Abstract
Squamous cell cancers comprise the most common type of human epithelial cancers. One subtype, esophageal squamous cell carcinoma (ESCC), is an aggressive cancer with poor prognosis due to late diagnosis and metastasis. Factors derived from the extracellular matrix (ECM) create an environment conducive to tumor growth and invasion. Specialized cancer-associated fibroblasts (CAFs) in the ECM influence tumorigenesis. We have shown previously that the nature and activation state of fibroblasts are critical in modulating the invasive ability of ESCC in an in vivo-like organotypic 3D cell culture, a form of human tissue engineering. Dramatic differences in invasion of transformed esophageal epithelial cells depended on the type of fibroblast in the matrix. We hypothesize that CAFs create an environment primed for growth and invasion through the secretion of factors. We find that fibroblast secretion of hepatocyte growth factor (HGF) fosters the ability of transformed esophageal epithelial cells to invade into the ECM, although other unidentified factors may cooperate with HGF. Genetic modifications of both HGF in fibroblasts and its receptor Met in epithelial cells, along with pharmacologic inhibition of HGF and Met, underscore the importance of this pathway in ESCC invasion and progression. Furthermore, Met activation is increased upon combinatorial overexpression of epidermal growth factor receptor (EGFR) and p53R175H, two common genetic mutations in ESCC. These results highlight the potential benefit of the therapeutic targeting of HGF/Met signaling in ESCC and potentially other squamous cancers where this pathway is deregulated.
Keywords: cancer-associated fibroblasts, esophageal cancer, tumor microenvironment, organotypic culture, c-met
Esophageal cancer comprises two subtypes: esophageal squamous cell carcinoma (ESCC) and adenocarcinoma. ESCC is one of the most aggressive forms of squamous cell cancer compared with types arising in other tissues including skin, head/neck, lung, and anogenital tract with risk factors for ESCC, including cigarette smoking, alcoholism, and certain mineral/vitamin deficiencies (1, 2). The reasons for the disparity in outcomes of squamous cancers in different tissues remain unclear. One premise is that differences in the tumor microenvironment, especially mesenchymal stromal fibroblasts, may exist within the various types of squamous cell cancers. Patients with ESCC often present at advanced stage and overall prognosis remains dismal (2, 3). The development of ESCC is a multistep, progressive process, and a number of canonical genetic alterations in the tumor cells have been identified (4, 5). Factors produced in the stroma assist in promoting disease progression by creating an environment conducive to tumor growth and invasion. Using a model of ESCC that recapitulates two common genetic alternations in ESCC—namely, EGFR overexpression and p53 mutation—we demonstrated previously that stromal fibroblasts are critical in regulating the invasive ability of ESCC in an organotypic 3D cell culture system (5). Fetal esophageal fibroblasts were found to be best suited for promoting tumor invasion when compared with adult esophageal fibroblasts or fetal skin fibroblasts (5). However, the effects imparted by esophageal cancer-associated fibroblasts (CAFs) and the molecular mechanism(s) by which CAFs promote ESCC invasion remain to be elucidated. These aspects are clearly critical for the development of targeted combinatorial therapeutics.
Prior studies have identified the importance of CAFs to tumor progression and collectively suggest that fibroblasts present in tumors have distinct biological features that distinguish them from their normal counterparts (6, 7). CAFs isolated from mammary and prostate tumors promoted normal epithelial cells to become tumorigenic, whereas fibroblasts from normal tissue converted malignant skin and prostate epithelial cells to morphologically benign lesions (8–12). Transgenic mice with fibroblasts deficient for transforming growth factor β (TGFβ) receptor II developed intraepithelia prostate neoplasia and invasive squamous cell carcinoma in the forestomach (8). The effects of tumor-activated fibroblasts on epithelial tumors have been shown to be mediated by the paracrine secretion of growth factors including hepatocyte growth factor (HGF), stromal derived factor-1 (SDF1), TGFβ, and others (11, 13, 14), as well as by protease- and force-mediated remodeling of the ECM initiated by stromal fibroblasts (15).
Studies investigating the role of fibroblasts in ESCC are limited (5, 16, 17). Here, we identify HGF as an important secreted factor from esophageal CAFs that acts in a paracrine manner to promote ESCC invasion. HGF (also known as scatter factor) was identified originally because of its ability to induce cell scattering and act as a mitogen of hepatocytes (18, 19). It is expressed ubiquitously and known to impact migration, proliferation, scattering, survival, and branching tubulogenesis. HGF is produced by mesenchymal cells including fibroblasts and binds to the receptor tyrosine kinase Met that is expressed on epithelial and endothelial cells, thereby providing a platform of cross-talk between the epithelial and stromal compartments (20). HGF/Met signaling activates a complex program of responses that has been described collectively as invasive growth necessary both during normal embryonic development and adult tissue repair (21, 22). HGF/Met signaling is well established to be important for cancer pathogenesis in a wide variety of tumor types largely through Met overexpression, although rare receptor mutations have been detected (23). Pharmacologic agents targeting this pathway are under clinical development with some promising preliminary results (21, 23, 24).
In ESCC, pretherapy serum HGF levels were found to be significantly elevated in patients when compared with control samples. Higher levels of serum HGF correlated with advanced tumor stage and survival (25, 26). HGF was identified as a secreted factor from ESCC-associated fibroblasts and reported to influence cell invasion and migration via activation of VEGF and IL8 expression (16, 25). We find that HGF is secreted exclusively from fibroblasts that promote matrix invasion of transformed esophageal epithelial cells in organotypic culture, and that HGF/Met signaling is activated in human ESCC samples and ESCC cell lines, and upon EGFR and p53R175H overexpression in our genetically defined model system. Novel genetic and pharmacologic inhibition of HGF/Met attenuates invasion in a dramatic fashion, indicating that this pathway is essential for tumor invasion; therefore, it may represent a novel approach to ESCC therapy targeting in the early steps of metastasis.
