Abstract
The ε4 allele of the apolipoprotein E (APOE) gene is the major genetic risk factor for Alzheimer’s disease (AD), but limited work has suggested that APOE genotype may modulate disease phenotype. Carriers of the ε4 allele have been reported to have greater medial temporal lobe (MTL) pathology and poorer memory than noncarriers. Less attention has focused on whether there are domains of cognition and neuroanatomical regions more affected in noncarriers. Further, a major potential confound of prior in vivo studies is the possibility of different rates of clinical misdiagnosis for carriers vs. noncarriers. We compared phenotypic differences in cognition and topography of regional cortical atrophy of ε4 carriers (n = 67) vs. noncarriers (n = 24) with mild AD from the Alzheimer’s Disease Neuroimaging Initiative, restricted to those with a cerebrospinal fluid (CSF) molecular profile consistent with AD. Between-group comparisons were made for psychometric tests and morphometric measures of cortical thickness and hippocampal volume. Carriers displayed significantly greater impairment on measures of memory retention, whereas noncarriers were more impaired on tests of working memory, executive control, and lexical access. Consistent with this cognitive dissociation, carriers exhibited greater MTL atrophy, whereas noncarriers had greater frontoparietal atrophy. Performance deficits in particular cognitive domains were associated with disproportionate regional brain atrophy within nodes of cortical networks thought to subserve these cognitive processes. These convergent cognitive and neuroanatomic findings in individuals with a CSF molecular profile consistent with AD support the hypothesis that APOE genotype modulates the clinical phenotype of AD through influence on specific large-scale brain networks.
Keywords: cognition, neuroimaging, dementia, cortical thickness, medial temporal lobe
Prototypically, Alzheimer’s disease (AD) presents clinically as a syndrome involving insidiously progressive episodic memory deficits accompanied by progressive impairment in several other cognitive domains, including executive functioning, language, visuospatial function, and praxis (1). This presentation reflects pathologic alterations within critical nodes of the large-scale neural network subserving episodic memory as well as alterations within other brain networks (2, 3). Although the prevailing view of AD as predominantly an episodic memory disorder is well supported (4), there are many clear examples of clinical and pathological heterogeneity (5–9). Although much of this work has focused on atypical focal presentations, such as visual variant of AD/posterior cortical atrophy (6), progressive aphasia (7, 10), or the executive variant of AD (5), phenotypic heterogeneity has been found even in less-selected AD populations (8, 9). Despite its potential importance for diagnosis, intervention, disease monitoring and, ultimately, our understanding of disease pathophysiology, there are surprisingly few data regarding potential genetic or environmental factors that may underlie clinical or pathologic heterogeneity in AD.
Apolipoprotein E (APOE) is the major genetic risk factor for AD. This gene on chromosome 19, which codes for a lipid transport protein, has three major alleles (ε2, ε3, and ε4). Carriers of at least one ε4 allele have an increased risk of developing AD, as well as an associated dose-related decrease in age of onset (11, 12). The mechanism by which this protein exerts its modulatory effect on AD remains unclear and may be related, among other hypotheses, to its function in cell membrane maintenance and repair, its effect on amyloid β (Aβ) deposition and clearance, and/or a potential regulatory role for tau phosphorylation (13–15).
Although APOE clearly affects disease risk, controversy exists as to whether APOE allelic variants are consistently associated with phenotypic variants of AD. Autopsy and amyloid imaging studies have reported greater Aβ plaque deposition in carriers of the ε4 allele, even after controlling for disease severity (16). Quantitative neuroimaging investigations have reported greater medial temporal lobe (MTL) atrophy, particularly involving the hippocampus, in AD patients who are ε4 carriers vs. noncarriers (17–21), although this has not been a universal finding (22, 23). Conflicting results have also been reported from the limited investigations of cortical anatomy, with some studies reporting no difference and others reporting more robust regional atrophy in ε4 carriers (20, 23). Finally, there are a few reports of greater atrophy in noncarriers either in select regions, such as the frontal lobe, or in global measures of brain volume (17, 19–21).
Data regarding APOE-related differences in the cognitive phenotype of AD have been similarly variable. Perhaps the most consistent finding is the presence of greater impairment of delayed recall on episodic memory tasks in ε4 carriers relative to noncarriers (24–27), although, again, conflicting results have been reported (17, 18). Findings are inconsistent regarding whether there are domains of greater cognitive impairment in noncarriers relative to carriers, with some indication that noncarriers may display greater difficulty on tasks of attention, executive, or verbal functions (17, 25, 26).
In addition to the presence of conflicting data in the literature, there are some important gaps. First, given the limited specificity of standard diagnostic approaches (28) and the potential for different rates of misdiagnosis in ε4 carriers vs. noncarriers, it is possible that some discrepant findings may be explained by the inclusion of patients with non-AD dementias. New in vivo biomarkers can be used to increase the likelihood that putative AD patient samples actually harbor AD neuropathology (29, 30). Second, although there have been previous investigations of cognitive and neuroanatomic differences between AD patients who are APOE ε4 carriers vs. noncarriers, no prior work has brought these lines of research together to identify an underlying neuroanatomic basis of these genetically influenced differences in the cognitive phenotype of AD.
In the present study, we investigated cognitive and neuroanatomic phenotypic variability between patients with mild AD who carry at least one APOE ε4 allele (“carriers”) vs. those who do not (“noncarriers”). To mitigate against concerns of misdiagnosis, this group of patients was restricted to those with a cerebrospinal fluid (CSF) molecular profile consistent with pathological AD. In addition to exploratory analyses, we used a hypothesis-driven approach to the measurement of neuroanatomic differences between APOE ε4 carriers and noncarriers, using a set of “cortical signature of AD” regions of interest (ROIs) previously defined from a separate sample of AD patients (31). We hypothesized that carriers would express more atrophy in cortical regions that are part of the episodic memory network, whereas noncarriers would express more atrophy in cortical regions that are part of networks subserving complex attention and executive function (32).
