Abstract
We have studied models of telomerase haploinsufficiency in humans and mice to analyze regulation of telomere length and the significance of “set points” in inheritance of telomere length. In three families with clinical syndromes associated with short telomeres resulting from haploinsufficient mutations in TERT, the gene encoding telomerase reverse transcriptase, we asked whether restoration of normal genotypes in offspring of affected individuals would elongate inherited short telomeres. Telomeres were shorter than normal in some but not all genotypically normal offspring of telomerase-mutant parents or grandparents. Analysis of these findings was complicated by heterogeneity of telomere length among individuals, as well as by the admixing of telomeres inherited from affected parents with those inherited from unaffected (“wild-type” TERT) parents. To understand further the inheritance of telomere length, we established a shortened-telomere mouse model. When Tert+/− heterozygous mice were successively cross-bred through 17 generations, telomere length shortened progressively. The late-generation Tert+/− mice were intercrossed to produce genotypically wild-type Tert+/+ mice, for which telomere length was characterized. Strikingly, telomere length in these Tert+/+ mice was not longer than that of their Tert+/− parents. Moreover, when successive crosses were carried out among these short-telomere Tert+/+ offspring mice, telomere length was stable, with no elongation up to six generations. This breeding strategy therefore has established a mouse strain, B6.ST (short telomeres), with C57BL/6 genotype and stable short telomeres. These findings suggest that the set point of telomere lengths of offspring is determined by the telomere lengths of their parents in the presence of normal expression of telomerase.
Keywords: dyskeratosis congenita, haploinsufficiency, telomerase, aplastic anemia, pulmonary fibrosis
Telomerase is an RNA-dependent DNA polymerase that consists of two essential components, a template RNA (TERC) and a catalytic reverse transcriptase (TERT). The telomerase holoenzyme adds telomeric DNA repeats at the termini of eukaryotic chromosomes, thus maintaining telomere length and function despite telomere attrition that normally occurs during chromosomal replication. Telomeric DNA repeats and specific associated proteins are assembled to form telomere structures that distinguish normal chromosome ends from DNA damage-induced double-strand breaks and thus subserve a critical function in maintaining chromosomal stability (1–3). The functional importance of telomere integrity and telomerase activity has been addressed in a variety of experimental model systems, notably including genetic alterations of telomerase and telomeric proteins in multiple eukaryotic species. Evidence for a physiologically important role of telomerase in humans first emerged from analysis of the genetic syndrome dyskeratosis congenita (DKC), characterized by failure of bone morrow and other rapidly dividing cell lineages. An X-linked form of DKC was the first human disease recognized to be related to telomerase deficiency and is caused by the mutation of the gene encoding dyskerin, a protein component of the telomerase complex (4–6). Subsequently, mutations of TERT and TERC have been shown to result in DKC and to be risk factors for a range of other human telomeric syndromes, including aplastic anemia, idiopathic pulmonary fibrosis, and acute myeloid leukemia (7). Syndromes resulting from mutations in TERT and TERC are inherited as autosomal dominant traits because of haploinsufficiency and the inability of a single normal copy of TERT or TERC to maintain telomere length (7).
The effect of altered telomerase expression on telomere length and function in mammalian cells has been studied extensively in mouse models. In the absence of telomerase, telomeres shorten progressively during successive generations of Terc- or Tert-deficient mice (8–10). No significant abnormal phenotypes were observed in early generations of telomerase-deficient laboratory mice, which have exceptionally long wild-type telomeres in comparison with humans (and with mice in the wild). The appearance of genetic instability and chromosomal abnormalities coincides with the progressive accumulation of critically short telomeres that are detected in late generations of Terc- (8) or Tert-deficient mice (10). Resultant functional consequences include male and female infertility as well as abnormalities in highly mitotic tissues. Haploinsufficiency has been shown in these mouse models in which progressive telomere shortening occurs in successive generations of Tert+/− (11, 12) and Terc+/− mice (13). Notably, in contrast to mice with complete absence of telomerase or to humans with heterozygous TERT or TERC mutation, telomerase activity in these mouse models of telomerase haploinsufficiency is sufficient to protect against the generation of critically short telomeres (12, 14), and no physiologic defects have been observed in these mice.