Results
Esophageal CAFs and Fetal Esophageal Fibroblasts Promote Matrix Invasion of Transformed Epithelial Cells.
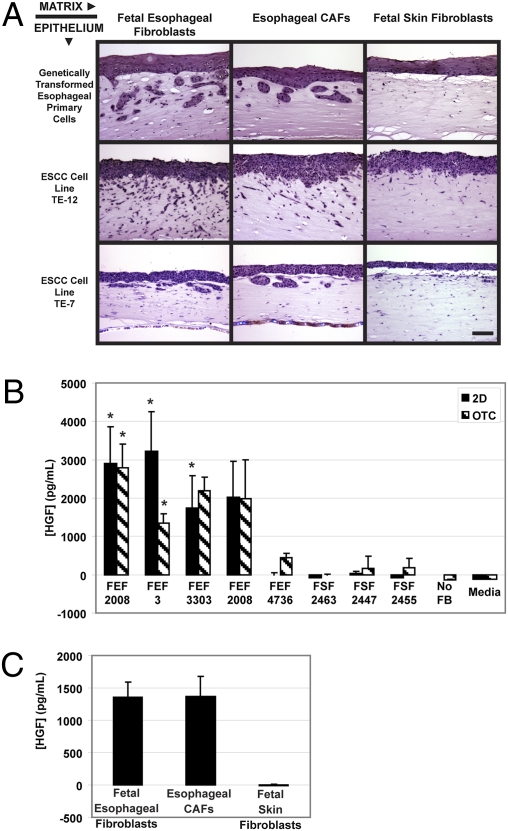
Using a panel of fetal esophageal fibroblasts and fetal skin fibroblasts seeded into the matrix of organotypic culture, we determined that the most aggressive invasion was promoted by fetal esophageal fibroblasts in the matrix because four of the five fetal esophageal fibroblast lines tested supported invasion (Fig. S1). This raised the possibility that fetal esophageal fibroblasts are primed or activated to support invasion and suggests that these closely resemble CAFs in vivo. To test this premise, a CAF line derived from a primary ESCC human tumor was obtained and tested for its ability to promote invasion of transformed EPC-hTERT-EGFR-p53R175H cells. Invasion of the epithelium into the ECM was observed when either the esophageal CAFs or fetal esophageal fibroblasts were seeded into the matrix, but not when fetal skin fibroblasts were used (Fig. 1A). We determined whether this fibroblast effect was limited only to our genetically transformed esophageal epithelial cells (EPC-hTERT-EGFR-p53R175H) by testing two established ESCC cell lines (TE7 and TE12) in organotypic culture seeded on top of matrices containing the respective fibroblast types. We observed the same pattern in both TE7 and TE12 cells with preferential invasion into matrices containing either esophageal CAFs or fetal esophageal fibroblasts when compared with fetal skin fibroblasts (Fig. 1A). Thus, the invasion-promoting effects of the fetal esophageal and esophageal CAFs are not limited to our genetically defined model system, but also hold true in human ESCC lines.
Fig. 1.
HGF is secreted from tumor esophageal and fetal esophageal fibroblasts that promote invasion of ESCC cells. (A) H&E-stained sections of organotypic culture where EPC-hTERT-EGFR-p53R175H (genetically transformed esophageal primary cells), TE12, or TE7 cells were seeded above matrices containing fetal esophageal fibroblasts (FEF3), fetal skin fibroblasts (FSF2463), or esophageal CAFs (TEF1947F1). (B) Level of human HGF detected in conditioned media collected from the indicated fibroblast sample set up either alone in 2D culture or in organotypic culture as measured by ELISA. (C) Level of HGF detected in conditioned media from the organotypic cultures containing fetal esophageal fibroblasts (FEF3), fetal skin fibroblasts (FSF2463), or esophageal CAFs (TEF1947F1). Error bars represent ± SEM. *, P ≤ 0.05 when compared with media (B; 2D), no fibroblasts (B; OTC), or fetal skin fibroblasts (C). (Scale bar: 100 μm.)
HGF Is Secreted Only by Fibroblasts Capable of Promoting Epithelial Cell Matrix Invasion.
To identify factors that are secreted differentially by the fibroblasts that promoted invasion, we collected conditioned media from the fibroblasts cultured in 2D culture and in organotypic culture. We chose to screen for two important growth factors in the tumor microenvironment—namely, HGF and SDF-1—previously identified in other tissues as fibroblast-secreted factors that promote tumor progression (8, 14). Interestingly, we observed that HGF was secreted only by the four fetal esophageal fibroblasts that promoted invasion in organotypic culture, but not by fetal skin fibroblasts or the one fetal esophageal fibroblast line (FEF4736) that did not promote invasion (Fig. 1B and Fig. S1). HGF was not detected in conditioned media from organotypic cultures established without fibroblasts, but could be detected in matrices established without epithelia, suggesting that the measured HGF is fibroblast-derived (Fig. 1B). The pattern of SDF-1 secretion did not mirror the invasive pattern, but was instead secreted preferentially by the fibroblasts that did not foster invasion (Fig. S2). We next measured HGF secretion from the esophageal CAF line and found HGF levels in conditioned media of esophageal CAFs to be comparable to the levels observed from fetal esophageal fibroblasts (Fig. 1C). Collectively, these results suggest that fibroblast-derived HGF, but not SDF-1, can promote esophageal tumor invasion, and prompted us to focus on HGF as well as the HGF-Met axis.
