Abstract
Background
Flavobacterium columnare causes columnaris disease in cultured and wild fish populations worldwide. Columnaris is the second most prevalent bacterial disease of commercial channel catfish industry in the United States. Despite its economic importance, little is known about the expressed proteins and virulence mechanisms of F. columnare. Here, we report the first high throughput proteomic analysis of F. columnare using 2-D LC ESI MS/MS and 2-DE MALDI TOF/TOF MS.
Results
Proteins identified in this study and predicted from the draft F. columnare genome were clustered into functional groups using clusters of orthologous groups (COGs), and their subcellular locations were predicted. Possible functional relations among the identified proteins were determined using pathway analysis. The total number of unique F. columnare proteins identified using both 2-D LC and 2-DE approaches was 621, of which 10.95% (68) were identified by both methods, while 77.29% (480) and 11.76% (73) were unique in 2-D LC and 2-DE, respectively. COG groupings and subcellular localizations were similar between our data set and proteins predicted from the whole genome. Twenty eight pathways were significantly represented in our dataset (P < 0.05).
Conclusion
Results from this study provide experimental evidence for many proteins that were predicted from the F. columnare genome annotation, and they should accelerate functional and comparative studies aimed at understanding virulence mechanisms of this important pathogen.
Background
Flavobacterium columnare is a long Gram-negative rod in the family Flavobacteriaceae, one of the main phyletic lines within the Bacteroidetes group from the domain Bacteria [1]. Several species in Flavobacteriaceae cause disease in fish. F. columnare is the causative agent of columnaris disease [2], which exists both in fresh and brackish water throughout the world [3]. Outbreaks may result in high mortality, especially during spring and autumn, and are most likely associated with poor environmental conditions causing stress [4,5]. Stressful conditions are common in commercial aquaculture where production is kept at maximum levels.
Columnaris disease generally begins as an external infection on the skin, fins, gills, or oral cavity [6]. On the skin and fins, lesions are characterized by dull, grayish-white or yellow erosive lesions that can progress to deep ulcers in the underlying muscle. External infection often is concurrent with systemic infection and subacute mortalities [3]. In some cases, systemic infection with little or no visible external or internal pathological signs may occur [7]. F. columnare infections can be chronic, but more often, the disease appears suddenly and causes mortalities within a few days [6].
A substantial amount of work has been done on F. columnare phylogeny [1,8], isolation, identification, and detection of F. columnare [9-18], and characterization of F. columnare strains [19-25]. In addition, genetic tools for the manipulation of F. columnare have recently been reported [26]. Efforts have been made to understand the mechanisms of virulence employed by the organism [27-30], but much remains poorly understood. F. columnare produces several extracellular proteases that are believed to be important virulence factors contributing to the branchial and cutaneous necrosis [28,31-33], but the role of the proteases has not been definitively elucidated. A surface capsular material that may be involved in adhesion has been described [34]. Lipopolysaccharide (LPS) may also play an important role in columnaris pathogenesis [35]. Chondroitin lyase activity was found to be significantly related to strain virulence in a temperature dependent manner [30].
Although, 14 F. columnare outer membrane related proteins were reported recently [36], little is known about the expressed F. columnare proteins. In the present study, we report the first protein expression analysis from F. columnare using the complementary technologies of 2-D LC ESI MS/MS and 2-DE MALDI TOF/TOF MS. Results of this study provide experimental evidence for the predicted F. columnare proteins and should accelerate functional and comparative studies to delineate pathogenic mechanisms of F. columnare.
Results
Protein identification using 2-D LC and 2-DE
2-D LC analysis yielded total 548 F. columnare proteins (Additional File 1: Table S1) representing 19.01% of the predicted F. columnare proteome (2,882 ORFs). 2-DE analysis of four CBB stained gels showed approximately 600 spots. Among the common spots, 192 were excised and analyzed from one of these gels. We were able to identify 182 (94.79%) of the excised spots (Additional File 2: Table S2), which represented 141 unique F. columnare proteins. In 2-DE analysis, 41 (22.53%) proteins were identified in multiple spots, probably due to post-translational modifications and processing. Together, 2-D LC and 2-DE analyses resulted in the identification of 621 F. columnare proteins, 480 (77.29%) of which were identified only by 2-D LC, while 73 (11.76%) were identified only by 2-DE (Figure 1), and 68 (10.95%) were detected by both approaches. Analysis of the functional role categories assigned by the J. Craig Venter Institute's Annotation Engine indicated some important categories under cell envelope and cellular processes. Sub categories under these main groups had potential to include proteins that may be involved in F. columnare pathogenesis (Table 1).
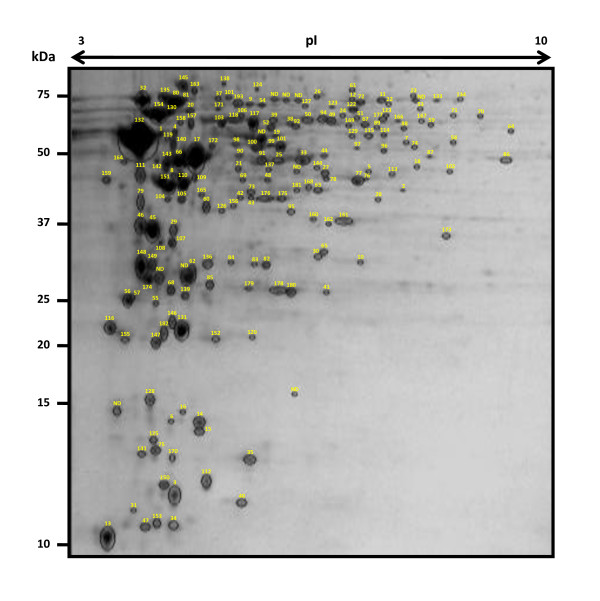
Figure 1.
2-D map of proteins extracted from Flavobacterium columnare. Circled spots were excised and analyzed by MS. Numbers indicate proteins with known IDs while NDs indicate unidentified proteins.
Table 1.
