Abstract
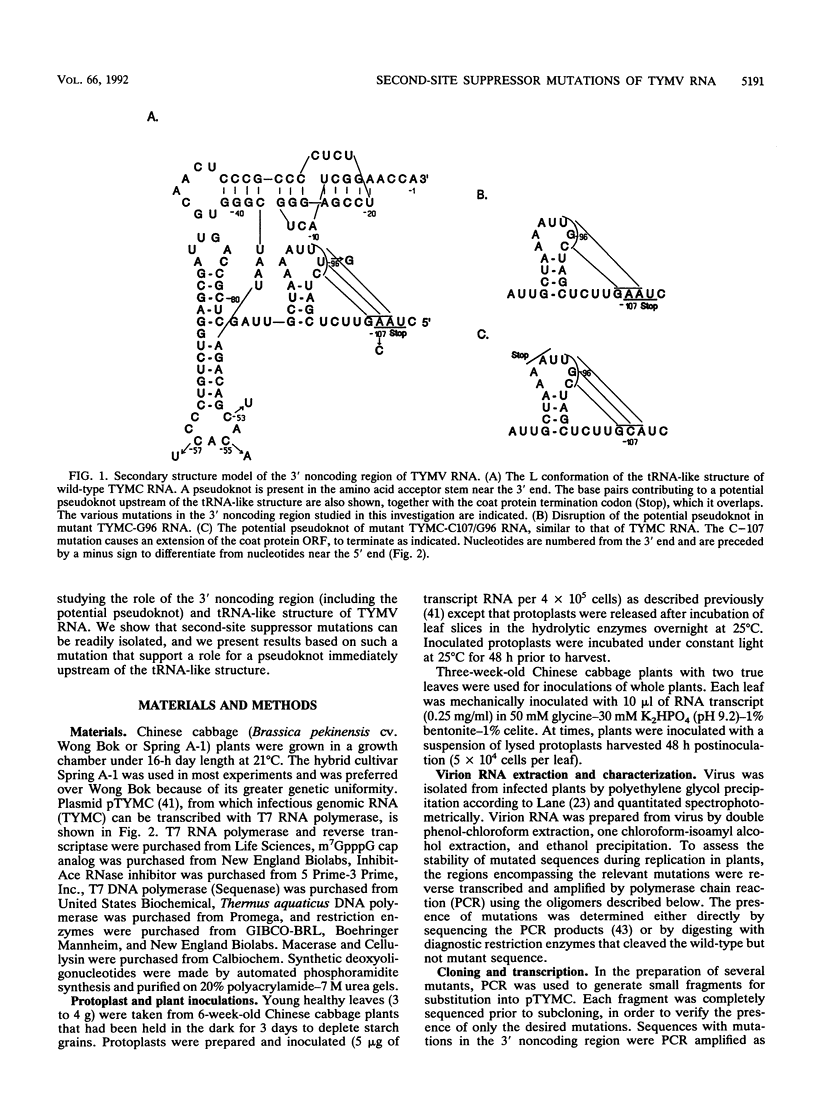
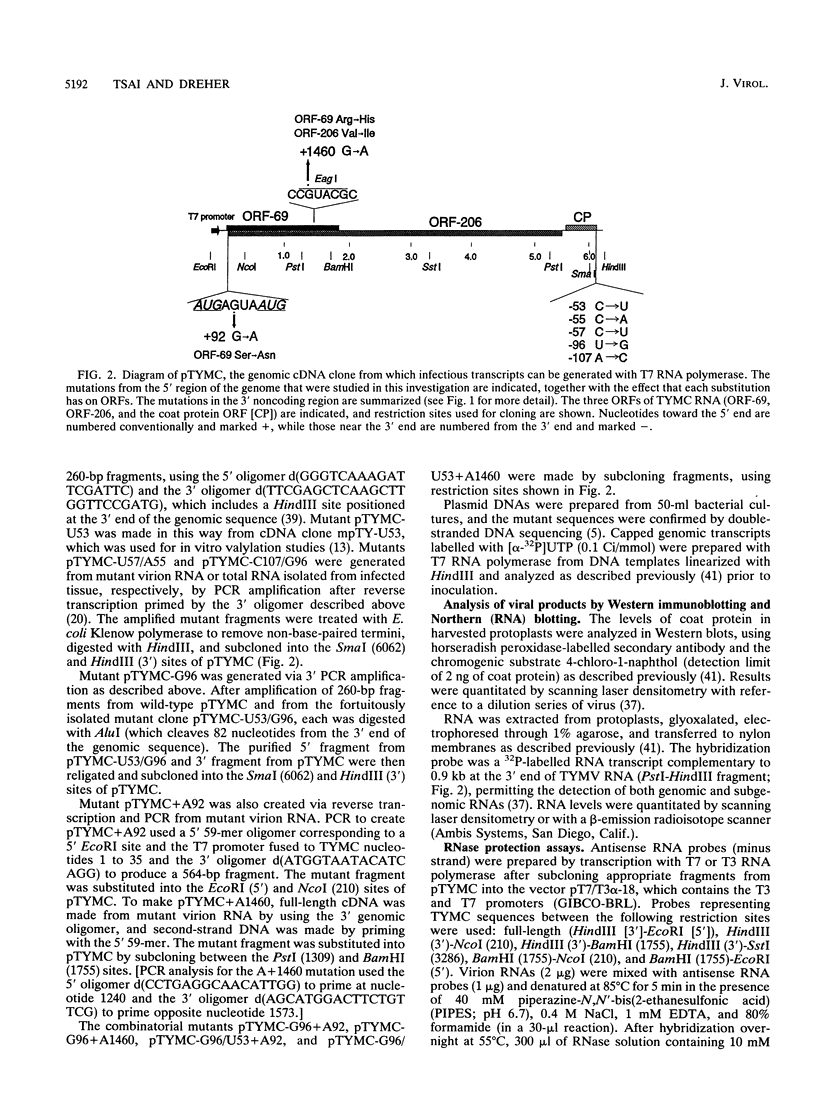
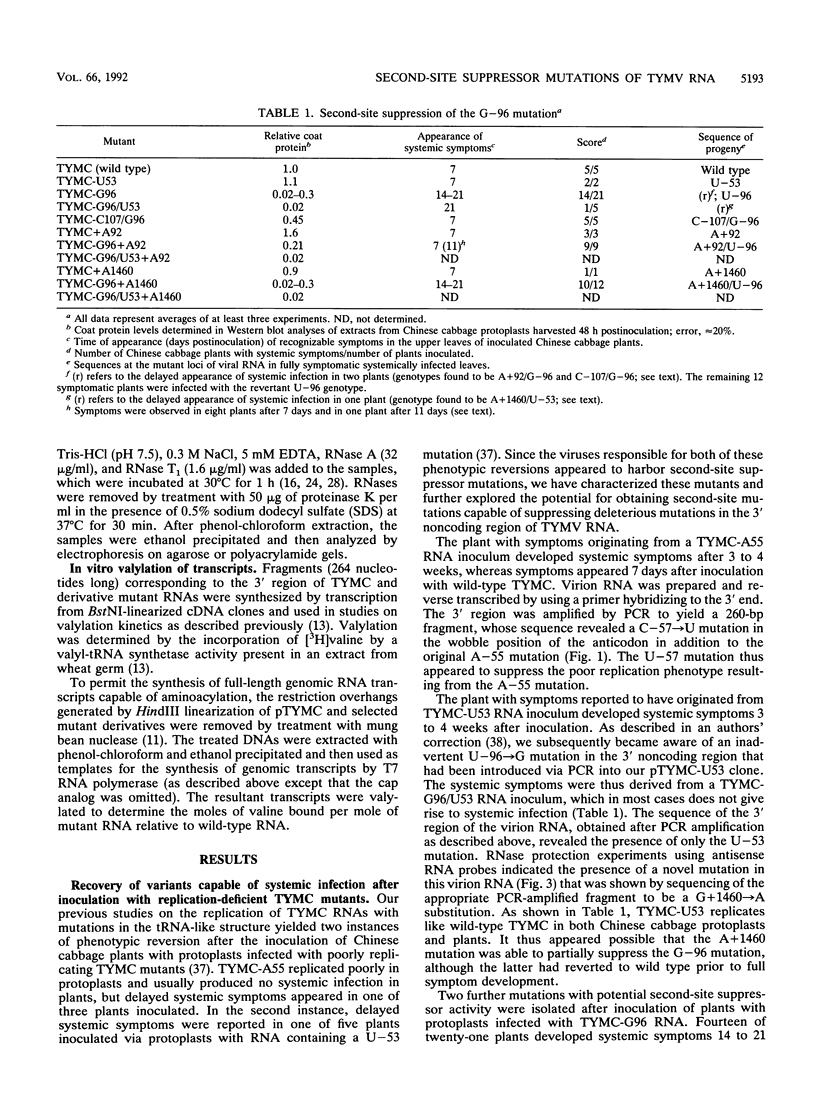
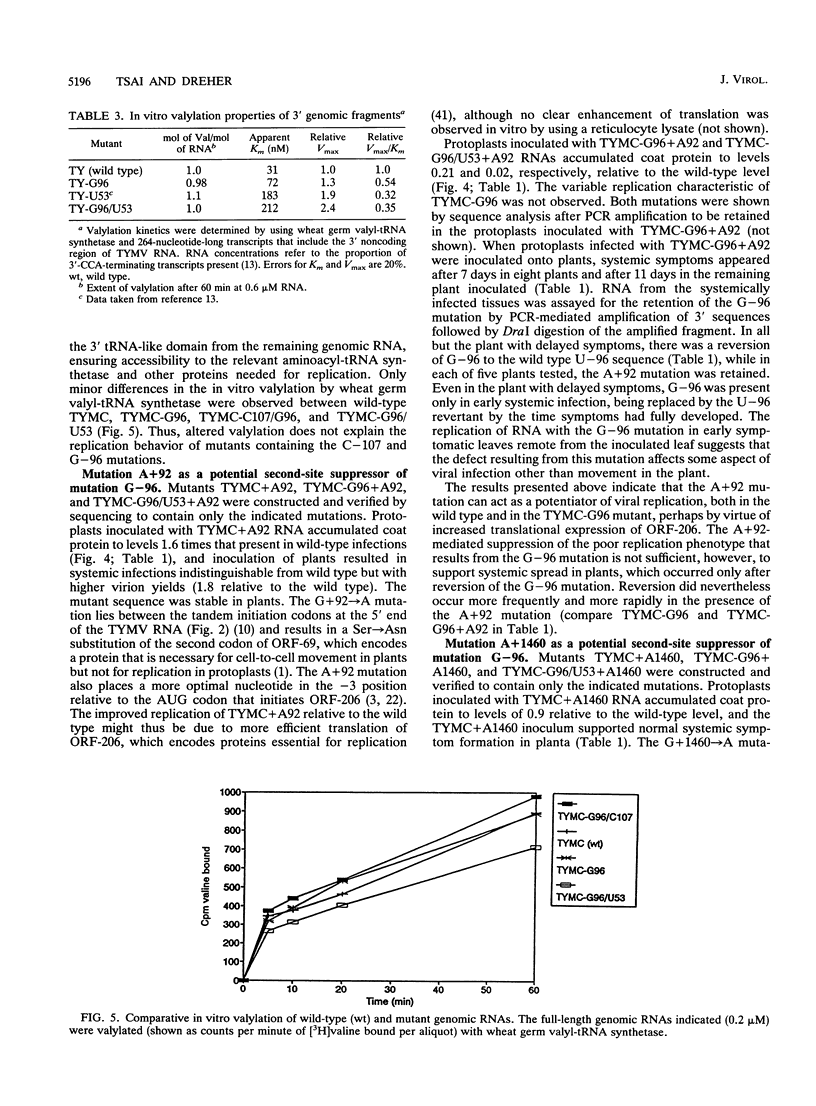
The 3' noncoding region of turnip yellow mosaic virus RNA includes an 82-nucleotide-long tRNA-like structure domain and a short upstream region that includes a potential pseudoknot overlapping the coat protein termination codon. Genomic RNAs with point mutations in the 3' noncoding region that result in poor replication in protoplasts and no systemic symptoms in planta were inoculated onto Chinese cabbage plants in an effort to obtain second-site suppressor mutations. Putative second-site suppressor mutations were identified by RNase protection and sequencing and were then introduced into genomic cDNA clones to permit their characterization. A C-57----U mutation in the tRNA-like structure was a strong suppressor of the C-55----A mutation which prevented both systemic infection and in vitro valylation of the viral RNA. Both of these phenotypes were rescued in the double mutant. An A-107----C mutation was a strong second-site suppressor of the U-96----G mutation, permitting the double mutant to establish systemic infection. The C-107 and G-96 mutations are located on opposite strands of one helix of a potential pseudoknot, and the results support a functional role for the pseudoknot structure. A mutation near the 5' end of the genome (G + 92----A), at position -3 relative to the initiation codon of the essential open reading frame 206, was found to be a general potentiator of viral replication, probably as a result of enhanced expression of open reading frame 206. The A + 92 mutation enhanced the replication of mutant TYMC-G96 in protoplasts but was not a sufficiently potent suppressor to permit systemic spread of the A + 92/G-96 double mutant in plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozarth C. S., Weiland J. J., Dreher T. W. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology. 1992 Mar;187(1):124–130. doi: 10.1016/0042-6822(92)90301-5. [DOI] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S. C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989 May 19;57(4):537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Ding S. W., Keese P., Gibbs A. Nucleotide sequence of the ononis yellow mosaic tymovirus genome. Virology. 1989 Oct;172(2):555–563. doi: 10.1016/0042-6822(89)90198-0. [DOI] [PubMed] [Google Scholar]
- Ding S., Keese P., Gibbs A. The nucleotide sequence of the genomic RNA of kennedya yellow mosaic tymovirus-Jervis Bay isolate: relationships with potex- and carlaviruses. J Gen Virol. 1990 Apr;71(Pt 4):925–931. doi: 10.1099/0022-1317-71-4-925. [DOI] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher T. W., Bransom K. L. Genomic RNA sequence of turnip yellow mosaic virus isolate TYMC, a cDNA-based clone with verified infectivity. Plant Mol Biol. 1992 Jan;18(2):403–406. doi: 10.1007/BF00034967. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Florentz C., Giege R. Valylation of tRNA-like transcripts from cloned cDNA of turnip yellow mosaic virus RNA demonstrate that the L-shaped region at the 3' end of the viral RNA is not sufficient for optimal aminoacylation. Biochimie. 1988 Dec;70(12):1719–1727. doi: 10.1016/0300-9084(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Florentz C., Briand J. P., Romby P., Hirth L., Ebel J. P., Glegé R. The tRNA-like structure of turnip yellow mosaic virus RNA: structural organization of the last 159 nucleotides from the 3' OH terminus. EMBO J. 1982;1(2):269–276. doi: 10.1002/j.1460-2075.1982.tb01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arenal F. Sequence and structure at the genome 3' end of the U2-strain of tobacco mosaic virus, a histidine-accepting tobamovirus. Virology. 1988 Nov;167(1):201–206. doi: 10.1016/0042-6822(88)90070-0. [DOI] [PubMed] [Google Scholar]
- Grange D. K., Gottesman G. S., Lewis M. B., Marini J. C. Detection of point mutations in type I collagen by RNase digestion of RNA/RNA hybrids. Nucleic Acids Res. 1990 Jul 25;18(14):4227–4236. doi: 10.1093/nar/18.14.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. L., Faulhammer H., Chapeville F., Sprinzl M., Haenni A. L. Aminoacyl RNA domain of turnip yellow mosaic virus Val-RNA interacting with elongation factor Tu. Nucleic Acids Res. 1984 Oct 11;12(19):7467–7478. doi: 10.1093/nar/12.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. L., Ravel J. M., Haenni A. L. Interaction of turnip yellow mosaic virus Val-RNA with eukaryotic elongation factor EF-1 [alpha]. Search for a function. EMBO J. 1986 Jun;5(6):1143–1148. doi: 10.1002/j.1460-2075.1986.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Length requirements for tRNA-specific enzymes and cleavage specificity at the 3' end of turnip yellow mosaic virus RNA. Nucleic Acids Res. 1982 Mar 25;10(6):1947–1962. doi: 10.1093/nar/10.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese P., Mackenzie A., Gibbs A. Nucleotide sequence of the genome of an Australian isolate of turnip yellow mosaic tymovirus. Virology. 