Abstract
Background
Animal mitochondrial genomes are potential models for molecular evolution and markers for phylogenetic and population studies. Previous research has shown interesting features in hymenopteran mitochondrial genomes. Here, we conducted a comparative study of mitochondrial genomes of the family Braconidae, one of the largest families of Hymenoptera, and assessed the utility of mitochondrial genomic data for phylogenetic inference at three different hierarchical levels, i.e., Braconidae, Hymenoptera, and Holometabola.
Results
Seven mitochondrial genomes from seven subfamilies of Braconidae were sequenced. Three of the four sequenced A+T-rich regions are shown to be inverted. Furthermore, all species showed reversal of strand asymmetry, suggesting that inversion of the A+T-rich region might be a synapomorphy of the Braconidae. Gene rearrangement events occurred in all braconid species, but gene rearrangement rates were not taxonomically correlated. Most rearranged genes were tRNAs, except those of Cotesia vestalis, in which 13 protein-coding genes and 14 tRNA genes changed positions or/and directions through three kinds of gene rearrangement events. Remote inversion is posited to be the result of two independent recombination events. Evolutionary rates were lower in species of the cyclostome group than those of noncyclostomes. Phylogenetic analyses based on complete mitochondrial genomes and secondary structure of rrnS supported a sister-group relationship between Aphidiinae and cyclostomes. Many well accepted relationships within Hymenoptera, such as paraphyly of Symphyta and Evaniomorpha, a sister-group relationship between Orussoidea and Apocrita, and monophyly of Proctotrupomorpha, Ichneumonoidea and Aculeata were robustly confirmed. New hypotheses, such as a sister-group relationship between Evanioidea and Aculeata, were generated. Among holometabolous insects, Hymenoptera was shown to be the sister to all other orders. Mecoptera was recovered as the sister-group of Diptera. Neuropterida (Neuroptera + Megaloptera), and a sister-group relationship with (Diptera + Mecoptera) were supported across all analyses.
Conclusions
Our comparative studies indicate that mitochondrial genomes are a useful phylogenetic tool at the ordinal level within Holometabola, at the superfamily within Hymenoptera and at the subfamily level within Braconidae. Variation at all of these hierarchical levels suggests that the utility of mitochondrial genomes is likely to be a valuable tool for systematics in other groups of arthropods.
Background
Most animal mitochondrial genomes are about 16 Kb in size and contain 37 genes: 13 protein-coding genes, 22 transfer RNA genes (tRNA) and two ribosomal RNA genes (rRNA) [1]. Additionally, an A+T-rich region is present which contains essential regulatory elements for transcription and replication. It is therefore referred to as the control region [2]. Complete mitochondrial genomes provide good models for molecular evolution and abundant molecular markers for phylogenetic and population studies [3-6].
Because mitochondrial genomes are highly economized, with few intergenic regions, gene rearrangements are rare [1]. However in some lineages they are more common. For example amongst arthropods, the following groups show significant gene rearrangements: Myriapoda [7], Hymenoptera [8], hemipteroids [9-11], Acari [12,13], Araneae [14,15], and Isopoda [16,17]. These lineages therefore make ideal candidates for the study of gene rearrangement mechanisms [8,18-21].
Mitogenomic studies of the Hymenoptera have revealed many interesting features: (i) gene arrangements are conserved in the basal Hymenoptera, i.e., the grade Symphyta, whereas frequent gene rearrangements are observed in the derived clade, Apocrita [8,22] with approximately equal amounts of gene shuffling, inversion, and translocation [8]; (ii) tRNA positions are selectively neutral in all studied hymenopteran mitochondrial genomes [23]; (iii) various gene rearrangement mechanisms are necessary to explain the derived gene arrangement patterns in Hymenoptera [8,24], whereas amongst vertebrates, most gene rearrangement events are best explained by tandem duplication [25]; (v) nucleotide substitution rates are extremely high in the mitochondrial genomes of three Nasonia (Hymenoptera: Chalcidoidea) species, about 30 times faster than nuclear protein-coding genes [26].
The number of hymenopteran mitochondrial genomes, though high relative to other taxa, is rather limited, especially in relation to the species-richness of the order [24,26-31]. The characterization of more hymenopteran mitochondrial genomes has promise in answering evolutionary questions such as mechanisms of remote inversion events of gene rearrangement [8] and the variation of strand-specific compositional bias between Braconidae and Ichneumonidae [8,32].
Here we sequenced seven complete mitochondrial genomes of members of Braconidae, representing the subfamilies, Doryctinae, Opiinae, Microgastrinae, Cheloninae, Aphidiinae, Macrocentrinae and Euphorinae. Braconidae was selected because it is one of the largest families in Hymenoptera, second only to Ichneumonoidea, and because no complete mitochondrial genome has been reported for the family, although one of the reported mitochondrial gene rearrangement hot spots has been examined in this family [8,20]; Due to variation within the family, Braconidae is an ideal group to study the evolution of modes of parasitism [33]. Members of the subfamily Doryctinae are mostly ectoparasitic idiobionts (the host does not recover after paralysis by an ovipositing wasp and the wasp larvae feeds immediately), whereas member of the other six subfamilies are endoparasitic koinobionts (the host recovers after oviposition and develops normally for some time before it is consumed by the parasitoid). Though many efforts have been focused on the phylogeny of Braconidae, it is still a problematic group with many unresolved relationships [34-41].
Holometabolous insects represent the most successful lineages of Metazoa with 11 orders encompassing more than half of all known animal species. However, the phylogenetic relationships among orders in holometabolous insects are still controversial. Studies based on morphology or single molecular markers are limited by character quantity and quality respectively [42,43], whereas those based on complete mitochondrial genome sequences or nuclear genomes are limited by taxon sampling [44,45].
In this study, we explored some evolutionary traits of braconid mitochondrial genomes, and consequently assessed the phylogenetic utility of mitogenomics at three hierarchical levels, i.e., Braconidae, Hymenoptera and Holometabola.
Results and Discussion
General description Braconidae mitochondrial genomes
Two complete mitochondrial genomes from Spathius agrili Yang and Cotesia vestalis Haliday and five nearly complete mitochondrial genomes from Aphidius gifuensis (Ashmead), Diachasmimorpha longicaudata (Ashmead), Phanerotoma flava Ashmead, Macrocentrus camphoraphilus He & Chen, and Meteorus pulchricornis (Wesmael) were sequenced, representing seven subfamilies of Braconidae (Table 1). The regions that we failed to sequence were usually located in or around gene nad2 and the A+T-rich region, where extremely high A+T content, frequent gene rearrangement and stable stem-and-loop structures may have disrupted PCR and sequencing reactions. This is a common problem in sequencing of hymenopteran mitochondrial genomes [28,29,46].
Table 1.
General information of the mitochondrial genomes from Ichneumonoidea
| Species | Length (bp) | Completeness | Family | Subfamily | Accession number | Resources |
|---|---|---|---|---|---|---|
| Diadegma semiclausum | 18728 | Complete | Ichneumonidae | Campopleginae | EU871947 | Wei et al., 2009 |
| Enicospilus sp. | 15300 | Incomplete | Ichneumonidae | Ophioninae | FJ478177 | Dowton et al., 2009 |
| Cotesia vestalis | 15543 | Complete | Braconidae | Microgastrinae | FJ154897 | This study |
| Spathius agrili | 15425 | Complete | Braconidae | Doryctinae | FJ387020 | This study |
| Phanerotoma flava | 10171 | Incomplete | Braconidae | Cheloninae | GU097654 | This study |
| Diachasmimorpha longicaudata | 13850 | Incomplete | Braconidae | Opiinae | GU097655 | This study |
| Macrocentrus camphoraphilus | 15801 | Incomplete | Braconidae | Macrocentrinae | GU097656 | This study |
| Meteorus pulchricornis | 10186 | Incomplete | Braconidae | Euphorinae | GU097657 | This study |
| Aphidius gifuensis | 11996 | Incomplete | Braconidae | Aphidiinae | GU097658 | This study |
All genes identified in the seven mitochondrial genomes are typical animal mitochondrial genes with normal gene sizes. In all, 37 genes and an A+T-rich region were identified in the two completely sequenced mitochondrial genomes of S. agrili and C. vestalis. In the mitochondrial genome of M. camphoraphilus, a region spanning partial sequence of cob and nad1 was duplicated and inserted downstream of the rrnS - trnM - trnI - A+T-rich region and reversed. This is the first report of protein-coding gene sequence duplication in Hymenoptera mitochondrial genomes. The duplicated region led to the failure of amplification of the sequence further downstream.
All protein-coding genes start with ATN start codons and stop with TAA termination codons or truncated termination codons TA or T. Gene nad1 has been found to employ TTG as a start codon in some species of Hymenoptera, Coleoptera and Lepidoptera, thus minimizing intergenic spacing and avoiding overlap with adjacent genes [47-49], however, we did not discover TTG start codons in nad1 genes for any Braconidae.