Results
Demographic and General Clinical Data.
Remarkably, despite ε4 carrier status being associated with an earlier age of onset, carriers and noncarriers did not differ in age [t(89) < 1.0] (Table 1); nor did the groups differ on global measures of disease severity [Mini-Mental State Examination (MMSE): t(89) < 1.0; Clinical Dementia Rating Scale Sum of Boxes (CDR-SB): t(89) = 1.5, P > 0.1]. Noncarriers were generally more educated than carriers [t(89) = 2.1, P < 0.05]. Of the carriers, 44 (65.7%) had one ε4 allele, whereas 23 (34.3%) had two. Finally, the t-tau/Aβ1–42 ratio did not differ between the groups [t(89) < 1.0].
Table 1.
Demographic and psychometric data of AD patients based on APOE carrier status
| Variable | APOE ε4 carriers (n = 67) | APOE ε4 noncarriers (n = 24) |
| Age (yr) | 74.9 (9.2) | 74.3 (7.3) |
| Gender, n (male/female) | 38/29 | 13/11 |
| t-tau/Aβ1–42 | 0.97 (0.42) | 1.04 (0.44) |
| Formal education (yr) | 14.8 (3.3) | 16.4 (2.9)* |
| MMSE | 23.5 (1.9) | 23.1 (1.9) |
| CDR-SB | 4.4 (1.6) | 3.9 (1.3) |
| AVLT trial 1 recall | 3.76 (1.4) | 3.08 (1.7)† |
| AVLT trial 5 recall | 5.39 (1.9) | 5.70 (3.07) |
| AVLT 5-min delayed recall | 1.80 (1.9) | 2.26 (2.8) |
| AVLT 30-min delayed recall | 0.64 (1.5) | 2.00 (2.8) * |
| AVLT percent retention | 8.8 (18.4) | 28.8 (36.0)‡ |
| AVLT recognition (d′) | 0.66 (0.62) | 1.19 (0.85)‡ |
| Digit Span Forward | 6.24 (1.0) | 6.25 (1.2) |
| Digit Span Backward | 3.97 (1.0) | 3.54 (1.2)* |
| Trails A (sec) | 65.5 (37.7) | 85.0 (43.4)* |
| Trails B (sec) | 193.5 (86.5) | 232.2 (85.2)* |
| Category fluency test | 21.5 (7.1) | 19.6 (8.2) |
| Digit Symbol | 27.9 (12.4) | 24.1 (13.4) |
| BNT | 23.4 (5.5) | 21.29 (7.7)‡ |
Values are presented as mean (SD) except where noted. Note that data were missing for four patients for Trails B, two patients for Digit Symbol and AVLT 5-min delayed recall, and one patient for BNT, Digit Span Backward, and AVLT trial 5 recall and percent retention.
*P < 0.05.
†P = 0.06.
‡P < 0.01.
Differences in Memory Performance.
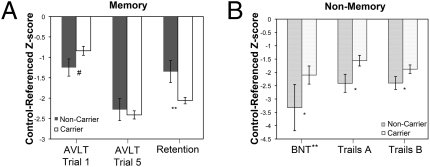
An interesting profile of memory performance distinguished the two groups (Fig. 1 and Table 1). On the first immediate recall trial of the Auditory Verbal Learning Test (AVLT), noncarriers showed a statistical trend toward poorer performance than the carriers [F(1, 86) = 3.66, P = 0.06]. However, by the fifth immediate recall trial the groups did not differ [F(1, 85) < 0.01], suggesting relatively equivalent overall learning. In stark contrast, 30-min delayed recall was markedly poorer in the carrier group [F(1, 86) = 6.8, P = 0.05]. Two measures thought to be particularly specific to temporo-limbic memory dysfunction, recognition memory (d′) and memory retention [percent retention = (delayed recall/trial 5 immediate recall) × 100] were also more impaired in the carriers [d′: F(1, 86) = 9.3, P < 0.01; percent retention: F(1, 85) = 9.7, P < 0.01].
Fig. 1.
Cognitive profile differences of APOE carriers versus noncarriers. Between-group comparison of psychometric performance on (A) memory and (B) nonmemory measures, presented as ADNI control-referenced z scores (Trails A/B and BNT were natural log and square root transformed, respectively). Error bars represent ±SEM; #, P = 0.06, *, P < 0.05, **, P < 0.01 for ANCOVA models.
Differences in Nonmemory Performance.
In the nonmemory domains, there was a general pattern of subtly poorer performance in the noncarriers, with several measures reaching statistical significance (Fig. 1 and Table 1). Noncarriers performed less well than carriers on Trails A [F(1, 86) = 4.6, P < 0.05] and Trails B [F(1, 82) = 7.0, P < 0.05], measures usually thought to represent visuomotor speed and executive function (sequencing), respectively. Backward Digit Span, another test of executive function, was also poorer in the noncarriers [F(1, 85) = 4.8, P < 0.05]. In addition, noncarriers displayed poorer naming on the 30-item Boston Naming Test (BNT) [F(1, 85) = 7.4, P < 0.01.]. None of the other measures reached statistical significance.
Differences in Regional Cortical Atrophy.