Met Is Overexpressed and Activated in ESCC and upon EGFR and Mutant p53R175H Overexpression.
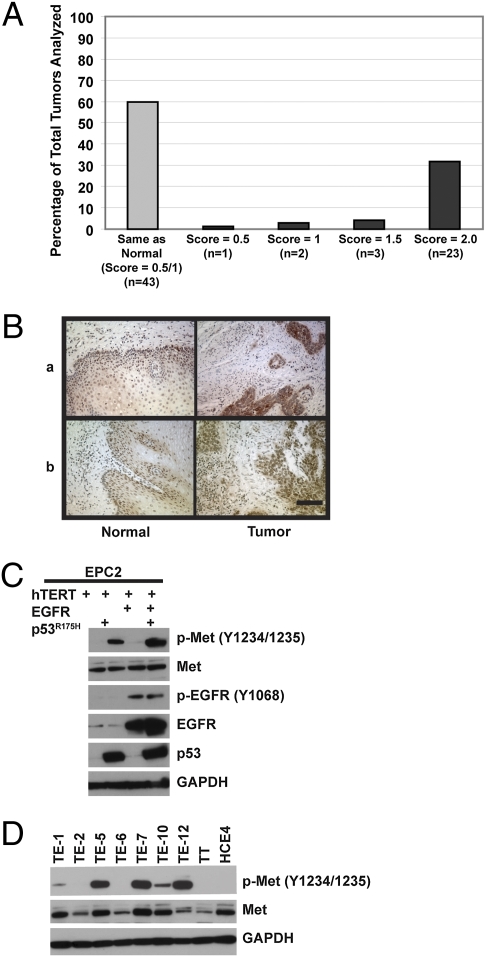
Forty percent of tumors showed increased Met levels in the tumor when compared with the paired normal tissue (Fig. 2 A and B) in a human ESCC tumor microarray. Previously, we have reported an increased invasive phenotype in esophageal primary cells transformed by overexpression of EGFR and mutant p53 (5) and now show that these cells preferentially invade in HGF-containing matrices (Fig. 1). We next interrogated whether there was a change in the level of Met expression in EPC-hTERT cells upon transformation with EGFR and p53R175H as Met overexpression is a hallmark of aggressive tumors. Interestingly, although we did not observe a difference in Met protein levels upon overexpression of EGFR and/or p53R175H in EPC-hTERT cells, an increase in Met phosphorylated at tyrosine residues 1234/1235 was evident upon expression of p53R175H and further enhanced by increased EGFR (Fig. 2C and Fig. S3). This suggests that the combination of mutant p53R175H and EGFR overexpression leads to increased Met activation and may result in increased dependence on HGF/Met signaling. Additionally, we observed an increase in both Met protein and its activity (phosphorylation) in a number of ESCC cell lines, including both TE-12 and TE-7 cells (Figs. 1A and 2D), which we identify as able to invade into HGF-rich matrices.
Fig. 2.
HGF receptor Met expression is elevated in human ESCC tumors and activated upon EGFR and p53R175H overexpression. (A) Met IHC staining results of 72 ESCC human tumor microarray samples. (B) Representative images of two ESCC human tumor samples with matched normal mucosa stained for Met. (Scale bar: 100 μm.) (C and D) Levels and phosphorylation status of the indicated proteins measured by Western blotting in whole-cell lysates of EPC-hTERT, EPC-hTERT-EGFR-puro, EPC-hTERT-p53R175H-neo, and EPC-hTERT-EGFR-p53R175H cells (C) or a panel of ESCC cell lines (D).
Fibroblast-Derived HGF Is Necessary for Transformed Esophageal Epithelial Cell Matrix Invasion.
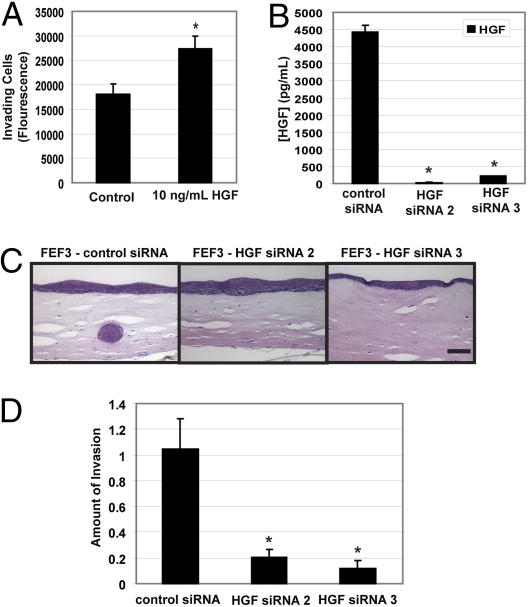
We next tested the direct effect of HGF as a chemoattractant in a Boyden chamber invasion assay. We observed significantly increased levels of invading EPC-hTERT-EGFR-p53R175H cells when stimulated by HGF (Fig. 3A). To determine whether HGF is necessary for invasion of EPC-hTERT-EGFR-p53R175H cells in organotypic culture, the expression level of HGF was engineered to be reduced in the fibroblasts by means of siRNA targeting HGF. Fetal esophageal fibroblasts (FEF3) with reduced HGF levels were generated after transfection with two independent siRNAs targeting HGF. HGF knockdown was monitored by ELISA of the respective fibroblast-conditioned media, and secreted levels were reduced >95% with either siRNA construct when compared with levels observed using the scrambled siRNA control fibroblasts (Fig. 3B). These HGF knockdown fibroblasts were embedded in the matrices of organotypic culture (24 h after knockdown), and the effect of HGF level on the ability of EPC-hTERT-EGFR-p53R175H to invade was evaluated. As demonstrated in Fig. 3 C and D, invasion was almost completely blocked upon HGF siRNA transfection in fetal esophageal fibroblasts with an 80% and 88% reduction (siRNA 2 and siRNA 3, respectively) in invading cells (EPC-hTERT-EGFR-p53R175H).