Flavobacterium columnare potential pathogenesis-related proteins.
| ORF # | Protein name | Approach/SNa) | Role categoryb) |
|---|---|---|---|
| ORF00560 | 3-deoxy-D-manno-octulosonate 8-phosphate phosphatase, YrbI family | 2-D LC | Cell envelope (A) |
| ORF01244 | 3-deoxy-D-manno-octulosonate cytidylyltransferase | 2-D LC | Cell envelope (A) |
| ORF02664 | Amidinotransferase family protein | 2-D LC | Cellular processes (E) |
| ORF01484 | CHU large protein; gliding motility-related protein; putative adhesin AidA-related | 2-D LC | Cellular processes (C) |
| ORF01824 | DTDP-4-dehydrorhamnose 3,5-epimerase | 2-DE/120 | Cell envelope (A) |
| ORF00198 | Extracellular elastinolytic metalloproteinase | 2-D LC | Cellular processes (D) |
| ORF01481 | Fibronectin type III domain protein | 2-D LC | Cellular processes (D) |
| ORF02336 | GldL | Both/152 | Cellular processes (C) |
| ORF02335 | GldM | 2-D LC | Cellular processes (C) |
| ORF02334 | GldN | 2-DE/151 | Cellular processes (C) |
| ORF02678 | Glucose-1-phosphate thymidylyltransferase | 2-D LC | Cell envelope (A) |
| ORF00396 | Glycosyl transferase, group 1 | 2-D LC | Cell envelope (A) |
| ORF02332 | Glycosyltransferase, putative | 2-D LC | Cell envelope (A) |
| ORF00532 | GTP-binding protein TypA/BipA | 2-DE/36, 37 | Cellular processes (F) |
| ORF01035 | Hemagglutinin | 2-D LC | Cellular processes (E) |
| ORF02801 | Lipid-A-disaccharide synthase | 2-D LC | Cell envelope (A) |
| ORF01136 | LPS biosynthesis protein , RfbU family | 2-D LC | Cell envelope (A) |
| ORF02060 | Mannosyltransferase | 2-D LC | Cell envelope (A) |
| ORF00475 | Membrane protein, putative | 2-D LC | Cellular processes (E) |
| ORF01989 | Monofunctional biosynthetic peptidoglycan transglycosylase | 2-D LC | Cell envelope (B) |
| ORF02568 | PepSY-associated TM helix family | 2-D LC | Cellular processes (C) |
| ORF02672 | Polysaccharide export outer membrane protein | 2-D LC | Cell envelope (A) |
| ORF01163 | Tetraacyldisaccharide 4'-kinase | 2-D LC | Cell envelope (A) |
| ORF01370 | UDP-3-0-acyl N-acetylglucosamine deacetylase | 2-D LC | Cell envelope (A) |
| ORF01367 | UDP-3-O-[3-hydroxymyristoyl] glucosamine N-acyltransferase | 2-D LC | Cell envelope (A) |
| ORF02676 | UDP-glucose 6-dehydrogenase | Both/169 | Cell envelope (A) |
| ORF02536 | UDP-N-acetylglucosamine 2-epimerase | 2-D LC | Cell envelope (A) |
| ORF00211 | Universal stress protein family protein | 2-DE/10 | Cellular processes (F) |
a) SN indicates spot number marked on the 2-DE image (Figure 1).
b) A, Biosynthesis and degradation of surface polysaccharides and lipopolysaccharides; B, Biosynthesis and degradation of murein sacculus and peptidoglycan; C, Chemotaxis and motility; D, Pathogenesis; E, Toxin production and resistance; F, Adaptations to atypical conditions.
Functional classification of identified proteins
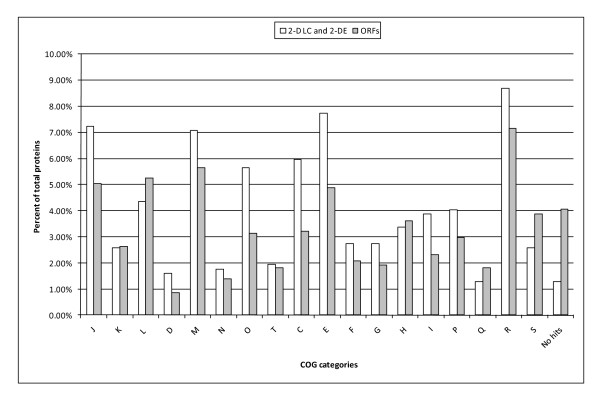
Functional classifications of the 621 identified unique proteins and the 2,882 predicted F. columnare proteins are summarized in Figure 2. A total of 88 (14.17%, 88/621) proteins from our dataset and 372 (12.91%, 372/2,882) proteins from the predicted whole proteome were classified as information storage and processing related categories (J, K, and L). Cellular processes and signaling related categories (D, M, N, O, and T) included 112 (18.04%) and 370 (12.84%) proteins from our protein dataset and the whole proteome database, respectively. A total of 197 (31.72%) and 658 (22.83%) proteins from our protein dataset and the predicted whole proteome, respectively, were represented in metabolism related categories (C, E, F, G, H, I, P, and Q). Poorly characterized COG groups (R and S) contained 70 (11.27%) and 318 (11.03%) proteins in our protein dataset and the predicted whole proteome, respectively. "No COGs" (proteins not belong to any of the currently-defined COGs) was the top represented category in our protein dataset (23.51%) and in the whole proteome (36.33%). Eight (1.29%) proteins from our protein dataset and 117 (4.06%) proteins from whole proteome were assigned to "No hit" (non-significant short or low-complexity sequences) category. A summary of Pfam domain analysis on proteins detected by 2-D LC and 2-DE, and predicted from the F. columnare draft genome are given in Additional Files 3, 4, and 5: Tables S3, S4 and S5, respectively.
Figure 2.
Comparison of COG categories. Proteins identified in this study (621 proteins in both 2-D LC and 2-DE analysis) and predicted from the Flavobacterium columnare draft genome (2,882 ORFs) were organized into COG functional groups. Percentages were calculated by dividing the number of proteins in the particular COG category by the number of unique proteins in each analysis. COG categories are as follows: J, translation, ribosomal structure and biogenesis; K, transcription; L, replication, recombination, and repair; D, cell cycle control, cell division, chromosome partitioning; M, cell wall/membrane/envelop biogenesis; N, cell motility; O, post-translational modification and protein turnover, chaperones; T, signal transduction mechanisms; U, intracellular trafficking, secretion, and vesicular transport; V, defense mechanisms; C, energy production and conversion; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G, carbohydrate transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolites biosynthesis, transport and catabolism; R, general function prediction only; S, function unknown. "No hit" group indicates non-significant short or low-complexity sequences. Query proteins not belong to any of the currently-defined COGs (23.51% in 2-D LC and 2-DE analysis and 36.33% in predicted ORFs) were not included in the figure for clarity.