Most transfer RNA (tRNA) genes have the usual cloverleaf structure and anticodons commonly found in insects. All trnK and trnS2 use TTT and TCT as anticodons rather than the normal CTT and GCT, respectively. The use of abnormal anticodons in these two tRNAs appears to be correlated with gene rearrangement [24].
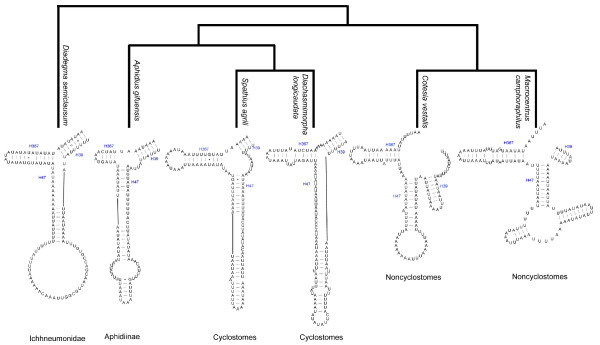
Two typical animal mitochondrial ribosomal RNAs (rrnL and rrnS) were sequenced in five species, i.e., A. gifuensis, S. agrili, D. longicaudata, C. vestalis, M. camphoraphilus. All of their rRNA genes conform to the secondary structure models proposed for other insects [24,50]. Compared to the secondary structure of D. semiclausum (Hymenoptera: Ichneumonidae) [24], these five species of Braconidae lack H9 and H17 in rrnS suggesting that it may be a widespread feature in Braconidae. In domain I, H37, H47 and H367 were variable among these five species. All the above three structures were present in A. gifuensis, S. agrili and D. longicaudata, as in D. semiclausum, but there was a big internal bulge at the bottom of H47 in A. gifuensis. In C. vestalis and M. camphoraphilus, H39 is formed by a continuous segment. The structures of H37, H47 and H367, suggest that Cyclostomes and Noncyclostomes are monophyletic and that Aphidiinae are the sister-group to the cyclostomes (Figure 1), congruent with the structures inferred from all protein coding genes (see below).
Figure 1.
Predicted phylogenetic relationships among five braconid species based on the secondary structures of H39, H47 and H367 in Domain I of rrnS. Base-pairing is indicated as follows: Watson-Crick pairs by lines, wobble GU pairs by dots and other noncanonical pairs by circles.
A+T-rich region
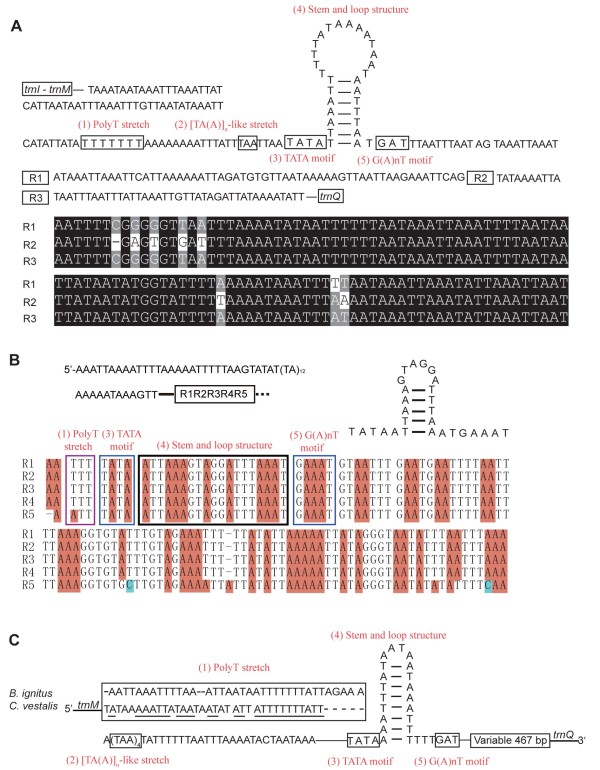
The A+T-rich region is believed to be involved in the regulation of transcription and control of DNA replication, characterized by five elements: (1) a polyT stretch at the 5'end of the A+T-rich region, which may be involved in the control of transcription and/or replication initiation; (2) a [TA(A)]n-like stretch following the polyT stretch; (3) a stem and loop structure, which may be associated with the second strand-replication origin; (4) a TATA motif and a G (A)nT motif flanking the stem and loop structure and (5) a G+A rich sequence downstream of the stem and loop structure [2].
A+T-rich regions were successfully sequenced for species, S. agrili, D. longicaudata, C. vestalis and M. camphoraphilus. Elements presumed to be involved in genome replication and transcription were found in all sequenced A+T-rich regions except that of M. camphoraphilus (Figure 2). The A+T-rich region in the mitochondrial genome of S. agrili is 578 bp long, with an A+T content of 93.6%, whereas in C. vestalis it is 571 bp long, with an A+T content of 92.6%. In the A+T-rich region of S. agrili, three repeat sequences are present downstream of the identified elements. In the A+T-rich region of D. longicaudata, identified elements are included in five repeat elements. However, all identified elements in the A+T-rich region of these three braconid species were found to be located in opposite directions and strands relative to those of other insects [2,30], indicating an inversion of the A+T-rich region in these species [16]. This is the first time that an inversion of the A+T-rich region has been demonstrated structurally for insects.
Figure 2.
Structural elements of A+T-rich region in three braconid mitochondrial genomes. (A) Structure of Spathius agrili mitochondrial A+T-rich region. Three repeat sequences are aligned. (B) Structure of Diachasmimorpha longicaudata mitochondrial A+T-rich region. Five repeat sequences including three elements were aligned. (C) Structure of Cotesia vestalis mitochondrial A+T-rich region. PolyT stretches were compared in C. vestalis and Bombus ignitus. Short dashes indicate gaps; underlines of the PolyT stretch sequence indicate conserved region between C. vestalis and B. ignitus.
Gene rearrangement
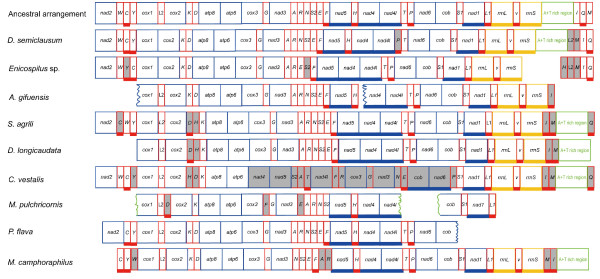
Gene rearrangement events occurred in all seven species of Braconidae (Figure 3). All rearranged genes were tRNA, except for C. vestalis, in which seven of the 13 protein-coding genes and 14 tRNA genes changed their positions or/and directions. Hymenoptera have been shown to have an accelerated rate of mitochondrial gene rearrangement [8,21], however, protein-coding gene rearrangement has only been found in the mitochondrial genomes of three Nasonia (Chalcidoidea:Pteromalidae) species, in which at least a large segment including six protein-coding genes and three tRNAs are inverted [26]. Gene rearrangement events in C. vestalis and the three Nasonia species indicate that large-scale protein-coding gene rearrangement are probably independent events in Hymenoptera.
Figure 3.
Gene arrangement of seven braconid mitochondrial genomes sequenced in this study. Abbreviations for the genes are as follows: cox1, cox2, and cox3 refer to the cytochrome oxidase subunits, cob refers to cytochrome b, and nad1-nad6 refer to NADH dehydrogenase components, rrnL and rrnS refer to ribosomal RNAs. Transfer RNA genes are denoted by one letter symbols according to the IPUC-IUB one-letter amino acid codes. L1, L2, S1, S2 denote tRNALeu(CUN), tRNALeu(UUR), tRNASer(AGY), tRNASer(UCN), respectively. Boxes with underscores indicate that the gene is encoded in minority strand. Shaded boxes indicate that the gene was rearranged compared with ancestral arrangement of Hexapoda.
We compared gene rearrangement rates among braconid mitochondrial genomes in the region from cox1 to trnH, which was successfully sequenced in all seven species; gene rearrangement rates were unequal among them. Gene arrangement pattern was conserved in A. gifuensis and P. flava, and derived in the other five species. Of the two microgastroid species P. flava and C. vestalis [20,41,51], the former is conserved while the latter was markedly rearranged in gene pattern. In conclusion, mitochondrial gene rearrangement rate was not taxonomically correlated at our level of investigation of the Braconidae.
Gene rearrangement events comprise three classes, i.e., translocation, local inversion (inverted but remaining in the position), and shuffling with remote inversion (translocated and inverted) [21]. All determined trnI and trnM were inverted and translocated, forming the arrangement pattern trnI(-) - trnM(-) - A+T-rich region - trnQ in S. agrili. D. longicaudata and C. vestalis, and trnM(-) - trnI(-) - A+T-rich region - trnQ in M. camphoraphilus.
In the three species S. agrili, D. longicaudata and C. vestalis, trnH was inverted and translocated (remote inversion) to the junction of cox2 and atp8. The arrangement pattern, trnD(-) - trnH - trnK or trnH(-) - trnD - trnK, has been found in many subfamilies of Braconidae. It has been reported that both the inversion of trnD and the remote inversion of trnH are independent evolutionary events in this family [8,20]. In the two reported species of Doryctinae, Jarra phorocantha and Heterospilus sp., the ancestral arrangement pattern of trnK - trnD occurs [8], which suggests that the gene rearrangement event in S. agrili occurred after the origin of the subfamily.