Although telomere length generally is maintained at a set point specific for a species or strain, the factors that determine the set point are not completely understood. Models of haploinsufficiency provide a model for studying the consequences of restoring a normal telomerase genotype on the length of inherited short telomeres. Genotypically wild-type children of telomerase-mutated parents were clinically free of any manifestations of human telomeric syndromes and were reported in one TERC-haploinsufficient DKC family to have telomeres that are shorter than normal (15, 16). It was noted that second-generation genotypically normal descendants (grandchildren) of affected individuals have normal overall telomere length measured by flow cytometry-FISH (flow-FISH) (16). However, the interpretation of these observations is complicated, because in these second-generation offspring only 25% of telomeres would be inherited from the affected grandparent, making it difficult to assess whether the expression of normal telomerase had elongated previously short telomeres. It therefore remains unclear whether normalized telomerase activity in the offspring of telomerase-mutated patients restores telomeres to a “normal” length. To understand the inheritance of telomere length, we first investigated three TERT-mutant families. Some but not all first- and second-generation genotypically wild-type offspring of affected TERT-mutated parents had shorter telomeres than did normal age-matched controls. Differences in observations made across generations in different clinically affected families might result from factors such as the heterogeneity in telomere length in human populations and the difficulty noted above in analyzing telomere length in admixtures of chromosomes from parental and grandparental generations. In the present study, we therefore analyzed telomere length in Tert+/+ mice that were generated by intercrossing Tert+/− mice for multiple generations. Successive crosses of Tert+/− mice for up to 17 generations resulted in progressive telomere shortening, without evidence of critically short dysfunctional telomeres. Notably, telomeres of genotypically wild-type Tert+/+ offspring of these late-generation Tert+/− mice were not longer than those of their Tert+/− parents. Moreover, there was no elongation of shortened telomeres after as many as six generations of crosses among these short-telomere genotypically wild-type offspring mice, although normalized Tert genotype did protect against further telomere shortening. Shortened telomeres generated in a haploinsufficient environment appear to be maintained without elongation or further shortening after restoration of wild-type telomerase, resulting in the generation of a substrain of mice with stable but short telomeres. These findings suggest a revised perspective on set-point regulation of telomere length.
Results
Inheritance of Telomere Length in TERT-Mutant Families.
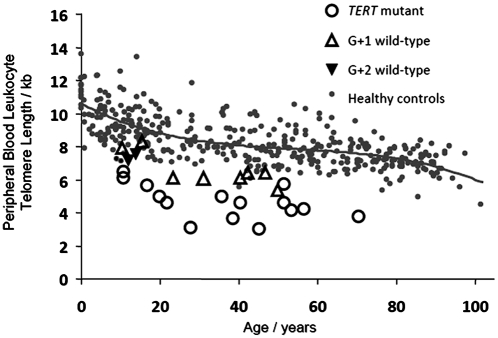
Telomere length in peripheral blood lymphocytes (PBLs) was analyzed in three families with TERT mutations and a history of aplastic anemia (17, 18). Telomere length was very short as measured by flow-FISH for individuals with TERT mutations from these three families, with all 15 below the 10th percentile for normal controls and 13 of 15 below the first percentile for controls (Fig. 1 and Fig. S1]. When genotypically normal-for-TERT offspring of affected individuals were analyzed, seven of nine children and one of two grandchildren studied had telomeres that were below the 10th percentile for age (Fig. 1 and Fig. S1). Taken together, the findings from these three families indicate that telomeres are short in some but not all genotypically normal offspring of parents or grandparents carrying TERT mutations. The heterogeneity of telomere length among individuals and the admixture of telomeres from normal parents in successive generations complicate the analysis of telomere length regulation in these human populations and make it difficult to determine whether restoration of normal telomerase actually elongates telomeres toward an actively maintained species set point. In the present study, therefore, we used a mouse model to address this question further.
Fig. 1.
Leukocyte telomere length and TERT genotype in families with aplastic anemia. Telomere length was measured by flow-FISH. Small gray circles represent the telomere lengths for 400 healthy volunteers, and the curve marks the 50th percentile for healthy controls as a function of age. Larger open circles represent telomere length of individuals carrying a TERT mutation, open triangles represent genotypically normal children of TERT mutation carriers, and inverted solid triangles represent their genotypically normal grandchildren.
Telomeres Shorten Progressively in Successive Generations of Tert+/− Mouse Intercrosses.
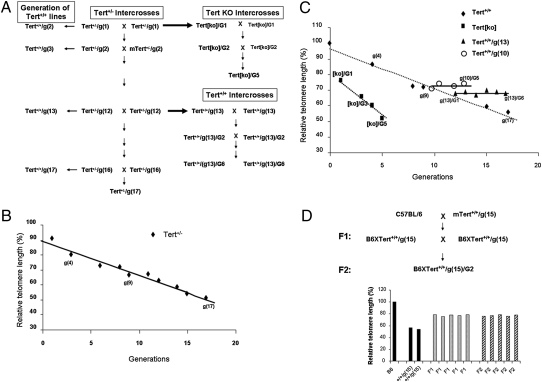
To analyze the effect of a normal telomerase genotype on the short telomeres that result from parental haploinsufficiency, we first bred successive generations of crosses between Tert+/− mice (Fig. 2A). PBL were prepared from C57BL/6 (B6) mice and from various generations of Tert+/− mice generated by successive Tert+/− × Tert+/− breeding, and relative telomere length was determined by flow-FISH. Consistent with previous reports (11), the telomeres of Tert+/− mice were shortened progressively in Tert+/− intercrosses, although at a slower rate of decrease than was observed in breeding of Tert−/− mice (Fig. 2B). Telomere length of 17th generation Tert+/− mice g(17), as determined by flow-FISH, was reduced to 52% of the mean fluorescence intensity of B6 mice. The calculated actual telomere lengths of B6 and Tert+/−/g(17) mice also were compared and similarly demonstrated a 54% reduction in telomere length in Tert+/−/g(17) mice (Fig. S2).
Fig. 2.
