol Chem. 1995;270:24769–24775. doi: 10.1074/jbc.270.42.24769. [DOI] [PubMed] [Google Scholar]
- 26.Riser BL, et al. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minchenko AG, et al. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson N, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoi H, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 31.Hocher B, et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuominen JT, Impivaara O, Puukka P, Rönnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan KA, et al. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couture R, Girolami JP. Putative roles of kinin receptors in the therapeutic effects of angiotensin 1-converting enzyme inhibitors in diabetes mellitus. Eur J Pharmacol. 2004;500:467–485. doi: 10.1016/j.ejphar.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Spillmann F, et al. Regulation of cardiac bradykinin B1- and B2-receptor mRNA in experimental ischemic, diabetic, and pressure-overload-induced cardiomyopathy. Int Immunopharmacol. 2002;2:1823–1832. doi: 10.1016/s1567-5769(02)00174-1. [DOI] [PubMed] [Google Scholar]
- 36.Duka I, et al. Vasoactive potential of the b(1) bradykinin receptor in normotension and hypertension. Circ Res. 2001;88:275–281. doi: 10.1161/01.res.88.3.275. [DOI] [PubMed] [Google Scholar]
- 37.Drummond GR, Cocks TM. Endothelium-dependent relaxations mediated by inducible B1 and constitutive B2 kinin receptors in the bovine isolated coronary artery. Br J Pharmacol. 1995;116:2473–2481. doi: 10.1111/j.1476-5381.1995.tb15098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangsree S, Brovkovych V, Minshall RD, Skidgel RA. Kininase I-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin B1 receptor agonists. Am J Physiol Heart Circ Physiol. 2003;284:H1959–H1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- 39.Savard M, et al. Expression of endogenous nuclear bradykinin B2 receptors mediating signaling in immediate early gene activation. J Cell Physiol. 2008;216:234–244. doi: 10.1002/jcp.21398. [DOI] [PubMed] [Google Scholar]
- 40.Schanstra JP, et al. Decreased renal NO excretion and reduced glomerular tuft area in mice lacking the bradykinin B2 receptor. Am J Physiol Heart Circ Physiol. 2003;284:H1904–H1908. doi: 10.1152/ajpheart.01150.2002. [DOI] [PubMed] [Google Scholar]
- 41.Brown GC, Bolaños JP, Heales SJ, Clark JB. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 42.Brunori M, et al. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Kakoki M, Smithies O. The kallikrein-kinin system in health and in diseases of the kidney. Kidney Int. 2009;75:1019–1030. doi: 10.1038/ki.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee I, et al. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 45.Piccoli C, et al. cAMP controls oxygen metabolism in mammalian cells. FEBS Lett. 2006;580:4539–4543. doi: 10.1016/j.febslet.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 46.Mikrut K, et al. The effect of bradykinin on the oxidative state of rats with acute hyperglycaemia. Diabetes Res Clin Pract. 2001;51:79–85. doi: 10.1016/s0168-8227(00)00222-9. [DOI] [PubMed] [Google Scholar]
- 47.Gabra BH, Berthiaume N, Sirois P, Nantel F, Battistini B. The kinin system mediates hyperalgesia through the inducible bradykinin B1 receptor subtype: Evidence in various experimental animal models of type 1 and type 2 diabetic neuropathy. Biol Chem. 2006;387:127–143. doi: 10.1515/BC.2006.018. [DOI] [PubMed] [Google Scholar]
- 48.Vileikyte L, et al. Diabetic peripheral neuropathy and depressive symptoms: The association revisited. Diabetes Care. 2005;28:2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 49.Brechter AB, Lerner UH. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum. 2007;56:910–923. doi: 10.1002/art.22445. [DOI] [PubMed] [Google Scholar]
- 50.Alexiou T, et al. Angiotensinogen and angiotensin-converting enzyme gene copy number and angiotensin and bradykinin peptide levels in mice. J Hypertens. 2005;23:945–954. doi: 10.1097/01.hjh.0000166834.32817.41. [DOI] [PubMed] [Google Scholar]
- 51.Duka I, et al. Role of the B(2) receptor of bradykinin in insulin sensitivity. Hypertension. 2001;38:1355–1360. doi: 10.1161/hy1201.096574. [DOI] [PubMed] [Google Scholar]
- 52.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 53.Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med. 2001;31:331–335. doi: 10.1016/s0891-5849(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 54.Wiggin TD, et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58:1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saito T, Sumithran E, Glasgow EF, Atkins RC. The enhancement of aminonucleoside nephrosis by the co-administration of protamine. Kidney Int. 1987;32:691–699. doi: 10.1038/ki.1987.262. [DOI] [PubMed] [Google Scholar]
- 56.Pichler RH, et al. Pathogenesis of cyclosporine nephropathy: Roles of angiotensin II and osteopontin. J Am Soc Nephrol. 1995;6:1186–1196. doi: 10.1681/ASN.V641186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.