Gene rearrangement mechanism
Mechanisms for the three classes of gene rearrangement events in hymenopteran mitochondrial genomes are widely discussed [8,21]. Inter/intro mitochondrial genome combination is presumed to be the most plausible explanation for local inversions. trnY in C. vestalis is the only locally inverted gene in these seven braconid species, except for the region from trnE to cob in C. vestalis. The duplication/random loss model, and the intramitochondrial genome recombination and duplication/nonrandom loss model are possible mechanisms to explain translocation. Of these, intramitochondrial genome recombination is presumed to be more common. Shuffling is thought to be the result of duplication/random loss. The tRNA clusters trnW - trnC - trnY and trnK - trnD are frequently shuffled regions. trnD was not only shuffled but also inverted. This may have been the result of two independent events caused by separate mechanisms.
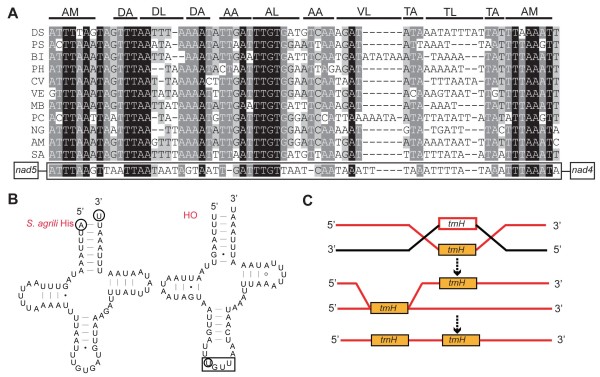
Remote inversion is a common rearrangement event in braconid mitochondrial genomes (trnH, trnI and trnM). However, remote inversion could not be deduced in previous studies due to incompleteness of sequence data [8,21]. In S. agrili, an inverted pseudo-trnH sequence is located between nad4 and nad5, the ancestral position of trnH (Figure 4A,B). Pseudo-genes are usually considered to be genomic evidence for the duplication and loss model of rearrangement [52-54]. The presence of the pseudo-trnH sequence in the same position and opposite direction to the ancestral trnH indicates that trnH was inverted before translocation. Both the inversion and translocation events may be the result of recombination as detailed above. During the inversion process, the two recombined sequences occur in opposite directions, whereas during translocation, the directions of the two recombined sequences are the same (Figure 4C). In C. vestalis, a 23 bp and in D. longicaudata a 52 bp intergenic region were found in the ancestral location of trnH, which might indicate a similar rearrangement process as trnH in S. agrili. The pseudo-trnH sequence in S. agrili is well conserved, whereas the ancestral sequence of trnH in C. vestalis and D. longicaudata were eliminated sometime after the loss of gene function [55]. Our results indicate that remote inversion may be caused by two separate recombination events.
Figure 4.
Mechanism of trnH remote inversion in Spathius agrili mitochondrial genome. (A) Presumed pseudo-trnH sequence (HO) and 10 hymenopteran trnH sequences are aligned according to their secondary structures. AM: Accepter arm, DA: D-loop arm, DL: D-loop, AA: Anticodon arm, AL: Anticodon loop, VL: Variable loop, TA: T Ψ C arm, TL: T Ψ C loop. (B) Secondary structure trnH is predicted in tRNAscan-SE search server [82] and HO is predicted manually. The inserted uracil in the anticodon is showed by a circle. (C) Recombination of two strands with opposite orientations leads the inversion of trnH, and the following recombination events lead the duplication of trnH. DS: Diadegma semiclausum, PS: Primeuchroeus spp., BI: Bombus ignites; PH: Polistes humilis, CV: Cotesia vestalis, VE: Vanhornia eucnemidarum, MB: Melipona bicolor, PC: Perga condei, NG: Nasonia giraulti, AM: Apis mellifera, SA: Spathius agrili.
Since trnI, trnM and the A+T-rich region were all inverted, separated remote inversions would make the rearrangement in this region extremely complicated. Therefore, it is more likely that trnI and trnM were inverted simultaneously. Thus, before the inversion of this region, trnQ would have been shuffled to form an intermediate pattern of A+T-rich region - trnM - trnI - trnQ. We observed a trnM - trnI - trnQ arrangement pattern in the mitochondrial genome of Diadegma semiclausum, an Ichneumonidae, the sister-group of Braconidae [24]. This suggests that trnM - trnI - trnQ is ancestral, and trnM - trnI - A+T-rich region is a derived pattern in Braconidae. Recombination is more likely to explain the inversion of A+T-rich region - trnM - trnI - trnQ based on the parsimony criterion.
In C. vestalis, it seems that all gene rearrangement events at atp6 - trnS1 junction are difficult to trace; however the parsimony criterion implies that three types of rearrangement events may have occurred (Figure 5): (1) Early large-scale inversion. Genes from trnE to cob were inverted as a whole before changing their relative positions. A large-scale inversion is present in the three sequenced Nasonia species, where either region nad3 to cox1 or trnF to cob is inverted. If an inverted region from trnE to cob is present in Ichneumonoidea, it may be a case of convergent evolution between the mitochondrial genomes of Chalcidoidea and Ichneumonoidea, because gene arrangement of this region is conserved in D. semiclausum (Ichneumonoidea) [24]. Since inversion cannot be explained by replication-slippage-based models [19], recombination is the most likely mechanism for this rearrangement event.
Figure 5.
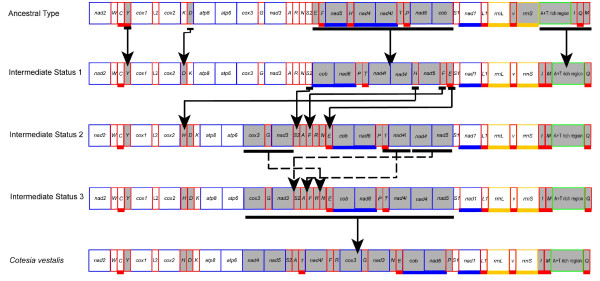
Putative gene rearrangement events in Cotesia vestalis mitochondrial genome. Four regions underwent gene rearrangements. Rearranged genes are shown in gray. The region from cox3 to cob experienced protein-coding gene rearrangements, and three types of rearrangement events might happen: large-scale inversion, tRNA rearrangement and small region rearrangement. The intermediate statuses are used to show different types of gene rearrangement events, but not the rearrangement process.
(2) tRNA translocations. The six tRNA genes between nad3 and nad5 in their ancestral position are heavily rearranged. And the most apparently conserved order of this tRNA cluster is trnA - trnR - trnN, which indicates that the derived relative positions of these tRNA genes are most likely the result of translocations of trnF and trnE and shuffling of trnS2. Nevertheless, trnH may have been inverted and translocated to the junction of cox2 and atp8 before the large-scale inversion event. The most possible mechanisms of gene translocation and shuffling are recombination, rather than tandem duplication followed by deletion (TDRL), which might change the remnant order of tRNA and the neighbouring protein-coding genes.
(3) Small-scale translocations. Four gene boundaries, i.e., nad4 - nad5, trnT - nad4l, cox3 - trnG - nad3 and cob - nad6 - trnP, were translocated after the large-scale inversion at the junction of atp6 - trnS1. Although tRNA gene rearrangement is more frequent than that of protein-coding genes, translocation of three gene boundaries is more parsimonious than tRNA gene rearrangement. Recombination is also favoured in these small-scale translocation events, because neither pseudo-genes nor large intergenic spacers are present in the boundaries of these rearranged genes, which are the intermediate state of the TDRL model [52].
Nucleotide composition
Braconidae mitochondrial genomes have high A+T content, a characteristic typical of other hymenopterans, with values from 82.4% to 87.2%.
Strand asymmetry (strand compositional bias) are usually reflected by AT skew, as expressed by (A-T)/(A+T), and GC skew, as expressed by (G-C)/(G+C) [56]. Strands of insect mitochondrial genome are discriminated as majority strand (encoding most genes) and minority strand (the other strand) [57]. In all braconid mitochondrial genomes, the signs of GC skew on the entire majority strand and all protein-coding genes were reversed relative to those of Ichneumonidae (Table 2).
Table 2.