Inheritance of telomere length in the offspring from successive generations of Tert+/− × Tert+/− mouse crosses. (A) Schematic representation of the mouse-breeding strategy. (B) Telomeres were progressively shortened in successive generations of Tert+/− crosses. PBLs were prepared from B6 and various generations of Tert+/− mice generated from successive Tert+/− × Tert+/− crosses, and relative telomere length was determined by flow-FISH. The FITC fluorescent signal of the cell-binding telomeric probe was converted to arbitrary MSEF units. The average of fluorescent intensities from each mouse was normalized to that of a B6 mouse (defined as 100). The relative telomere length is plotted against each generation of mice. (C) Telomeres were progressively shortened in Tert+/+ offspring of successive Tert+/− crosses (diamonds). Rapid telomere shortening occurred in successive generations of Tert[ko] mice (squares) originated from Tert−/− crosses. In contrast, telomeres were neither shortened nor elongated in successive generations of genotypically wild-type offspring of haploinsufficient Tert+/− mice: Tert+/+/g(13) (triangles) and Tert+/+/g(10) (circles). PBLs were prepared from B6, from various generations of Tert+/+ mice generated from successive Tert+/− × Tert+/−crosses, from various generations of Tert[ko] generated from successive Tert−/− × Tert−/−crosses, from various generations of Tert+/+/g(13) mice generated from successive Tert+/+/g(13) × Tert+/+/g(13) crosses, and from various generations of Tert+/+/g(10) mice generated from successive Tert+/+/g(10) × Tert+/+/g(10) crosses. Relative telomere length was determined by flow-FISH as above. (D) Inheritance of shortened telomeres does not segregate. The upper panel shows the breeding strategy for B6 × Tert+/+/g(15) mice. PBLs were prepared from B6, Tert+/+/g(15), F1, and F2 mice, and relative telomere length was determined by flow-FISH as above.
Telomeres Are Not Elongated in Genotypically Wild-Type Offspring of Haploinsufficient Tert+/− Mice.
To determine the effect of restoring a normal Tert genotype, telomere length was measured in Tert+/+ offspring at successive generations in breeding of Tert+/− mice (Fig. 2A). Telomeres in genotypically wild-type Tert+/+ mice were not longer than those of their Tert+/− parents at each generation tested (Fig. 2C, diamonds, and Fig. S2). Telomere length measured by flow-FISH in 17th-generation Tert+/− mice or their Tert+/+ littermates was comparable to that of the infertile last-generation Tert[ko]/G5 mice derived from Tert−/− × Tert−/− crosses (Fig.2C, squares). To test more rigorously whether elongation of the short telomeres in these Tert+/+ mice occurred in response to restored telomerase genotype, the late-generation Tert+/+ offspring from Tert+/− breeding were interbred for multiple generations. Telomere length of Tert+/+/g(13) mice that originated from Tert+/−g(12) crosses was not detectably elongated in six generations of interbreeding (Fig. 2C). To test the reproducibility of this finding, a second independent multigenerational cross was carried out for five generations, with the same result (Fig. 2C). Thus, even prolonged breeding of genotypically normal mice did not restore the reduced telomere length to levels observed in the parental B6 strain, although it should be noted that restoration of Tert+/+ genotype was effective in preventing the additional telomere loss that was seen in successive generations of Tert+/− mice.
The stable inheritance of shortened telomeres in late-generation Tert+/+ mice raised the question of whether a genetic or epigenetic change had occurred during derivation of these mice and was responsible for shortened telomeres. The likelihood of a random event causing this change was lessened by the observation of an identical outcome in two independent multigenerational breeding pedigrees. In addition, the failure to observe telomere elongation in Tert+/+ offspring even in early generations of haploinsufficient breeding suggested that this phenotype did not emerge late in the breeding process. To characterize further the inheritance of telomere length, three lines of shortened-telomere Tert+/+ mice were backcrossed to B6 mice that had the long telomere length characteristic of this strain, and the resulting F1 mice then were intercrossed (Fig. 2D). Telomere length in individual F1 mice resulting from crosses of B6 and Tert+/+g(15) mice was intermediate between that of the parental strains, consistent with admixture of the two parental telomere populations. Intercrosses between these F1 mice generated an F2 population with remarkably uniform telomere length in individual F2 mice, which did not differ from those of F1 parents (Fig. 2D). Thus there was no segregation of telomere length as a trait in these mice, indicating that the short telomeres of Tert+/+ mice were not the result of a simple segregating genetic or epigenetic change.
Shortened Telomeres in Tert+/+ Mice Have No Apparent Physiological Defects.