Fig. 3.
HGF promotes invasion and HGF knockdown in fetal esophageal fibroblasts blocks invasion of transformed esophageal epithelial cells. (A) Fluorescence measurements representing invading cells on the bottom filters of a Matrigel-coated Boyden chamber where HGF was added as the chemoattractant of EPC-hTERT-EGFR-p53R175H cells seeded atop the filter. (B) Level of secreted HGF in conditioned media from FEF3 fibroblasts ± HGF siRNA set up in organotypic culture and detected by ELISA. (C) H&E-stained sections of organotypic cultures of FEF3 fibroblasts ± HGF siRNA seeded into the matrix with EPC-hTERT-EGFR-p53R175H grown above. (Scale bar: 100 μm.) (D) Quantification of invasion in entire organotypic culture imaged in C. Error bars represent ± SEM. *, P ≤ 0.05 when compared with the control.
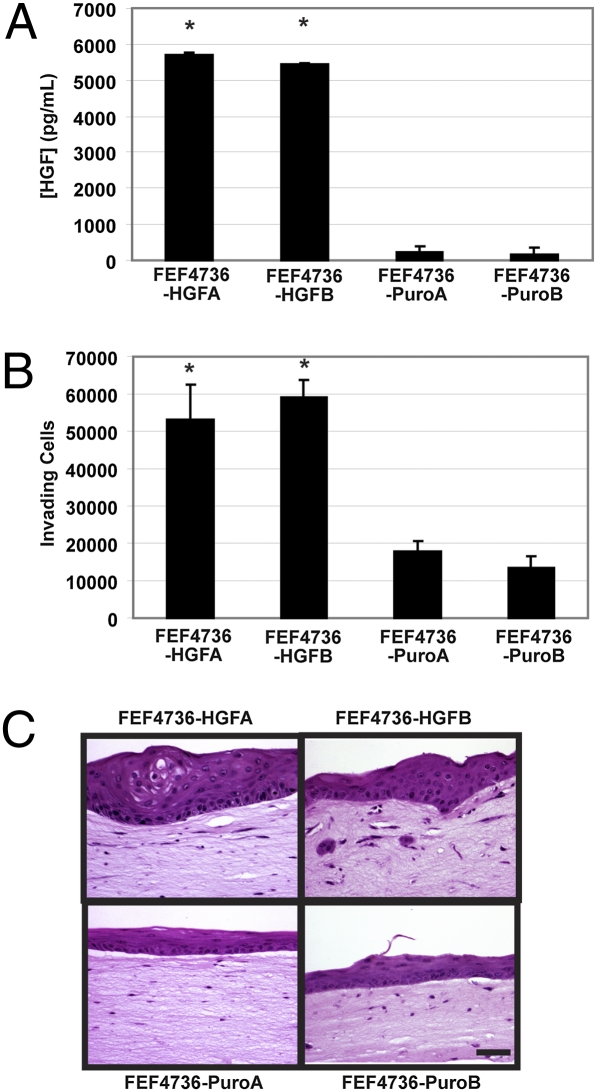
As shown in Fig. S1, one fetal esophageal fibroblast line (FEF4736) did not secrete HGF or support invasion of ESCC cells. We used these fibroblasts to generate two independent cell lines that stably overexpressed HGF and compared them to two empty vector-expressing cell lines (Fig. 4A). Conditioned media collected from FEF4736-HGF dramatically enhanced invasion of EPC-hTERT-EGFR-p53R175H cells compared with FEF4736-puro-conditioned media in a Boyden chamber invasion assay (Fig. 4B). These fibroblasts were seeded in organotypic culture matrices and tested for their ability to promote invasion of EPC-hTERT-EGFR-p53R175H cells. HGF overexpression in FEF4736 matrix fibroblasts did not result in conversion to the invasive phenotype observed with the four other HGF-secreting fetal esophageal fibroblasts, highlighting the apparent diversity of fibroblasts obtained from the same tissue. However, increased keratin pearl formation (consistent with carcinoma in situ) and dysplasia (Fig. 4C) in the epithelium were apparent in the cells seeded atop matrices containing FEF4736-HGF. Small regions of invading areas were also evident (Fig. 4C). In combination with the knockdown studies, these results suggest that HGF is necessary for ESCC invasion.
Fig. 4.
Overexpression of fibroblast-secreted HGF promotes transformed esophageal epithelial cell proliferation and invasion. (A) Level of secreted HGF in conditioned media from FEF4736 fibroblasts ± HGF overexpression set up in organotypic culture and detected by ELISA. (B) Conditioned media from FEF4736 fetal esophageal fibroblasts ± HGF or empty vector (puro) promotes invasion of transformed esophageal epithelial cells. Fluorescence measurements representing invading cells on the bottom filters of Matrigel-coated Boyden chambers with the indicated fibroblast derived conditioned media sample used as the chemoattractant of EPC-hTERT-EGFR-p53R175H cells seeded atop the filter. (C) H&E-stained sections of organotypic cultures of FEF4736 fibroblasts ± HGF seeded into the matrix with EPC-hTERT-EGFR-p53R175H grown above. (Scale bar: 100 μm.) Error bars represent ± SEM. *, P ≤ 0.05 compared with either empty vector cell line.
Constitutive Activation of Met Enhances Invasion Dramatically.