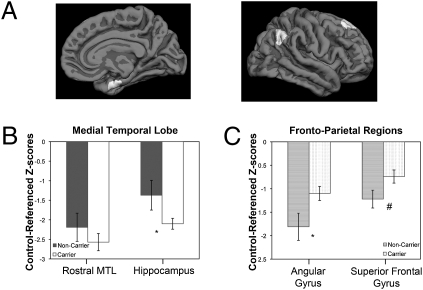
For the between-group ROI analysis, covariates of age and disease severity (CDR-SB) were applied. All ROI values were converted to z scores on the basis of morphometric measures of the control cohort in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset. The carriers displayed reduced cortical thickness in the MTL and had smaller hippocampal volume than the noncarriers. However, only the latter comparison reached statistical significance [F(1, 65) = 7.1, P < 0.01] (Fig. 2). In contrast, almost all other ROIs and total mean cortical thickness were reduced in the noncarriers (Table S1). This difference reached statistical significance in several regions, including the superior parietal lobule [F(1, 65) = 4.2, P < 0.05], precuneus [F(1, 65) = 4.7, P < 0.05], and angular gyrus [F(1, 65) = 6.3, P < 0.05]. Additionally, the superior frontal gyrus [F(1, 65) = 3.5, P = 0.07] and overall mean cortical thickness [F(1, 65) = 3.8, P = 0.06] approached significance.
Fig. 2.
Atrophy pattern profile differences of APOE carriers versus noncarriers. Between-group comparison of morphometric values for specific ROIs, including (B) MTL ROIs (rostral medial temporal cortex and hippocampus) and (C) isocortical ROIs (angular gyrus and superior frontal gyrus). Error bars represent ±SEM. Cortical surface inset illustrates localization of ROIs (A); #, P = 0.07, *, P < 0.05 for ANCOVA models.
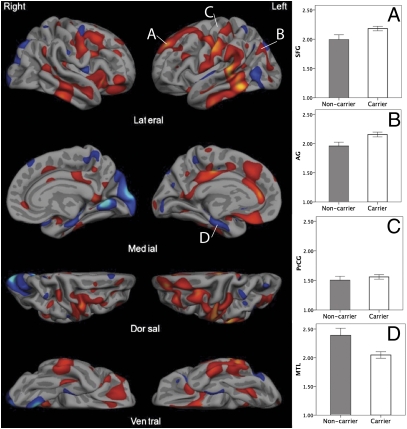
In addition to the ROI analysis, an exploratory between-group comparison was performed across the entire cortical mantle (Fig. 3). Consistent with the above findings, a broad set of superior and midline parietal and dorsolateral frontal regions, along with lateral temporal regions, were thinner in the noncarrier group. Carriers showed more prominent thinning in medial temporal and caudal temporal and occipital regions relative to the noncarrier group. Note that because this analysis only interrogates thickness of the cortex, the hippocampus itself is not included.
Fig. 3.
Exploratory analysis across the entire cerebral cortex of regions in which mild AD ε4 carriers demonstrated thinner cortex than noncarriers (blue) and in which noncarriers demonstrated thinner cortex than carriers (red-yellow). Map is thresholded at P < 0.1 to illustrate relatively subtle effects. Bar graphs illustrate group differences in (A) superior frontal gyrus, (B) angular gyrus, and (D) MTL defined on these maps. The precentral gyrus (C) is included as a control region to illustrate similarities between groups. Error bars represent ±SEM.
Regional Cortical Atrophy–Behavioral Relationships.
The above analyses suggest that ε4 carrier status modulates both the cognitive and neuroanatomic phenotype of AD. To better understand how the cognitive differences relate to the underlying neuroanatomy, a series of stepwise regressions were performed. In each case the dependent variable was one of the cognitive measures that statistically differed between the two groups (AVLT trial 1 recall, AVLT 30-min delayed recall, AVLT recognition memory, BNT, Backward Digit Span, Trails A, and Trails B), and the independent variables were the above ROIs. To avoid the influence of APOE-genotype group differences driving these correlations, APOE status was entered first as a covariate in the model, along with age and years of formal education. In each case, the best model included one ROI. In the case of tasks performed less well by the carriers (30-min delayed recall, recognition memory), hippocampus (β = 0.42, P < 0.01) and MTL (β = 0.25, P < 0.05) were retained in the respective models. In contrast, non-MTL structures correlated with performance on the cognitive tests in which the noncarriers performed less well. For BNT, the best model included inferior frontal sulcus (β = 0.32, P < 0.01), although there was also a trend for temporal pole in this model (β = 0.22, P = 0.09). For Trails A, Trails B, and AVLT trial 1 recall, inclusion of angular gyrus produced the best models (β = −0.38, P < 0.01; β = −0.46, P < 0.001; and β = 0.38, P < 0.01, respectively). No ROIs were included in the Backward Digit Span regression.
Discussion
APOE genotype seems to modify not only the risk for AD but also the cognitive and neuroanatomic phenotype of the disease. With respect to cognition, ε4 carriers in this cohort were impaired to a greater extent than noncarriers on specific episodic memory measures usually considered to be dependent on the MTL memory system. In contrast, noncarriers displayed greater impairment in cognitive processes requiring sustained attention, working memory, executive function, and lexical access. The two groups demonstrated anatomic dissociations consistent with these cognitive differences: ε4 carrier AD patients exhibited more prominent MTL atrophy, whereas noncarriers expressed more robust atrophy in a broad set of cortical regions, including some that subserve the nonmnemonic cognitive functions in which they were more impaired.