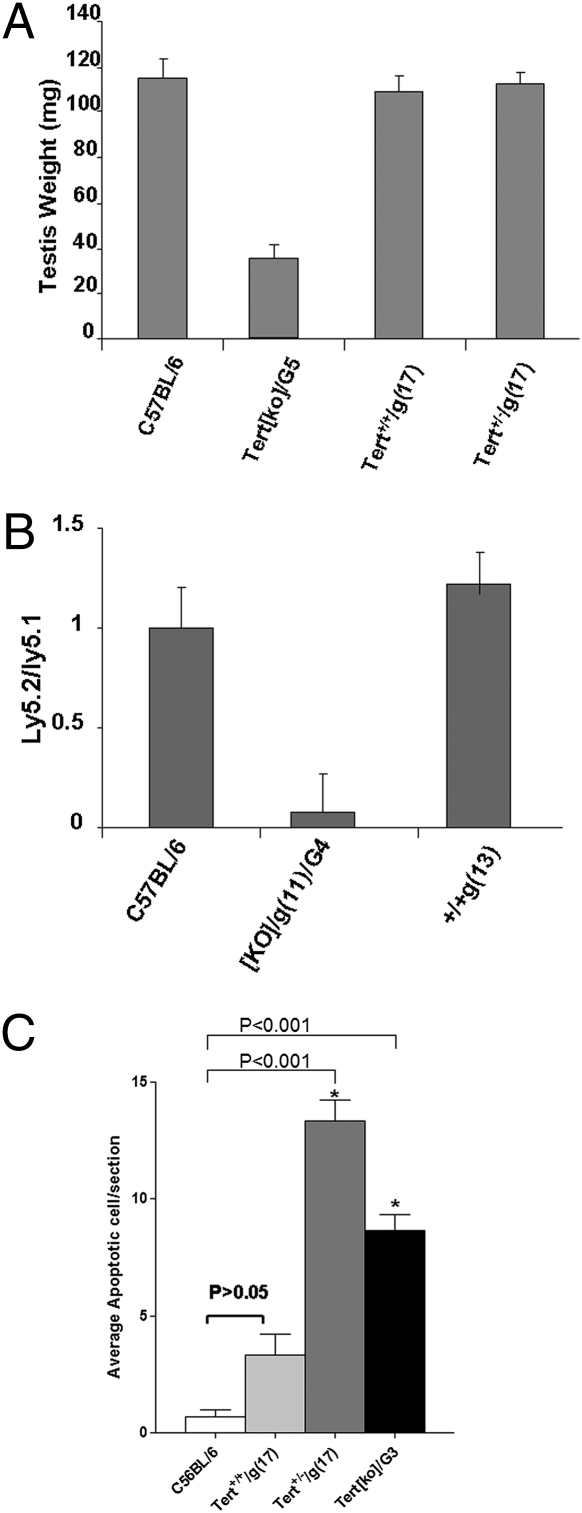
These results indicated that the breeding strategy employed resulted in generation of a strain of mice genetically identical to B6 with the exception of shortened telomeres. These mice are referred to here as “B6.ST.” (Nomenclature is under discussion by the nomenclature committee at Mouse Genome Informatics.) We next investigated if B6.ST mice, late-generation Tert+/+ mice originated from Tert+/− crosses, exhibited any of the physiological defects associated with critically short telomeres in late-generation telomerase-knockout mice. Infertility resulting from a gross defect in spermatogenesis is the most obvious physiological defect observed in the late-generation telomerase-deficient mice. The weight of testes from Tert[ko]/G5 male mice was in fact dramatically decreased in comparison with wild-type males (Fig. 3A). In contrast, Tert+/+/g(17) and Tert+/−/g(17) males, which had equally short telomeres by flow-FISH, had normal testis weight.
Fig. 3.
Tert+/+ mice with shortened telomeres have no apparent physiological defects, whereas infertile generation Tert[ko] mice do have physiological defects. (A) The testis weight of male B6, Tert+/+/g(17), and Tert[ko]/G5 mice was measured and presented as mean ± SEM (n = 5 for each genotype). (B) Results of competitive bone marrow transfers. PBLs were prepared from chimeras. The ratio between test PBL (Ly5.2 cells of B6 or Tert+/+/g(13) or Tert[ko]/G5 origin) and Ly5.1 control PBLs was calculated and presented as mean ± SEM (n = 9–13 for the three chimera groups). (C) TUNEL assay of apoptosis in small intestines. Small intestines were collected from C57BL/6, Tert+/+g(17), Tert+/−/g(17), and Tert[ko]/G3/g(17) mice, fixed in formalin, and embedded in paraffin. TUNEL assays were performed. Three mice were analyzed from each group, and 15–20 sections per mouse were scored.
Competitive bone marrow transfers were carried out to test hematopoietic stem cell activity as a measure of telomere-sensitive function in bone marrow stem cell repopulation. Bone marrow from normal CD45-congenic B6.Ly5.1 mice (long telomeres) was used as a competitor and mixed with Ly5.2 bone marrow from mice with short telomeres for reconstitution of lethally irradiated B6.Ly5.1 × B6.Ly5.2 recipients. PBL from chimeric mice were analyzed for the ratio of lymphocytes derived from the Ly5.2 test bone marrow to lymphocytes derived from the wild-type competitor B6.Ly5.1 bone marrow. Infertile mice with critically short telomeres were generated by intercrossing Tert−/−/g(11) knockout mice (derived from crosses of Tert+/−/g(10) parents) for four generations {Tert[ko]/g(11)/G4}. Bone marrow derived from these Tert[ko]/g(11)/G4 mice was profoundly deficient in its ability to compete with wild-type bone marrow (Fig. 3B). In contrast, Tert+/+/g(13) bone marrow competed effectively with wild-type control bone marrow for reconstitution, reflecting the absence of a physiologic defect caused by telomere shortening in these mice. Similar experiments carried out with Tert+/+/g(17) and Tert+/−/g(17) bone marrow gave equivalent results. Thus there is no physiologic defect detectable in short-telomere Tert+/+ and Tert+/− bone marrow in this sensitive competitive assay of competence for bone marrow reconstitution.