Subcellular localization of identified proteins
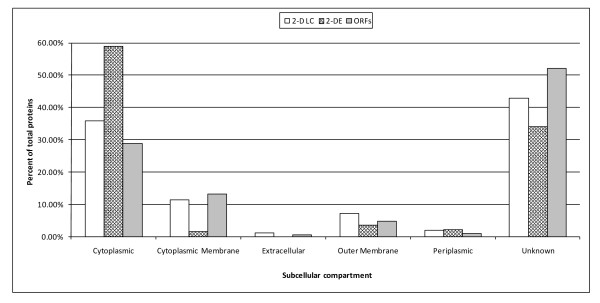
Subcellular locations of F. columnare proteins identified in this study and predicted from the draft genome were determined (Figure 3). 2-DE and 2-D LC generally resulted in similar coverage of subcellular compartments compared to the predicted proteome, except 2-DE gave better coverage of cytoplasmic proteins and poorer coverage of cytoplasmic membrane proteins. 2-D LC had better representation of proteins from both membrane compartments and extracellular proteins. Both 2-D LC and 2-DE had lower representation of the "unknown" subcellular location proteins than the percentage from the predicted proteome. In this study, 42 proteins were predicted to locate in the F. columnare outer membrane by PSORTb (Table 2).
Figure 3.
Subcellular locations of Flavobacterium columnare proteins determined by PSORTb prediction. Percentages were calculated by dividing the number of proteins in the particular subcellular location by the number of unique proteins in each analysis (548 proteins in 2D-LC, 141 proteins in 2-DE, and 2,882 ORFs predicted from the draft genome). 2-D LC and 2-DE indicates proteins identified in this study, while ORFs indicates proteins predicted from the whole genome. Unknown category includes proteins with multiple subcellular locations or unknown location.
Table 2.
Outer membrane proteins of Flavobacterium columnare predicted by PSORTb.
| Protein name | ORF # | COGa) | Approach/SNb) |
|---|---|---|---|
| Conserved hypothetical protein | ORF02881 | - | 2-D LC |
| Conserved hypothetical protein | ORF00226 | - | 2-D LC |
| Conserved hypothetical protein | ORF01938 | D | 2-D LC |
| Conserved hypothetical protein | ORF00224 | - | 2-D LC |
| Conserved hypothetical protein | ORF02143 | - | 2-D LC |
| Conserved hypothetical protein | ORF02328 | R | 2-D LC |
| Extracellular elastinolytic metalloproteinase | ORF00198 | - | 2-D LC |
| Fjo24 | ORF02628 | - | 2-D LC |
| Hypothetical protein | ORF02366 | - | 2-D LC |
| Hypothetical protein | ORF01273 | - | 2-D LC |
| Hypothetical protein | ORF01272 | - | 2-D LC |
| Hypothetical protein | ORF01485 | - | 2-D LC |
| Hypothetical protein | ORF02042 | P | 2-D LC |
| Lipoprotein, putative | ORF01143 | - | 2-D LC |
| Lipoprotein, putative | ORF02619 | - | Both/166, 167 |
| Omp assembly complex, YaeT protein | ORF02858 | M | 2-D LC |
| OmpA | ORF02005 | M | Both/132 |
| OmpA | ORF00341 | M | 2-D LC |
| OmpA family protein | ORF00085 | M | 2-D LC |
| OmpA/MotB family | ORF00937 | M | 2-D LC |
| OmpA/MotB family | ORF01639 | N | 2-D LC |
| OmpA/MotB family | ORF00225 | M | 2-DE/11, 12 |
| Outer membrane efflux protein | ORF00073 | MN | 2-D LC |
| Outer membrane insertion C- signal domain protein | ORF00187 | - | 2-D LC |
| Peptidyl-prolyl cis-trans isomerase C | ORF02829 | O | 2-D LC |
| Putative TonB-dependent receptor | ORF00266 | P | 2-D LC |
| RagA protein, putative | ORF00258 | P | 2-D LC |
| Secreted peptidase, family M23 | ORF01434 | M | 2-D LC |
| Secreted protein | ORF02176 | - | 2-DE/141 |
| Snf2 family helicase | ORF00742 | KL | 2-D LC |
| Tetratricopeptide repeat domain protein | ORF01855 | R | 2-D LC |
| TonB-dependent outer membrane receptor | ORF00779 | P | 2-D LC |
| TonB-dependent outer membrane receptor | ORF02179 | P | 2-D LC |
| TonB-dependent outer membrane receptor | ORF00147 | P | 2-D LC |
| TonB-dependent outer membrane receptor | ORF02172 | P | 2-D LC |
| TonB-dependent outer membrane receptor | ORF02232 | P | 2-D LC |
| TonB-dependent outer membrane receptor | ORF01690 | P | 2-D LC |
| TonB-dependent outer membrane receptor, putative | ORF00704 | P | 2-D LC |
| TonB-dependent outer membrane receptor, putative | ORF02424 | P | 2-D LC |
| TonB-dependent receptor plug domain protein | ORF01433 | P | 2-D LC |
| TonB-dependent receptor, putative | ORF01511 | P | 2-D LC |
| Transcriptional regulator, AraC family protein | ORF01879 | R | 2-D LC |
a) COG categories are as follows: M, cell wall/membrane/envelop biogenesis; D, cell cycle control, cell division, chromosome partitioning; P, inorganic ion transport and metabolism; S, function unknown; N, cell motility; R, general function prediction only; O, post-translational modification and protein turnover, chaperones; K, transcription; L, replication, recombination and repair; -, "No COGs".
b) SN indicates spot number marked on the 2-DE image (Figure 1).
Pathway analysis
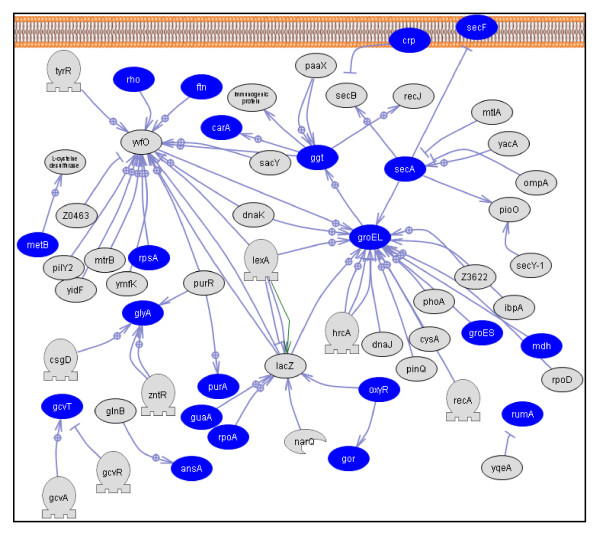
We used Pathway Studio analysis to gain insight into various pathways represented in F. columnare proteins identified by 2-D LC and 2-DE analysis. Twenty seven pathways belonging to metabolism and one pathway in genetic information processing were significantly represented (P < 0.05, Additional File 6: Table S6). Out of 28 pathways, eight related to amino acid metabolism (Figure 4), five carbohydrate metabolism, four cofactor and vitamin metabolism, four xenobiotics biodegradation and metabolism, two glycan biosynthesis and metabolism, two nucleotide metabolism, one energy metabolism, one biosynthesis of secondary metabolites, and one related to translational process. 277 proteins from our dataset were integral to these pathways. Of the significantly represented pathways, purine metabolism contained the highest number of proteins (24 proteins), while biotin metabolism pathway had the fewest (4 proteins). Of all the pathways, folate biosynthesis pathway was the most significant pathway in our dataset (P = 2.25E-09), which was followed by purine metabolism pathway (P = 1.44E-07).