Perhaps the most important advance of the present work relative to prior in vivo studies is that our cohort was restricted to a relatively large sample of mild AD patients with a CSF molecular profile consistent with AD based on a previously established cutoff with high diagnostic accuracy for autopsy-determined cases of dementia (30). Although the pathologic diagnosis cannot be confirmed in the present cohort, the inclusion of only patients with a CSF t-tau/Aβ1–42 in the “AD range” should significantly mitigate the concern of misdiagnosis that may be present in samples defined on a purely clinical basis. For example, a reasonable criticism of prior work demonstrating poorer memory and greater hippocampal atrophy in ε4 carriers relative to noncarriers is that the former group is associated with a greater proportion of patients with true pathologic AD, whereas the latter group may contain more individuals with non-AD pathologies, which may be expected to affect memory and MTL structures less prominently. Restriction of the cohort in this manner may have excluded some patients with pathological AD given the imperfect sensitivity of the test (30); however, this minority of AD patients is unlikely to significantly alter the present results unless it is postulated that APOE genotype would have a differential effect on these patients relative to those with an AD CSF profile.
Modulatory Influence of APOE on Expression of AD.
The present findings echo threads of some of the extant literature. Several studies have reported that ε4 carriers display poorer episodic memory (24–27) and smaller hippocampal or other MTL volumes (17–21, 33) than noncarriers. Within the present CSF-restricted sample in which carriers and noncarriers were well matched for age and mild disease severity, we observed similar findings.
It stands to reason that if carriers and noncarriers are indeed well matched for disease severity, but ε4 carriers have poorer memory, that noncarriers should display greater impairment in nonmemory cognitive domains. Although several studies have not found psychometric measures favoring carriers (18, 20, 27), a few reports accord well with the present data. For example, van der Vlies et al. (25) reported remarkably similar findings in which noncarriers performed more poorly than carriers on an object naming task and parts A and B of the Trail Making Test. Two additional reports of poorer scores in noncarriers on measures of attention/concentration, performance intelligence quotient, and other verbal tasks, including naming, provide further support for this differential pattern of cognitive impairment (17, 26).
APOE ε4 noncarriers displayed greater cortical thinning in frontoparietal regions that form nodes of two interacting networks that have been referred to as the “dorsal attention network” and the “frontoparietal control” system (32, 34). Although these networks may index partially dissociable cognitive operations, a variety of working memory and complex attention tasks are associated with activation in both systems. As would be predicted by such involvement, noncarriers performed most poorly on tasks that depend on these processes. Although almost all prior studies examining the role of APOE genotype on structural imaging alterations have examined a few select regions, usually focused on MTL structures or measures of whole brain volume (17, 33), Pievani et al. (18) recently reported data from a survey of the entire cortical mantle that produced anatomic results similar to those of the present work. Consistent with our findings, they reported greater dorsal fronto-parietal atrophy in the noncarrier group but did not find concomitant psychometric differences, possibly owing to small sample size. In contrast, another recent study surveying the whole brain using voxel-based morphometry identified relatively more prominent MTL atrophy in carriers but did not find areas where gray matter volume was greater in ε4 carriers than noncarriers (35). Finally, two older studies of select regions reported evidence of smaller frontal lobe volumes in noncarriers relative to carriers (19, 21), but again with no psychometric differences, likely owing to small sample size.
One implication of the present findings is that the clinical phenotype of AD reflects an amalgamation of relatively selective regional brain pathology, the distribution of which may be influenced in part by APOE genotype. In previous work, we demonstrated that a “signature” set of localized cortical regions is atrophied consistently across multiple samples of very mild and mild clinically defined AD patients (31, 36). Although these regions seem to be affected in a generalizable way across AD patients, the present work suggests that there is variability in the degree to which these regions are affected and that one factor influencing the relative balance of regional atrophy is APOE genotype. As in other neurodegenerative diseases with heterogeneity of clinico-pathologic relationships, such as frontotemporal dementia, the cognitive phenotype seems to reflect the topographic distribution and severity of regional brain pathology rather than the type of pathology (37). Yet, as demonstrated in this study and another recent investigation (20), molecular influences driven at least in part by genetic variants dictate the degree to which distinct brain systems are affected by a class of neuropathology. Of course, other genetic and nongenetic factors may further influence the cognitive profile and topography of brain involvement in AD, including education, cerebrovascular disease, and other comorbid medical conditions, and, perhaps, individual differences in premorbid cognitive capacities.
Differential Effects of APOE on Distinct Memory Processes.
Episodic memory, often thought of as a monolithic process, perhaps best illustrates this amalgamation in that both groups displayed impaired memory but in qualitatively different ways. The ability to learn from repetition on a supraspan list learning task such as the AVLT is likely dependent on auditory–verbal working memory, strategic–control processes for elaborative encoding, and the transfer of this information into the long-term store (38). Although these processes have all been shown to be impaired in AD (39, 40), there have been few demonstrations of subgroups of AD patients with dissociated deficits in these component processes of memory (41); for example, one recent comprehensive investigation reported that some AD patients present with more prominent amnesic deficits on tests of delayed recall and recognition, whereas others present with more prominent working memory deficits (42). In the present study, despite the poorer delayed memory of ε4 carriers, the noncarriers actually performed less well on the AVLT initial learning trial. The first immediate recall trial of a supraspan word list learning task is probably more dependent on auditory–verbal working memory (“short-term memory”) and possibly executive–strategic processes that might be used during encoding rather than specific episodic (“long-term”) memory processes. Consistent with this notion, impairment on this measure did not correlate with MTL atrophy but rather was most strongly associated with atrophy of the angular gyrus in the posterior parietal cortex/inferior parietal lobule. This localization is congruent with findings from lesion studies of auditory–verbal short-term memory impairment (43, 44) and current concepts from functional neuroimaging regarding the phonologic store in working memory (45).