It has been reported that apoptosis in epithelial cells of small intestinal crypts is increased in late-generation telomerase-deficient mice as a consequence of telomere dysfunction. We therefore used the TUNEL assay to measure apoptosis in small intestines of B6, Tert+/+/g(17), and Tert+/−/g(17) mice. No statistically significant difference in apoptosis was detected in epithelial cells of small intestinal crypts of B6 and Tert+/+/g(17) mice. Of interest, however, the Tert+/−/g(17) littermates of Tert+/+/g(17) mice had increased numbers of apoptotic cells (Fig. 3C).
Tert+/+ Mice Originating from Tert+/− Crosses Do Not Have Critically Short Telomeres by Quantitative FISH Analysis.
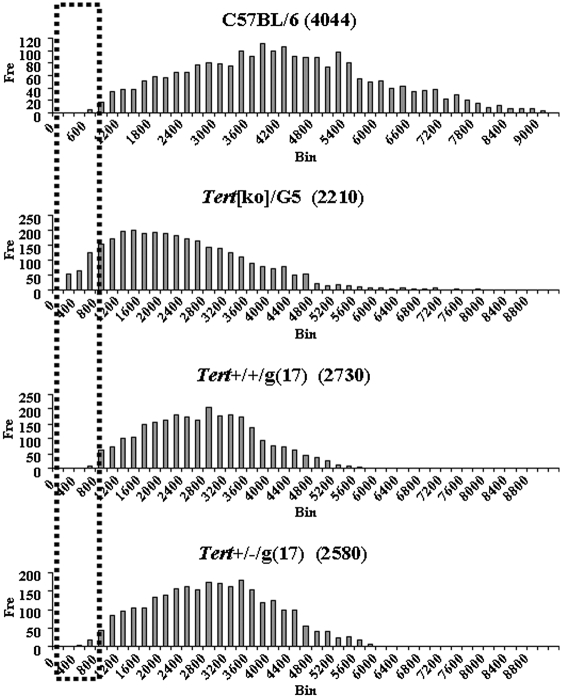
Overall telomere length measured by flow-FISH of Tert+/+ mice originating from multigeneration Tert+/− mice was similar to that of late-generation Tert[ko] mice originated from successive Tert−/− crosses. However, the short-telomere late-generation Tert+/+ mice did not have detectable physiological defects, whereas late-generation Tert[ko] mice had multiple abnormalities. Given the previously demonstrated association of functional defects with the accumulation of critically short individual telomeres, rather than with overall or average telomere length, we carried out quantitative FISH (Q-FISH) analysis to measure the individual telomere length for Tert+/+/g(17) mice. The distribution of telomere length in metaphases from Tert[ko]/G5 mice revealed a skewing toward extremely short telomeres with little or no signal when hybridized with the telomere-specific probe (Fig. 4). In contrast, telomeres from Tert+/+/g(17) metaphases did not have the extremely short telomeres that were observed in Tert[ko]/G5 mice, consistent with the absence of functional defects in these mice. Also, no extremely short telomeres were observed in Tert+/−/g(17) metaphases (Fig. 4).
Fig. 4.
Q-FISH analysis. Spleen cells from B6, Tert[ko]/G5, Tert+/+/g(17), and Tert+/−/g(17) mice were stimulated in vitro. Metaphase preparations derived from these activated cells were analyzed by quantitative fluorescence hybridization with Cy3-labeled telomere-specific DNA probe. Telomere fluorescence intensity was calculated from digital images. The distribution of fluorescent intensities corresponding to individual telomeres was plotted as a histogram. The dashed rectangle indicates extremely short telomeres.
Discussion
Although inheritance of telomere length has been reported in human families, the mechanisms underlying telomere-length inheritance remain unclear (19–22).We have analyzed telomere-length regulation and inheritance in human pedigrees and mouse models, studying telomere shortening in circumstances of telomerase haploinsufficiency and the regulation of telomere length upon restoration of a normal telomerase genotype. In families with TERT haploinsufficiency manifest as aplastic anemia, we observed that some but not all genotypically normal offspring of a haploinsufficient parent or grandparent retained short telomeres. It remained unclear from these studies whether restoration of a normal telomerase genotype is sufficient to restore telomere length in the short telomeres inherited from affected parents or grandparents. Interpretation of differences in telomere length in studies of human families is complicated by at least two factors. The first of these factors is heterogeneity in telomere length among individuals, requiring analysis within the context of telomere length of all parents, affected and unaffected, with data not always available. Even more challenging is the complexity that results from the fact that telomeres in any individual represent a mixture of telomeres inherited from both parents. In the families carrying telomerase mutations, short telomeres from the affected parent are admixed with generally longer telomeres from the unaffected parent. Determining the presence or absence of elongation of short telomeres in the context of this admixture is difficult, although in concept this question could be approached by tracking of telomere length on individual chromosomes. To assess further telomerase haploinsufficiency and the regulation of telomere length, we therefore established a mouse model to address these questions. We extended the previous demonstration, from our own laboratory and others, that mouse Tert haploinsufficiency results in progressive telomere shortening (12, 23). We then analyzed the genotypically normal Tert+/+ offspring of breeding between Tert+/− mice and found that telomeres were not elongated. Breeding of short-telomere Tert+/+ mice for up to six generations failed to result in detectable telomere elongation. Thus short telomeres resulting from telomerase haploinsufficiency result in a stable and inheritable phenotype despite normal genotype. Telomeres were protected from further shortening but were not elongated toward the wild-type strain/species-specific set point of B6 mice. Crossing these short-telomere Tert+/+ mice with B6 wild-type mice and subsequent intercrossing failed to show any segregation of telomere length, indicating that persistent short telomeres in these mice do not represent a simple heritable genetic or epigenetic trait. These findings suggest a revised understanding of telomere-length regulation and species-specific set points.