Nucleotide composition of Ichneumonoidea mitochondrial genomes
| Species | Whole genome sequences | All protein-coding genes | |||||
|---|---|---|---|---|---|---|---|
| AT skew | GC skew | A+T% | AT skew | GC skew | A+T% | ||
| Diadegma semiclausum | 0.01 | -0.20 | 87.40 | -0.12 | -0.03 | 84.75 | |
| Enicospilus sp. | -0.02 | -0.18 | 85.10 | -0.11 | -0.02 | 85.12 | |
| Aphidius gifuensis | -0.06 | 0.05 | 84.70 | -0.16 | 0.09 | 84.80 | |
| Spathius agrili | -0.07 | 0.19 | 84.00 | -0.15 | 0.16 | 83.25 | |
| Diachasmimorpha longicaudata | -0.09 | 0.22 | 82.40 | -0.16 | 0.11 | 81.52 | |
| Cotesia vestalis | -0.09 | 0.10 | 87.20 | -0.16 | 0.06 | 86.81 | |
| Phanerotoma flava | -0.07 | 0.28 | 84.50 | -0.16 | 0.19 | 85.38 | |
| Macrocentrus camphoraphilus | -0.05 | 0.09 | 86.60 | -0.13 | 0.10 | 86.47 | |
| Meteorus pulchricornis | -0.06 | 0.14 | 83.30 | -0.16 | 0.11 | 83.10 | |
Hassanin et al. (2005) suggested that strand asymmetry is best reflected in the GC skew. Hence, all braconid species in the present study show reversal of strand asymmetry, as in some other arthropods [16,58-61], flatworms [62], brachiopods [63], echinoderms [64] and fish [65].
Inversion of replication origin located in the A+T-rich region would lead to reversal of strand asymmetry [16,59,66], which were proved by examination of regulatory elements in A+T rich region in three sequenced braconid mitochondrial genomes. Although further sampling is necessary the present evidence suggests that reversal of strand asymmetry and inversion of the A+T-rich region is a synapomorphy for members of Braconidae.
Evolutionary rate
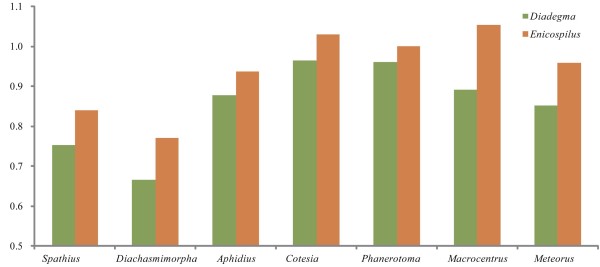
The rate of non-synonymous substitutions (Ka), the rate of synonymous substitutions (Ks) and the ratio of the rate of non-synonymous substitutions to the rate of synonymous substitutions (Ka/Ks) were calculated for each braconid mitochondrial genome using D. semiclausum or Enicospilus sp. (Hymenoptera: Ichneumonidae) as reference sequences (Figure 6). Species of non-cyclostomes showed higher evolutionary rates than those of cyclostomes. C. vestalis, P. flava and M. camphoraphilus showed the highest Ka/Ks ratios, indicating that the mitochondrial genomes with high gene rearrangement rates have high evolutionary rates, but those with low gene rearrangement rate are not constrained in their evolutionary rate.
Figure 6.
Evolutionary rates of braconid mitochondrial genomes. The ration of the number of nonsynonymous substitutions per nonsynonymous site (Ka) and the number of synonymous substitutions per synonymous site (Ks) for each braconid mitochondrial genomes, using that of Diadegma semiclausum or Enicospilus sp. as reference sequences.
Phylogenomics of Braconidae
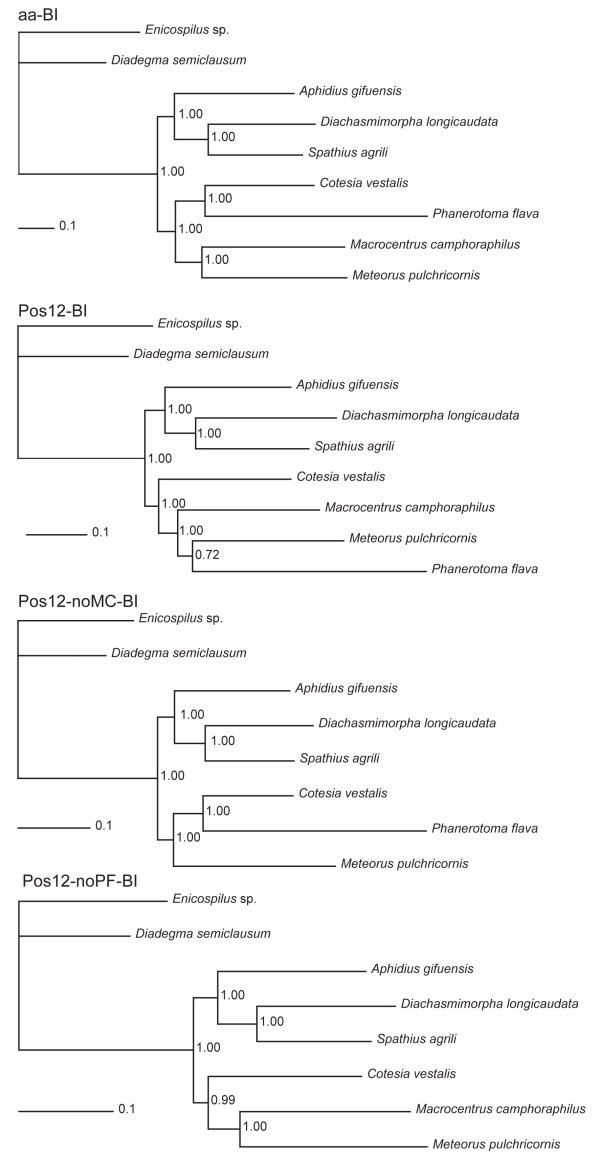
A sister-group relationship between S. agrili (subfamily Doryctinae) and D. longicaudata (subfamily Opiinae) were recovered in all analyses employing different inference methods and data. This is consistent with the widely accepted group, the cyclostomes. A. gifuensis (subfamily Aphidiinae) are firmly placed as the sister-group to the cyclostomes. Four species C. vestalis (Microgastrinae), P. flava (Cheloninae), M. camphoraphilus (Macrocentrinae) and M. pulchricornis (Euphorinae) constitute the other major group, the noncyclostomes. However, the internal relationships among noncyclostome subfamilies were not well resolved. In previous studies, Microgastrinae and Cheloninae were generally recovered in a clade referred to as microgastroids, while Macrocentrinae and Euphorinae form part of the clade, helconoids [20]. The latter group was recovered in our study in our Bayes analyses based on all amino acid sequences and it was also recovered in most likelihood analyses based on the first and second codon positions of protein-coding genes. In other analysis, Cheloninae was recovered as the sister-group of Macrocentrinae or Euphorinae, with Microgastrinae sister to the remaining noncyclostomes. Support values for the relationships Cheloninae + Euphorinae or Cheloninae + Macrocentrinae were higher than Microgastrinae + Cheloninae (well corroborated in many other analyses) and Macrocentrinae + Euphorinae. When M. camphoraphilus or P. flava were excluded from the Bayes analyses, both microgastroids and helconoids were recovered with improved support values (Figure 7, Additional file 1).
Figure 7.
Braconidae phylogeny based on complete mitochondrial genome sequences. Bayes phylogenetic trees for all seven braconid species based on amino acid sequence (aa) and nucleotide sequences of first and second codon positions (Pos12-BI) of all protein-coding genes except nad2, for species without Macrocentrus camphoraphilus (Pos12-noMC-BI) or Phanerotoma flava (Pos12-noPF-BI) were present. Bootstrap support values followed by Bayesian posterior probabilities (BPP) are shown at the right of respective nodes.
Subfamilies of braconid have traditionally been divided into two groups, cyclostomes and noncyclostomes [20,35,39]. These clades were firmly resolved in all our analyses of seven representative subfamilies. Aphidiinae was also recovered as sister-group to cyclostomes by Dowton (2002) and Zaldivar-Riverón (2006). Our results obviously support the sister group relationship between Aphidiinae and cyclostomes. Sampling of only seven of the 40 subfamilies of Braconidae may be the cause of the misplacement of Cheloninae in some analyses. Our analyses indicate that mitochondrial genome sequence data has the potential to resolve the phylogenetic relationships among braconid subfamilies with increased taxon sampling.
Phylogenomics of Holometabola with an emphasis on Hymenoptera
We performed 12 phylogenetic analyses using combinations of four datasets and three analytical methods to test the utility of mitochondrial genome sequences among hymenopteran superfamilies and holometabolus orders (Additional file 2). The third codon position has proved to be less restricted by purifying selection [67] and easily saturated with substitutions, thus it is usually ignored in phylogenetic analyses [68], and, Cameron et al. (2007b) found it to be a major source of homoplasy. In our analyses, the exclusion of the third codon improved the topology of the tree, furthermore, the recoding of purines and pyrimidines into R and Y showed improvement of both topology and support values (Figure 8). Baysian (BI) trees tend to reflect accepted topologies better than maximum likelihood (ML) and maximum parsimony (MP) trees. In the MP trees, many nodes were unresolved, and in ML, many nodes had low support values.
Figure 8.
A combined holometabolous phylogenetic tree based on all mitochondrial protein-coding genes. Bootstrap support values for the nodes inferred from four analyses (by Bayes inference method based on first, second and RY-coded third codon positions, first and second codon positions, and amino acid sequences, and by most likelihood method based on first, second and RY-coded third codon positions of all mitochondrial protein-coding genes) were shown sequentially separated by "/". "*" indicates that the node were fully supported by all four inferences; "-" indicates that the node was not recovered by the corresponding inference.