Figure 4.
Amino acid metabolism related pathways in Flavobacterium columnare. Amino acid metabolism related pathways include glutamate; histidine; selenoamino acid; alanine and aspartate; glycine, serine, and threonine metabolism; lysine biosynthesis; lysine degradation; and valine, leucine, and isoleucine degradation pathways. Entities shown in oval represent genes involved in this pathway. Filled ovals represent proteins detected in this study. Unconnected genes were removed from the figure to reduce complexity.
Discussion
This research analyzed the soluble cell proteome of Flavobacterium columnare using complementary technologies of 2-D LC and 2-DE. Proteomic data from this study has provided experimental evidence for the expression of F. columnare proteins that were previously only predicted from the draft genome sequence. Data from this study provides a picture of the expressed F. columnare proteome during log-phase growth in FCGM along with a 2-D reference map, which will provide an important basis for comparison under other environmental conditions.
Currently, the most popular methods employed to explore protein expression utilize protein separation by 2-D LC (gel-free) or 2-DE (gel-based) followed by MS analysis [37]. Use of more than one methodology in proteomic investigations improves proteome coverage because each method identifies a set of unique proteins [38,39]. Therefore, in the present study, we employed the complementary techniques of 2-DE and 2-D LC to identify 621 unique proteins from F. columnare (480 unique to 2-D LC, 73 unique to 2-DE, and 68 common to both), which provided experimental evidence for more than one fifth (21.55%) of the predicted 2,882 F. columnare ORFs.
Ribosomal proteins, one of the most abundantly expressed protein types in cells, were detected in high numbers. In the draft F. columnare genome sequence, 60 proteins were predicted as ribosomal proteins. In the present study, we identified 14 and 3 unique ribosomal proteins using 2-D LC and 2-DE, respectively. Along with ribosomal proteins, we also identified several proteins involved in translational machinery, including several aminoacyl-tRNA synthases, translation initiation factors such as translation initiation factor IF-2 (InfB) (ORF00214), and elongation factors (EF) such as transcription elongation factor GreA (ORF00178), translation elongation factor Tu (ORF00244), translation elongation factor Ts (ORF00695), translation elongation factor P (ORF01368), and translation elongation factor G (ORF02228).
Outer membrane proteins (OMPs) often act as important virulence factors in Gram-negative bacteria. Therefore, identification of such molecules provides targets for future studies to increase our understanding of F. columnare pathogenesis. Moreover, OMPs may also play a crucial role in interacting with other molecules. In the present study, we identified 42 unique OMPs using 2-D LC and 2-DE analysis, which includes seven of the 14 OMPs previously identified by Liu et al. (p7, p8, p9, p10, p11, p12, p16) [36]. TonB-dependent outer membrane receptors function in the import of iron-siderophore complexes and vitamin B12 across the outer membrane in Gram-negative bacteria [40,41]. We identified six OmpA related proteins, which are the most abundant protein components of outer membranes of Gram-negative bacteria [42]. OmpA is predicted to be a nonspecific diffusion channel, allowing small solutes to cross the outer membrane [43].
In F. columnare, proteases, adherence factors, and chondroitin AC lyase have been reported as virulence factors [28,44]. The F. columnare genome encodes eight putative secreted metalloproteases/peptidases probably involved in virulence and/or in the destruction of host tissues. In this study, we identified only one extracellular elastinolytic metalloproteinase (ORF00198) using 2-D LC, which is probably due to the absence of host/host factors during F. columnare growth and/or isolation of the bacterial cell proteins, but not the secreted proteins. Chondroitin AC lyase was not detected, but we did detect fibronectin type III domain protein (ORF01481), a potential adherence factor that may be involved in cell surface binding of F. columnare. LemA (ORF00319) is also shown to be a putative virulence factor in several animal and plant bacteria especially in Pseudomonas [45,46].
F. columnare moves over surfaces by gliding motility. Thirteen gliding motility related proteins are encoded in the F. columnare draft genome, out of which we detected four (GldN, GldM, GldL and gliding motility-related protein). Detection of only four gliding motility proteins may be due to planktonic growth of F. columnare in broth culture.
Production of reactive oxygen species (ROS) by macrophages is one of the most effective defense mechanisms of host against bacterial pathogens [47]. Superoxide dismutases, bifunctional catalase-peroxidases, thiol peroxidases, and proteins of the peroxy-redoxin family are important bacterial defense mechanisms allowing survival of F. psychrophilum against phagocytes [48-52]. In the present study, we identified copper/zinc superoxide dismutase (SodC) (ORF02131), superoxide dismutase [Mn] (ORF02002), catalase/peroxidase HPI (KatG) (ORF02145), cytochrome c551 peroxidase (ORF01733), thiol peroxidase (ORF00815), and glutathione peroxidase (ORF00555). SodC is a periplasmic enzyme that converts superoxide radicals to hydrogen peroxide and water. Sod [Mn] is expressed only under aerobic conditions [53] and is believed to be involved in effective prevention of DNA damage in Escherichia coli [54]. Expression of these proteins in F. columnare suggests its ability to resist ROS.
The predicted F. columnare proteins were classified into COGs to assign functions. The majority of proteins identified in the current study (31.72%) were housekeeping proteins in "metabolism" related categories ('metabolism', 'cellular processes and signaling', and 'information storage and processing'). This was not surprising because we harvested proteins from bacteria when they were metabolically active at mid log phase. As compared to the predicted whole proteome, our experimental dataset had relatively lower coverage of the F. columnare proteins that were not assigned to COG groups. Proteins with unknown function may be expressed only under specialized conditions like host environment or stressed conditions and may not be expressed under laboratory culture conditions. Analysis of metabolism-related proteins in our list implies that amino acids, instead of carbohydrates, could be the major sources of carbon or energy. The FCGM medium we used for bacterial growth is a good source of amino acids, nitrogen, and certain salts like MgSO4 and CaCl2 but it lacks readily available carbohydrate sources. Most of the metabolic proteins indentified in our study were amino acid kinases, including five histidine kinases and five aminotransferases. Only three carbohydrate kinases involved in core carbohydrate metabolisms were identified. We found 18 peptidases, which may be useful in utilizing available amino acids as a major source of carbon. Previous studies on F. psychrophilum also suggest that this bacterium was unable to use readily available carbohydrates as a major energy source [55].