The critical role of telomerase in maintaining telomere length has been well established in multiple species, including mouse and humans. The phenomenon of haploinsufficiency also has been well described in human patients with heritable defects in telomerase components (6, 7, 18) as well as in Tert+/− and Terc+/− heterozygous mice (11–13). Thus successive breeding of Tert+/− mice results in progressive telomere shortening. In the present study, breeding of Tert+/− mice for up to 17 generations continues to generate fertile mice without evidence of telomere dysfunction as measured by testis weight and bone marrow reconstituting capacity, despite an overall telomere length measured by flow-FISH that is equivalent to that achieved in late-generation Tert−/− mice that have reached a point of infertility. The finding of increased apoptosis in intestinal crypts of Tert+/−/g(17) but not of Tert+/+/g(17) mice might be the earliest indication of telomere dysfunction during progressive breeding of haploinsufficient mice. Overall, these results are consistent with the finding that limiting amounts of telomerase may act selectively to maintain the shortest of telomeres, thus protecting against dysfunction (12, 14). What has been less well addressed in previous studies is the ability of telomerase to maintain or extend overall telomere length in cells that have inherited abnormally short telomeres from telomerase-deficient progenitors. The present data demonstrate that short telomeres can represent a heritable phenotype that is stable for at least six generations in absence of any apparent or segregating change in genotype, in effect creating a strain of mice with short telomeres. These results contrast with those which we previously reported based on intercrosses between two species of mice: Mus musculus domesticus, a species with long telomeres, and M. spretus, a species of feral mice with much shorter telomeres (24). Interbreeding and segregation analysis between these species revealed that short telomeres of M. spretus origin were elongated in F1 mice and that elongation segregated as a dominant trait mapped to a single locus on distal chromosome 2 (24). Subsequent studies mapped this phenotype to the gene encoding the Rec helicase (Rtel) (25, 26). The precise mechanism of action of this gene product remains to be elucidated. Thus, elongation of overall telomere lengths, beyond protection of critically short telomeres (14), can occur in interspecies crosses. In contrast, the current work indicates that, in genetically homogeneous B6 mice, telomeres that have been shortened by haploinsufficiency are not elongated by restoration of telomerase genotype. This observation was made in two independent lines of mice, indicating that the phenomenon is unlikely to represent a chance mutation. Moreover, the failure to observe any segregation of telomere length in a cross with wild-type B6 mice indicates that no simple genetic or epigenetic change accounts for failure of telomere elongation.
What then explains the apparent stability of telomere length within a species or strain but the failure to elongate telomeres rendered short in this strain? The newly established B6.ST strain appears otherwise to be genetically identical to the parental B6 line, but the two express substantially different telomere lengths. It should be emphasized that in both wild-type B6 and B6.ST mice, maintenance of telomere length is strictly telomerase-dependent. Heterozygosity for Tert deficiency results in telomere shortening in both B6 and B.ST mice, and homozygous intact Tert is necessary for maintenance of telomere length. We conclude that the species-specific set point for telomere length is not determined by genotype alone but reflects historic events in the species, in the current model including telomerase haploinsufficiency in past generations. These results provide an interesting and surprising extension of the previous observations that telomerase acts selectively to elongate critically short telomeres (12, 14). There may be no efficient mechanism for targeting telomerase-dependent telomere elongation over a wide range of telomere shortening when there is no sensing of “criticality” in telomere length (14). Telomerase activity is otherwise regulated to provide, on average, approximate compensation for the telomere loss that occurs with chromosomal replication and cell division. It has been demonstrated previously that telomerase-dependent regulation of telomere length can be modulated by alterations that affect other components of the telomere. Thus, although wild-type telomerase will not extend telomere length under otherwise normal conditions, deletion or down-regulation of telomere-associated proteins can result in telomere elongation beyond the species- or strain-characteristic length (1, 3).