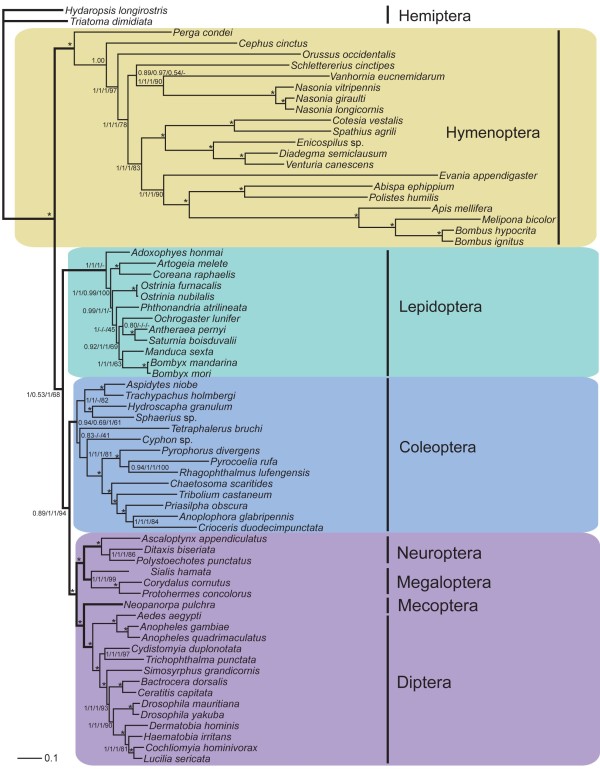
Monophyly of the seven in-group orders was recovered in most analysis. Hymenoptera was recovered at the base of all homometabolous lineages, except in most likelihood analyses based on amino acid and protein-coding positions, in which Hymenoptera and Lepidoptera were recovered as sister-groups with low support value. In this study, we included mitochondrial genome sequences from Neuroptera and Mecoptera for the first time. Mecoptera was recovered as a sister-group of Diptera. Neuropterida (Neuroptera + Megaloptera in this study) is supported across all analyses, forming the sister-group to (Diptera + Mecoptera), differing from the presently preferred sister-group relationship of Neuropterida and Coleoptera [43].
In the previous analysis using complete mitochondrial genome sequence data, the relationships among holometabolous orders were not well resolved [45,68]. Here, increased taxon sampling generated more stable phylogenetic relationships. The basal position of Hymenoptera is congruent with the analysis based on whole nuclear genome sequences [44].
Within Hymenoptera, three species of Symphyta representing three superfamilies and three families and 17 species of Apocrita representing seven superfamilies and eight families were used in analysis. Symphyta was shown to be paraphyletic; Apocrita was monophyletic with Orussoidea as its sister-group. Two major clades were recovered within Apocrita: Stephanoidea + Proctotrupomorpha, and Ichneumonoidea + (Evanioidea + Aculeata). Proctotrupomorpha (Proctotrupoidea + Chalcidoidea in this study), Ichneumonoidea (Ichneumonidae + Braconidae) and Aculeata ((Eumeninae + Vespinae) + Apoidea) in this study) were strongly supported in all analysis. Evaniomorpha proposed in [69] was not recovered: Evanoidea was recovered as sister-group to Aculeata, while Stephanoidea was sister to Proctotrupomorpha. Ichneumonoidea was the sister group of (Evanoidea + Aculeata). All nodes among Hymenoptera were perfectly supported in BI analyses except that of Stephanoidea + Proctotrupomorpha. In the topology based on amino acid sequences using BI, Stephanoidea was recovered as the sister-group of (Ichneumonoidea + (Evanoidea + Aculeata)), with a low support value of 0.54. In all analyses the ancestral position of Nasonia vitripennis among three Nasonia species is supported, which is congruent with the analyses based on nuclear gene sequence data and phylogeny of Wolbachia bacteria that they host [26,70].
Phylogenetic relationships among Hymenoptera at the superfamily level remain controversial [71]. Many well accepted phylogenetic relationships were recovered, such as the sister group relationship between Orussoidea and Apocrita, Apocrita, Proctotrupomorpha, Aculeata (or Vespomorpha) [69,72-78]. Evaniomorpha was frequently recovered as polyphyletic [71,75,76,79]. Castro and Dowton (2006) recovered a similar relationship among Ichneumonoidea, Evaniidae and Aculeata. The difference is that a group including Stephanidae was sister to Aculeata.
Within Diptera, well established relationships were recovered in nearly all of the analyses. Suborder relationships within Coleoptera were less stable. Adephaga and Myxophaga were recovered as sister group and then sister to Polyphaga. However, Archostemata was either recovered as sister-group to Polyphaga, or to Adephaga. Monophyly of the suborder Polyphaga is recovered in most analyses with internal relationships of Cyphon + (Elateroidea + (Chaetosoma + (Tribolium+ (Priasilpha + (Anoplophora + Crioceris))))). The intraordinal relationships of Lepidoptera are also unstable among datasets and methods. BI analyses supported early divergent of Rhopalocera (Lycaenidae + Pieridae in this study) most ML and MP analyses supported that of Tortricidae (Tortricoidea). Monophyly of Bombycoidea (Saturniidae + (Sphingidae + Bombycidae) in this study) was firmly supported in most analysis. The position of Lepidoptera and its internal relationships is likely negatively affected that the small sample of all taxa all of which were restricted to the clade Apoditrysia. We will not discuss the relationships among Coleoptera and Lepidoptera further since phylogenetic relationships are not robustly supported. However, our results might be of service to future researchers.
Conclusions
In this study, we reported seven complete or nearly complete mitochondrial genomes representing seven subfamilies of Braconidae. Four sequenced A+T-rich regions were shown to be inverted, as reported for species of Philopteridae (Phthiraptera) and Aleyrodidae (Hemiptera). Reversal of strand asymmetry was found in all seven sequenced mitochondrial genomes, which is correlated with the inversion of the A+T-rich region, indicating that inversion of A+T-rich region might be a ground-plan feature of braconid mitochondrial genomes. Mitochondrial gene rearrangement rates differed markedly among Braconidae, revealing that gene rearrangement might be more diverse than that previously reported [8,21,22,29]. Among different rearrangement events, remote inversion was common in Braconidae.
Noncyclostome species have a higher evolutionary rate in mitochondrial genome sequences than those of cyclostome species. Those species with high gene rearrangement rates also have high nucleotide sequence evolutionary rates.
Phylogenetic analysis using complete mitochondrial genomes sequences recovered most of the well corroborated phylogenetic relationships among major lineages of Braconidae, depending on the method and data matrix used. Cyclostomes and noncyclostomes were recovered in all analysis, and Aphidiinae was firmly recovered as be a sister-group to the cyclostomes.
Within Hymenoptera, many well accepted relationships, such as the paraphyly of Symphyta and Evaniomorpha, the sister-group relationship between Orussoidea and Apocrita, and the taxa Proctotrupomorpha, Ichneumonoidea and Aculeata, were recovered with high support values. New views among major groups in Hymenoptera are suggested, such as the sister-group relationship between Evaniidae and Aculeata. Within Diptera, relationships were very stable across analysis, but not stable among suborders of Coleoptera and major lineages of Lepidoptera.
Relationships among holometabolous orders were improved with the increase of sampling based on complete mitochondrial genome sequences and the recently suggested basal position of Hymenoptera was supported in most analysis.
In conclusion, complete mitochondrial genome data have obvious potential to infer phylogenetic relationships at the subfamily, family, ordinal levels.
Methods
DNA extraction, PCR amplification and sequencing
For each species, one male adult or a leg was homogenized in liquid nitrogen and total genomic DNA was extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany) following manufacturer protocols.
A range of universal insect mitochondrial primers and hymenopteran mitochondrial primers modified from universal insect mitochondrial primers were used [40,57,80,81]. When necessary, species-specific primers were designed based on sequenced fragments and combined in various ways to bridge gaps. PCR and sequencing reactions were conducted following Wei at al. (2009).
Genome annotation
Protein-coding and rRNA genes were initially identified using BLAST searches in GenBank and subsequently by alignment with genes of other insects. Protein-coding genes were translated using invertebrate mitochondrial genetic code. The tRNA searches were carried out with tRNAscan-SE search server [82]. The parameters for the tRNA scan were set for Mito/Chloromast as the source, and the Invertebrate Mito genetic code was used. When long tracts of non-coding sequence were apparent and tRNA genes were not detected using default settings, the cove cutoff score was reduced and the search repeated. Finally, those tRNA genes that could not be identified by tRNAscan-SE were inspected by eye. rRNA structures were constructed by comparison with those in other insects and algorithm-based methods as in [24,49]. All secondary structures were drawn in XRNA (developed by B. Weiser and available at http://rna.ucsc.edu/rnacenter/xrna/xrna.html).