1989 Oct;172(2):536–546. doi: 10.1016/0042-6822(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Galindez C., Lopez J. A., Melero J. A., de la Fuente L., Martinez C., Ortin J., Perucho M. Analysis of genetic variability and mapping of point mutations in influenza virus by the RNase A mismatch cleavage method. Proc Natl Acad Sci U S A. 1988 May;85(10):3522–3526. doi: 10.1073/pnas.85.10.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans R. M., Pleij C. W., Bosch L. tRNA-like structures. Structure, function and evolutionary significance. Eur J Biochem. 1991 Oct 15;201(2):303–324. doi: 10.1111/j.1432-1033.1991.tb16288.x. [DOI] [PubMed] [Google Scholar]
- Mans R. M., Verlaan P. W., Pleij C. W., Bosch L. Aminoacylation of 3' terminal tRNA-like fragments of turnip yellow mosaic virus RNA: the influence of 5' nonviral sequences. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):186–192. doi: 10.1016/0167-4781(90)90164-w. [DOI] [PubMed] [Google Scholar]
- Morch M. D., Boyer J. C., Haenni A. L. Overlapping open reading frames revealed by complete nucleotide sequencing of turnip yellow mosaic virus genomic RNA. Nucleic Acids Res. 1988 Jul 11;16(13):6157–6173. doi: 10.1093/nar/16.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Osorio-Keese M. E., Keese P., Gibbs A. Nucleotide sequence of the genome of eggplant mosaic tymovirus. Virology. 1989 Oct;172(2):547–554. doi: 10.1016/0042-6822(89)90197-9. [DOI] [PubMed] [Google Scholar]
- Pleij C. W. Pseudoknots: a new motif in the RNA game. Trends Biochem Sci. 1990 Apr;15(4):143–147. doi: 10.1016/0968-0004(90)90214-v. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Steitz T. A. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature. 1991 Jul 18;352(6332):213–218. doi: 10.1038/352213a0. [DOI] [PubMed] [Google Scholar]