Following submission of this paper, Meznikova et al. (27) reported an analysis of multigenerational breeding of Tert heterozygous mice. They employed a breeding strategy that differed from that used in the studies reported here. Rather than successive breeding between Tert+/− mice, Meznikova et al. repeatedly crossed Tert+/− mice with wild-type B6 Tert+/+ mice (27). They reported that this strategy resulted in telomere shortening through 10 generations, after which continued breeding resulted in elongation of telomeres through generation 14. The mechanism of initial progressive telomere shortening in this strategy is not clear, because breeding involves introduction of long B6 parental telomeres at each generation. In our own colony, we have repeatedly backcrossed Tert+/− mice to B6 to maintain a stable B6 genotype. Through 35 generations of breeding, we have not observed telomere shortening. It should be noted that our studies and those of Meznikova et al. employed different Tert-mutant lines. We constructed an altered Tert allele by disruption of exon 1, with documented absence of detectable Tert mRNA (9). In contrast, the Tert mutation used in the study of Meznikova et al. (27) involved the replacement of a portion of the Tert gene extending from within exon 4 to a point within exon 7 (10), an alteration that might be expected to allow transcription of a truncated Tert mRNA, although it was reported that no transcripts were detected by quantitative RT-PCR (12). Also after submission of our paper, Armanios et al. (28) reported studies of heterozygous Tert+/− mice that had been backcrossed to a CAST/EiJ background with telomeres substantially shorter than those of B6 mice. They reported that successive generations of Tert+/− CAST/EiJ breeding resulted in progressive telomere shortening, in this case sufficient to result in telomere dysfunction. When Tert+/− CAST/EiJ mice were intercrossed to generate Tert+/+ CAST/EiJ mice, successive intercrosses of these genotypically wild-type mice resulted in telomere elongation. Taken together, the results of Armanios et al. (28) and those which we have reported here are consistent with our proposed model, in which telomere length is inherited as a stable phenotype, with elongation of telomeres in the presence of wild-type telomerase occurring only when telomeres are recognized as critically short. Thus, functionally critical levels of telomere shortening may have been generated by haploinsufficiency in CAST/EiJ mice but not yet in the B6 mice that we have studied. In our previous studies of crosses between M. musculus and M. spretus, M. spretus telomeres appear to be recognized in M. musculus × M. spretus F1 mice as shorter than the threshold length that triggers telomere elongation, and we observed elongation of M. spretus telomeres in these interspecies F1 mice (24). The short telomeres of late-generation B6.ST mice are still longer than those of M. spretus (24) and do not trigger elongation. The model proposed here would predict that further shortening of telomeres in continued breeding of Tert+/− mice eventually would result in functionally critical shortening that would trigger telomere elongation when a normal Tert+/+ genotype is restored. The B6.ST mouse represents a strain that may be useful in studies of telomere dynamics and insufficiency on the commonly used B6 genetic background. Breeding of Tert+/− mice will be continued to generate variant strains with progressively shorter telomeres and to monitor functional status under these conditions.
Materials and Methods
Mice.
The generation and characterization of Tert-deficient mice was reported previously (9). Tert mutants were backcrossed to B6 for at least 18 generations before use in studies reported here. Fig. 2A shows the breeding strategy used in generation of Tert-deficient mice. All animals were housed at Bioqual (Rockville, MD).
Q-FISH.
Mouse spleen lymphocytes were stimulated with Con A (5 μg/mL), LPS (15 μg/mL), and recombinant IL-2 (150 IU/mL) for 48 h. Activated cells were treated with colcemide and fixed with 3:1 methanol:acetic acid. Metaphase cells were dropped on slides that were then hybridized with Cy3-labeled (CCCTAA)3 oligonucleotides, and chromosomes were stained with DAPI. Fluorescent signals were collected and analyzed as previously described (9). Using FluoreSphere beads (Molecular Probes) as standards, we fluorescent signal intensities using Q-FISH software (29).
Human Peripheral Blood Leukocyte Separation.
Peripheral blood was collected after informed consent was obtained in accordance with the Declaration of Helsinki and the research protocol approved by the National Heart, Lung, and Blood Institute Institutional Review Board (ClinicalTrials.gov NCT00071045). Twenty milliliters of peripheral blood was collected from patients with TERT mutation and from healthy volunteers. DNA was extracted from total peripheral blood leukocytes using the Qiagen DNAeasy kit (Qiagen). Telomere length of peripheral blood leukocytes was measured after red cell lysis with ammonium chloride solution by flow-FISH as reported previously (30, 31).
Flow-FISH Analysis of Mouse Lymphocytes.
Mouse spleen lymphocytes were prepared and incubated with FITC-labeled (CCCTAA)3 oligonucleotide in buffer containing 20 mM Tris, 75% formamide, 1% BSA, and 20 mM NaCl at 86 °C for 15 min, followed by room temperature incubation for 2 h in the same buffer. Cells were used for FACS analysis after being washed four times with buffer containing 20 mM Tris, 75% formamide, 1% BSA, and 0.1% Tween 20 (Sigma) (32). The FITC fluorescent signal of the cell-binding telomeric probe was converted to arbitrary units of molecule equivalents of soluble fluorescence (MSEF). The average of fluorescent intensities from each mouse was normalized to that of a B6 mouse (defined as 100).
Bone Marrow Transplantation.
Radiation bone morrow chimeras were prepared as described previously (33). (B6.Ly5.1 × B6.Ly5.2) F1 recipient mice were lethally irradiated with 1,000 rad and reconstituted with a mixture of cell-depleted bone marrow cells containing 3 × 106 CD45-congenic B6.Ly5.1 wild-type bone marrow cells and 3 × 106 Ly5.2 bone marrow cells from test mice. The ratio of Ly5.2 to Ly5.1 PBL from chimeras was analyzed by FACS after 6–10 weeks of reconstitution.
TUNEL Assay of Apoptosis.