Evolutionary rates
The software packages DnaSP 4.0 (Rozas et al. 2003) was used to compute the number of synonymous substitutions per synonymous site (Ks) and the number of nonsynonymous substitutions per nonsynonymous site (Ka) for each braconid mitochondrial genomes, using that of D. semiclausum or Enicospilus sp. as reference sequences.
Phylogenetic inference
For construction of the phylogenetic relationships among Braconidae, seven species with mitochondrial genomes sequenced in this study were used, representing seven subfamilies of Braconidae. D. semiclausum and Enicospilus sp., both Ichneumonidae, the family commonly accepted as the sister-group of the Braconidae, were employed as outgroups.
For construction of phylogenetic relationships among holometabolous insects, 69 species with complete or nearly complete mitochondrial genome sequences were used (Additional file 2). Twenty species were chosen from Hymenoptera, representing 10 superfamilies and 11 families [71]. Among Braconidae, S. agrili representing the cyclostomes and C. vestalis representing the noncyclostomes were used. Twelve species in nine families of the Lepidoptera, 14 species in 14 families of Coleoptera and 14 species in nine families of Diptera were included. Additionally, three species of Neuroptera, three species of Megaloptera and one species of Mecoptera were used to improve the sampling of orders among Holometabola. Hydaropsis longirostris and Triatoma dimidiata (Hemiptera) were used as outgroup taxa.
Amino acid sequences of protein-coding genes were aligned independently using ClustalX version 2.0.7 [83] with default parameters. Alignment of protein-coding genes was inferred from amino acid alignment using RevTrans [84]. Regions especially in the boundaries of genes that were aligned ambiguously were excluded in MacClade ver4.06 [85]. All protein-coding genes were concatenated following their ancestral order in insect mitochondrial genomes. Data were partitioned based on first, second and third codon positions.
Four datasets were employed in the phylogenetic analyses: amino acid sequence (aa), nucleotide sequences of first and second codon positions (Pos12), all codon positions (Pos123), first and second codon positions, and RY-coded (purines coded by R and pyrimidines coded by Y) third codon position (Pos12RY3) of all protein-coding genes. Gene nad2 was excluded in construction of the phylogenetic relationships of braconid subfamilies, because this gene failed to amplify in four of the seven braconid species.
Phylogenetic analyses were performed using Maximum Parsimony (MP) with PAUP* 4.0b10 [86], Maximum Likelihood (ML) with PhyML [87,88], and Bayesian Inference (BI) with MrBayes v3.1.2 [89]. The MP analyses were run with default heuristic search options except that 100 replicates of random stepwise additions were used. Bootstrap proportions (BPs) were obtained after 1000 replicates by using 10 replicates of random stepwise additions of taxa. Models of DNA substitution were estimated in Modeltest 3.7 [90]. For ML, we used a GTR+I+G model for nucleotide sequences and MtArt model for amino acid sequences with all parameters estimated. For BI, we used GTR+I+G model for four-state nucleotide sequences and a two-state substitution model with parameter I+G for RY-coded third codon position. All Bayesian analyses were conducted with four independent Markov chains run for 1,000,000 to 5,000,000 metropolis-coupled MCMC generations, with tree sampling every 100 to 500 generations and a burn-in of 2500 trees.
Authors' contributions
Conceived and designed the experiments: SJW and MS. Performed the experiments: SJW. Analyzed the data: SJW and MS. Wrote the paper: SJW. Intellectual contributions during the design and implementation of this study, and during the writing of the manuscript: XXC, MJS and CVA. Provided funding in support of this study: XXC and MJS. All authors read and approved the final manuscript.
Supplementary Material
Phylogenetic trees constructed in this study
Species used in phylogenetic reconstruction of Holometabola
Contributor Information
Shu-jun Wei, Email: shujun268@163.com.
Min Shi, Email: shimin0623@zju.edu.cn.
Michael J Sharkey, Email: msharkey@uky.edu.
Cornelis van Achterberg, Email: achterberg@naturalis.nnm.nl.
Xue-xin Chen, Email: xxchen@zju.edu.cn.
Acknowledgements
We thank Professor He Jun-Hua for his identification of the specimens used in this study. Dr. Zhu Chao-Dong from China Academy of Sciences, the Computational Biology Service Unit from Cornell University which is partially funded by Microsoft Corporation helped to carry some computation work. We also thank two anonymous reviewers for their valuable comments and suggestions. Funding for this study was provided jointly by the National Science Fund for Distinguished Young Scholars (30625006), the 973 Program (2006CB102005), the National Science Foundation of China (No. 30970384, 30700063, 30499341), and the National Special Basic Research Funds (2006FY110500-3, 2006FY120100) to XXC and the NSF (EF-0337220) to MJS.
References
- Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27(8):1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Hewitt GM. Insect mitochondrial control region: a review of its structure, evolution and usefulness in evolutionary studies. Bio-chem Syst Ecol. 1997;25(2):99–120. doi: 10.1016/S0305-1978(96)00042-7. [DOI] [Google Scholar]
- Miller W, Drautz DI, Janecka JE, Lesk AM, Ratan A, Tomsho LP, Packard M, Zhang Y, McClellan LR, Qi J. et al. The mitochondrial genome sequence of the Tasmanian tiger (Thylacinus cynocephalus) Genome Res. 2009;19(2):213–220. doi: 10.1101/gr.082628.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton RE, Milam JE, Roe BA. The complete sequence of the zebrafish (Danio rerio) mitochondrial genome and evolutionary patterns in vertebrate mitochondrial DNA. Genome Res. 2001;11(11):1958–1967. doi: 10.1101/gr.156801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingman M, Kaessmann H, Paabo S, Gyllensten U. Mitochondrial genome variation and the origin of modern humans. Nature. 2000;408(6813):708–713. doi: 10.1038/35047064. [DOI] [PubMed] [Google Scholar]
- Meng XF, Shi MIN, Chen X. Population genetic structure of Chilo suppressalis (Walker) (Lepidoptera: Crambidae): strong subdivision in China inferred from microsatellite markers and mtDNA gene sequences. Mol Ecol. 2008;17(12):2880–2897. doi: 10.1111/j.1365-294X.2008.03792.x. [DOI] [PubMed] [Google Scholar]
- Negrisolo E, Minelli A, Valle G. Extensive gene order rearrangement in the mitochondrial genome of the centipede Scutigera coleoptrata. J Mol Evol. 2004;58(4):413–423. doi: 10.1007/s00239-003-2563-x. [DOI] [PubMed] [Google Scholar]
- Dowton M, Austin AD. Evolutionary dynamics of a mitochondrial rearrangement "hot spot" in the Hymenoptera. Mol Biol Evol. 1999;16(2):298–309. doi: 10.1093/oxfordjournals.molbev.a026111. [DOI] [PubMed] [Google Scholar]
- Shao RF, Barker SC. The highly rearranged mitochondrial genome of the plague thrips, Thrips imaginis (Insecta: Thysanoptera): convergence of two novel gene boundaries and an extraordinary arrangement of rRNA genes. Mol Biol Evol. 2003;20(3):362–370. doi: 10.1093/molbev/msg045. [DOI] [PubMed] [Google Scholar]
- Shao R, Campbell NJH, Barker SC. Numerous gene rearrangements in the mitochondrial genome of the wallaby louse, Heterodoxus macropus (Phthiraptera) Mol Biol Evol. 2001;18(5):858–865. doi: 10.1093/oxfordjournals.molbev.a003867. [DOI] [PubMed] [Google Scholar]
- Shao RF, Campbell NJH, Schmidt ER, Barker SC. Increased rate of gene rearrangement in the mitochondrial genomes of three orders of hemipteroid insects. Mol Biol Evol. 2001;18(9):1828–1832. doi: 10.1093/oxfordjournals.molbev.a003970. [DOI] [PubMed] [Google Scholar]
- Navajas M, Le Conte Y, Solignac M, Cros-Arteil S, Cornuet JM. The complete sequence of the mitochondrial genome of the honeybee ectoparasite mite Varroa destructor (Acari: Mesostigmata) Mol Biol Evol. 2002;19(12):2313–2317. doi: 10.1093/oxfordjournals.molbev.a004055. [DOI] [PubMed] [Google Scholar]
- Shao RF, Barker SC, Mitani H, Takahashi M, Fukunaga M. Molecular mechanisms for the variation of mitochondrial gene content and gene arrangement among chigger mites of the genus Leptotrombidium (Acari: Acariformes) J Mol Evol. 2006;63(2):251–261. doi: 10.1007/s00239-005-0196-y. [DOI] [PubMed] [Google Scholar]