In contrast, despite equivalent learning after repetition as measured by the fifth immediate recall trial, ε4 carriers displayed a more rapid rate of forgetting over the delay interval. As expected, delayed memory measures were strongly associated with MTL structures, the region most affected in carriers. Thus, although the two genetic subgroups of AD patients both demonstrate memory impairment and actually display similar learning, their memory deficits are likely due to different underlying mechanisms, which we believe arise from the differential genetic effects on the frontoparietal working memory/executive control system(s) vs. the MTL episodic memory system.
MRI Methodology.
The quantitative MRI-based neuroanatomic analytic approach taken here has both strengths and limitations. The primary analyses used a priori ROIs, defined using an entirely separate sample from the present study and obtained from an exploratory map across the entire cerebral cortex that identified regions that were prominently atrophic in mild AD compared with age-matched controls. As opposed to traditional methods that use a priori ROIs defined using macroscopic anatomic landmarks, these “AD-signature” ROIs were defined on the average cortical surface template from foci of atrophy at the group level, and these ROIs were then mapped using the surface-based spherical coordinate registration to new individual subjects. This is a unique approach that is conservative because it restricts the analysis to ROIs where the average disease effect is relatively large; regions where the AD-related atrophy is not as prominent or where variance is higher are not included in the analysis, potentially leading to false-negative findings. Furthermore, if the localization of genotype-related atrophy effects is not fully overlapping with the disease-signature ROIs, the effects may be underestimated or missed. Yet effects that are found are likely to be generalizable, because they are identified using an unbiased set of ROIs. Additionally, disease-specific ROIs have the advantage over traditional landmark-based approaches in that disease processes may not respect these boundaries. Secondarily, we used a more liberal exploratory analysis to survey the entire cerebral cortex. Although subject to false-positive results due to nongeneralizable biologic features of the sample, this approach allows for the possibility that regionally specific effects may be near to but not exactly colocalized with a priori ROIs, providing complementary information.
Although most of these points are still theoretical, not having yet received systematic study, we have shown in two studies that the generation of spatially localized ROIs from exploratory analyses in one sample can be powerful in predicting effects in another sample (36), sometimes detecting effects not obvious from an exploratory analysis alone (46). Finally, we and others have demonstrated that traditional volumetric measures of MTL structures may conflate age-related changes in surface area and AD-related changes in cortical thickness (measured here) because these types of changes may be at least partly independent but both contribute in a nonredundant fashion to volumetric atrophy of a cortical structure (47, 48) or to genetically related variance in the volume of the structure (49).
Conclusions
We found that the presence or absence of the APOE ε4 allele influences the cognitive and anatomic phenotypic expression of AD in a dissociable manner. The mechanism by which APOE produces this dissociation is unclear. A variety of lines of evidence support the role of the ε4 allele in facilitating Aβ production and deposition, as well as reducing the effectiveness of neuronal repair mechanisms in the setting of toxic insults (50). However, more specific effects of ε4 carrier status on memory and MTL dysfunction may result from Aβ-independent pathways. The ε4 isoform is more susceptible to proteolytic cleavage in neurons leading to toxic fragments that have been demonstrated to directly contribute to neurodegeneration and are associated with memory loss in mouse models (51). Associated mitochondrial dysfunction and impaired glucose utilization may alter neural recruitment during memory processes in carriers (50, 52). Further, the ε4 allele seems to promote tau phosphorylation and neurofibrillary tangle (NFT) production (53). Given the predilection of NFT deposition in MTL structures early in AD it is, perhaps, not surprising that ε4 carriers would display poorer memory with greater MTL atrophy than noncarriers.
Less clear are mechanisms that actually support or enhance cognitive function and cortical integrity in ε4 carriers. Amyloid imaging and autopsy studies have demonstrated a similar topographic distribution, but with more extensive and greater Aβ plaque deposition in isocortical regions, including frontal and parietal lobes, in carriers relative to noncarriers (23, 54). Thus, the less-prominent atrophy of these brain regions in ε4 carriers may be mediated through other mechanisms, perhaps related to differential response of isocortical neurons to AD pathology or even developmental influences of APOE genotype, which may relate to individual differences in cognitive performance (55, 56), neuroanatomy (57), or brain function (52) at a young age. For example, several reports in young adults or children have suggested that noncarriers perform less well on measures of executive functioning and processing speed but better on tests of memory, foreshadowing the more prominent dissociation in the context of AD described here (55, 56, 58). These observations hint that APOE genotype may work in complex dissociable ways to modulate functional–anatomic brain networks subserving cognition throughout the lifespan and also the differential vulnerability of these networks to AD later in life (59). More work is clearly needed in this area. Regardless, the foregoing results have important implications for the early detection and monitoring of AD, because APOE carrier status seems to exert a strong influence on the cognitive and anatomic expression of the disease.
Materials and Methods
Participants, psychometric testing, and MRI analytic methods are summarized briefly here, with details provided in SI Materials and Methods.
We selected patients with a diagnosis of very mild to mild AD (n = 193), further limited to patients who had CSF testing consistent with AD (t-tau/Aβ1–42 ≥ 0.39) as previously established in ADNI and an autopsy-based dataset (30), and then divided into those with at least one APOE ε4 allele (“carriers”, n = 67) and those without (“noncarriers”, n = 24).