Small intestines were resected, flushed, fixed in formalin, and embedded in paraffin. Apoptotic cells were evaluated in tissue sections by TUNEL to detect fragmented DNA, using the TACS 2 TdT-Fluor In Situ Apoptosis Detection kit (Trevigen Inc.), according to the manufacturer's instructions. The analysis of stained slides followed the procedures of Meznikova et al. (27), and sections of the small intestine were used to determine incidence of apoptosis in intestinal crypts. Three mice from each genotype were used, and 15 to 20 sections per mouse were analyzed. One-way ANOVA followed by Bonferroni's multiple comparison test was used to analyze the frequency of apoptotic cells in small intestine sections. The measurements were made on coded samples by an observer who did not know the identity of these samples.
Supplementary Material
Acknowledgments
We thank Genevieve Sanchez-Howard and the staff at Bioqual for expert animal care and breeding. We thank Michael Kruhlak for helping with Q-FISH analysis, Irma Vulto for helping measure actual telomere length, David Winkler and Jeffrey Hammer for helping isolate mouse-tail DNAs, and Susan Sharrow for helping in FACS analysis. We thank Nan-ping Weng for his critical reading and helpful comments. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913125107/-/DCSupplemental.
References
- 1.Blackburn EH. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Greider CW. Telomerase RNA levels limit the telomere length equilibrium. Cold Spring Harb Symp Quant Biol. 2006;71:225–229. doi: 10.1101/sqb.2006.71.063. [DOI] [PubMed] [Google Scholar]
- 4.Kirwan M, Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim Biophys Acta. 2009;1792:371–379. doi: 10.1016/j.bbadis.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safa WF, Lestringant GG, Frossard PM. X-linked dyskeratosis congenita: Restrictive pulmonary disease and a novel mutation. Thorax. 2001;56:891–894. doi: 10.1136/thorax.56.11.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessler M, Du HY, Gu B, Mason PJ. Dysfunctional telomeres and dyskeratosis congenita. Haematologica. 2007;92:1009–1012. doi: 10.3324/haematol.11221. [DOI] [PubMed] [Google Scholar]
- 7.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 9.Chiang YJ, et al. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Mol Cell Biol. 2004;24:7024–7031. doi: 10.1128/MCB.24.16.7024-7031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, et al. The telomerase reverse transcriptase is limiting and necessary for telomerase function in vivo. Curr Biol. 2000;10:1459–1462. doi: 10.1016/s0960-9822(00)00805-8. [DOI] [PubMed] [Google Scholar]
- 11.Hathcock KS, Jeffrey Chiang Y, Hodes RJ. In vivo regulation of telomerase activity and telomere length. Immunol Rev. 2005;205:104–113. doi: 10.1111/j.0105-2896.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Kha H, Ungrin M, Robinson MO, Harrington L. Preferential maintenance of critically short telomeres in mammalian cells heterozygous for mTert. Proc Natl Acad Sci USA. 2002;99:3597–3602. doi: 10.1073/pnas.062549199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hathcock KS, et al. Haploinsufficiency of mTR results in defects in telomere elongation. Proc Natl Acad Sci USA. 2002;99:3591–3596. doi: 10.1073/pnas.012549799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 15.Du HY, et al. Telomerase reverse transcriptase haploinsufficiency and telomere length in individuals with 5p- syndrome. Aging Cell. 2007;6:689–697. doi: 10.1111/j.1474-9726.2007.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman F, et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci USA. 2005;102:17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calado RT, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci USA. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 19.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: A twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 20.Njajou OT, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci USA. 2007;104:12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atzmon G, et al. Evolution in Health and Medicine Sackler Colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci U S A. 107(Suppl 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrew T, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington L. Making the most of a little: Dosage effects in eukaryotic telomere length maintenance. Chromosome Res. 2005;13:493–504. doi: 10.1007/s10577-005-0994-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, et al. Telomere length regulation in mice is linked to a novel chromosome locus. Proc Natl Acad Sci USA. 1998;95:8648–8653. doi: 10.1073/pnas.95.15.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding H, et al. Regulation of murine telomere length by Rtel: An essential gene encoding a helicase-like protein. Cell. 2004;117:873–886. doi: 10.1016/j.cell.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Flanary B. Regulation of murine telomere length via Rtel. Rejuvenation Res. 2004;7:168–170. doi: 10.1089/rej.2004.7.168. [DOI] [PubMed] [Google Scholar]
- 27.Meznikova M, Erdmann N, Allsopp R, Harrington LA. Telomerase reverse transcriptase-dependent telomere equilibration mitigates tissue dysfunction in mTert heterozygotes. Dis Model Mech. 2009;2:620–626. doi: 10.1242/dmm.004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armanios M, et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zijlmans JM, et al. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc Natl Acad Sci USA. 1997;94:7423–7428. doi: 10.1073/pnas.94.14.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin ZT, et al. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood. 2007;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- 31.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 32.Hathcock KS, Hodes RJ, Weng NP. Analysis of telomere length and telomerase activity. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im1030s62. Chapter 10, Unit 10 30. [DOI] [PubMed] [Google Scholar]
- 33.Singer A, Hathcock KS, Hodes RJ. Cellular and genetic control of antibody responses. V. Helper T-cell recognition of H-2 determinants on accessory cells but not B cells. J Exp Med. 1979;149:1208–1226. doi: 10.1084/jem.149.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.