- Masta SE, Boore JL. The complete mitochondrial genome sequence of the spider Habronattus oregonensis reveals rearranged and extremely truncated tRNAs. Mol Biol Evol. 2004;21(5):893–902. doi: 10.1093/molbev/msh096. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Song DX, Zhou KY, Sun HY. The mitochondrial sequences of Heptathela hangzhouensis and Ornithoctonus huwena reveal unique gene arrangements and atypical tRNAs. J Mol Evol. 2005;60(1):57–71. doi: 10.1007/s00239-004-0010-2. [DOI] [PubMed] [Google Scholar]
- Kilpert F, Podsiadlowski L. The complete mitochondrial genome of the common sea slater, Ligia oceanica (Crustacea, Isopoda) bears a novel gene order and unusual control region features. BMC Genomics. 2006;7:241. doi: 10.1186/1471-2164-7-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlowski L, Bartolomaeus T. Major rearrangements characterize the mitochondrial genome of the isopod Idotea baltica (Crustacea: Peracarida) Mol Phylogenet Evol. 2006;40(3):893–899. doi: 10.1016/j.ympev.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Lavrov DV, Boore JL, Brown WM. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: duplication and nonrandom loss. Mol Biol Evol. 2002;19(2):163–169. doi: 10.1093/oxfordjournals.molbev.a004068. [DOI] [PubMed] [Google Scholar]
- Dowton M, Campbell NJH. Intramitochondrial recombination - is it why some mitochondrial genes sleep around? Trends Ecol Evol. 2001;16(6):269–271. doi: 10.1016/S0169-5347(01)02182-6. [DOI] [PubMed] [Google Scholar]
- Dowton M. Simultaneous molecular and morphological analysis of braconid relationships (Insecta: Hymenoptera: Braconidae) indicates independent mt-tRNA gene inversions within a single wasp family. J Mol Evol. 2002;54(2):210–226. doi: 10.1007/s00239-001-0003-3. [DOI] [PubMed] [Google Scholar]
- Dowton M, Castro LR, Campbell SL, Bargon SD, Austin AD. Frequent mitochondrial gene rearrangements at the hymenopteran nad3-nad5 junction. J Mol Evol. 2003;56(5):517–526. doi: 10.1007/s00239-002-2420-3. [DOI] [PubMed] [Google Scholar]
- Dowton M, Castro LR, Austin AD. Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: the examination of genome 'morphology'. Invertebr Syst. 2002;16(3):345–356. doi: 10.1071/IS02003. [DOI] [Google Scholar]
- Dowton M, Cameron SL, Dowavic JI, Austin AD, Whiting MF. Characterization of 67 mitochondrial tRNA gene rearrangements in the Hymenoptera suggests that mitochondrial tRNA gene position is selectively neutral. Molecular Biology and Evolution. 2009;26(7):1607. doi: 10.1093/molbev/msp072. [DOI] [PubMed] [Google Scholar]
- Wei SJ, Shi M, He JH, Sharkey MJ, Chen XX. The complete mitochondrial genome of Diadegma semiclausum (Hymenoptera: Ichneumonidae) indicates extensive independent evolutionary events. Genome. 2009;52:308–319. doi: 10.1139/G09-008. [DOI] [PubMed] [Google Scholar]
- San Mauro D, Gower DJ, Zardoya R, Wilkinson M. A hotspot of gene order rearrangement by tandem duplication and random loss in the vertebrate mitochondrial genome. Mol Biol Evol. 2006;23(1):227–234. doi: 10.1093/molbev/msj025. [DOI] [PubMed] [Google Scholar]
- Oliveira DCSG, Rhitoban R, Lavrov DV, Werren JH. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae) Mol Biol Evol. 2008;25(10):2167–2180. doi: 10.1093/molbev/msn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RH, Crozier YC. The mitochondrial genome of the honeybee Apis mellifera: complete sequence and genome organization. Genetics. 1993;133(1):97–117. doi: 10.1093/genetics/133.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro LR, Ruberu K, Dowton M. Mitochondrial genomes of Vanhornia eucnemidarum (Apocrita: Vanhorniidae) and Primeuchroeus spp. (Aculeata: Chrysididae): evidence of rearranged mitochondrial genomes within the Apocrita (Insecta: Hymenoptera) Genome. 2006;49(7):752–766. doi: 10.1139/G06-030. [DOI] [PubMed] [Google Scholar]
- Castro LR, Dowton M. The position of the Hymenoptera within the Holometabola as inferred from the mitochondrial genome of Perga condei (Hymenoptera: Symphyta: Pergidae) Molecular Phylogenetics and Evolution. 2005;34(3):469–479. doi: 10.1016/j.ympev.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Cha SY, Yoon HJ, Lee EM, Yoon MH, Hwang JS, Jin BR, Han YS, Kim I. The complete nucleotide sequence and gene organization of the mitochondrial genome of the bumblebee, Bombus ignitus (Hymenoptera: Apidae) Gene. 2007;392(1-2):206–220. doi: 10.1016/j.gene.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Silvestre D, Arias MC. Mitochondrial tRNA gene translocations in highly eusocial bees. Genet Mol Bio. 2006;29(3):572–575. [Google Scholar]
- Dowton M, Austin AD. The evolution of strand-specific compositional bias. A case study in the Hymenopteran mitochondrial 16S rRNA gene. SMBE. 1997;14:109. doi: 10.1093/oxfordjournals.molbev.a025696. [DOI] [PubMed] [Google Scholar]
- Gauld I. Evolutionary patterns of host utilization by ichneumonoid parasitoids (Hymenoptera: Ichneumonidae and Braconidae) Biol J Linn Soc. 1988;35(4):351–377. doi: 10.1111/j.1095-8312.1988.tb00476.x. [DOI] [Google Scholar]
- Achterberg C. A preliminary key to the subfamilies of the Braconidae (Hymenoptera) Tijdschrift voor Entomologie. 1976;119(3):33–78. [Google Scholar]
- Achterberg C. Essay on the phylogeny of Braconidae (Hymenoptera: Ichneumonoidea) Entomologisk Tidskrift. 1984;105:41–58. [Google Scholar]
- Achterberg C. Parallelisms in the Braconidae (Hymenoptera) with special reference to the biology. Advances Parasitic Hymenoptera Research. 1988. pp. 85–115.
- Achterberg K, Quicke DLJ. Phylogeny of the subfamilies of the family Braconidae: a reassessment assessed. Cladistics. 1992;8(3):237–264. doi: 10.1111/j.1096-0031.1992.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Dowton M, Austin AD. Phylogenetic relationships among the microgastroid wasps (Hymenoptera: Braconidae): combined analysis of 16S and 28S rDNA genes and morphological data. Molecular Phylogenetics and Evolution. 1998;10(3):354–366. doi: 10.1006/mpev.1998.0533. [DOI] [PubMed] [Google Scholar]
- Shi M, Chen XX, van Achterberg C. Phylogenetic relationships among the Braconidae (Hymenoptera: Ichneumonoidea) inferred from partial 16S rDNA, 28S rDNA D2, 18S rDNA gene sequences and morphological characters. Molecular Phylogenetics and Evolution. 2005;37(1):104–116. doi: 10.1016/j.ympev.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Zaldivar-Riverón A, Mori M, Quicke DLJ. Systematics of the cyclostome subfamilies of braconid parasitic wasps (Hymenoptera: Ichneumonoidea): A simultaneous molecular and morphological Bayesian approach. Mol Phylogenet Evol. 2006;38(1):130–145. doi: 10.1016/j.ympev.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Murphy N, Banks JC, Whitfield JB, Austin AD. Phylogeny of the parasitic microgastroid subfamilies (Hymenoptera: Braconidae) based on sequence data from seven genes, with an improved time estimate of the origin of the lineage. Molecular Phylogenetics and Evolution. 2008;47(1):378–395. doi: 10.1016/j.ympev.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Kristensen NP. Phylogeny of endopteryygote insects, the most successful lineage of living organisms. European Journal of Entomology. 1999;96:237–254. [Google Scholar]
- Whiting MF. Phylogeny of the holometabolous insect orders: molecular evidence. Zoologica Scripta. 2002;31(1):3–15. doi: 10.1046/j.0300-3256.2001.00093.x. [DOI] [Google Scholar]
- Savard J, Tautz D, Richards S, Weinstock GM, Gibbs RA, Werren JH, Tettelin H, Lercher MJ. Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the base of the radiation of Holometabolous insects. Genome Res. 2006;16(11):1334. doi: 10.1101/gr.5204306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SL, Lambkin CL, Barker SC, Whiting MF. A mitochondrial genome phylogeny of Diptera: whole genome sequence data accurately resolve relationships over broad timescales with high precision. Syst Entomol. 2007;32(1):40–59. doi: 10.1111/j.1365-3113.2006.00355.x. [DOI] [Google Scholar]
- Cameron SL, Dowton M, Castro LR, Ruberu K, Whiting MF, Austin AD, Diement K, Stevens J. Mitochondrial genome organization and phylogeny of two vespid wasps. Genome. 2008;51(10):800–808. doi: 10.1139/G08-066. [DOI] [PubMed] [Google Scholar]