We examined baseline cognitive testing, which included the Rey AVLT, the Trail Making Test, Digit Symbol Substitution Test, Digit Span, category fluency test [Animals and Vegetables], and BNT. On the basis of prior work suggesting a greater memory deficit in ε4 carriers, we were particularly interested in examination of the AVLT, which allows for fractionation of different aspects of episodic memory. The AVLT consists of five learning trials in which a list of 15 words is read and the subject is asked to immediately recall as many items as possible. After an interference list of 15 novel words is read and recalled, subjects are then asked to recall words from the initial list (5-min delayed recall). A 30-min delayed recall trial and recognition test follow. For the recognition test, subjects are presented with a list of the 15 studied words and 15 nonstudied foils and are asked to circle all words previously studied. To account for false alarms (FA) to nonstudied items, we calculated a measure of discriminability, d-prime (d′), in a standard fashion based on classic signal detection theory (60). Because d′ is undefined when either proportion is 0 or 1, we used standard formulas to convert these values: Hits = (no. of hits + 0.5)/(no. of studied items + 1) and FA = (no. of FA + 0.5)/(no. of unstudied items + 1).
T1-weighted MRI data were analyzed using a cortical surface-based reconstruction method to generate measures of cortical thickness, which were then analyzed using two complementary approaches. First we examined group differences in hippocampal volume and thickness of ROIs previously determined to be reliably associated with AD, constituting the “cortical signature” of AD (31, 36). Unlike most ROI analyses, these regions were defined in a data-driven manner on the basis of analysis of several datasets, as opposed to being determined strictly by anatomic boundaries. These ROIs include medial temporal cortex, inferior temporal gyrus, temporal pole, angular gyrus, supramarginal gyrus, superior parietal lobule, precuneus, superior frontal gyrus, and inferior frontal sulcus. In addition to the ROI approach, an exploratory analysis across the entire cortical mantle was pursued.
Statistical analyses were performed in a standard fashion using SPSS, using analysis of covariance (ANCOVA) with age, years of formal education, and CDR-SB as covariates. Stepwise linear regression analyses were performed by entering age, education, and group status (carrier, noncarrier) into the models with anatomic ROIs as independent variables. Statistical analysis of the whole-cortex comparison was performed as described previously using a general linear model (31, 36).
Supplementary Material
Acknowledgments
This work was primarily funded by the ADNI (Principal Investigator: Michael Weiner; National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging (NIA), National Institute of Biomedical Imaging and Bioengineering, and the Foundation for the National Institutes of Health, through generous contributions from the following companies and organizations: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, the Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the US Food and Drug Administration. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. This analysis was also supported by NIA Grants R01-AG29411, R21-AG29840, P50-AG005134, K23-AG028018, and P30AG010124 and the Alzheimer’s Association.
Footnotes
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu\ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators is available at www.loni.ucla.edu/ADNI/Collaboration/ADNI_Citation.shtml.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001412107/-/DCSupplemental.
Contributor Information
Michael Weiner, ADNI.
Marilyn Aiello, ADNI.
Paul Aisen, ADNI.
Marilyn S. Albert, ADNI
Gene Alexander, ADNI.
Heather S. Anderson, ADNI
Karen Anderson, ADNI.
Liana Apostolova, ADNI.
Steve Arnold, ADNI.
Wes Ashford, ADNI.
Michele Assaly, ADNI.
Sanjay Asthana, ADNI.
Dan Bandy, ADNI.
Rob Bartha, ADNI.
Vernice Bates, ADNI.
Laurel Beckett, ADNI.
Karen L. Bell, ADNI
Amanda L. Benincasa, ADNI
Howard Bergman, ADNI.
Charles Bernick, ADNI.
Matthew Bernstein, ADNI.
Sandra Black, ADNI.
Karen Blank, ADNI.
Michael Borrie, ADNI.
Connie Brand, ADNI.
James Brewer, ADNI.
Alice D. Brown, ADNI
Jeffrey M. Burns, ADNI
Nigel J. Cairns, ADNI
Curtis Caldwell, ADNI.
Horacio Capote, ADNI.
Cynthia M. Carlsson, ADNI
Owen Carmichael, ADNI.
Janet S. Cellar, ADNI
Dzintra Celmins, ADNI.
Kewei Chen, ADNI.
Howard Chertkow, ADNI.
Munir Chowdhury, ADNI.
David Clark, ADNI.
Donald Connor, ADNI.
Stephen Correia, ADNI.
Karen Crawford, ADNI.
Anders Dale, ADNI.
Mony J de Leon, ADNI.
Susan M De Santi, ADNI.
Charles DeCarli, ADNI.
Leyla deToledo-Morrell, ADNI.
Michael DeVous, ADNI.
Ramon Diaz-Arrastia, ADNI.
Sara Dolen, ADNI.
Michael Donohue, ADNI.
Rachelle S. Doody, ADNI
P. Murali Doraiswamy, ADNI.
Ranjan Duara, ADNI.
Jessica Englert, ADNI.
Martin Farlow, ADNI.
Howard Feldman, ADNI.
Joel Felmlee, ADNI.
Adam Fleisher, ADNI.
Evan Fletcher, ADNI.
Tatiana M. Foroud, ADNI
Norm Foster, ADNI.
Nick Fox, ADNI.
Richard Frank, ADNI.
Anthony Gamst, ADNI.
Curtis A. Given, II, ADNI.
Neill R Graff-Radford, ADNI.
Robert C. Green, ADNI
Randall Griffith, ADNI.
Hillel Grossman, ADNI.
Ann M. Hake, ADNI
Peter Hardy, ADNI.
Danielle Harvey, ADNI.
Judith L. Heidebrink, ADNI
Barry A. Hendin, ADNI
Scott Herring, ADNI.
Lawrence S. Honig, ADNI
Chris Hosein, ADNI.
Ging-Yuek Robin Hsiung, ADNI.
