The evolution of brain structure and function has long fascinated biologists. This fascination was initially prompted by the observation that allometric relationships exist between the size of the brain—or brain region—and body size across a wide range of vertebrates (1). Besides random drift, two main ideas have been advanced to explain how brains evolve, and both have found considerable support. From a purely adaptationist point of view, selection on a specific set of behavior patterns or sensory specializations is thought to result in “mosaic” changes in only the brain regions that mediate these processes (2). In contrast, selection on any single brain region would cause the brain to change as a whole unit owing to developmental constraints and processes that regulate the formation and growth of a range of brain regions overall (3).
Beyond the “just so” stories that often characterize the interpretation of the causes and origins of brain diversity (4), two problems have vexed this line of research. First, it is not at all obvious how an increase in (relative) size would give rise to functional differences (e.g., increased cognitive abilities, novel sensory specializations, or behavioral complexity). Although a larger number of neurons and/or synapses might well result in greater processing power and/or speed, there is no clear relationship between such measures and behavioral or cognitive outcomes. Second, our understanding of the developmental mechanisms that give rise to the observed variation in brain structure is still very limited, and most studies have suggested that neurogenesis later in development generates diversity, which might result in the differential expansion of various brain areas (3). In this context it is also important to keep in mind that differences in brain structure and function can be as much a consequence of genetic and/or developmental control as they can be the result of (developmental) plasticity in response to the environment (5, 6).
It is this developmental problem for which a new study (7) establishes a ground-breaking paradigm. Similar to the basic patterning processes that specify the main body axes across all metazoans, the overall spatial and temporal activity patterns of transcription factor networks that establish the main compartments during early brain development are highly conserved (8). Could small variations in these expression profiles potentially result in large changes in overall brain structure? Surprisingly, this possibility has not yet been explored within an evolutionary developmental framework. The new study elegantly applies this “evo-devo” approach to gain insights into the developmental processes that might give rise to brain diversity by examining the activity of several of the genes that delineate compartments very early during the development of the nervous system.
The authors exploit the remarkable phenotypic diversity found in the cichlid fishes from east Africa's Great Lakes region, which provide an ideal model system for uncovering the ecological and behavioral forces that sculpt neural phenotypes. Cichlids display the most rapid and extensive adaptive radiations known for vertebrates (9), yet they have produced an astonishing array of phenotypes with little genetic diversification (10, 11). The extraordinary ecological (e.g., habitat, feeding specialization) and behavioral (e.g., color preferences by females, mating and parental care systems) diversity is correlated with variation in brain structure of a magnitude that exceeds that of all mammals and facilitates comparisons across large social and physical gradients in closely related species of cichlids (12, 13).
The new study (7) takes advantage of this “natural mutant screen” (9) by investigating brain development across a range of ecologically distinct cichlids from Lake Malawi. Specifically, the authors examine the expression patterns of a gene regulatory circuit involving WNT signaling that is important across vertebrates for specifying the anterior–posterior orientation of the developing brain and for determining the boundaries between its major compartments. There is considerable variation in the expression patterns of these genes between rock-dwelling mbuna and sand-dwelling nonmbuna cichlids, consistent with the differences observed in the relative size of fore- and midbrain structures in adult fish.
When the WNT signaling pathway is chemically perturbed in the developing embryo, alterations in this coexpression network are sufficient to give rise to the observed differences in brain development, resulting for instance in a rock-dweller with the forebrain shaped and sized like that of a sand-dweller.
Finally, a SNP in the irx1b gene, which mediates WNT signaling and is required for proper fore- and midbrain development, is fixed between rock- and sand-dwelling cichlids. Although it is not yet clear whether this substitution causes the observed shift in the developing brain compartments, it is likely the result of natural selection, because the vast majority of SNPs in Malawi cichlids are not fixed (11).
These neuroanatomical, developmental, and genomic results strongly support the conclusion that evolutionary changes in the patterning of developing brain compartments can establish ecologically and behaviorally relevant differences in the brain. Variation in subsequent neurogenesis, which until now has been thought to be the main source of variation in brain structure across species, can then elaborate the construction of diverse brains.
It will be interesting to reevaluate some of the ongoing debates on this topic in light of the new study. For example, the evolutionary expansion of the cerebral cortex in mammals has—not surprisingly—attracted much attention, particularly in primates (3, 14). The increase in cortex size in the lineage leading to humans has been interpreted as the result of variation in neurogenesis later in development, when cells in preestablished compartments proliferate, die, and/or differentiate into mature neurons and glia cells. According to the radial unit hypothesis, simply altering the first of the three phases of cell division that produce cortical excitatory neurons can scale the size of the cortex (15). In contrast, the intermediate progenitor hypothesis, which seems to have stronger support, suggests that in the evolutionary expansion of the cortex proportionately more neurogenesis occurs during the third and final phase of proliferation (16, 17). What, however, is the relative importance of these processes compared with the initial delineation of the future cortical sheet much earlier during development? The insights presented in the new study (7) will hopefully spur new investigations in this direction.