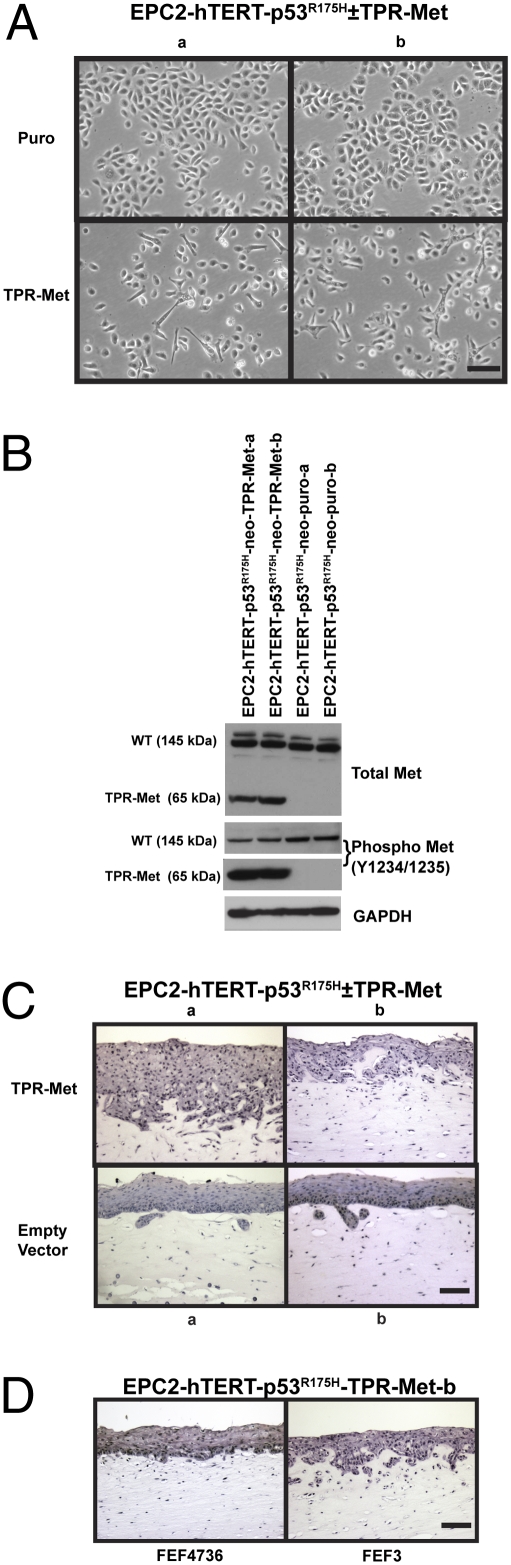
Met was identified originally as part of the oncogene TPR-Met that is the product of a chromosomal translocation induced in carcinogen-treated human osteosarcoma cells that fuses translocated promoter region (TPR) to the kinase domain and carboxyl terminus of MET. TPR encodes a dimerization leucine zipper motif that when fused to the cytosolic region of MET results in a ligand-independent, constitutively active dimerized Met protein (20). TPR-Met has been detected in rare cases of human gastric cancer and has been used widely to study the effects of Met constitutive activation (27). We tested the effect of expressing TPR-Met in the noninvasive EPC-hTERT-p53R175H cells (5) as another approach to investigate tumor cell invasion in 3D organotypic culture. Two independent lines of stable EPC-hTERT-p53R175H-TPR-Met cells were generated along with empty vector control cells (EPC-hTERT-p53R175H-puro) for comparison. Stable cell lines could not be generated without the coexpression of mutant p53 due to the oncogenic strength of TPR-Met and subsequent induction of both apoptosis and senescence. In 2D culture, TPR-Met overexpression resulted in a phenotypic change in the cells with decreased cell–cell contact and increased mesenchymal features such as elongated cellular morphologies (Fig. 5A). Expression of TPR-Met was confirmed by Western blotting and shown to be constitutively active (Fig. 5B). Using the EPC-hTERT cells expressing p53R175H and TPR-Met, we tested whether constitutive active Met could promote invasion in conjunction with p53R175H similar to the EGFR/p53R175H combination (5). As shown in Fig. 5C, matrix invasion was dramatically increased upon expression of TPR-Met when compared with the empty vector control. The epithelium formed by control cells (empty vector) showed a few well differentiated and organized epithelial down growths into the matrix, whereas cells expressing TPR-Met showed a diffuse pattern of invasion characterized by the presence of dysplastic cells penetrating the subepithelial matrix on a broad front and in a haphazard orientation. This pattern of invasion was aggressive and reminiscent of undifferentiated transformed ESCC tumor cells. Interestingly, EPC-hTERT-p53R175H-TPR-Met cells did not invade into matrices containing the low HGF-expressing FEF4736 fetal esophageal fibroblasts (Fig. 5D).
Fig. 5.
Expression of constitutively active Met enhances invasion of transformed esophageal epithelial cells. (A) Phase contrast images of EPC-hTERT-p53R175H-TPR-Met or EPC-hTERT-p53R175H-puro grown in 2D on tissue culture plastic. Images a and b represent independently generated cell lines with the same genotype. (Scale bar: 100 μm.) (B) Western blot of EPC-hTERT-p53R175H-TPR-Met or EPC-hTERT-p53R175H-puro whole-cell lysates for detection of wild-type Met (145 kDa), TPR-Met (65 kDa), and phosphorylation status. (C and D) H&E-stained sections of organotypic culture of EPC-hTERT-p53R175H-TPR-Met or EPC-hTERT-p53R175H-puro cells seeded above matrices containing FEF3 (C and D) or FEF4736 (D) fetal esophageal fibroblasts. (Scale bar: 100 μm.)