Leon Hudson, ADNI.
M. Saleem Ismail, ADNI.
Clifford R. Jack, Jr., ADNI
Sandra Jacobson, ADNI.
William Jagust, ADNI.
Annapurni Jayam-Trouth, ADNI.
Kris Johnson, ADNI.
Heather Johnson, ADNI.
Nancy Johnson, ADNI.
Kathleen Johnson, ADNI.
Keith A. Johnson, ADNI
Sterling Johnson, ADNI.
Zaven Kachaturian, ADNI.
Jason H. Karlawish, ADNI
Maria Kataki, ADNI.
Jeffrey Kaye, ADNI.
Andrew Kertesz, ADNI.
Ronald Killiany, ADNI.
Smita Kittur, ADNI.
Robert A. Koeppe, ADNI
Magdalena Korecka, ADNI.
John Kornak, ADNI.
Nicholas Kozauer, ADNI.
James J. Lah, ADNI
Mary M. Laubinger, ADNI
Virginia M.-Y. Lee, ADNI
T.-Y. Lee, ADNI
Alan Lerner, ADNI.
Allan I. Levey, ADNI
Crystal Flynn Longmire, ADNI.
Oscar L. Lopez, ADNI
Joanne L. Lord, ADNI
Po H. Lu, ADNI
Martha G. MacAvoy, ADNI
Paul Malloy, ADNI.
Daniel Marson, ADNI.
Kristen Martin-Cook, ADNI.
Walter Martinez, ADNI.
George Marzloff, ADNI.
Chet Mathis, ADNI.
Catherine Mc-Adams-Ortiz, ADNI.
Marsel Mesulam, ADNI.
Bruce L. Miller, ADNI
Mark A. Mintun, ADNI
Jacobo Mintzer, ADNI.
Susan Molchan, ADNI.
Tom Montine, ADNI.
John Morris, ADNI.
Ruth A. Mulnard, ADNI
Donna Munic, ADNI.
Anil Nair, ADNI.
Scott Neu, ADNI.
Dana Nguyen, ADNI.
Alexander Norbash, ADNI.
MaryAnn Oakley, ADNI.
Thomas O. Obisesan, ADNI
Paula Ogrocki, ADNI.
Brian R. Ott, ADNI
Francine Parfitt, ADNI.
Sonia Pawluczyk, ADNI.
Godfrey Pearlson, ADNI.
Ronald Petersen, ADNI.
Jeffrey R. Petrella, ADNI
Steven Potkin, ADNI.
William Z. Potter, ADNI
Adrian Preda, ADNI.
Joseph Quinn, ADNI.
Michelle Rainka, ADNI.
Stephanie Reeder, ADNI.
Eric M. Reiman, ADNI
Dorene M. Rentz, ADNI
Brigid Reynolds, ADNI.
Jennifer Richard, ADNI.
Peggy Roberts, ADNI.
John Rogers, ADNI.
Allyson Rosen, ADNI.
Howard J. Rosen, ADNI
Henry Rusinek, ADNI.
Marwan Sabbagh, ADNI.
Carl Sadowsky, ADNI.
Stephen Salloway, ADNI.
Robert B. Santulli, ADNI
Andrew J. Saykin, ADNI
Douglas W. Scharre, ADNI
Lon Schneider, ADNI.
Stacy Schneider, ADNI.
Norbert Schuff, ADNI.
Raj C. Shah, ADNI
Les Shaw, ADNI.
Li Shen, ADNI.
Daniel H.S. Silverman, ADNI
Donna M. Simpson, ADNI
Kaycee M. Sink, ADNI
Charles D. Smith, ADNI
Peter J. Snyder, ADNI
Bryan M. Spann, ADNI
Reisa A. Sperling, ADNI
Kenneth Spicer, ADNI.
Bojana Stefanovic, ADNI.
Yaakov Stern, ADNI.
Edward Stopa, ADNI.
Cheuk Tang, ADNI.
Pierre Tariot, ADNI.
Lisa Taylor-Reinwald, ADNI.
Gaby Thai, ADNI.
Ronald G. Thomas, ADNI
Paul Thompson, ADNI.
Jared Tinklenberg, ADNI.
Arthur W. Toga, ADNI
Geoffrey Tremont, ADNI.
J.Q. Trojanowki, ADNI
Dick Trost, ADNI.
Raymond Scott Turner, ADNI.
Christopher H. van Dyck, ADNI
Helen Vanderswag, ADNI.
Daniel Varon, ADNI.
Javier Villanueva-Meyer, ADNI.
Teresa Villena, ADNI.
Sarah Walter, ADNI.
Paul Wang, ADNI.
Franklin Watkins, ADNI.
Michael Weiner, ADNI.
Jeff D. Williamson, ADNI
David Wolk, ADNI.
Chuang-Kuo Wu, ADNI.
Maria Zerrate, ADNI.