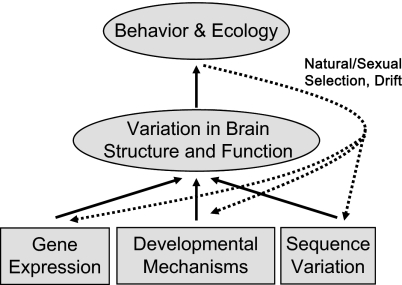
The study also provides new avenues for exploring the causes and functional implications that underlie the diversity in brain structure; meaningful insights have been difficult to come by owing to the correlative nature of neuroanatomical comparisons and the often ill-defined categorizations of habitat and behavior (4, 18). A recent essay (18) outlined four approaches that will move the field of brain evolution beyond these shortcomings. Importantly, the new study uses three of them in that it (i) analyzes the developmental mechanisms underlying neuroanatomical and behavioral differences; (ii) applies genomic comparisons such as gene expression studies to identify the genetic basis for phenotypic differences; and (iii) examines variation in DNA sequence that can provide clues about possibly causative and adaptive genetic changes that affect the nervous system (Fig. 1). The stage is now set for applying the fourth approach to explaining brain diversity in Malawi cichlids: we need to establish robust and unbiased assays to characterize relevant cross-species differences in behavior and ecology,
Most studies have suggested that neurogenesis later in development generates diversity.
so as to begin to understand the functional consequences of, for instance, an increased telencephalon in the rock-dwelling mbuna cichlids. For example, does maneuvering in a visually complex environment benefit from a larger forebrain? Is it easier to find food, defend territories, or attract mates as a consequence of this expansion?
Fig. 1.
Concept map illustrating three approaches to brain evolution studies used in the new study (7): evolutionary changes at the level of gene expression can occur as a consequence of DNA sequence variation and affect developmental mechanisms underlying differences in brain structure and behavior. Together with careful quantitative cross-species comparisons of behavior and ecology, this framework can considerably advance our understanding of brain evolution at all levels of biological organization.
Understanding how variation in the early patterning of the developing nervous system can give rise to diverse brains may ultimately help us understand why many cichlid clades have so rapidly diversified to such a remarkable extent. Are changes in the WNT signaling network necessary to produce forebrain differences, or can they come about in other ways as well? More specifically, does variation in irx1b signaling also underlie variation in brain structure in other groups of cichlids, such as the Ectodini of Lake Tanganyika? In this clade, multiple independent transitions from a polygamous to a monogamous mating system have occurred over the past 1.5 million years (19), and monogamous males have a larger forebrain than polygamous males (13). It will be exciting to see whether variation in this signaling pathway has been recruited in this clade as well. Finally, the experimental manipulation of developmental transcriptional networks opens up the possibility to address a fundamental problem in brain evolution: does a mutation that results in a larger telencephalon facilitate more complex neural processing and, as a consequence, allow its carrier to exhibit novel behavior patterns and/or exploit previously unsuitable habitats? Or will selection for improved performance in the existing social and ecological context favor the spread of the novel allele through the population? It will be interesting to see whether, for example, a sand-dwelling cichlid with an experimentally increased telencephalon displays a preference for a visually complex habitat. Whatever the outcomes of these future studies, we are in for a thrilling ride.
Acknowledgments
Work on the evolution of brain and behavior in my laboratory has been supported by National Science Foundation Grants IOS-021795 and IOS-0843712, an Alfred P. Sloan Foundation Fellowship, and a Dwight W. and Blanche Faye Reeder Centennial Fellowship in Systematic and Evolutionary Biology.
Footnotes
The author declares no conflict of interest.
See companion article on page 9718 in issue 21 of volume 107.
References
- 1.Striedter GF. Principles of Brain Evolution. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- 2.Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- 3.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 4.Healy SD, Rowe C. A critique of comparative studies of brain size. Proc Biol Sci. 2007;274:453–464. doi: 10.1098/rspb.2006.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann HA. Functional genomics of neural and behavioral plasticity. J Neurobiol. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- 6.Shumway CA. Habitat complexity, brain, and behavior. Brain Behav Evol. 2008;72:123–134. doi: 10.1159/000151472. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester JB, et al. Brain diversity develops at the boundaries. Proc Natl Acad Sci USA. 2010;107:9718–9723. doi: 10.1073/pnas.1000395107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puelles L, Rubenstein JLR. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 9.Kocher TD. Adaptive evolution and explosive speciation: The cichlid fish model. Nat Genet. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 10.Renn SC, Aubin-Horth N, Hofmann HA. Biologically meaningful expression profiling across species using heterologous hybridization to a cDNA microarray. BMC Genomics. 2004;5:42. doi: 10.1186/1471-2164-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh YHE, et al. Comparative analysis reveals signatures of differentiation amid genomic polymorphism in Lake Malawi cichlids. Genome Biol. 2008;9:R113. doi: 10.1186/gb-2008-9-7-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber R, van Staaden MJ, Kaufman LS, Liem KF. Microhabitat use, trophic patterns, and the evolution of brain structure in African cichlids. Brain Behav Evol. 1997;50:167–182. doi: 10.1159/000113330. [DOI] [PubMed] [Google Scholar]
- 13.Pollen AA, et al. Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain Behav Evol. 2007;70:21–39. doi: 10.1159/000101067. [DOI] [PubMed] [Google Scholar]
- 14.Reader SM, Laland KN. Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 16.Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- 17.Kriegstein AR, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 18.Pollen AA, Hofmann HA. Beyond neuroanatomy: Novel approaches to studying brain evolution. Brain Behav Evol. 2008;72:145–158. doi: 10.1159/000151474. [DOI] [PubMed] [Google Scholar]
- 19.Koblmüller S, Salzburger W, Sturmbauer C. Evolutionary relationships in the sand-dwelling cichlid lineage of Lake Tanganyika suggest multiple colonization of rocky habitats and convergent origin of biparental mouthbrooding. J Mol Evol. 2004;58:79–96. doi: 10.1007/s00239-003-2527-1. [DOI] [PubMed] [Google Scholar]