We used the PSORTb algorithm to predict the subcellular locations of F. columnare proteins. Many proteins were mapped to the cytoplasm, which is expected because these proteins are not hydrophobic, and thus solubility does not hinder protein isolation and separation. 2-DE was particularly effective at detection of cytoplasmic proteins. By contrast, cytoplasmic and outer membrane proteins were identified in higher percentages using 2-D LC when compared to 2-DE. The same buffer solution containing 7M urea and 2% CHAPSO was used to solubilize proteins during isolations for both 2D-LC and 2-DE, so it appears that the difference is not due to the isolation procedure, but rather the separation techniques. Other studies have reported that hydrophobic membrane proteins are typically difficult to identify using 2-DE. Hydrophobic proteins were found to be under-represented in 2-DE based proteome analyses of membrane protein fractions from E. coli [56-58] and Bacillus subtilis [39,59,60]. It is important to keep in mind that we chose to analyze only 192 common spots (30.81%) out of approximately 600 spots identified on our gels, so the bias toward cytoplasmic proteins in our 2-DE results may be because these proteins were more abundant or consistently present in gels. However, for future functional studies of membrane proteins, specialized isolation techniques may be needed. The ease and higher sensitivity of 2-D LC for detection of hydrophobic membrane proteins may make this the preferred method for studying membrane proteins.
From the Pathway Studio analysis, we identified pathways that were significantly represented in our protein dataset. Folate biosynthesis was the most significantly represented metabolic pathway, which was followed by purine metabolism pathway. Higher representation of metabolic and translational process related pathways is expected because proteins were isolated from F. columnare during a metabolically active state. Amino acid metabolism related pathways (glutamate; histidine; selenoamino acid; alanine and aspartate; glycine, serine, and threonine metabolism; lysine biosynthesis; lysine degradation; and valine, leucine, and isoleucine degradation) are highly represented in our dataset compared to carbohydrate metabolism. Moreover, we have identified 18 peptidases in our protein list suggesting breakdown of host proteins and usage of resultant amino acids as a major source of energy, carbon, and nitrogen by F. columnare. Similarly, Glycosylphosphatidylinositol (GPI)-anchor biosynthesis and LPS biosynthesis pathways were significantly represented in our dataset, which may play an important role in cell wall synthesis. Moreover, LPS biosynthesis may be one of the important virulence-related systems in F. columnare pathogenesis. LPS is an important component of the cell envelope in Gram-negative bacteria and is an immunodominant antigen.
Conclusions
Our main objective was to identify the expressed proteins of F. columnare during normal growth. We showed for the first time the expression of 621 proteins from F. columnare, which provides experimental evidence for many proteins that were predicted from the F. columnare genome annotation. We expect this information could accelerate functional and comparative studies aimed at understanding virulence mechanisms of this important pathogen. For example, comparative protein expression analysis between low and high virulence F. columnare strains could help determine proteins involved in virulence. Furthermore, orthologous protein mapping of identified F. columnare proteins to well studied bacteria would reveal protein targets for mutational analysis to understand gene function as well as to develop live attenuated vaccines.
Methods
Protein extraction
F. columnare growth medium (FCGM) [tryptone (8.00 g), yeast extract (0.80 g), MgSO4 7 H2O (1.00 g), CaCl2 2 H2O (0.74 g), NaCl (5.00 g), and sodium citrate (1.50 g) per liter] was used to grow F. columnare. Isolate ATCC 49512 was streaked on FCGM agar plates (FCGM medium plus 8.00 g agar per liter) and incubated at 30°C for 48 h. Four colonies were grown in 12 ml FCGM broth separately at 30°C under continuous shaking at 200 rpm. Growth of bacteria was monitored by measuring optical density at 600 nm (OD600), and bacteria were harvested at mid-exponential phase (OD600 0.6) by centrifugation at 3,750 rpm for 15 min at 30°C.
The four bacterial pellets were separately resuspended in cold urea-CHAPS buffer (7 M urea, 50 mM tris-HCl, 2% CHAPS, 8 mM PMSF pH 8.0), and cells were lysed immediately on ice by applying ten intermittent pulses of 10 s with a sonicator. Bacterial homogenates were centrifuged at 14,000 rpm for 5 min at 4°C to remove cell debris and unbroken cells. Proteins from supernatant were precipitated by trichloroacetic acid/Acetone, and the resultant protein pellets were resuspended in rehydration buffer (7 M urea, 20 mM tris-HCl, 5 mM EDTA, 5 mM MgCl2, 4% CHAPS, and 5 mM PMSF pH 8.0). Protein concentrations were estimated using a 2-D Quant Kit (GE Healthcare, Piscataway, NJ).
2-D LC ESI MS/MS analysis
100 μg of protein was digested with trypsin (1:50 w/w) at 37°C for 16 h. The resultant peptides were desalted, dried in a vacuum centrifuge, and resuspended in 20 μL of 5% acetonitrile and 0.1% formic acid. Mass spectrometric analysis was accomplished using a ProteomeX Workstation (Thermo Scientific, Waltham, MA) as described previously [61]. The F. columnare draft genome was annotated using Annotation Engine at the J. Craig Venter Institute, which uses the Glimmer algorithm for gene prediction [62,63]. The F. columnare protein database with 2,882 open reading frames (ORFs) is available on our website http://www.miangel.msstate.edu, while the F. columnare genome project is hosted at The Oklahoma University Health Sciences Center http://microgen.ouhsc.edu. To identify proteins, mass spectra and tandem mass spectra were searched against the in silico trypsin digested F. columnare protein database as previously reported [64]. Protein identifications and associated MS data were submitted to the PRIDE database [Accession number: 9749].
2-DE and MALDI MS analysis
Proteins were extracted from four mid-exponential phase bacterial cultures as described in section 2.1. Protein pellets were resuspended in 350 μL of freshly prepared rehydration buffer (7 M urea, 2 M thio urea, 2% CHAPS, 1:50 carrier ampholytes, 0.3% DTT) and quantified using 2-D Quant Kit (GE Healthcare). For in-gel rehydration, 800-1000 μg of solubilized proteins were loaded onto each IPG strip (17 cm pH 3-10 NL). IEF, in-gel trypsin digestion, and MALDI MS analysis were performed as previously described [64,65].