Pharmacologic or Genetic Inhibition of Met Blocks Invasion.
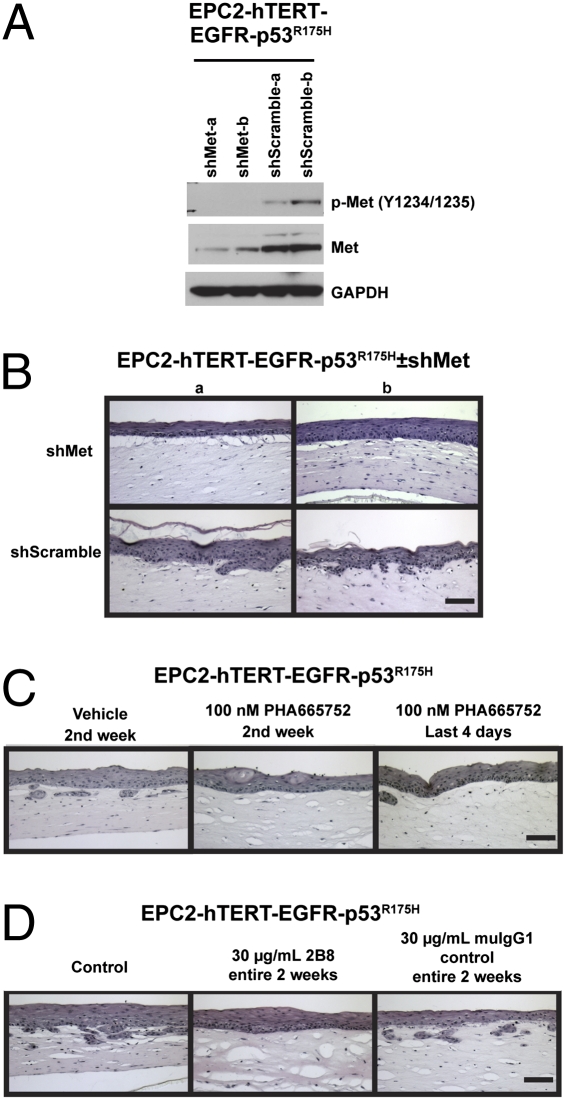
To test the functional importance of Met directly, experiments using shRNA targeting Met were conducted. Stable EPC-hTERT-EGFR-p53R175H-shMet cells were generated with decreased levels of Met protein and activation (Fig. 6A). Two independent lines with Met knockdown were assessed in organotypic culture and compared with lines expressing the shScramble control. As shown in Fig. 6B, although the overall invasion was reduced in these experiments, it is clearly revealed that knockdown of Met decreased the amount of invasion by the EPC-hTERT-EGFR-p53R175H cells ± shMet.
Fig. 6.
Inhibition of HGF/Met pharmacologically or by genetic knockdown in transformed esophageal epithelial cells reduces invasion. (A) Western blot of EPC-hTERT-EGFR-p53R175H-shMet or EPC-hTERT-EGFR-p53R175H-shScramble cells (two independent lines of each genotype) to determine extent of Met knockdown of total protein and activation state. (B) H&E-stained sections of organotypic culture of EPC-hTERT-EGFR-p53R175H-shMet or EPC-hTERT-EGFR-p53R175H-shScramble cells seeded above matrices containing FEF3 fetal esophageal fibroblasts. (C) H&E-stained sections of organotypic cultures of EPC-hTERT-EGFR-p53R175H cells seeded above FEF3 matrices treated with DMSO vehicle (days 7–15) or 100 nM PHA665752 (days 7–15 or day 11–15) as indicated. (D) H&E-stained sections of organotypic cultures of EPC-hTERT-EGFR-p53R175H cells seeded above FEF3 matrices treated with 30 μg/mL 2B8 or muIgG1 control antibody (days 0–15) as indicated. (Scale bars: 100 μm.)
To test whether invasion can be affected by therapeutically targeting HGF/Met, two independent means of pharmacologic inhibition were pursued. A Met receptor tyrosine kinase inhibitor, PHA665752, inhibited invasion of EPC-hTERT-EGFR-p53R175H cells when treated for 7 days following epithelial cell seeding or for the final 4 days when invasion occurs (Fig. 6C) (5). In parallel, 2B8, a mouse anti-human HGF antibody (IgG1) generated from a hybridoma, was tested in organotypic culture. Treatment with 2B8 blocked matrix invasion of EPC-hTERT-EGFR-p53R175H cells when added to the organotypic culture for the entire 2-week protocol, but not if added only for the final 4 days when invasion occurs, whereas the control mouse IgG1 antibody had no effect on invasion (Fig. 6D). The timing difference of the two therapeutics is likely due to the technical aspects of the organotypic culture protocol. HGF acts as a trigger to catalyze cell invasion via its effector receptor Met. Because HGF is secreted by the matrix-embedded fibroblasts that reside within these cultures throughout the 2-week protocol, 2B8 treatment was necessary for the entire time to penetrate the ECM protein-rich matrix and constitutively inhibit HGF, whereas treatment with the small molecule PHA665752, which inhibits the epithelial receptor, was only necessary in week 2 when the Met-expressing epithelial cells were seeded on top of the fibroblast-embedded matrices.