Earl A. Zimmerman., ADNI
References
- 1.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 6.Hof PR, Bouras C, Constantinidis J, Morrison JH. Balint’s syndrome in Alzheimer’s disease: Specific disruption of the occipito-parietal visual pathway. Brain Res. 1989;493:368–375. doi: 10.1016/0006-8993(89)91173-6. [DOI] [PubMed] [Google Scholar]
- 7.Alladi S, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 8.Kanne SM, Balota DA, Storandt M, McKeel DW, Jr, Morris JC. Relating anatomy to function in Alzheimer’s disease: Neuropsychological profiles predict regional neuropathology 5 years later. Neurology. 1998;50:979–985. doi: 10.1212/wnl.50.4.979. [DOI] [PubMed] [Google Scholar]
- 9.Stopford CL, Snowden JS, Thompson JC, Neary D. Variability in cognitive presentation of Alzheimer’s disease. Cortex. 2008;44:185–195. doi: 10.1016/j.cortex.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam M. Primary progressive aphasia pathology. Ann Neurol. 2008;63:124–125. doi: 10.1002/ana.20940. [DOI] [PubMed] [Google Scholar]
- 11.Strittmatter WJ, et al. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirier J, et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 13.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 14.Holtzman DM, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strittmatter WJ, et al. Hypothesis: Microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- 16.Polvikoski T, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M, et al. Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer’s disease. Neurology. 2001;57:1461–1466. doi: 10.1212/wnl.57.8.1461. [DOI] [PubMed] [Google Scholar]
- 18.Pievani M, et al. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer’s disease in vivo. Neuroimage. 2009;45:1090–1098. doi: 10.1016/j.neuroimage.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geroldi C, et al. APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology. 1999;53:1825–1832. doi: 10.1212/wnl.53.8.1825. [DOI] [PubMed] [Google Scholar]
- 20.Agosta F, et al. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc Natl Acad Sci USA. 2009;106:2018–2022. doi: 10.1073/pnas.0812697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehtovirta M, et al. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience. 1995;67:65–72. doi: 10.1016/0306-4522(95)00014-a. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drzezga A, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 24.Marra C, et al. Apolipoprotein E epsilon4 allele differently affects the patterns of neuropsychological presentation in early- and late-onset Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2004;18:125–131. doi: 10.1159/000079191. [DOI] [PubMed] [Google Scholar]
- 25.van der Vlies AE, et al. Cognitive impairment in Alzheimer’s disease is modified by APOE genotype. Dement Geriatr Cogn Disord. 2007;24:98–103. doi: 10.1159/000104467. [DOI] [PubMed] [Google Scholar]
- 26.Lehtovirta M, et al. Clinical and neuropsychological characteristics in familial and sporadic Alzheimer’s disease: Relation to apolipoprotein E polymorphism. Neurology. 1996;46:413–419. doi: 10.1212/wnl.46.2.413. [DOI] [PubMed] [Google Scholar]
- 27.Smith GE, et al. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer’s disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- 28.Varma AR, et al. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1999;66:184–188. doi: 10.1136/jnnp.66.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 30.Shaw LM, et al. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickerson BC, et al. The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen PJ, Sr, Soininen H. Major decrease in the volume of the entorhinal cortex in patients with Alzheimer’s disease carrying the apolipoprotein E epsilon4 allele. J Neurol Neurosurg Psychiatry. 1998;65:322–327. doi: 10.1136/jnnp.65.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 35.Filippini N, et al. Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer’s disease. Neuroimage. 2009;44:724–728. doi: 10.1016/j.neuroimage.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: Regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain. 2009;132:2906–2908. doi: 10.1093/brain/awp286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: Performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–1503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- 39.Miller E. Short- and long-term memory in patients with presenile dementia (Alzheimer’s disease) Psychol Med. 1973;3:221–224. doi: 10.1017/s003329170004856x. [DOI] [PubMed] [Google Scholar]
- 40.Becker JT, Bajulaiye O, Smith C. Longitudinal analysis of a two-component model of the memory deficit in Alzheimer’s disease. Psychol Med. 1992;22:437–445. doi: 10.1017/s0033291700030385. [DOI] [PubMed] [Google Scholar]
- 41.Baddeley A, Della Sala S, Spinnler H. The two-component hypothesis of memory deficit in Alzheimer’s disease. J Clin Exp Neuropsychol. 1991;13:372–380. doi: 10.1080/01688639108401051. [DOI] [PubMed] [Google Scholar]
- 42.Stopford CL, Snowden JS, Thompson JC, Neary D. Distinct memory profiles in Alzheimer’s disease. Cortex. 2007;43:846–857. doi: 10.1016/s0010-9452(08)70684-1. [DOI] [PubMed] [Google Scholar]
- 43.Shallice T, Warrington EK. Independent functioning of verbal memory stores: A neuropsychological study. Q J Exp Psychol. 1970;22:261–273. doi: 10.1080/00335557043000203. [DOI] [PubMed] [Google Scholar]
- 44.Markowitsch HJ, et al. Short-term memory deficit after focal parietal damage. J Clin Exp Neuropsychol. 1999;21:784–797. doi: 10.1076/jcen.21.6.784.853. [DOI] [PubMed] [Google Scholar]
- 45.Buchsbaum BR, D’Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- 46.Dickerson BC, et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickerson BC, et al. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feczko E, Augustinack JC, Fischl B, Dickerson BC. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiol Aging. 2009;30:420–431. doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler AM, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.12.028. 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris FM, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarmeas N, et al. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brecht WJ, et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beffert U, et al. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- 55.Acevedo SF, Piper BJ, Craytor MJ, Benice TS, Raber J. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr Res. 2010;67:293–299. doi: 10.1203/PDR.0b013e3181cb8e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci Lett. 2000;294:179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]
- 57.Shaw P, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: An observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 58.Marchant NL, King SL, Tabet N, Rusted JM. Positive effects of cholinergic stimulation favor young APOE epsilon4 carriers. Neuropsychopharmacology. 2010;35:1090–1096. doi: 10.1038/npp.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickerson BC. The entorhinal cortex: An anatomical mediator of genetic vulnerability to Alzheimer’s disease? Lancet Neurol. 2007;6:471–473. doi: 10.1016/S1474-4422(07)70112-6. [DOI] [PubMed] [Google Scholar]
- 60.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.