- Sheffield NC, Song H, Cameron SL, Whiting MF. A comparative analysis of mitochondrial genomes in Coleoptera (Arthropoda: Insecta) and genome descriptions of six new beetles. Molecular Biology and Evolution. 2008;25(11):2499–2509. doi: 10.1093/molbev/msn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JS, Kim I, Sohn HD, Jin BR. The mitochondrial genome of the firefly, Pyrocoelia rufa: complete DNA sequence, genome organization, and phylogenetic analysis with other insects. Molecular Phylogenetics and Evolution. 2004;32(3):978–985. doi: 10.1016/j.ympev.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wei SJ, Tang P, Zheng LH, Shi M, Chen XX. The complete mitochondrial genome of Evania appendigaster (Hymenoptera: Evaniidae) has low A+ T content and a long intergenic spacer between atp8 and atp6. Molecular Biology Reports. 2010;37(4):1931–42. doi: 10.1007/s11033-009-9640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannone JJ, Subramanian S, Schnare MN, Collett JR, D'Souza LM, Du Y, Feng B, Lin N, Madabusi LV, Muller KM. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 2002;3(2):1471–2105. doi: 10.1186/1471-2105-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Chen XX. Molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae) based on DNA sequences of 16S rRNA, 18S rDNA and ATPase 6 genes. European Journal of Entomology. 2005;102(2):133–138. [Google Scholar]
- Mueller RL, Boore JL. Molecular mechanisms of extensive mitochondrial gene rearrangement in plethodontid salamanders. Mol Biol Evol. 2005;22(10):2104–2112. doi: 10.1093/molbev/msi204. [DOI] [PubMed] [Google Scholar]
- Arndt A, Smith MJ. Mitochondrial gene rearrangement in the sea cucumber genus Cucumaria. Mol Biol Evol. 1998;15(8):1009–1016. doi: 10.1093/oxfordjournals.molbev.a025999. [DOI] [PubMed] [Google Scholar]
- Macey JR, Schulte JA, Larson A, Papenfuss TJ. Tandem duplication via light-strand synthesis may provide a precursor for mitochondrial genomic rearrangement. Mol Biol Evol. 1998;15(1):71–75. doi: 10.1093/oxfordjournals.molbev.a025849. [DOI] [PubMed] [Google Scholar]
- Moritz C, Dowling TE, Brown WM. Evolution of animal mitochondrial-DNA - relevance for population biology and systematics. Annual Review of Ecology and Systematics. 1987;18:269–292. doi: 10.1146/annurev.es.18.110187.001413. [DOI] [Google Scholar]
- Perna NT, Kocher TD. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J Mol Evol. 1995;41(3):353–358. doi: 10.1007/BF01215182. [DOI] [PubMed] [Google Scholar]
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87(6):651–701. [Google Scholar]
- Cameron SL, Johnson KP, Whiting MF. The mitochondrial genome of the screamer louse Bothriometopus (Phthiraptera: Ischnocera): effects of extensive gene rearrangements on the evolution of the genome. J Mol Evol. 2007;65(5):589–604. doi: 10.1007/s00239-007-9042-8. [DOI] [PubMed] [Google Scholar]
- Hassanin A, Leger N, Deutsch J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of Metazoa, and consequences for phylogenetic inferences. Syst Biol. 2005;54(2):277–298. doi: 10.1080/10635150590947843. [DOI] [PubMed] [Google Scholar]
- Masta SE, Longhorn SJ, Boore JL. Arachnid relationships based on mitochondrial genomes: Asymmetric nucleotide and amino acid bias affects phylogenetic analyses. Mol Phylogenet Evol. 2009;50(1):117–128. doi: 10.1016/j.ympev.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Hassanin A. Phylogeny of Arthropoda inferred from mitochondrial sequences: strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution. Mol Phylogenet Evol. 2006;38(1):100–116. doi: 10.1016/j.ympev.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Min XJ, Hickey DA. DNA asymmetric strand bias affects the amino acid composition of mitochondrial proteins. DNA Res. 2007;14(5):201. doi: 10.1093/dnares/dsm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfenbein KG, Brown WM, Boore JL. The complete mitochondrial genome of the articulate brachiopod Terebratalia transversa. Mol Biol Evol. 2001;18(9):1734–1744. doi: 10.1093/oxfordjournals.molbev.a003961. [DOI] [PubMed] [Google Scholar]
- Scouras A, Smith MJ. The complete mitochondrial genomes of the sea lily Gymnocrinus richeri and the feather star Phanogenia gracilis: Signature nucleotide bias and unique nad4L gene rearrangement within crinoids. Mol Phylogenet Evol. 2006;39(2):323–334. doi: 10.1016/j.ympev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Wang J, He SP, Mayden RL. The complete mitochondrial genome of the Chinese hook snout carp Opsariichthys bidens (Actinopterygii: Cyprinifonnes) and an altemative pattem of mitogenomic evolution in vertebrate. Gene. 2007;399(1):11–19. doi: 10.1016/j.gene.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Fonseca MM, Posada D, Harris DJ. Inverted replication of vertebrate mitochondria. Mol Biol Evol. 2008;25(5):805. doi: 10.1093/molbev/msn050. [DOI] [PubMed] [Google Scholar]
- Jermiin LS, Graur D, Lowe RM, Crozier RH. Analysis of directional mutation pressure and nucleotide content in mitochondrial cytochrome b genes. J Mol Evol. 1994;39(2):160–173. doi: 10.1007/BF00163805. [DOI] [PubMed] [Google Scholar]
- Castro LR, Dowton M. Mitochondrial genomes in the Hymenoptera and their utility as phylogenetic markers. Syst Entomol. 2007;32(1):60–69. doi: 10.1111/j.1365-3113.2006.00356.x. [DOI] [Google Scholar]
- Rasnitsyn AP. An outline of evolution of the hymenopterous insects. Oriental Insects. 1988;22(1):115–145. [Google Scholar]
- Breeuwer JAJ, Stouthamer R, Barns SM, Pelletier DA, Weisburg WG, Werren JH. Phylogeny of cytoplasmic incompatibility microorganisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol Biol. 1993;1(1):25–36. doi: 10.1111/j.1365-2583.1993.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Sharkey MJ. Phylogeny and classification of Hymenoptera. Zootaxa. 2007;1668:521–548. [Google Scholar]
- Vilhelmsen L. The phylogeny of lower Hymenoptera (Insecta), with a summary of the early evolutionary history of the order. Journal of Zoological Systematics and Evolutionary Research. 1997;35:49–70. [Google Scholar]
- Dowton M, Austin AD. Molecular phylogeny of the insect order Hymenoptera: Apocritan relationships. Proceedings of the National Academy of Sciences. 1994;91(21):9911–9915. doi: 10.1073/pnas.91.21.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowton M, Austin A, Dillon N, Bartowsky E. Molecular phylogeny of the apocritan wasps: the Proctotrupomorpha and Evaniomorpha. Systematic Entomology. 1997;22(3):245–255. doi: 10.1046/j.1365-3113.1997.d01-42.x. [DOI] [Google Scholar]
- Dowton M, Austin AD. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita-evolutionary transitions among parasitic wasps. Biological Journal of the Linnean Society. 2001;74(1):87–111. [Google Scholar]
- Castro LR, Dowton M. Molecular analyses of the Apocrita (Insecta: Hymenoptera) suggest that the Chalcidoidea are sister to the diaprioid complex. Invertebrate Systematics. 2006;20(5):603–614. doi: 10.1071/IS06002. [DOI] [Google Scholar]
- Rasnitsyn AP. In: History of Insects. Rasnitsyn AP, Quicke DLJ, editor. Dordrecht: Kluwer Academic Publishers; 2002. Superorder Vespidea Laicharting, 1781. Order Hymenoptera Linné, 1758 (Vespida Laicharting, 1781) pp. 242–254. [Google Scholar]
- Rasnitsyn AP, Basibuyuk HH, Quicke DLJ. A basal chalcidoid (Insecta: Hymenoptera) from the earliest Cretaceous or latest Jurassic of Mongolia. Insect Systematics and Evolution. 2004;35(2):123–135. [Google Scholar]
- Ronquist F. Phylogeny, classification and evolution of the Cynipoidea. Zoologica Scripta. 1999;28(1-2):139–164. doi: 10.1046/j.1463-6409.1999.00022.x. [DOI] [Google Scholar]
- Simon C, Buckley TR, Frati F, Stewart JB, Beckenbach AT. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu Rev Ecol Syst. 2006;37:545–579. doi: 10.1146/annurev.ecolsys.37.091305.110018. [DOI] [Google Scholar]
- Barau JG, De Azeredo-Espin AML, Lessinger AC. Conservation and versatility of a new set of primers for long-PCR amplification of complete insect mitochondrial genomes based on Haematobia irritans mtDNA sequences. Molecular Ecology Notes. 2005;5(4):885–887. doi: 10.1111/j.1471-8286.2005.01103.x. [DOI] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Wernersson R, Pedersen AG. RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res. 2003;31(13):3537. doi: 10.1093/nar/gkg609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4.06: Analysis of phylogeny and character evolution. Sinauer Associates, Sunderland, Massachusetts; 2003. [Google Scholar]
- Swofford DL PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods), Version 4 2003Sinauer Associates, Sunderland, Massachusetts; 18428704 [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O. PHYML Online-a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005;33(suppl 2):W557–W559. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Posada D. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic trees constructed in this study
Species used in phylogenetic reconstruction of Holometabola