Identification of COGs, domains, protein locations, and pathways
All identified proteins from our dataset and the predicted whole F. columnare proteome were organized into COG functional groups using COGnitor tool [66-68]. Similarly, protein domain analysis was conducted by using batch Pfam analysis [69]. The subcellular location of proteins from our dataset as well as from the whole F. columnare proteome were predicted using PSORTb v2.0.4 [70,71]. Pathways with significant protein representation in our experimentally derived protein dataset were identified using Pathway Studio (Ariadne Genomics, Rockville, MD). Due to lack of a molecular interaction database for F. columnare in Pathway Studio, all F. columnare proteins were mapped to their orthologs in E. coli, F. psychrophilum, and F. johnsoniae by identifying reciprocal-best-BLAST hits. The resulting ortholog map file was used to predict pathways in F. columnare proteins. We used P ≤ 0.05 to select pathways with significant protein coverage.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PRD carried out the experiments, analysis and interpretation of the data, and preparation of the manuscript. NG participated in the experiments and preparation of the manuscript. MLL contributed to preparation and critical review of the manuscript. AK contributed to the overall conception and design of the project as well as preparation and critical review of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Table S1 - Flavobacterium columnare proteins identified by 2-D LC analysis.
Table S2 - Flavobacterium columnare proteins identified by 2-DE analysis.
Table S3 - Pfam analysis of proteins identified by 2-D LC analysis.
Table S4 - Pfam analysis of proteins identified by 2-DE analysis.
Table S5 - Pfam analysis of proteins predicted from the Flavobacterium columnare genome.
Table S6 - Pathways significantly represented in Flavobacterium columnare protein dataset.
Contributor Information
Pradeep R Dumpala, Email: pdumpala@cvm.msstate.edu.
Nagihan Gülsoy, Email: nagehan@marmara.edu.tr.
Mark L Lawrence, Email: lawrence@cvm.msstate.edu.
Attila Karsi, Email: karsi@cvm.msstate.edu.
Acknowledgements
The authors would like to thank The J. Craig Venter Institute for providing automated annotation. We thank Edward Zaitshik, Dr. Allison Gillaspy, and Dr. David Dyer at the Laboratory for Genomics and Bioinformatics at the University of Oklahoma Health Sciences Center for maintaining the annotation in Manatee. We also thank Tibor Pechan and Dr. Erdogan Memili for technical assistance, and Divya Swetha Peddinti for support with data analysis. Mass spectrometry was conducted at the Life Sciences and Biotechnology Institute, Mississippi State University. This project was partially supported by competitive grants from the National Research Initiative of the United States Department of Agriculture Cooperative State Research, Education and Extension Service (2006-35600-16571 and 2007-35204-18404). Approved for publication as Journal Article No J-11703 of the Mississippi Agricultural and Forestry Experiment Station, Mississippi State University.
References
- Bernardet J, Segers P, Cancanneyt M, Berthe M, Kersters K, Vandamme P. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (Basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46:128–148. doi: 10.1099/00207713-46-1-128. [DOI] [Google Scholar]
- Bernardet JF. Immunization with bacterial antigens: Flavobacterium and Flexibacter infections. Dev Biol Stand. 1997;90:179–188. [PubMed] [Google Scholar]
- Plumb J. Health maintenance and microbial diseases of cultured fishes. Iowa State University Press, Ames, IA. 1999.
- Moore A, Eimers ME, Cardella NA. Attempts to control Flexibacter columnaris epizootics in pond-reared channel catfish by vaccination. J Aquat Anim Health. 1990;2:109–111. doi: 10.1577/1548-8667(1990)002<0109:ATCCEI>2.3.CO;2. [DOI] [Google Scholar]
- Wakabayashi H. Effect of environmental conditions on the infectivity of Flexibacter columnaris to fish. J Fish Dis. 1991;14:279–290. doi: 10.1111/j.1365-2761.1991.tb00825.x. [DOI] [Google Scholar]
- Austin B, Austin DA. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish. Edinburgh, UK: Heriot-Watt University; 1999. [Google Scholar]
- Hawke JP, Thune RL. Systemic isolation and antimicrobial susceptibility of Cytophaga columnaris from commercially reared channel catfish. J Aquat Anim Health. 1992;4:109–113. doi: 10.1577/1548-8667(1992)004<0109:SIAASO>2.3.CO;2. [DOI] [Google Scholar]
- Bernardet JF, Grimont PAD. Deoxyribonucleic acid relatedness and phenotypic characterization of Flexibacter columnaris sp. nov., nom. rev., Flexibacter psychrophilus sp. nov., nom. rev., and Flexibacter maritimus Wakabayashi, Hikida, and Masumura 1986. Int J Syst Bacteriol. 1989;39:346–354. doi: 10.1099/00207713-39-3-346. [DOI] [Google Scholar]
- Ordal EJ, Rucker RR. Pathogenic myxobacteria. Proc Soc Exp Biol Med. 1944;56:15–18. [Google Scholar]
- Fijan FJ. Antibiotic additives for the isolation of Chondrococcus columnaris from fish. Appl Microbiol. 1969;17:333–334. doi: 10.1128/am.17.2.333-334.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decostere A, Haesebrouck F, Devriese LA. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J Clin Microbiol. 1997;35:322–324. doi: 10.1128/jcm.35.1.322-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin BR. A Simple Procedure for Identification of Cytophaga columnaris. J Aquat Anim Health. 1992;4:63–66. doi: 10.1577/1548-8667(1992)004<0063:ASPFIO>2.3.CO;2. [DOI] [Google Scholar]
- Bader JA, Shoemaker CA, Klesius PH. Rapid detection of columnaris disease in channel catfish (Ictalurus punctatus) with a new species-specific 16-S rRNA gene-based PCR primer for Flavobacterium columnare. J Microbiol Methods. 2003;52:209–220. doi: 10.1016/S0167-7012(02)00208-7. [DOI] [PubMed] [Google Scholar]
- Darwish AM, Ismaiel AA, Newton JC, Tang J. Identification of Flavobacterium columnare by a species-specific polymerase chain reaction and renaming of ATCC43622 strain to Flavobacterium johnsoniae. Mol Cell Probes. 2004;18:421–427. doi: 10.1016/j.mcp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Shoemaker CA, Arias CR, Klesius PH, Welker TL. Technique for identifying Flavobacterium columnare using whole-cell fatty acid profiles. J Aquat Anim Health. 2005;17:267–274. doi: 10.1577/H04-034.1. [DOI] [Google Scholar]