Discussion
We demonstrated that HGF secretion from both esophageal CAFs and fetal esophageal fibroblasts promotes invasion of EPC-hTERT-EGFR-p53R175H cells and ESCC cells into the ECM. Using genetic and pharmacologic methods to inhibit HGF and its receptor Met, we have established the functional importance of this signaling pathway to ESCC invasion. Collectively, our results highlight the probable dependency of ESCC tumor invasion on stromal HGF and that HGF/Met signaling is potentiated by EGFR and mutant p53R175H. These findings suggest that ESCC and potentially other squamous cancers are excellent candidates for HGF/Met therapeutic studies. This is of importance because the 5-year survival rate for patients with esophageal cancer is <20%, and the extent of tumor invasion and metastasis at the time of initial diagnosis influences dramatically the survival and treatment options (28, 29). Thus, targeting tumor invasion by HGF/Met inhibition may be meritorious.
Fibroblasts are associated with tumors at all stages of cancer development and are key determinants in tumor progression (7). CAFs have been studied in a number of different tumor types and found to have diverse functions that affect tumor cells (6, 7). These include ECM protein deposition and remodeling, activation of angiogenesis, regulation of epithelial cell proliferation, differentiation, and invasion into the ECM mediated by the secretion of cytokines and matrix proteases (6, 7). Evidence of similarities between CAFs and fetal fibroblasts has been reported, and both types of fibroblasts are thought to express and secrete similar proteins (30, 31). Our findings that fetal skin fibroblasts do not secrete HGF highlight the tissue-specific heterogeneity that exists between stromal fibroblasts (32).
HGF is expressed ubiquitously and plays an important role in the tumor stromal microenvironment (21), apart from its functions in organogenesis, regeneration, and wound healing. Secreted by mesenchymal cells, HGF acts on epithelial and endothelial cells. TGF-β1 is a known suppressor of HGF. Increased HGF expression from Tgfbr2FSPKO fibroblasts was identified as one mechanism for promoting carcinoma in the mouse forestomach and mammary gland (8, 13, 33). Genetic modification of human mammary fibroblasts to express HGF before xenograft implantation with clinically normal mammary epithelial cells resulted in outgrowth of malignant lesions (11, 25, 26, 34). The HGF/Met signaling pathway has been found to be activated in a number of human tumor types, including ESCC where elevated HGF serum levels correlate with disease severity (23). Our HGF knockdown studies reveal that the stromal produced HGF is an important component of transformed esophageal epithelial cell invasion. However, this does not preclude the likely involvement of other growth factors and/or cytokines. For example, overexpression of HGF in the FEF4736 esophageal fibroblast line (the single esophageal fibroblast line that was not permissive for invasion) did not result in tumor cell invasion in organotypic culture, and EPC-hTERT- p53R175H-TPR-Met cells could not invade into FEF4736 containing matrices. Another compelling finding is the enhanced Met activation that is obtained upon EGFR overexpression and p53 mutation in transformed esophageal cells. Moreover, Met phosphorylation is increased in genetically engineered cells expressing p53R175H. This phosphorylation is augmented further by EGFR coexpression and is activated in multiple ESCC cell lines derived from tumors. Evidence exists in the literature to support cross-talk between EGFR and Met (23). Most intriguingly, up-regulation of Met signaling has been identified as a mechanism of resistance to EGFR tyrosine kinase inhibition where treatment with a Met inhibitor could rescue the resistance phenotype (23, 24). The mechanism for increased Met phosphorylation upon p53R175H and EGFR expression in esophageal cells is currently unknown. Autocrine HGF production was not detected in EPC-hTERT-EGFR- p53R175H cells, nor was Met phosphorylation inhibited upon treatment with the AG1478 EGFR kinase inhibitor (data not shown).
Agents to target the HGF/Met signaling pathway are in preclinical and clinical development (21). We present data that invasion can be blocked by a Met tyrosine kinase inhibitor (PHA665752), which has a closely related compound that is in clinical development for human treatment (21), and by an antibody directed at HGF (2B8), whose humanized version, SCH900105, is in clinical development. Our data argue for clinical trials using HGF/Met inhibition in combination with standard chemotherapy or other receptor tyrosine kinase-directed inhibitors (e.g., EGFR inhibition) as in ESCC and potentially other similar squamous cell cancers.
Methods
See SI Methods for further information.
Organotypic Culture.
The employment of EPC cells in organotypic culture was done as described in refs. 5 and 35. The cultures were harvested and fixed for 1 h or overnight in 10% buffered formalin phosphate (Fisher) before being paraffin-embedded and sectioned (Leica RM2155 microtome; Leica Microsystems) for hematoxylin and eosin (H&E) staining. The amount of invasion was determined by taking three ×40 images for each sample spanning the entire culture. Photoshop (Adobe) was used to cut out the invading regions and create a composite image of invasion from all three images that was quantified using Scion Image software (Scion) and expressed as a fold change normalized to control.
Conditioned Medium and ELISA.
Conditioned media was collected from fibroblasts cultured in 2D tissue culture dishes for 48 h (invasion) or 72 h (ELISA), or from 3D organotypic cultures on harvest day 15 (48 h). For invasion assay conditioned media, 8 × 104 fibroblasts were plated in six-well plates, and for 2D ELISA, 3 × 105 fibroblasts were plated in 10-cm dishes. The levels of human HGF and SDF1 were determined by using the DuoSet ELISA Development System (R&D Systems) specific to each cytokine according to the manufacturer's protocol.
Genetic Knockdown and Overexpression Studies.