- Schimmel P. RNA pseudoknots that interact with components of the translation apparatus. Cell. 1989 Jul 14;58(1):9–12. doi: 10.1016/0092-8674(89)90395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu N., Watanabe Y., Meshi T., Okada Y. Mutational analysis of the pseudoknot region in the 3' noncoding region of tobacco mosaic virus RNA. J Virol. 1990 Aug;64(8):3686–3693. doi: 10.1128/jvi.64.8.3686-3693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. K., Draper D. E. Unusual mRNA pseudoknot structure is recognized by a protein translational repressor. Cell. 1989 May 19;57(4):531–536. doi: 10.1016/0092-8674(89)90123-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. H., Dreher T. W. Turnip yellow mosaic virus RNAs with anticodon loop substitutions that result in decreased valylation fail to replicate efficiently. J Virol. 1991 Jun;65(6):3060–3067. doi: 10.1128/jvi.65.6.3060-3067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. J., Dreher T. W. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acids Res. 1989 Jun 26;17(12):4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills N. M., Gesteland R. F., Atkins J. F. Evidence that a downstream pseudoknot is required for translational read-through of the Moloney murine leukemia virus gag stop codon. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6991–6995. doi: 10.1073/pnas.88.16.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Abrahams J. P., Pleij C. W., Bosch L. Five pseudoknots are present at the 204 nucleotides long 3' noncoding region of tobacco mosaic virus RNA. Nucleic Acids Res. 1985 Nov 11;13(21):7673–7686. doi: 10.1093/nar/13.21.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]