- Panangala VS, Shelby RA, Shoemaker CA, Klesius PH, Mitra A, Morrison EE. Immunofluorescent test for simultaneous detection of Edwardsiella ictaluri and Flavobacterium columnare. Dis Aquat Org. 2006;68:197–207. doi: 10.3354/dao068197. [DOI] [PubMed] [Google Scholar]
- Panangala VS, Shoemaker CA, Klesius PH. TaqMan real-time polymerase chain reaction assay for rapid detection of Flavobacterium columnare. Aquac Res. 2007;38:508–517. doi: 10.1111/j.1365-2109.2007.01695.x. [DOI] [Google Scholar]
- Yeh HY, Shoemaker CA, Klesius PH. Sensitive and rapid detection of Flavobacterium columnare in channel catfish Ictalurus punctatus by a loop-mediated isothermal amplification method. J Appl Microbiol. 2006;100:919–925. doi: 10.1111/j.1365-2672.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- Shamsudin MN, Plumb JA. Morphological, biochemical and physiological characterization of Flexibacter columnaris isolates from four fish species of fish. J Aquat Anim Health. 1996;8:335–382. doi: 10.1577/1548-8667(1996)008<0335:MBAPCO>2.3.CO;2. [DOI] [Google Scholar]
- Decostere A, Haesebrouck F, Devriese LA. Characterization of four Flavobacterium columnare (Flexibacter columnaris) strains isolated from tropical fish. Vet Microbiol. 1998;62:35–45. doi: 10.1016/S0378-1135(98)00196-5. [DOI] [PubMed] [Google Scholar]
- Darwish AM, Ismaiel AA. Genetic diversity of Flavobacterium columnare examined by restriction fragment length polymorphism RNA gene and the and sequencing of the 16S ribosomal 16S-23S rDNA spacer. Mol Cell Probes. 2005;19:267–274. doi: 10.1016/j.mcp.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Thomas-Jinu S, Goodwin AE. Morphological and genetic characteristics of Flavobacterium columnare isolates: correlations with virulence in fish. J Fish Dis. 2004;27:29–35. doi: 10.1046/j.1365-2761.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- Schneck JL, Caslake LF. Genetic diversity of Flavobacterium columnare isolated from fish collected from warm and cold water. J Fish Dis. 2006;29:245–248. doi: 10.1111/j.1365-2761.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- Olivares-Fuster O, Shoemaker CA, Klesius PH, Arias CR. Molecular typing of isolates of the fish pathogen, Flavobacterium columnare, by single-strand conformation polymorphism analysis. FEMS Microbiol Lett. 2007;269:63–69. doi: 10.1111/j.1574-6968.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- Soto E, Mauel MJ, Karsi A, Lawrence ML. Genetic and virulence characterization of Flavobacterium columnare from channel catfish (Ictalurus punctatus) J Appl Microbiol. 2008;104(5):1302–10. doi: 10.1111/j.1365-2672.2007.03632.x. [DOI] [PubMed] [Google Scholar]
- Staroscik AM, Hunnicutt DW, Archibald KE, Nelson DR. Development of methods for the genetic manipulation of Flavobacterium columnare. BMC Microbiol. 2008;8:115. doi: 10.1186/1471-2180-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader JA, Shoemaker CA, Klesius PH. Production, characterization and evaluation of virulence of an adhesion defective mutant of Flavobacterium columnare produced by beta-lactam selection. Letters in applied microbiology. 2005;40:123–127. doi: 10.1111/j.1472-765X.2004.01641.x. [DOI] [PubMed] [Google Scholar]
- Newton J, Wood TM, Hartley MM. Isolation and partial characterization of extracellular proteases produced by isolates of Flavobacterium columnare derived from channel catfish. J Aquat Anim Health. 1997;9:75–85. doi: 10.1577/1548-8667(1997)009<0075:IAPCOE>2.3.CO;2. [DOI] [Google Scholar]
- Stringer-Roth KM, Yunghans W, Caslake LF. Differences in chondroitin AC lyase activity of Flavobacterium columnare isolates. J Fish Dis. 2002;25:687–691. doi: 10.1046/j.1365-2761.2002.00421.x. [DOI] [Google Scholar]
- Suomalainen LR, Tiirola M, Valtonen ET. Chondroitin AC lyase activity is related to virulence of fish pathogenic Flavobacterium columnare. J Fish Dis. 2006;29:757–763. doi: 10.1111/j.1365-2761.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- Bertolini JM, Rohovec JS. Electrophoretic detection of proteases from different Flavobacterium columnare strains and assessment of their variability. Dis Aquat Org. 1992;12:121–128. doi: 10.3354/dao012121. [DOI] [Google Scholar]
- Christison J, Martin SM. Isolation and preliminary characterization of an extracellular protease of Cytophaga sp. Can J Microbiol. 1971;17:1207–1216. doi: 10.1139/m71-193. [DOI] [PubMed] [Google Scholar]
- Griffin BR. Characteristics of a chondrotin AC lyase produced by Cytophaga columnais. Trans Am Fish Soc. 1991;120:391–395. doi: 10.1577/1548-8659(1991)120<0391:COACAL>2.3.CO;2. [DOI] [Google Scholar]
- Pate JL, Ordal EJ. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967;35:37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Arias CR, Shoemaker CA, Klesius PH. Comparison of lipopolysaccharide and protein profiles between Flavobacterium columnare strains from different genomovars. J Fish Dis. 2006;29:657–663. doi: 10.1111/j.1365-2761.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- Liu GY, Nie P, Zhang J, Li N. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Flavobacterium columnare. J Fish Dis. 2008;31:269–276. doi: 10.1111/j.1365-2761.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- Gorg A, Weiss W, Dunn MJ. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4:3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- Salzano AM, Arena S, Renzone G, D'Ambrosio C, Rullo R, Bruschi M, Ledda L, Maglione G, Candiano G, Ferrara L, Scaloni A. A widespread picture of the Streptococcus thermophilus proteome by cell lysate fractionation and gel-based/gel-free approaches. Proteomics. 2007;7:1420–1433. doi: 10.1002/pmic.200601030. [DOI] [PubMed] [Google Scholar]
- Wolff S, Otto A, Albrecht D, Zeng JS, Buttner K, Gluckmann M, Hecker M, Becher D. Gel-free and gel-based proteomics in Bacillus subtilis: a comparative study. Mol Cell Proteomics. 2006;5:1183–1192. doi: 10.1074/mcp.M600069-MCP200. [DOI] [PubMed] [Google Scholar]
- Braun V, Hantke K, Koster W, (Ed) Bacterial iron transport: mechanisms, genetics, and regulation. In: Metal Ions in Biological Systems: Iron Transport and Storage in Microorganisms, Plants and Animals. New York: Marcel Dekker Inc; 1998. [PubMed] [Google Scholar]
- Letain TE, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- Molloy MP, Phadke ND, Maddock JR, Andrews PC. Two-dimensional electrophoresis and peptide mass fingerprinting of bacterial outer membrane proteins. Electrophoresis. 2001;22:1686–1696. doi: 10.1002/1522-2683(200105)22:9<1686::AID-ELPS1686>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Sugawara E, Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992;267:2507–2511. [PubMed] [Google Scholar]