Stable transduction of primary esophageal cells with viral vectors is described in refs. 5 and 35. Human lentiviral shRNAmir against Met (shMet clone V2HS_76544) and nonsilencing control shRNAmir retroviral vector (shScramble) were purchased from Open Biosystems. Human pBABE-TPR-Met and pBABE-HGF were purchased from Addgene. Two different Silencer Select Predesigned siRNA targeting HGF (siHGFs: siRNA ID# s6529, siHGF3: siRNA ID# s6530) were purchased from Applied Biosystems along with the Silencer Select Negative Control #1 siRNA (siControl). For HGF knockdown studies, early-passage fibroblasts were transfected with 10 nM siRNA the day before embedding in organotypic culture matrix using Lipofectamine 2000 (Invitrogen) according to the manufacturers’ recommendations. For HGF overexpression, early-passage fibroblasts were infected with pBABE-HGF or pBABE-puro. Overexpression cells were selected for using puromycin (0.5 μg/mL) for >72 h. Overexpression and knockdown of HGF secretion was monitored by ELISA. EPC-hTERT-EGFR-p53R175H cells were infected with pGIPZ-shMet or pSM2c-shScramble. Cells were selected for with puromycin (shScramble) or by flow cytometry cell sorting for GFP (shMet) on a FACSVantage SE with FACSDiva Option (BD Biosciences). Met knockdown was monitored by Western blot. EPC-hTERT-p53R175H-neo, EPC-hTERT-EGFR-zeo, and EPC-hTERT-neo-zeo cells were infected with pBABE-TPR-Met and pBABE-puro. Cells were selected for with puromycin as above and overexpression monitored by Western blot.
Supplementary Material
Acknowledgments
We thank the Morphology Core (S. Mitchell, D. Budo, and G. Swain), the Molecular Biology/Gene Expression Core (G. Wu and S. Keilbaugh), the Biostatistics Core (P. Gimotty and R. Hammond), the Cell Culture Core (R. Carroll), and members of the Rustgi laboratory for helpful discussions and E. Cukierman for excellent suggestions. This work was supported by National Institutes of Health/National Cancer Institute Grant P01-CA098101 (to K.D.G., C.Z.M., A.J.K.-S., J.A.D., M.H., H.N., and A.K.R.), National Institutes of Health/National Research Service Award Grant F32DK-082149-01 (to K.D.G.), and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Center for Molecular Studies in Digestive and Liver Diseases Grant P30-DK050306.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914295107/-/DCSupplemental.
References
- 1.Okano J, Snyder L, Rustgi AK. Genetic alterations in esophageal cancer. Methods Mol Biol. 2003;222:131–145. doi: 10.1385/1-59259-328-3:131. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK. Models of esophageal carcinogenesis. Semin Oncol. 2006;33(6, Suppl 11):S57–S58. doi: 10.1053/j.seminoncol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Koliopanos A, et al. Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg. 2002;26:420–427. doi: 10.1007/s00268-001-0242-x. [DOI] [PubMed] [Google Scholar]
- 4.Lehrbach DM, Nita ME, Cecconello I. Molecular aspects of esophageal squamous cell carcinoma carcinogenesis. Arq Gastroenterol. 2003;40:256–261. doi: 10.1590/s0004-28032003000400011. [DOI] [PubMed] [Google Scholar]
- 5.Okawa T, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmick NA, et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 9.Cooper M, Pinkus H. Intrauterine transplantation of rat basal cell carcinoma as a model for reconversion of malignant to benign growth. Cancer Res. 1977;37:2544–2552. [PubMed] [Google Scholar]
- 10.Hayashi N, Cunha GR. Mesenchyme-induced changes in the neoplastic characteristics of the Dunning prostatic adenocarcinoma. Cancer Res. 1991;51:4924–4930. [PubMed] [Google Scholar]
- 11.Kuperwasser C, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Enhanced hepatocyte growth factor signaling by type II transforming growth factor-β receptor knockout fibroblasts promotes mammary tumorigenesis. Cancer Res. 2007;67:4869–4877. doi: 10.1158/0008-5472.CAN-06-3381. [DOI] [PubMed] [Google Scholar]
- 14.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 16.Iwazawa T, et al. Primary human fibroblasts induce diverse tumor invasiveness: Involvement of HGF as an important paracrine factor. Jpn J Cancer Res. 1996;87:1134–1142. doi: 10.1111/j.1349-7006.1996.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noma K, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–1993. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 20.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–1285. doi: 10.1038/sj.onc.1210201. [DOI] [PubMed] [Google Scholar]
- 21.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 22.Comoglio PM, Trusolino L. Invasive growth: From development to metastasis. J Clin Invest. 2002;109:857–862. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen BS, Vande Woude G. Showering c-MET-dependent cancers with drugs. Curr Opin Genet Dev. 2008;18:87–96. doi: 10.1016/j.gde.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer. Eur J Cancer. 2010;46:1260–1270. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: A prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11:6190–6197. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- 26.Takada N, et al. Expression of immunoreactive human hepatocyte growth factor in human esophageal squamous cell carcinomas. Cancer Lett. 1995;97:145–148. doi: 10.1016/0304-3835(95)03967-2. [DOI] [PubMed] [Google Scholar]
- 27.Soman NR, Correa P, Ruiz BA, Wogan GN. The TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesions. Proc Natl Acad Sci USA. 1991;88:4892–4896. doi: 10.1073/pnas.88.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anonymous . American Cancer Society; 2007. Esophageal cancer. [Google Scholar]
- 29.Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58:343–351. doi: 10.1136/jcp.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schor SL, et al. Migration-stimulating factor: A genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 2003;63:8827–8836. [PubMed] [Google Scholar]
- 31.Yoshikawa Y, Ignjatovic J, Bauer H. Tissue-specific expression of onco-fetal antigens during embryogenesis. Differentiation. 1979;15:41–47. doi: 10.1111/j.1432-0436.1979.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 32.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 33.Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Transforming growth factor-β signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Mol Cancer Res. 2008;6:1521–1533. doi: 10.1158/1541-7786.MCR-07-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 35.Andl CD, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.