- Decostere A, Haesebrouck F, Van Driessche E, Charlier G, Ducatelle R. Characterization of the adhesion of Flavobacterium columnare (Flexibacter columnaris) to gill tissue. J Fish Dis. 1999;22:465–474. doi: 10.1046/j.1365-2761.1999.00198.x. [DOI] [Google Scholar]
- Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak EM, Willis DK. The lemA gene required for pathogenicity of Pseudomonas syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992;174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secombes CJ. The nonspecific immune system: cellular defences. in The Fish Immune System: Organism, Pathogen and Environment. San Diego: Academic Press; 1996. [Google Scholar]
- Kawai Y, Okawarab AI, Okuyama H, Kura F, Suzuki K. Modulation of chemotaxis, O(2)(-) production and myeloperoxidase release from human polymorphonuclear leukocytes by the ornithine-containing lipid and the serineglycine-containing lipid of Flavobacterium. FEMS Immunol Med Microbiol. 2000;28:205–209. doi: 10.1111/j.1574-695X.2000.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Nematollahi A, Decostere A, Pasmans F, Haesebrouck F. Flavobacterium psychrophilum infections in salmonid fish. J Fish Dis. 2003;26:563–574. doi: 10.1046/j.1365-2761.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- Nematollahi A, Pasmans F, Haesebrouck F, Decostere A. Early interactions of Flavobacterium psychrophilum with macrophages of rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 2005;64:23–28. doi: 10.3354/dao064023. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno M, Monteoliva-Sanchez M, Ortega F, Ramos-Cormenzana A, Monteoliva M. Superoxide dismutase in strains of the genus Flavobacterium: isolation and characterization. Arch Microbiol. 1989;152:407–410. doi: 10.1007/BF00425182. [DOI] [PubMed] [Google Scholar]
- Duchaud E, Boussaha M, Loux V, Bernardet JF, Michel C, Kerouault B, Mondot S, Nicolas P, Bossy R, Caron C, Bessières P, Gibrat JF, Claverol S, Dumetz F, Le Hénaff M, Benmansour A. Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat Biotechnol. 2007;25:763–769. doi: 10.1038/nbt1313. [DOI] [PubMed] [Google Scholar]
- Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988;170:2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkin KA, Papazian MA, Steinman HM. Functional differences between manganese and iron superoxide dismutases in Escherichia coli K-12. J Biol Chem. 1992;267:24253–24258. [PubMed] [Google Scholar]
- Bernardet JF, Kerouault B. Phenotypic and genomic studies of "Cytophaga psychrophila" isolated from diseased rainbow trout (Oncorhynchus mykiss) in France. Appl Environ Microbiol. 1989;55:1796–1800. doi: 10.1128/aem.55.7.1796-1800.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis M, Gasser R. Proteomic analysis of the cell envelope fraction of Escherichia coli. Amino Acids. 2003;24:19–41. doi: 10.1007/s00726-002-0339-z. [DOI] [PubMed] [Google Scholar]
- Lai EM, Nair U, Phadke ND, Maddock JR. Proteomic screening and identification of differentially distributed membrane proteins in Escherichia coli. Mol Microbiol. 2004;52:1029–1044. doi: 10.1111/j.1365-2958.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- Molloy MP, Herbert BR, Slade MB, Rabilloud T, Nouwens AS, Williams KL, Gooley AA. Proteomic analysis of the Escherichia coli outer membrane. Eur J Biochem. 2000;267:2871–2881. doi: 10.1046/j.1432-1327.2000.01296.x. [DOI] [PubMed] [Google Scholar]
- Bunai K, Yamane K. Effectiveness and limitation of two-dimensional gel electrophoresis in bacterial membrane protein proteomics and perspectives. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:227–236. doi: 10.1016/j.jchromb.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Eymann C, Dreisbach A, Albrecht D, Bernhardt J, Becher D, Gentner S, Tam le T, Buttner K, Buurman G, Scharf C, Venz S, Völker U, Hecker M. A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics. 2004;4:2849–2876. doi: 10.1002/pmic.200400907. [DOI] [PubMed] [Google Scholar]
- McCarthy FM, Burgess SC, van den Berg BH, Koter MD, Pharr GT. Differential detergent fractionation for non-electrophoretic eukaryote cell proteomics. J Proteome Res. 2005;4:316–324. doi: 10.1021/pr049842d. [DOI] [PubMed] [Google Scholar]
- Salzberg SL, Delcher AL, Kasif S, White O. Microbial gene identification using interpolated Markov models. Nucleic acids research. 1998;26:544–548. doi: 10.1093/nar/26.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic acids research. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumpala PR, Lawrence ML, Karsi A. Proteome analysis of Edwardsiella ictaluri. Proteomics. 2009;9:1353–1363. doi: 10.1002/pmic.200800652. [DOI] [PubMed] [Google Scholar]
- Chaudhary A, Pechan T, Willett KL. Differential protein expression of peroxiredoxin I and II by benzo(a)pyrene and quercetin treatment in 22Rv1 and PrEC prostate cell lines. Toxicol Appl Pharmacol. 2007;220:197–210. doi: 10.1016/j.taap.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Natale DA, Garkavtsev IV, Tatusova TA, Shankavaram UT, Rao BS, Kiryutin B, Galperin MY, Fedorova ND, Koonin EV. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001;29:22–28. doi: 10.1093/nar/29.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K. The Pfam protein families database. Nucleic acids research. 2010;38:D211–222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Ester M, Brinkman FS. PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21:617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- Gardy JL, Spencer C, Wang K, Ester M, Tusnady GE, Simon I, Hua S, deFays K, Lambert C, Nakai K, Brinkman FS. PSORT-B: Improving protein subcellular localization prediction for Gram-negative bacteria. Nucleic Acids Res. 2003;31:3613–3617. doi: 10.1093/nar/gkg602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 - Flavobacterium columnare proteins identified by 2-D LC analysis.
Table S2 - Flavobacterium columnare proteins identified by 2-DE analysis.
Table S3 - Pfam analysis of proteins identified by 2-D LC analysis.
Table S4 - Pfam analysis of proteins identified by 2-DE analysis.
Table S5 - Pfam analysis of proteins predicted from the Flavobacterium columnare genome.
Table S6 - Pathways significantly represented in Flavobacterium columnare protein dataset.